Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders
Abstract
1. Introduction
2. Results and Discussion
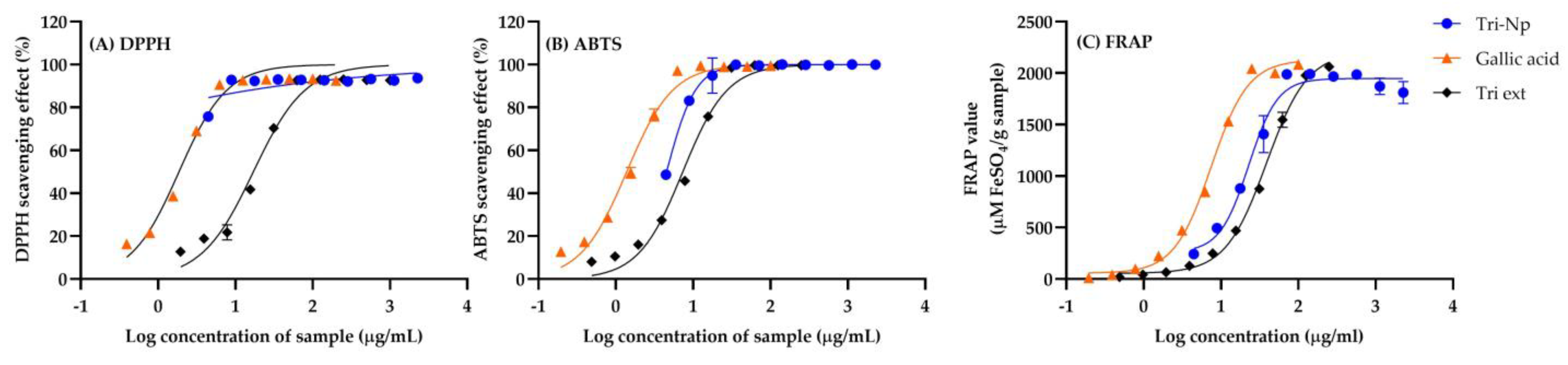
2.1. Antioxidant Activities of Triphala Extract and Nanotirphala
2.2. Antimicrobial Activities of Triphala Extract and Nanotriphala
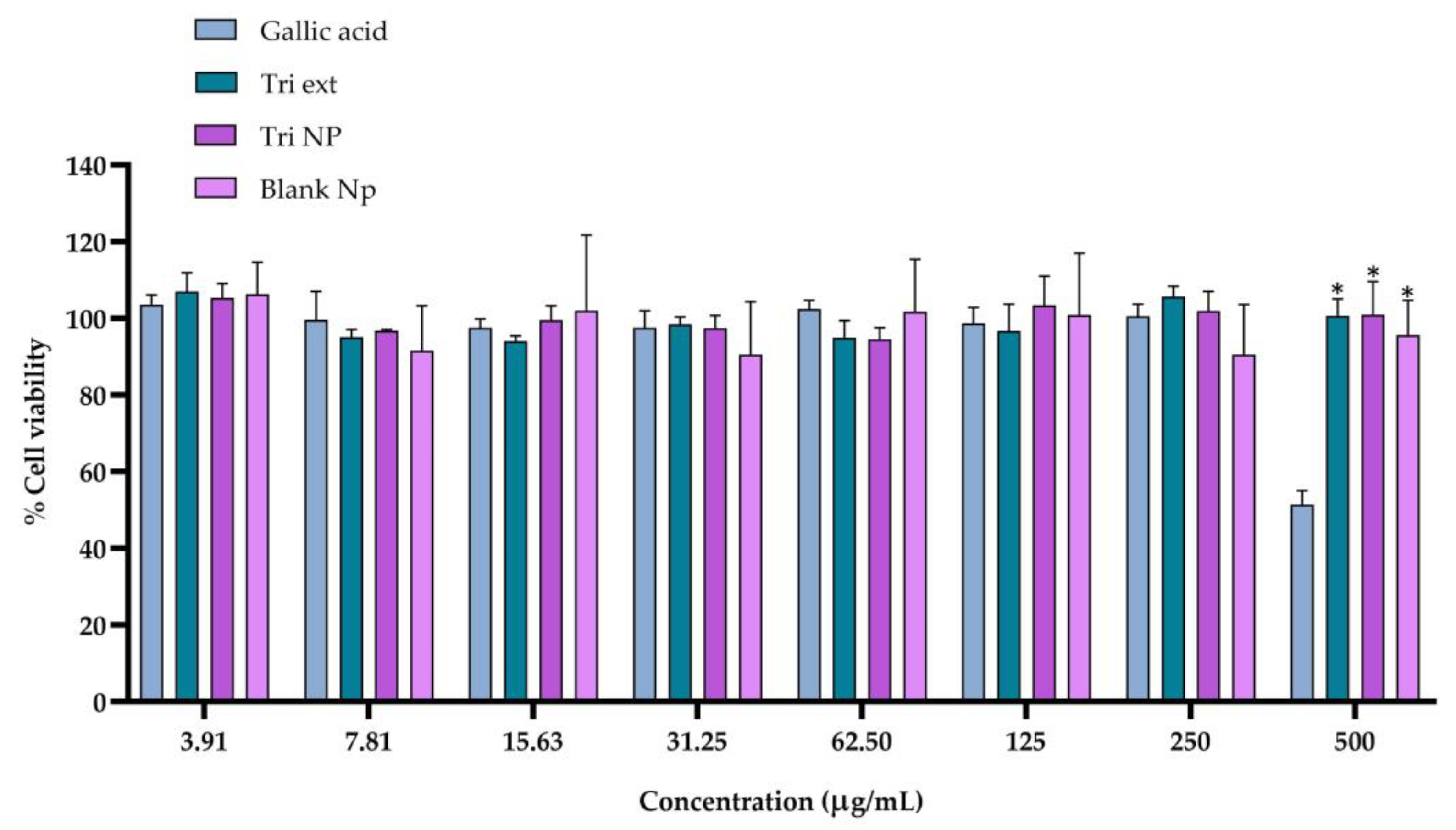
2.3. Cytotoxicity of Triphala Extract and Nanotriphala
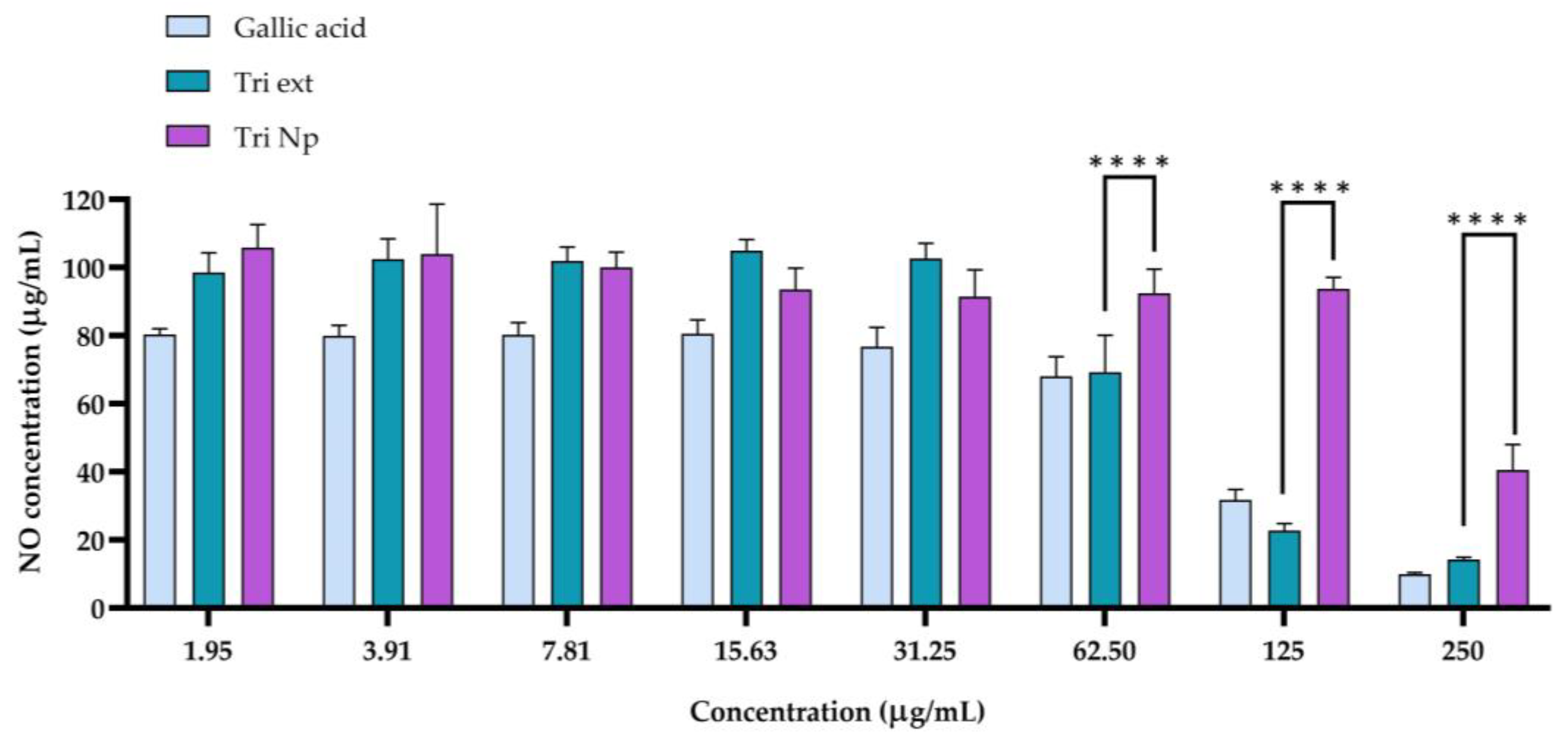
2.4. Anti-Inflammatory Activity of Nanotriphala
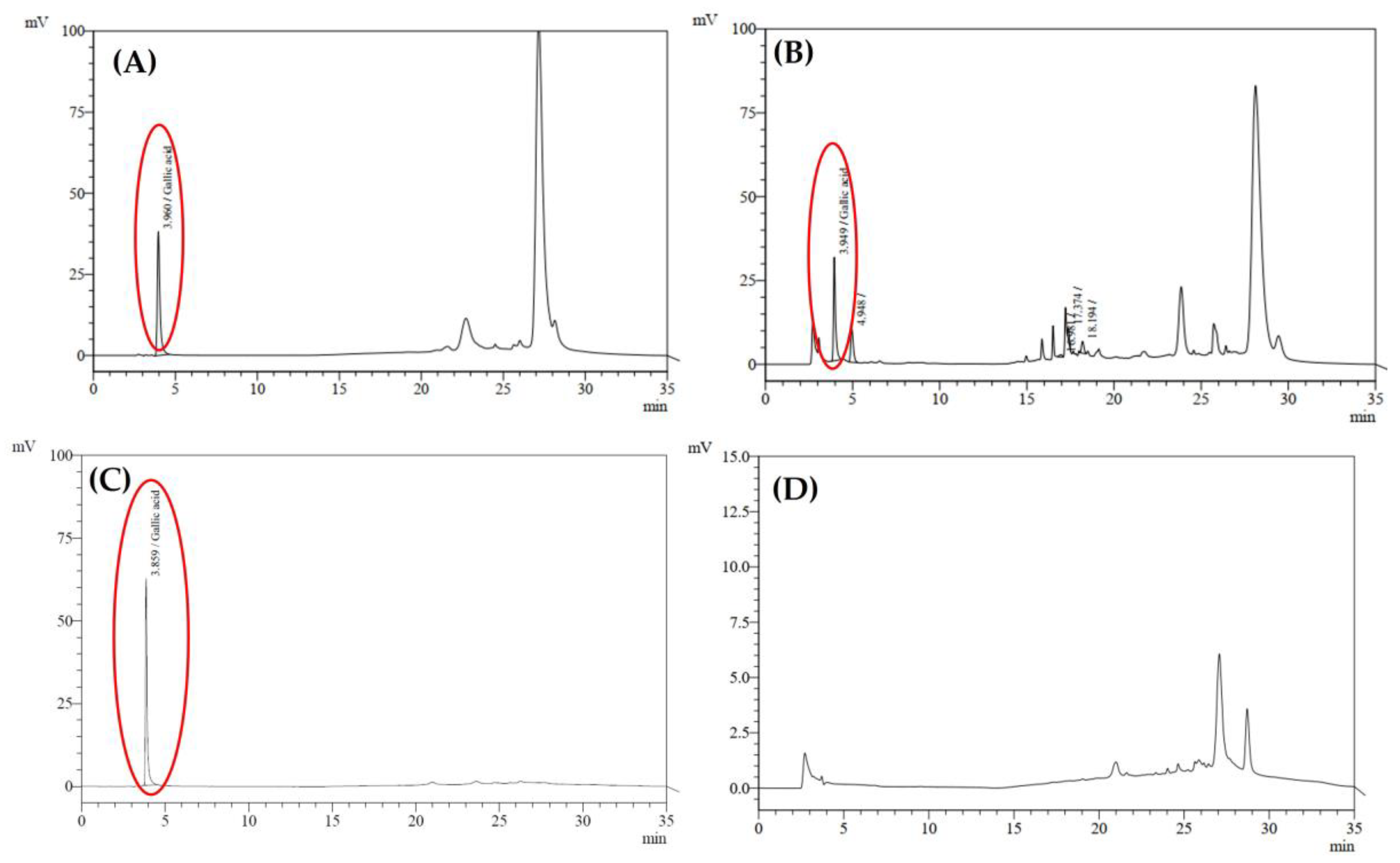
2.5. Physicochemical Properties of In Situ Gel Loaded with Nanotriphala
2.6. Texture Profile Analysis
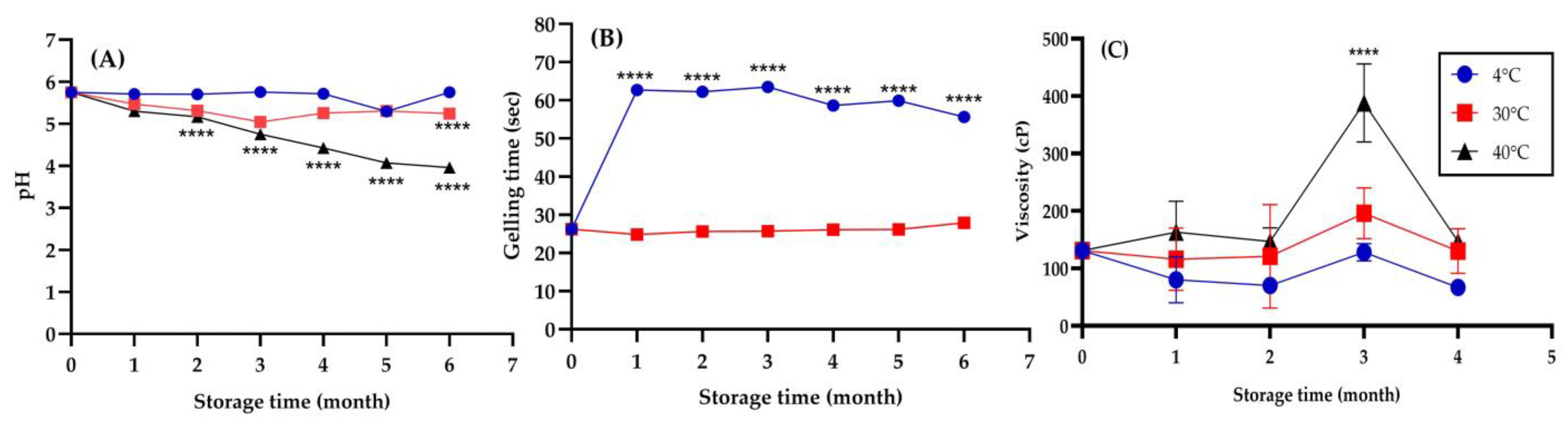
2.7. Physical Stability of In Situ Hydrogel Loaded with Nanotriphala
2.8. Antimicrobial Activities of In Situ Hydrogel Loaded with Nanotriphala
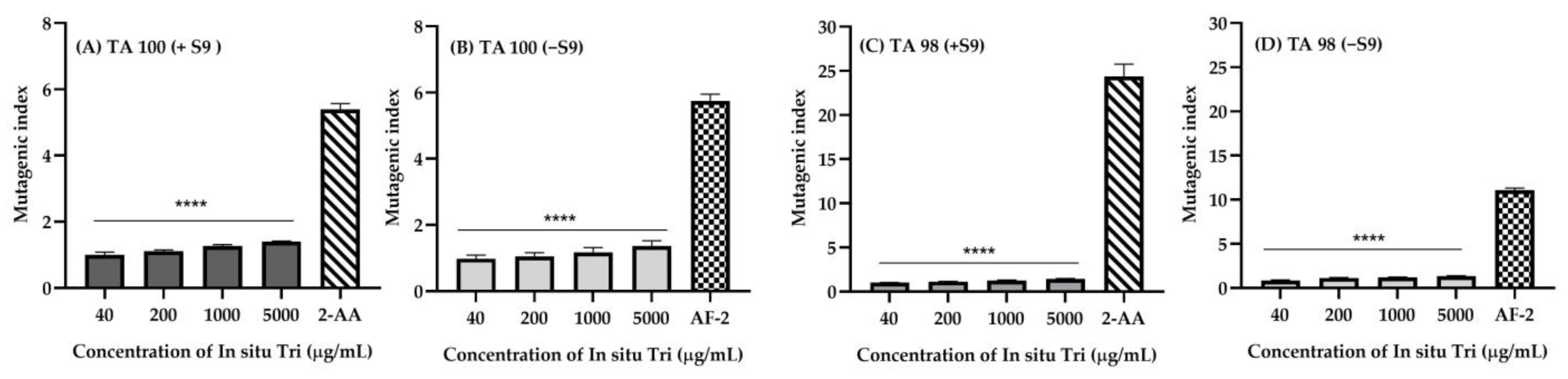
2.9. Mutagenicity of In Situ Hydrogel Loaded with Nanotriphala
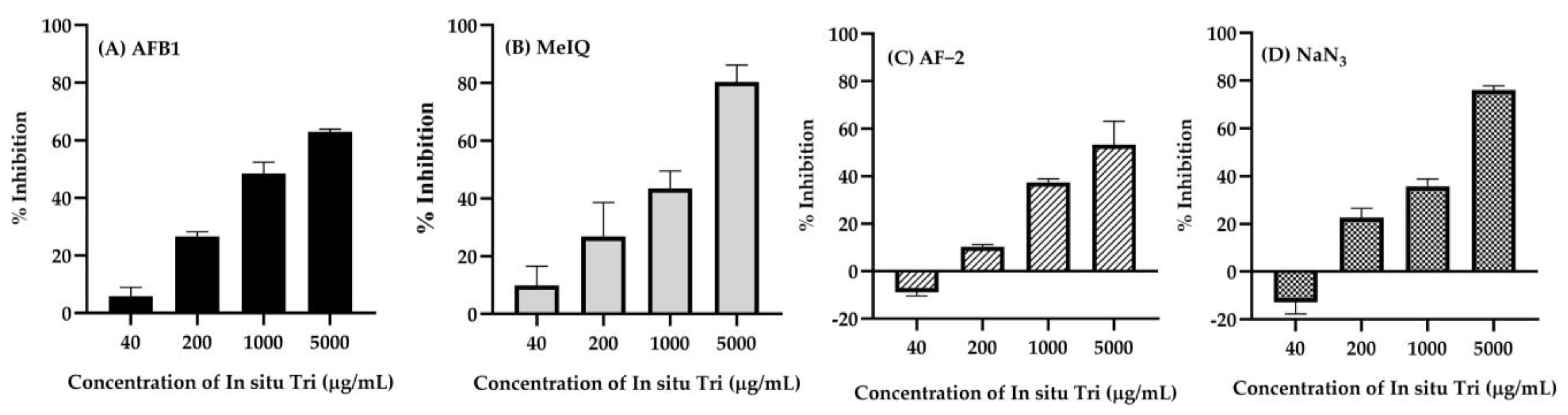
2.10. Antimutagenic Activity of In Situ Hydrogel Loaded with Nanotriphala
3. Conclusions
4. Materials and Methods
4.1. Preparation of Nanotriphala
4.2. Antioxidant Activities of Nanotriphala
4.2.1. DPPH Free Radical-Scavenging Activity Assay
4.2.2. ABTS Free Radical-Scavenging Activity Assay
4.2.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.3. Antimicrobial Activity of Triphala Extract and Nanotriphala
4.4. Cell Culture
4.5. Cytotoxicity Assay of Triphala Extract and Nanotriphala Against RAW264.7 Cells
4.6. Anti-Inflammation Activity Assay of Triphala Extract and Nanotriphala
4.7. Preparation of In Situ Hydrogel Loaded with Nanotriphala
4.8. Characterization of In Situ Hydrogel Loaded with Nanotriphala
4.8.1. Gelation Temperature
4.8.2. Gelation Time
4.8.3. pH
4.8.4. Rheology and Viscosity
4.8.5. Ex Vivo Spreadability
4.8.6. Quantification of Gallic Acid in In Situ Hydrogel Loaded with Nanotriphala Formulation by High-Performance Liquid Chromatography (HPLC)
4.9. Texture Profile Analysis of In Situ Hydrogel Loaded with Nanotriphala
4.10. Physical Stability of In Situ Hydrogel Loaded with Nanotriphala
4.11. Antimicrobial and Microbiological Stability of In Situ Hydrogel Loaded with Nanotriphala
4.12. Mutagenicity Determination of In Situ Hydrogel Loaded with Nanotriphala
4.13. Antimutagenicity Test of In Situ Hydrogel Loaded with Nanotriphala
4.14. Statical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Shin, J.-M.; Park, J.; Han, M.; Kim, T.H. Oxidative stress and antioxidants in chronic rhinosinusitis with nasal polyps. Antioxidants 2023, 12, 195. [Google Scholar] [CrossRef]
