Chromatophoromas in Reptiles
Abstract
:1. Background
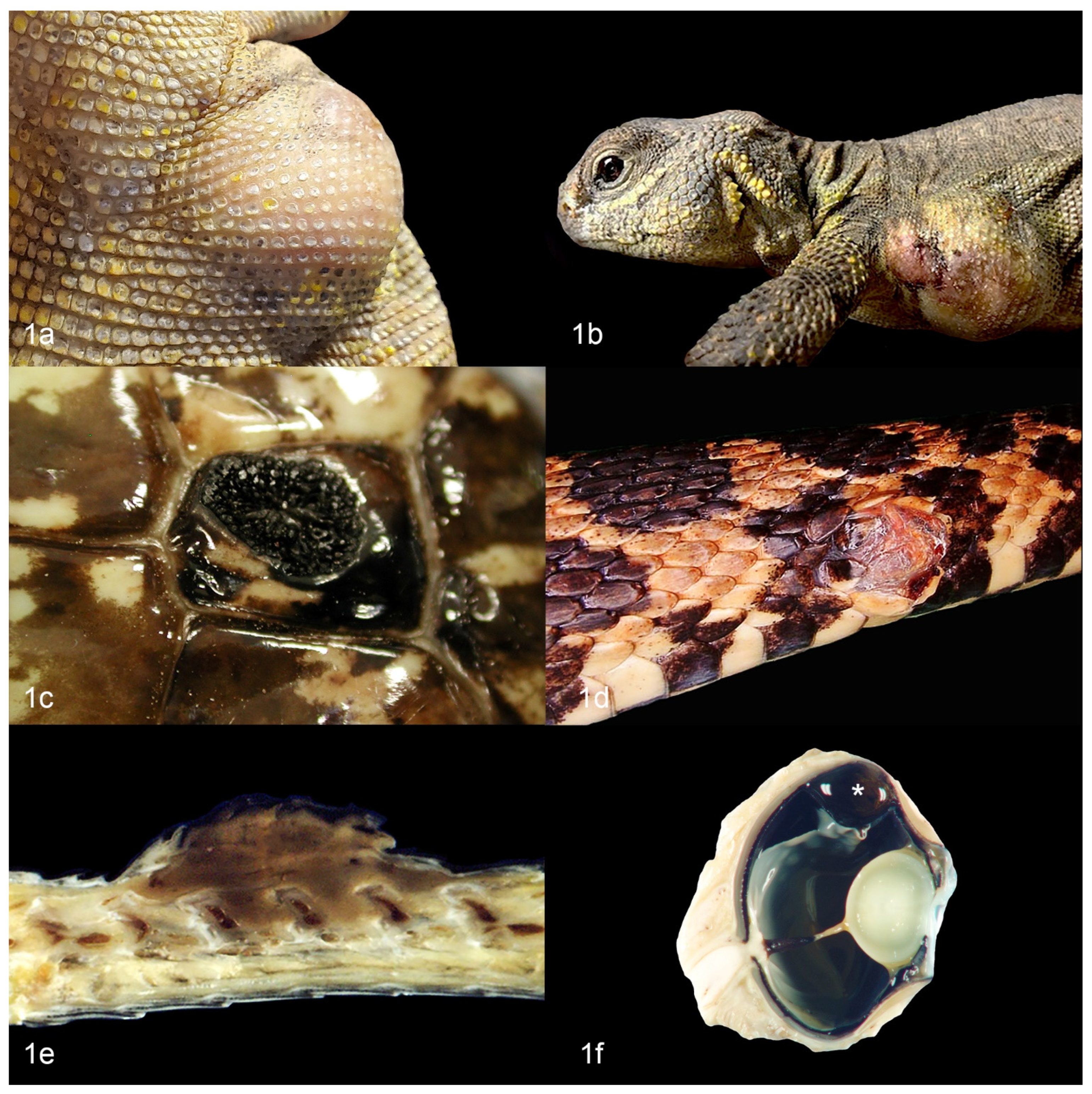
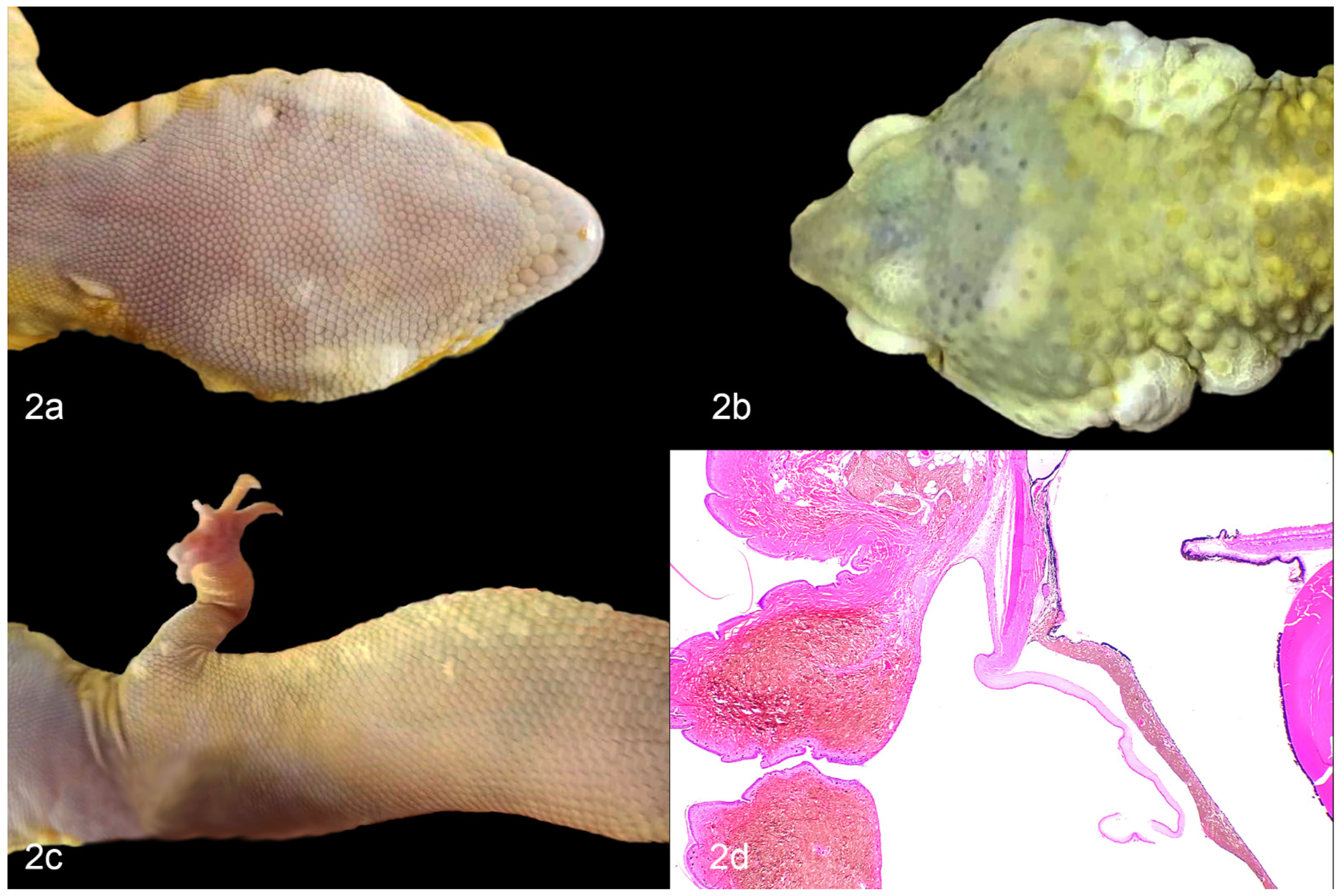
2. Chromatophoromas in Reptiles: Overview and Gross Appearance
3. Etiology of Chromatophoroma in Reptiles
3.1. UV Radiation
3.2. Genetics
3.3. Mutations in Cancer Genes
4. Diagnosis
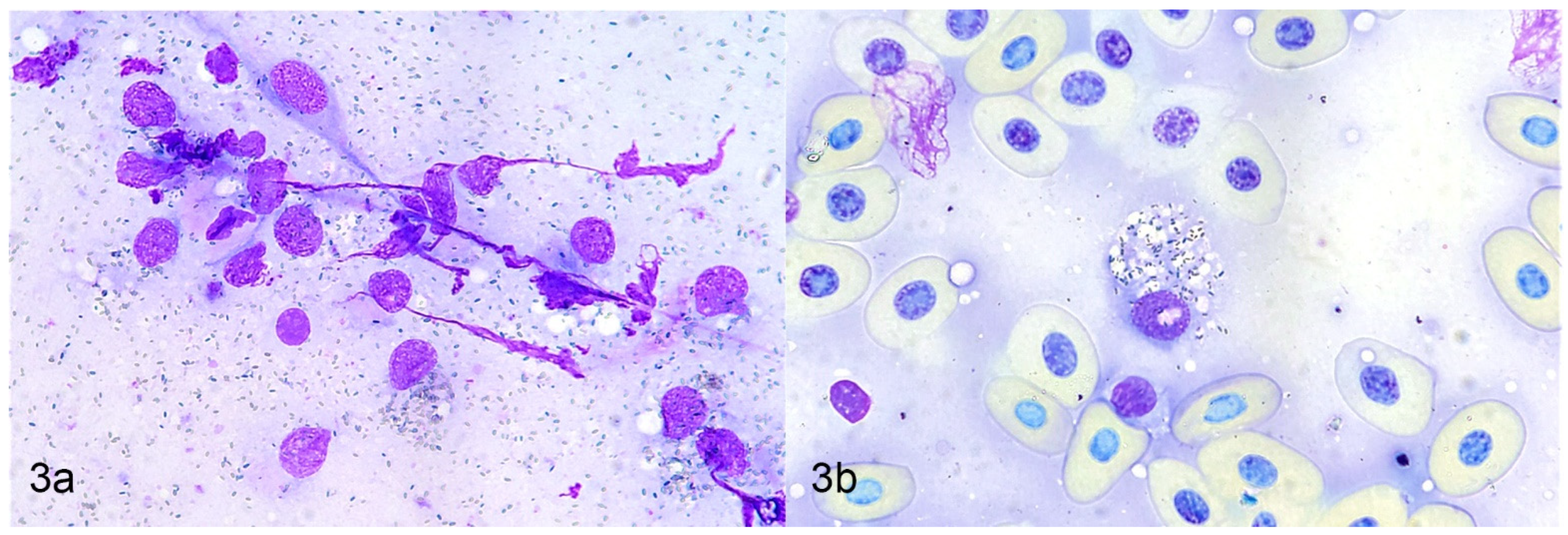
4.1. Cytology
4.2. Histology
4.3. Immunohistochemistry
4.4. Electron Microscopy
5. Chromatophoromas in Lacertilla
5.1. Family: Agamidae
5.2. Family: Iguanidae
5.3. Family: Eublepharidae
5.4. Family: Chamaeleonidae
5.5. Family: Helodermatidae
5.6. Family: Varanidae
5.7. Family: Scincideae
5.8. Family: Gekkonidae
5.9. Families: Diplodacylidae, Corytophanidae, Dactyloidae
6. Chromatophoromas in Serpentes
6.1. Family: Colubridae
6.2. Family: Viperidae
6.3. Family: Pythonidae
6.4. Family: Boidae
6.5. Family: Elapidae
6.6. Family: Xenopeltidae
7. Chromatophoromas in Chelonia
7.1. Family: Testudinidae
7.2. Family: Emydidae
8. Clinical Staging and Treatment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schartl, M.; LaRue, L.; Goda, M.; Bosenberg, M.W.; Hashimoto, H.; Kelsh, R. What is a vertebrate pigment cell? Pigment Cell Melanoma Res. 2015, 29, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hadley, M.E.; Oldman, J.M.G. Physiological Color Changes in Reptiles. Am. Zool. 1969, 9, 489–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnara, J.T.; Taylor, J.D.; Hadley, M.E. The Dermal Chromatophore Unit. J. Cell Biol. 1968, 38, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Ligon, R.A.; McCartney, K.L. Biochemical regulation of pigment motility in vertebrate chromatophores: A review of physiological color change mechanisms. Curr. Zool. 2016, 62, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Miyaji, K.; Sugimoto, M.; Hasegawa, M. Ultrastructure of the Dermal Chromatophores in a Lizard (Scincidae: Plestiodon latiscutatus) with Conspicuous Body and Tail Coloration. Zool. Sci. 2006, 23, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bagnara, J.T.; Matsumoto, J.; Ferris, W.; Frost, S.K.; Turner, W.A.; Tchen, T.T.; Taylor, J.D. Common Origin of Pigment Cells. Science 1979, 203, 410–415. [Google Scholar] [CrossRef] [PubMed]
- De Mello, P.L.H.; Hime, P.M.; Glor, R.E. Transcriptomic Analysis of Skin Color in Anole Lizards. Genome Biol. Evol. 2021, 13, evab110. [Google Scholar] [CrossRef] [PubMed]
- Heckers, K.O.; Aupperle, H. Pigment-forming tumors in reptiles: Light regime and its dark sides. In Proceedings of the 21st Annual Conference of the Association of Reptilian and Amphibian Veterinarians, Orlando, FL, USA, 18–24 October 2014; pp. 31–35. [Google Scholar]
- Heckers, K.; Aupperle, H.; Schmidt, V.; Pees, M. Melanophoromas and Iridophoromas in Reptiles. J. Comp. Pathol. 2012, 146, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.M.; Trupkiewicz, J.G. Reptile Neoplasia at the Philadelphia Zoological Garden, 1901–2002. J. Zoo Wildl. Med. 2006, 37, 11–19. [Google Scholar] [CrossRef]