- Holguin, F. Oxidative stress in airway diseases. Ann. Am. Thorac. Soc. 2013, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, O.K.; Kuzu, S.; Altıntaş, M.; Vurmaz, A.; Çelik, S. The effect of nasal steroid and antihistamine use on total oxidative stress and antioxidant status in the treatment of allergic rhinitis. Am. J. Rhinol. Allergy 2021, 35, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Arnaudova Danevska, I.; Jakjovska, T.; Atanasovska, E.; Petrushevska, M.; Dzekova Vidimliski, P.; Krstevska Balkanov, S.; Balkanov, T.; Zendelovska, D. Effect of nasal corticosteriods on oxidative stress parameters in children with allergic rhinitis. J. Morphol. Sci. 2023, 6, 1–7. [Google Scholar]
- Tai, J.; Lee, K.; Kim, T.H. Current perspective on nasal delivery systems for chronic rhinosinusitis. Pharmaceutics 2021, 13, 246. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
- Charoenchai, L.; Pathompak, P.; Madaka, F.; Settharaksa, S.; Saingam, W. HPLC-MS profiles and quantitative analysis of triphala formulation. Interprof. J. Health Sci. 2016, 14, 57–67. [Google Scholar]
- Nariya, M.; Shukla, V.; Jain, S.; Ravishankar, B. Comparison of enteroprotective efficacy of triphala formulations (Indian Herbal Drug) on methotrexate-induced small intestinal damage in rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1092–1098. [Google Scholar] [CrossRef]
- Prasad, S.; Srivastava, S.K. Oxidative stress and cancer: Chemopreventive and therapeutic role of triphala. Antioxidants 2020, 9, 72. [Google Scholar] [CrossRef]
- Omran, Z.; Bader, A.; Porta, A.; Vandamme, T.; Anton, N.; Alehaideb, Z.; El-Said, H.; Faidah, H.; Essa, A.; Vassallo, A.; et al. Evaluation of antimicrobial activity of Triphala constituents and nanoformulation. Evid.-Based Complement. Altern. Med. 2020, 2020, 6976973. [Google Scholar] [CrossRef]
- Kalaiselvan, S.; Rasool, M.K. Triphala herbal extract suppresses inflammatory responses in LPS-stimulated RAW 264.7 macrophages and adjuvant-induced arthritic rats via inhibition of NF-κB pathway. J. Immunotoxicol. 2016, 13, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Jantrapirom, S.; Hirunsatitpron, P.; Potikanond, S.; Nimlamool, W.; Hanprasertpong, N. Pharmacological benefits of Triphala: A perspective for allergic rhinitis. Front. Pharmacol. 2021, 12, 628198. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Z.; Zhao, S.-Y.; Ke, X.-M.; Lin, J.-Z.; Huang, S.-S.; Xu, R.-C.; Ma, H.-Y.; Zhang, Y.; Han, L.; Zhang, D.-K. Study on the stability control strategy of Triphala solution based on the balance of physical stability and chemical stabilities. J. Pharm. Biomed. Anal. 2018, 158, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, X.-H.; Yang, X.; Hu, J.-Q.; Zhu, Y.-Z.; Yan, P.-Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.; Ma, J.; Guo, S.; Gao, L.; Jia, Y.; Li, X.; Zhang, Q. In situ gel-forming system: An attractive alternative for nasal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Nazar, H.; Fatouros, D.; van der Merwe, S.; Bouropoulos, N.; Avgouropoulos, G.; Tsibouklis, J.; Roldo, M. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2011, 77, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Karnaki, A.; Siamidi, A.; Karalis, V.; Lagopati, N.; Pippa, N.; Vlachou, M. Thermoresponsive Hydrogels: Current Status and Future Perspectives. In Bioinspired Technology and Biomechanics; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Kumar, R. Nanotechnology in herbal medicine: Challenges and future perspectives. In Nanotechnology in Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 515–548. [Google Scholar]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef]
- Namuga, C.; Ocan, M.; Kinengyere, A.A.; Richard, S.; Namisango, E.; Muwonge, H.; Kirabira, J.B.; Lawrence, M.; Obuku, E.A. Efficacy of nano encapsulated herbal extracts in the treatment of induced wounds in animal models: A systematic review protocol. Syst. Rev. 2023, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.C.; Vargas, V.M.F. Antimutagenic activity of extracts of natural substances in the Salmonella/microsome assay. Mutagenesis 2003, 18, 113–118. [Google Scholar] [CrossRef]
- Chittasupho, C.; Umsumarng, S.; Srisawad, K.; Arjsri, P.; Phongpradist, R.; Samee, W.; Tingya, W.; Ampasavate, C.; Dejkriengkraikul, P. Inhibition of SARS-CoV-2-Induced NLRP3 Inflammasome-Mediated Lung Cell Inflammation by Triphala-Loaded Nanoparticle Targeting Spike Glycoprotein S1. Pharmaceutics 2024, 16, 751. [Google Scholar] [CrossRef] [PubMed]
- Lofts, A.; Campea, M.A.; Winterhelt, E.; Rigg, N.; Rivera, N.P.; Macdonald, C.; Frey, B.N.; Mishra, R.K.; Hoare, T. In situ-gelling hydrophobized starch nanoparticle-based nanoparticle network hydrogels for the effective delivery of intranasal olanzapine to treat brain disorders. Int. J. Biol. Macromol. 2024, 277, 134385. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.A.A.; Perdomo, C.A.A.; Núnez, L.A.H.; Rivera-Flores, O.; Sánchez-Barahona, M.; Guerrero, A.; Romero, A. Lychee peel extract-based magnetic iron oxide nanoparticles: Sustainable synthesis, multifaceted antioxidant system, and prowess in eco-friendly food preservation. Food Bioprod. Process. 2024, 145, 148–157. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Fisher, D.R.; Zheng, T.; Bielinski, D.F.; Kelly, M.E.; Cahoon, D.S.; Shukitt-Hale, B. Phytochemical combination is more effective than individual components in reducing stress signaling in rat hippocampal neurons and microglia in vitro. Int. J. Mol. Sci. 2022, 23, 12651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Sam, J.H.; Jeevanandam, J.; Chan, Y.S.; Nandong, J. Thermal degradation of antioxidant compounds: Effects of parameters, thermal degradation kinetics, and formulation strategies. Food Bioprocess Technol. 2022, 15, 1919–1935. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Muteeb, G. Nanotechnology—A light of hope for combating antibiotic resistance. Microorganisms 2023, 11, 1489. [Google Scholar] [CrossRef]
- Culas, M.; Popovich, D.; Rashidinejad, A. Recent advances in encapsulation techniques for cinnamon bioactive compounds: A review on stability, effectiveness, and potential applications. Food Biosci. 2023, 57, 103470. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ghosal, S.; Bhattacharya, S. Antioxidant activity of tannoid principles of Emblica officinalis (amla) in chronic stress induced changes in rat brain. Indian J. Exp. Biol. 2000, 38, 877–880. [Google Scholar] [PubMed]
- Mishra, S.; Anuradha, J.; Tripathi, S.; Kumar, S. In vitro antioxidant and antimicrobial efficacy of Triphala constituents: Emblica officinalis, Terminalia belerica and Terminalia chebula. J. Pharmacogn. Phytochem. 2016, 5, 273–277. [Google Scholar]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic uses of triphala in ayurvedic medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-C.; Su, C.-H.; Cheng, F.-Y.; Weng, J.-C.; Chen, J.-H.; Tsai, T.-L.; Yeh, C.-S.; Su, W.-C.; Hwu, J.R.; Tzeng, Y.; et al. Modularly assembled magnetite nanoparticles enhance in vivo targeting for magnetic resonance cancer imaging. Bioconjug. Chem. 2008, 19, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jun, C.-D.; Suk, K.; Choi, B.-J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.-Y.; Kim, D.-K.; Shin, T.-Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Oyawaluja, B.; Oyawaluja, A.; Babasanmi, J.; Soneye, O. Phytochemistry and antioxidant assays of Entandrophragma angolense (Welw) C. DC.(meliaceae) using DPPH and nitric oxide free radical scavenging methods. Niger. J. Pharm. Res. 2019, 15, 229–235. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Shabbir, M.; Khan, M.R. Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker. Chem. Cent. J. 2018, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, S.; Rasool, M.K. The anti-inflammatory effect of triphala in arthritic-induced rats. Pharm. Biol. 2015, 53, 51–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parveen, R.; Shamsi, T.N.; Singh, G.; Athar, T.; Fatima, S. Phytochemical analysis and in-vitro biochemical characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotechnol. Rep. 2018, 17, 126–136. [Google Scholar] [CrossRef]
- Conte, R.; Marturano, V.; Peluso, G.; Calarco, A.; Cerruti, P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. Int. J. Mol. Sci. 2017, 18, 709. [Google Scholar] [CrossRef]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef]
- Hinchcliffe, M. The Novel Application of Chitosan for the Intranasal Delivery of Insulin. Doctoral Dissertation, University of Nottingham, Nottingham, UK, 1996. [Google Scholar]
- Behl, C.; Pimplaskar, H.; Sileno, A.; Demeireles, J.; Romeo, V. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 89–116. [Google Scholar] [CrossRef]