- Garner, M.M.; Hernandez-Divers, S.M.; Raymond, J.T. Reptile neoplasia: A retrospective study of case submissions to a specialty diagnostic service. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gutiérrez, J.F.; Garner, M.M.; Kiupel, M. Cutaneous Chromatophoromas in Captive Snakes. Veter. Pathol. 2016, 53, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Monahan, C.F.; Meyer, A.; Garner, M.M.; Kiupel, M. Gross, histologic, and immunohistochemical characteristics of cutaneous chromatophoromas in captive bearded dragons. J. Veter. Diagn. Investig. 2021, 33, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Lanckneus, A.; Martel, A.; Bosseler, L.; Hellebuyck, T. Chromatoforoma’s bij reptielen. Vlaams Diergeneeskd. Tijdschr. 2017, 86, 5–15. [Google Scholar] [CrossRef]
- Frye, F.L. Diagnosis and surgical treatment of reptilian neoplasms with a compilation of cases 1966–1993. In Vivo 1994, 8, 885–892. [Google Scholar]
- Lewis, N.; Martinson, S.; Wadowska, D.; Desmarchelier, M. Malignant Mixed Chromatophoroma with Cutaneous, Pulmonary, and Testicular Metastases in a Veiled Chameleon (Chamaeleo calyptratus). J. Herpetol. Med. Surg. 2015, 25, 16. [Google Scholar] [CrossRef]
- Thompson, K.A.; Campbell, M.; Levens, G.; Agnew, D. Bilaterally Symmetrical Oral Amelanotic Melanoma in a Boa Constrictor (Boa Constrictor Constrictor). J. Zoo Wildl. Med. 2015, 46, 629–632. [Google Scholar] [CrossRef]
- Ball, H.A. Melanosarcoma and rhabdomyoma in two pine snakes (Pituophis melanoleucus). Cancer Res. 1946, 6, 134–138. [Google Scholar]
- Bielli, M.; Forlani, A.; Nardini, G.; Avallone, G. Mucinous Melanophoroma in a Northern Red-Bellied Cooter (Pseudemys rubriventris). J. Exot. Pet Med. 2015, 24, 71–75. [Google Scholar] [CrossRef]
- Heckers, K.O.; Schmidt, V.; Krastel, D.; Hildebrandt, G.; Kiefer, I.; Pees, M. Malignant melanophoroma in a Hermann’s tortoise (Testudo hermanni). A case report. Tierarztl. Prax. Ausg. K Klientiere Heimtiere 2011, 39, 45–50. [Google Scholar]
- De Santi, M.; Cruz, N.; Barranco, G.; Lima, G.; Menezes, M.; Matiz, O.S.; Freitas, P.; Atiê, L.; Armani, D.; Santana, A.; et al. Cutaneous melanoma in a red-footed tortoise (Chelonoidis carbonaria). J. Exot. Pet Med. 2020, 34, 44–47. [Google Scholar] [CrossRef]
- Schlumberger, H.G.; Lucke, B.H.E.W. Tumors of fishes, amphibians, and reptiles. Cancer Res. 1948, 8, 657–753. [Google Scholar] [PubMed]
- Tong, L.J.; Ong, W.; Hulst, F.; Tobias, G.; Herrin, K.V.; Vogelnest, L. Clinical, Diagnostic, and Pathological Features of 2 Cases of Metastatic Iridophoroma in a Veiled Chameleon (Chamaeleo calyptratus) and a Red-Barred Dragon (Ctenophorus vadnappa). J. Exot. Pet Med. 2018, 27, 53–60. [Google Scholar] [CrossRef]
- Taggart, P.L.; Woolford, L.; Dunstan, N.; Allen, L.; Buote, M.; Lindsay, S.A. Cutaneous Chromatophoromas in Four Species of Australian Elapid Snake. J. Comp. Pathol. 2021, 183, 33–38. [Google Scholar] [CrossRef]