- Salatin, S.; Alami-Milani, M.; Daneshgar, R.; Jelvehgari, M. Box–Behnken experimental design for preparation and optimization of the intranasal gels of selegiline hydrochloride. Drug Dev. Ind. Pharm. 2018, 44, 1613–1621. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Wang, M.; Ma, X.; Zong, S.; Su, Y.; Su, R.; Zhang, H.; Liu, Y.; Wang, C.; Li, Y. The prescription design and key properties of nasal gel for CNS drug delivery: A review. Eur. J. Pharm. Sci. 2024, 192, 106623. [Google Scholar] [CrossRef] [PubMed]
- González, N.N.; Rassu, G.; Cossu, M.; Catenacci, L.; Sorrenti, M.L.; Cama, E.S.; Serri, C.; Giunchedi, P.; Gavini, E. A thermosensitive chitosan hydrogel: An attempt for the nasal delivery of dimethyl fumarate. Int. J. Biol. Macromol. 2024, 278, 134908. [Google Scholar] [CrossRef]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Vilhelmova-Ilieva, N.; Bratoeva, K.; Stoyanov, G.; Andonova, V. In Situ Gelling Behavior and Biopharmaceutical Characterization of Nano-Silver-Loaded Poloxamer Matrices Designed for Nasal Drug Delivery. Gels 2024, 10, 385. [Google Scholar] [CrossRef]
- Majithiya, R.J.; Ghosh, P.K.; Umrethia, M.L.; Murthy, R.S. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS Pharmscitech 2006, 7, E80–E86. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Nair, A.B.; Chaudhary, S.; Shah, H.; Jacob, S.; Mewada, V.; Shinu, P.; Aldhubiab, B.; Sreeharsha, N.; Venugopala, K.N.; Attimarad, M.; et al. Intranasal delivery of darunavir-loaded mucoadhesive in situ gel: Experimental design, in vitro evaluation, and pharmacokinetic studies. Gels 2022, 8, 342. [Google Scholar] [CrossRef]
- Lin, H.; Gebhardt, M.; Bian, S.; Kwon, K.A.; Shim, C.-K.; Chung, S.-J.; Kim, D.D. Enhancing effect of surfactants on fexofenadineĚHCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 2007, 330, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Phongpradist, R.; Thongchai, W.; Thongkorn, K.; Lekawanvijit, S.; Chittasupho, C. Surface modification of curcumin microemulsions by coupling of KLVFF peptide: A prototype for targeted bifunctional microemulsions. Polymers 2022, 14, 443. [Google Scholar] [CrossRef]
- Phongpradist, R.; Jiaranaikulwanitch, J.; Thongkorn, K.; Lekawanvijit, S.; Sirilun, S.; Chittasupho, C.; Poomanee, W. KLVFF Conjugated Curcumin Microemulsion-Based Hydrogel for Transnasal Route: Formulation Development, Optimization, Physicochemical Characterization, and Ex Vivo Evaluation. Gels 2023, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Taha, M.; Orlu, M.; Araman, A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Maity, S. Preparation and characterisation of mucoadhesive nasal gel of venlafaxine hydrochloride for treatment of anxiety disorders. Indian J. Pharm. Sci. 2012, 74, 428. [Google Scholar] [CrossRef]
- Basu, S.; Bandyopadhyay, A.K. Characterization of mucoadhesive nasal gels containing midazolam hydrochloride prepared from Linum usitatissimum L. mucilage. Braz. J. Pharm. Sci. 2011, 47, 817–823. [Google Scholar] [CrossRef]
- Saudagar, R.B.; Deore, S.B. In-Situ Nasal Gel Drug Delivery: An Overview. Res. J. Pharm. Dos. Forms Technol. 2016, 8, 9–14. [Google Scholar] [CrossRef]
- Dawre, S.; Waghela, S.; Saraogi, G. Statistically designed vitamin D3 Encapsulated PLGA microspheres dispersed in thermoresponsive in-situ gel for nasal delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103688. [Google Scholar] [CrossRef]
- Tichota, D.M.; Silva, A.C.; Sousa Lobo, J.M.; Amaral, M.H. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int. J. Nanomed. 2014, 9, 3855–3864. [Google Scholar]
- Çelik, Y.S.; Örenli, B.; Al-Mohaya, M.; Mesut, B.; Özsoy, Y. Nasal in situ gels as a drug delivery system: An overview of literature and clinical studies. J. Res. Pharm. 2023, 27, 1875. [Google Scholar]
- Hanafy, N.A.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Makade, C.S.; Shenoi, P.R.; Bhongade, B.A.; Shingane, S.A.; Ambulkar, P.C.; Shewale, A.M. Estimation of MBC: MIC Ratio of Herbal Extracts against Common Endodontic Pathogens. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 2), S1414–S1416. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Elumalai, S.; Kumar, V.; Kumar, S.; Sangwan, R.S. Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity. Microb. Pathog. 2018, 114, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Biradar, Y.S.; Jagatap, S.; Khandelwal, K.; Singhania, S.S. Exploring of antimicrobial activity of Triphala Mashi—An ayurvedic formulation. Evid.-Based Complement. Altern. Med. 2008, 5, 107–113. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface modification of nano-drug delivery systems for enhancing antibiotic delivery and activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1758. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sime, F.B.; Cabot, P.J.; Maqbool, F.; Roberts, J.A.; Falconer, J.R. Solid nanoparticles for oral antimicrobial drug delivery: A review. Drug Discov. Today 2019, 24, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, V.S.; Espuri, P.F.; Alves, R.B.; de Camargos, L.F.; dos Santos, F.V.; de Souza Judice, W.A.; Marques, M.J.; Freitas, R.P. Leishmanicidal, antiproteolytic, and mutagenic evaluation of alkyltriazoles and alkylphosphocholines. Eur. J. Med. Chem. 2015, 101, 24–33. [Google Scholar] [CrossRef]
- Silva, V.A.; Gonçalves, G.F.; Pereira, M.S.; Gomes, I.F.; Freitas, A.F.; Diniz, M.F.; Pessôa, H.L. Assessment of mutagenic, antimutagenic and genotoxicity effects of Mimosa tenuiflora. Rev. Bras. Farmacogn. 2013, 23, 329–334. [Google Scholar] [CrossRef]