- Guo, L.; Bloom, J.; Sykes, S.; Huang, E.; Kashif, Z.; Pham, E.; Ho, K.; Alcaraz, A.; Xiao, X.G.; Duarte-Vogel, S.; et al. Genetics of white color and iridophoroma in “Lemon Frost” leopard geckos. PLoS Genet. 2021, 17, e1009580. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Hill, D.L.; Whitney, G.D. Malignant Chromatophoroma in a Gopher Snake. Veter. Pathol. 1981, 18, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Crane, M.M.; McManamon, R.; Gregory, C.R. Surgical Treatment of Pulmonary Melanophoroma in a Beaded Lizard (Heloderma horridum exasperatum). J. Zoo Wildl. Med. 2015, 46, 397–399. [Google Scholar] [CrossRef]
- Catão-Dias, J.L.; Nichols, D.K. Neoplasia in snakes at the National Zoological Park, Washington, DC (1978–1997). J. Comp. Pathol. 1999, 120, 89–95. [Google Scholar] [CrossRef]
- Boniol, M.; Autier, P.; Boyle, P.; Gandini, S. Cutaneous melanoma attributable to sunbed use: Systematic review and meta-analysis. BMJ 2012, 345, e4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellebuyck, T.; Pasmans, F.; Haesebrouck, F.; Martel, A. Dermatological diseases in lizards. Veter. J. 2012, 193, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.L. Reptile Wellness Management. Veter. Clin. N. Am. Exot. Anim. Pr. 2015, 18, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Szydłowski, P.; Madej, J.P.; Duda, M.; Madej, J.A.; Sikorska-Kopyłowicz, A.; Chełmońska-Soyta, A.; Ilnicka, L.; Duda, P. Iridophoroma associated with the Lemon Frost colour morph of the leopard gecko (Eublepharis macularius). Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, H.; Kawaguchi, M.; Fukushima, T.; Shimomura, T. Hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2): Emerging key players in epithelial integrity and cancer. Pathol. Int. 2018, 68, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Abenza, E.; Ibáñez-Molero, S.; García-Moreno, D.; Fuentes, I.; Zon, L.I.; Mione, M.C.; Cayuela, M.L.; Gabellini, C.; Mulero, V. Zebrafish modeling reveals that SPINT1 regulates the aggressiveness of skin cutaneous melanoma and its crosstalk with tumor immune microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korabiowska, M.; Brinck, U.; Frye, F.L.; Harshbarger, J.C.; Droese, M.; Kaiser, H.E. Expression of growth arrest and DNA damage genes and DNA mismatch repair genes in snake melanomas. In Vivo 1998, 12, 539–542. [Google Scholar]
- Rousselet, E.; Souza, C.H.d.; Wellehan, J.F.X.; Epperson, E.D.; Dark, M.J.; Wamsley, H.L. Cutaneous iridophoroma in a Green iguana (Iguana iguana). Vet. Clin. Pathol. 2017, 46, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Irizarry-Rovira, A.R.; Wolf, A.; Ramos-Vara, J.A. Cutaneous melanophoroma in a green iguana (Iguana iguana). Vet. Clin. Pathol. 2006, 35, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.W.; Nichols, D.K.; Hartsell, W.; Torgerson, R.W. Radiation Therapy of a Malignant Chromatophoroma in a Yellow Rat Snake (Elaphe obsoleta quadrivittata). J. Zoo Wildl. Med. 1991, 22, 241–244. [Google Scholar]
- Gregory, C.R.; Harmon, B.G.; Latimer, K.S.; Hafner, S.; Campagnoli, R.P.; McManamon, R.M.; Steffens, W.L. Malignant chromatophoroma in a canebrake rattlesnake (Crotalus horridus atricaudatus). J. Zoo Wildl. Med. 1997, 28, 198–203. [Google Scholar] [PubMed]