- Borges, F.F.V.; Silva, C.R.; Véras, J.H.; Cardoso, C.G.; da Cruz, A.D.; Chen, L.C. Antimutagenic, antigenotoxic, and anticytotoxic activities of Silybum marianum [L.] Gaertn assessed by the Salmonella mutagenicity assay (Ames test) and the micronucleus test in mice bone marrow. Nutr. Cancer 2016, 68, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Arora, S.; Kaur, K.; Kumar, S. The in vitro antimutagenic activity of Triphala—An Indian herbal drug. Food Chem. Toxicol. 2002, 40, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; Ferrarini, L.; Franzoni, S.; Galati, S.; Lazzaretti, M.; Mussi, F.; Northfleet de Albuquerque, C.; Maria Araújo Domingues Zucchi, T.; Poli, P. Genotoxicity revaluation of three commercial nitroheterocyclic drugs: Nifurtimox, benznidazole, and metronidazole. J. Parasitol. Res. 2009, 2009, 463575. [Google Scholar] [CrossRef] [PubMed]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef]
- Hour, T.-C.; Liang, Y.-C.; Chu, I.-S.; Lin, J.-K. Inhibition of eleven mutagens by various tea extracts, (−) epigallocatechin-3-gallate, gallic acid and caffeine. Food Chem. Toxicol. 1999, 37, 569–579. [Google Scholar] [CrossRef]
- Chittasupho, C.; Athikomkulchai, S.; Samee, W.; Na Takuathung, M.; Yooin, W.; Sawangrat, K.; Saenjum, C. Phenylethanoid Glycoside-Enriched Extract Prepared from Clerodendrum chinense Leaf Inhibits A549 Lung Cancer Cell Migration and Apoptosis Induction through Enhancing ROS Production. Antioxidants 2023, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chidrawar, V.R.; Hermawan, D.; Nwabor, O.F.; Olatunde, O.O.; Jayeoye, T.J.; Samee, W.; Ontong, J.C.; Chittasupho, C. Solvent-assisted dechlorophyllization of Psidium guajava leaf extract: Effects on the polyphenol content, cytocompatibility, antibacterial, anti-inflammatory, and anticancer activities. S. Afr. J. Bot. 2023, 158, 166–179. [Google Scholar] [CrossRef]
- Chittasupho, C.; Kengtrong, K.; Chalermnithiwong, S.; Sarisuta, N. Anti-angiogenesis by dual action of R5K peptide conjugated itraconazole nanoparticles. AAPS PharmSciTech 2020, 21, 74. [Google Scholar] [CrossRef]
- Angsusing, J.; Singh, S.; Samee, W.; Tadtong, S.; Stokes, L.; O’connell, M.; Bielecka, H.; Toolmal, N.; Mangmool, S.; Chittasupho, C. Anti-inflammatory activities of Yataprasen Thai traditional formulary and its active compounds, beta-amyrin and stigmasterol, in RAW264. 7 and THP-1 cells. Pharmaceuticals 2024, 17, 1018. [Google Scholar] [CrossRef] [PubMed]
- Sherafudeen, S.P.; Vasantha, P.V. Development and evaluation of in situ nasal gel formulations of loratadine. Res. Pharm. Sci. 2015, 10, 466–476. [Google Scholar] [PubMed]
- Xu, X.; Shen, Y.; Wang, W.; Sun, C.; Li, C.; Xiong, Y.; Tu, J. Preparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochloride. Eur. J. Pharm. Biopharm. 2014, 88, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar] [CrossRef] [PubMed]
| Sample | MIC:MBC (µg/mL) | |||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | S. epidermidis | |
| Tri ext | 128:1024 | >1024:>1024 | 1024:>1024 | 1024:>1024 |
| Tri Np | 390:1562 | 12,500:25,000 | 1562:12,500 | 1562:6250 |
| Antibiotics | 0.5:0.5 Gen | 0.5:1 Gen | 0.5: 0.5 VA | 0.5:1 VA |
| Sample | pH | Gelation Time (s) | Gelation Temperature (°C) | Viscosity (cP) | Spreadability (cm) | Gallic Acid Content (µg/100 mL) |
|---|---|---|---|---|---|---|
| In situ Tri | 5.75 ±0.01 | 26.27 ± 1.16 | 34.0 ± 0.0 | 227 ± 22 | 10.25 ± 0.28 | 0.5796 ± 0.0218 |
| Hardness (g) | Adhesiveness (g·s) | |
|---|---|---|
| In situ Tri | 14.05 ± 0.35 | −13.84 ± 1.48 |
| Microorganism | µg/mL | |||
|---|---|---|---|---|
| MIC | MBC | MBC/MIC | Activity | |
| E. coli | 128 | 32 | <4 | Bactericidal |
| P. aeruginosa | 4 | 2 | <4 | Bactericidal |
| S. aureus | 32 | 4 | <4 | Bactericidal |
| S. epidermidis | 32 | 8 | <4 | Bactericidal |
| Treatment | Concentration (µg/Plate) | Average of Revertant Colonies | |||
|---|---|---|---|---|---|
| TA 98 | TA 100 | ||||
| −S9 | +S9 | −S9 | +S9 | ||
| DMSO | 50 (µL/plate) | 31.56 ± 0.56 | 28.89 ± 0.48 | 136.89 ± 2.23 | 144.67 ± 1.92 |
| 2AA | 0.5 | N/A | 703.11 ± 13.18 | N/A | 778.67 ± 4.81 |
| AF-2 | 0.1 | 349.00 ± 2.22 | N/A | 786.67 ± 14.05 | N/A |
| Blank hydrogel | 5000 | 29.78 ± 0.95 | 28.22 ± 0.44 | 169.89 ± 1.22 | 179.33 ± 2.85 |
| In situ Tri | 40 | 27.22 ± 0.95 | 28.78 ± 1.18 | 134.33 ± 7.43 | 145.11 ± 5.10 |
| 200 | 36.44 ± 0.97 | 32.89 ± 0.99 | 143.00 ± 8.19 | 160.56 ± 1.25 | |
| 1000 | 38.11 ± 0.56 | 36.67 ± 1.07 | 161.22 ± 9.88 | 182.22 ± 1.46 | |
| 5000 | 42.22 ± 0.40 | 41.56 ± 0.97 | 188.56 ± 2.23 | 202.67 ± 2.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phongpradist, R.; Chittasupho, C.; Singh, S.; Ontong, J.C.; Tadtong, S.; Akachaipaibul, P.; Punvittayagul, C.; Thongkorn, K.; Dejkriengkraikul, P.; Jiaranaikulwanitch, J.; et al. Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders. Gels 2025, 11, 106. https://doi.org/10.3390/gels11020106
Phongpradist R, Chittasupho C, Singh S, Ontong JC, Tadtong S, Akachaipaibul P, Punvittayagul C, Thongkorn K, Dejkriengkraikul P, Jiaranaikulwanitch J, et al. Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders. Gels. 2025; 11(2):106. https://doi.org/10.3390/gels11020106
Chicago/Turabian StylePhongpradist, Rungsinee, Chuda Chittasupho, Sudarshan Singh, Julalak Chorachoo Ontong, Sarin Tadtong, Puriputt Akachaipaibul, Charatda Punvittayagul, Kriangkrai Thongkorn, Pornngarm Dejkriengkraikul, Jutamas Jiaranaikulwanitch, and et al. 2025. "Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders" Gels 11, no. 2: 106. https://doi.org/10.3390/gels11020106
APA StylePhongpradist, R., Chittasupho, C., Singh, S., Ontong, J. C., Tadtong, S., Akachaipaibul, P., Punvittayagul, C., Thongkorn, K., Dejkriengkraikul, P., Jiaranaikulwanitch, J., Chansakaow, S., & Hongwiset, D. (2025). Investigation of a Thermoresponsive In Situ Hydrogel Loaded with Nanotriphala: Implications for Antioxidant, Anti-Inflammatory, and Antimicrobial Therapy in Nasal Disorders. Gels, 11(2), 106. https://doi.org/10.3390/gels11020106