- De Brot, S.; Sydler, T.; Nufer, L.; Ruetten, M. Histologic, Immunohistochemical, and Electron Microscopic Characterization of a Malignant Iridophoroma in a Dwarf Bearded Dragon (Pogona henrylawsoni). J. Zoo Wildl. Med. 2015, 46, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Kusewitt, D.F.; Reece, R.L.; Miska, K.B. S-100 immunoreactivity in melanomas of two marsupials, a bird, and a reptile. Veter. Pathol. 1997, 34, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korabiowska, M.; Brinck, U.; Frye, F.F.; Harshbarger, J.C.; Schauer, A.; Kaiser, H.E. Immunohistochemical and photometric analysis of snake-melanomas. In Vivo 1998, 11, 415–419. [Google Scholar]
- Jacobson, E.; Ferris, W.; Bagnara, J.; Iverson, W. Chromatophoromas in a Pine Snake. Pigment Cell Res. 1989, 2, 26–33. [Google Scholar] [CrossRef]
- De Macêdo, I.L.; DE Sousa, D.E.; Hirano, L.Q.L.; Name, K.P.O.; Báo, S.N.; de Castro, M.B. Nasal Melanophoroma in a Captive Green Iguana (Iguana iguana). Top. Companion Anim. Med. 2020, 41, 100463. [Google Scholar] [CrossRef] [PubMed]
- Suedmeyer, W.; Bryan, J.N.; Johnson, G.; Freeman, A. Diagnosis and Clinical Management of Multiple Chromatophoromas in an Eastern Yellowbelly Racer (Coluber constrictor flaviventris). J. Zoo Wildl. Med. 2007, 38, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Beissenherz, M.E.; Miller, M.A.; Johnson, G.C.; Pace, L.W.; Fard, A.; Kottler, S.J. Retrospective Study of 338 Canine Oral Melanomas with Clinical, Histologic, and Immunohistochemical Review of 129 Cases. Veter. Pathol. 2000, 37, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Vara, J.A.; Frank, C.B.; DuSold, D.; Miller, M.A. Immunohistochemical Expression of Melanocytic Antigen PNL2, Melan A, S100, and PGP 9.5 in Equine Melanocytic Neoplasms. Veter. Pathol. 2013, 51, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Smedley, R.C.; Lamoureux, J.; Sledge, D.G.; Kiupel, M. Immunohistochemical Diagnosis of Canine Oral Amelanotic Melanocytic Neoplasms. Veter. Pathol. 2010, 48, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.E.; Smedley, R.; Anthony, S.; Garner, M.M. Malignant Melanoma in the Penguin: Characterization of the Clinical, Histologic, and Immunohistochemical Features of Malignant Melanoma in 10 Individuals from Three Species of Penguin. J. Zoo Wildl. Med. 2014, 45, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ukaj, S.; Hochleithner, M.; Richter, B.; Brandstetter, D.; Knotek, Z. A survey of diseases in captive bearded dragons: A retrospective study of 529 patients. Veter. Med. 2017, 62, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, M.; Denk, D.; Stidworthy, M.F. Retrospective review of neoplasms of captive lizards in the United Kingdom. Veter. Rec. 2020, 186, 28. [Google Scholar] [CrossRef]
- Magnotti, J.M.; Garner, M.M.; Stahl, S.J.; Corbin, E.M.; LaDouceur, E.E.B. Retrospective Review of Histologic Findings in Captive Gila Monsters (Heloderma suspectum) and Beaded Lizards (Heloderma horridum). J. Zoo Wildl. Med. 2021, 52, 166–175. [Google Scholar] [CrossRef]
- Elkan, E. Malignant melanoma in a Snake. J. Comp. Pathol. 1974, 84, 51–57. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Hahne, M.; Leach, K.; Murphy, H.; Lock, B.; Rivera, S. Neoplasia in Snakes at Zoo Atlanta during 1992–2012. J. Zoo Wildl. Med. 2017, 48, 521–524. [Google Scholar] [CrossRef]
- Van Der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 2020, 252, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.; Devau, M.; Wilson-Robles, H.; Hoppes, S.; Rech, R.; Russell, K.E.; Heatley, J.J. Oncology of Reptiles. Veter. Clin. N. Am. Exot. Anim. Pr. 2017, 20, 87–110. [Google Scholar] [CrossRef]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Dwarf bearded dragon (Pogona henrylawsoni) | Cutaneous and oral | Malignant iridophoroma | Yes | Heart, liver, kidney, intestine, pharyngeal and sublingual mucosa, visceral fat | [40] |
| Red-barred dragon (Ctenophorus vadnappa) | Cutaneous | Malignant iridophoroma | Yes; tumor microembolic in pulmonary arterial vessels | Lung | [23] |
| Bearded Dragon (Pogona sp). | Cutaneous or lip | Melanoma (4 cases) | No | [8,9,13,51,52] | |
| Melanophoroma (26) | Yes, 2 cases | Kidneys, liver, lungs, intestine, fat bodies | |||
| Myxoid melanophoroma (24) | No | ||||
| Iridophoroma (8) | Yes, 1 case | 11 different (unspecified) organs | |||
| Mixed chromatophoroma (melanophoroma and iridophoroma) (5) | No | ||||
| Nonpigmented chromatophoroma (6) | No | ||||
| Generic Agamid | Cutaneous | Melanoma | Not reported | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Citation |
|---|---|---|---|---|
| Green iguana (Iguana iguana) | Cutaneous, nasal, or unspecified | Iridophoroma (1 case) | No | [8,36,37,44] |
| Melanophoroma (3) | No |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Leopard gecko (Eublepharis macularius) | Cutaneous | Malignant iridophoroma (4 cases) | Yes, 1 case; No, 3 cases | Liver, eye, muscle | [8,9,25,32] |
| Melanophoroma (1) | Yes | Lung |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Veiled chameleon (Chamaeleo calyptratus) | Cutaneous | Malignant mixed chromatophoroma (iridophoroma and xanthophoroma) (1 case) | Yes | Skin, lung, testicle | [8,9,16,23,52] |
| Metastatic iridophoroma (1) | Yes | Skin, liver, spleen, kidney, abdominal fat, lung, stomach, peritoneum, omentum, myocardium, epicardium, great vessels, testicle, skeletal muscle | |||
| Melanoma (1) | Not reported | ||||
| Melanophoroma (3) | Yes; 1 case | Heart, lung, tongue, stomach, gut, liver, spleen, kidneys, bone, fat bodies, parietal serosa of the coelomic cavity, brain | |||
| Iridophoroma (3) | No | ||||
| Erythro-/xanthophoroma (1) | No | ||||
| Mixed tumors (melanophoroma and iridophoroma) (1) | No | ||||
| Generic chameleon | Cutaneous | Melanoma | Not reported | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Citation |
|---|---|---|---|---|
| Rio Fuerte beaded lizard (Heloderma horridum exasperatum) | Pulmonary | Melanophoroma | No | [27] |
| Gila monster (Heloderma suspectum) | Not specified | Melanoma | Not reported | [53] |
| Beaded lizard (Heloderma horridum) | Body wall | Melanoma | Not reported | [53] |
| Mexican beaded lizard (Heloderma horridum) | Cutaneous | Chromatophoroma | No | [10] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Ornate monitor (Varanus ornatus) | Cutaneous | Malignant melanoma | Not reported | [52] | |
| Savanna monitor (Varanus exanthematicus) | Cutaneous | Iridophoroma | Not reported | [8,9] | |
| Generic monitors | Cutaneous | Melanoma (2 cases) | Yes (1 case) | Not reported | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Pine snake (Pituophis melanoleucus) | Cutaneous or lip | Malignant melanoma (1 case) | Yes | Cloaca, coelomic cavity, liver | [10,18] |
| Melanosarcoma (1) | No | ||||
| Malignant chromatophoroma (1) | Not reported | ||||
| Northern pine snake (Pituophis melanoleucus melanoleucus) | Cutaneous | Combined iridophoroma and melanophoroma | Yes | Thyroid gland, subepicardium, liver, spleen, lung, kidneys | [43] |
| Florida pine snake (everglade snake; Elaphe obsoleta rossalleni) | Cutaneous | Malignant melanoma | Yes | Skeletal muscle, bone marrow of vertebral bodies, pulp of maxillary teeth, dental ridges, lung, fat body, liver, spleen, kidneys, testes, hemipenes, pancreas | [54] |
| Gopher snake (bullsnake; Pituophis catenifer var. sayi) | Cutaneous or unspecified | Malignant chromatophoroma (mosaic, xanthophore and melanophores) (1) | No | [10,12,15,26,35,42] | |
| Melanoma (2) | Yes, 1 case; other not reported | Liver | |||
| Chromatophoroma (1) | Not reported | ||||
| Mixed chromatophoroma (melanophoroma and iridophoroma) (2) | No | ||||
| Iridophoroma (1) | No | ||||
| Yellow rat snake (Elaphe obsoleta quadrivittata) | Cutaneous | Melanin-producing malignant chromatophoroma (1) | Yes | Coelomic cavity | [12,38] |
| Melanocytoma (1) | No | ||||
| Eastern yellowbelly racer (Coluber constrictor flaviventris) | Cutaneous | Chromatophoroma | No | [45] | |
| Northern water snake (Nerodia sipedon sipedon) | Cutaneous | Chromatophoroma and benign dermal melanoma | Not reported | [10] | |
| Mexican hognose snake (Heterodon nasicus kennerlyi) | Cutaneous | Chromatophoroma | Not reported | [10] | |
| Garter snake (Thamnophis sirtalis terrestrism) | Cutaneous or unspecified | Malignant mixed chromatophoroma (1) | Not reported | [8,9,15] | |
| Melanophoroma (2) | Yes, 1 case; No, 1 case | Not reported | |||
| Erythro-/ Xanthophoroma (4) | Yes in 4 cases | Widespread: specific organs not reported | |||
| Southern water snake (Nerodia fasciata) | Unspecified | Melanophoroma | No | [8,9] | |
| Corn snake (Pantherophis guttatus) | Cutaneous | Malignant mixed chromatophoroma (melanophoroma and iridiophoroma) (1) | Yes | Not reported | [12] |
| Iridophoroma (1) | No | ||||
| San Francisco garter snake (Thamnophis sirtalis tetrataenia) | Cutaneous | Mixed chromatophoroma (melanophoroma and iridiophoroma) (2) | No | [12] | |
| Melanocytoma (1) | No | ||||
| Iridophoroma (1) | No | ||||
| Eastern king snake (Lampropeltis getula) | Cutaneous | Mixed chromatophoroma (melanophoroma and iridiophoroma) | No | [12] | |
| Tricolor hognose snake (Xenodon pulcher) | Cutaneous | Mixed chromatophoroma (melanophoroma and iridiophoroma) (1) | No | [12] | |
| Malignant iridophoroma (1) | Yes | Not reported | |||
| California king snake (Lampropeltis getula californiae) | Cutaneous | Mixed chromatophoroma (melanophoroma and iridiophoroma) | No | [12] | |
| Lyre snake (Trimorphodon biscutatus) | Cutaneous | Melanocytoma | No | [12] | |
| Eastern hognose snake (Heterodon platirhinos) | Cutaneous | Melanocytoma | No | [12] | |
| Honduran milk snake (Lampropeltis triangulum hondurensis) | Cutaneous | Melanocytoma | No | [12] | |
| Western fox snake (Pantherophis spp.) | Cutaneous | Melanocytoma | No | [12] | |
| Eastern indigo snake (Drymarchon couperi) | Cutaneous | Melanocytoma | No | [12] | |
| Green vine snake (Ahaetulla nasuta) | Cutaneous | Melanocytoma | No | [12] | |
| Rat snake (Elaphe [Pantherophis] obsolete) | Cutaneous | Melanocytoma | No | [12] | |
| Great plains rat snake (Pantherophis emoryi) | Cutaneous | Malignant melanoma | Yes | Not reported | [12] |
| Sierra mountain kingsnake (Lampropeltis zonata) | Cutaneous | Malignant melanoma | Yes | Not reported | [12] |
| Generic colubrids | Cutaneous | Melanoma (11) | No | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Canebreak rattlesnake (Crotalus horridus atricaudatus) | Cutaneous | Malignant chromatophoroma | Not reported | [39] | |
| Jumping viper (Atropoides picadoi) | Cutaneous | Chromatophoroma | Not reported | [35,42] | |
| Pigmy rattlesnake (Sistrurus spp.) | Not specified | Melanophoroma | No | [8,9] | |
| Bush viper (Atheris spp.) | Cutaneous | Xanthophoroma (1 case) | No | [12] | |
| Iridophoroma (1) | No | ||||
| Northern pacific rattlesnake (Crotalus oreganus) | Cutaneous | Melanocytoma | No | [12] | |
| Mojave Desert sidewinder (Crotalus cerastes) | Cutaneous | Melanocytoma (1) | No | [12] | |
| Malignant melanoma (1) | Yes | Not reported | |||
| Neotropical rattlesnake (Crotalus durissus) | Cutaneous | Melanocytoma | No | [12] | |
| Broad-banded copperhead (Agkistrodon laticinctus) | Cutaneous | Melanocytoma | No | [12] | |
| Temple viper (Tropidolaemus wagleri) | Cutaneous | Malignant melanoma | Yes | Not reported | [12] |
| Prairie rattlesnake (Crotalus viridis) | Cutaneous | Malignant melanoma | Yes | Not reported | [12] |
| Generic vipers and crotalids | Cutaneous | Melanoma (4) | Yes (1 case) | Not reported | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Reticulated python (Python reticulatus)) | Cutaneous | Melanoma (1 case) | Yes | Coelomic cavity, kidneys | [22] |
| Multiple non-malignant melanomas (1) | No | ||||
| Green tree python (Morelia (previously Chondropython) viridis) | Small intestine; cutaneous | Chromatophoroma (1) | Not reported | [12,28] | |
| Malignant iridophoroma (1) | Yes | Not reported | |||
| Burmese python (Python molurus) | Unspecified | Melanophoroma | Not reported | [8] | |
| Woma python (Aspidites ramsayi) | Cutaneous | Melanocytoma | No | [12] | |
| Carpet python (Morelia spilota) | Cutaneous | Melanocytoma | No | [12] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Boa constrictor (boa constrictor constrictor) | Oral; unspecified | Metastatic malignant amelanotic melanoma (1 case) | Yes | Liver, spleen | [8,9,17] |
| Melanophoroma (1) | No | ||||
| Amazon tree boa (Corallus hortulana) | Cutaneous | Malignant melanophoroma | Vascular dissemination | Not reported | [55] |
| Yellow anaconda (Eunectes notaceus) | Cutaneous | Melanophoroma | Yes | Heart, stomach, gut, pancreas, kidneys, uterus, ovaries, fat body | [8,9] |
| Rainbow boa (Epicrates cenchria) | Cutaneous | Melanocytoma | No | [12] | |
| Anaconda (Eunectes murinus) | Cutaneous | Melanocytoma | No | [12] | |
| Rubber boa (Charina bottae) | Cutaneous | Melanocytoma | No | [12] | |
| Rosy boa (Charina trivirgata) | Cutaneous | Melanocytoma (1) | No | [12] | |
| Malignant melanoma (1) | Yes | Not reported | |||
| Dumeril’s ground boa (Acrantophis dumerili) | Cutaneous | Malignant melanoma | Yes | Not reported | [12] |
| Generic boids | Cutaneous | Melanoma (3) | No | [11] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Common death adder (Acanthophis antarcticus) | Cutaneous | Melanoma | Yes | Adventitia of esophagus, pericardium | [41] |
| Strap-snouted brown snake (Pseudonaja aspidorhynchus) | Cutaneous | Malignant melanophoroma | Yes | Esophagus, intestine, liver, descending aorta | [24] |
| Tiger snake (Notechis scutatus) | Cutaneous | Malignant melanophoroma | No | [24] | |
| Eastern brown snake (Pseudonaja textilis) | Cutaneous | Melanophoroma | No | [24] | |
| Mengden’s brown snake (Pseudonaja mengdeni) | Cutaneous | Malignant melanophoroma | No | [24] | |
| Black mamba (Dendroaspis polylepis) | Disseminated, specific sites unspecified | Malignant melanoma | Not reported | [55] | |
| Cape cobra (Naja nivea) | Cutaneous | Mixed chromatophoroma (melanophoroma and iridophoroma) | No | [12] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Hermann’s tortoise (Testudo hermanni) | Cutaneous (carapace) | Malignant melanophoroma | Yes | Lungs, liver, spleen, kidneys, adrenal glands, thyroid gland, ductus deferens | [8,9,20] |
| Red-footed tortoise (Chelonoidis carbonaria) | Cutaneous | Melanoma | No | [21] |
| Species | Site | Diagnosis | Presence of Visceral Metastases | Organs Affected by Metastases | Citation |
|---|---|---|---|---|---|
| Northern red-bellied cooter (Pseudemys rubriventris) | Cutaneous | Mucinous variant of melanophoroma | Not reported | [19] | |
| Red-eared slider turtle (Trachemys scripta elegans) | Cutaneous (shell) | Metastatic melanophoroma | Yes | Not reported | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monahan, C.F.; Garner, M.M.; Kiupel, M. Chromatophoromas in Reptiles. Vet. Sci. 2022, 9, 115. https://doi.org/10.3390/vetsci9030115
Monahan CF, Garner MM, Kiupel M. Chromatophoromas in Reptiles. Veterinary Sciences. 2022; 9(3):115. https://doi.org/10.3390/vetsci9030115
Chicago/Turabian StyleMonahan, Colleen F., Michael M. Garner, and Matti Kiupel. 2022. "Chromatophoromas in Reptiles" Veterinary Sciences 9, no. 3: 115. https://doi.org/10.3390/vetsci9030115
APA StyleMonahan, C. F., Garner, M. M., & Kiupel, M. (2022). Chromatophoromas in Reptiles. Veterinary Sciences, 9(3), 115. https://doi.org/10.3390/vetsci9030115






