Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
2.2. Antigen Preparation
2.3. Serological Diagnosis by ELISA
2.3.1. Leishmania infantum ELISA
2.3.2. KMP11 ELISA
2.4. Whole Blood Assay
2.5. Sandwich ELISA for the Determination of IFN-γ
2.6. Statistical Analysis
3. Results
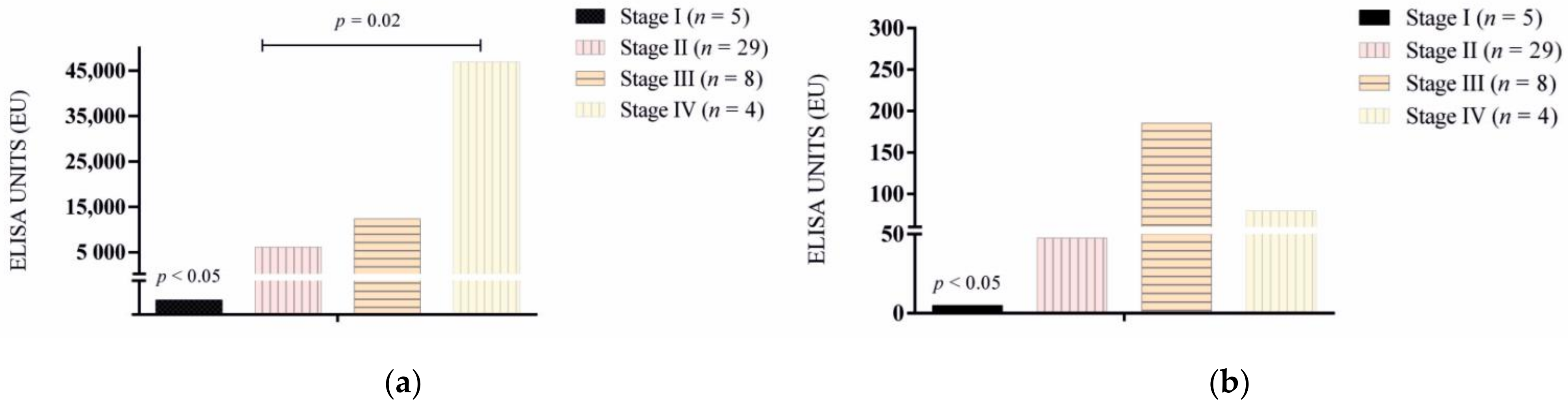
3.1. Antibody Response
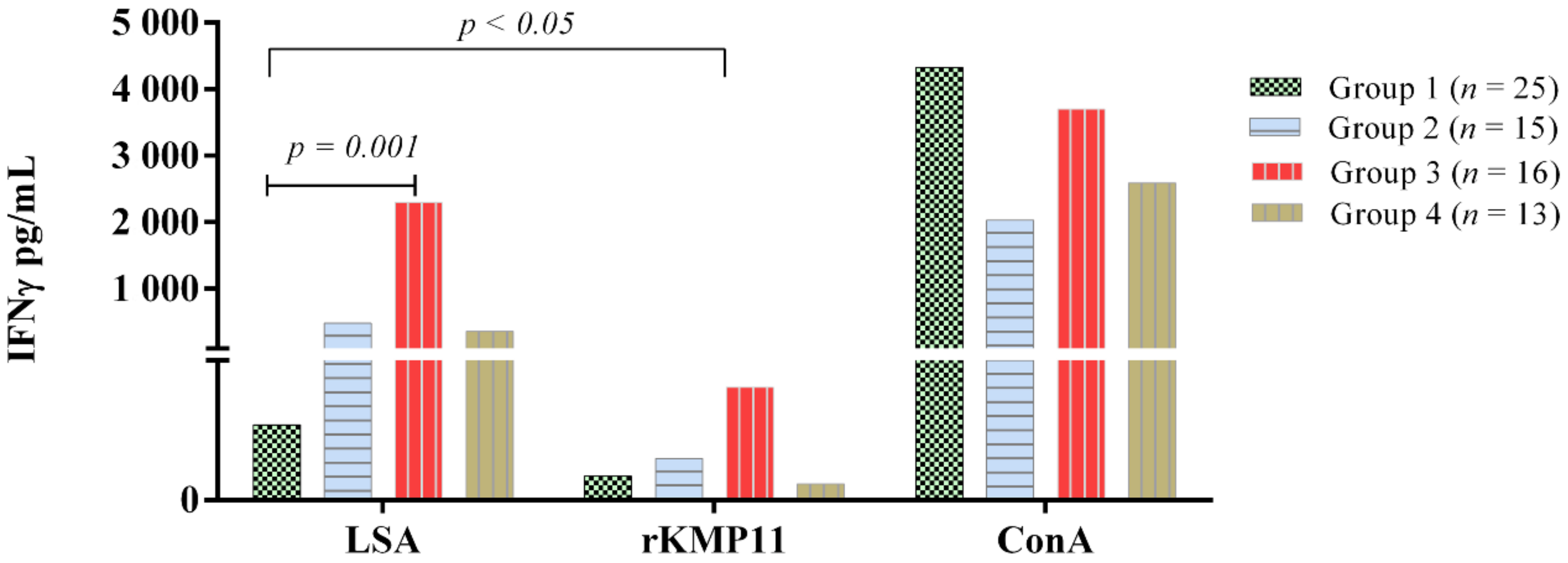
3.2. Ex Vivo IFN-γ-Release Whole Blood Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noli, C.; Saridomichelakis, M.N. An update on the diagnosis and treatment of canine leishmaniosis caused by Leishmania infantum (syn. L. chagasi). Vet. J. 2014, 202, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Leishmaniasis emergence in Europe. Eurosurveillance 2010, 15, 1–11. [Google Scholar] [CrossRef]
- Velez, R.; Ballart, C.; Domenech, E.; Abras, A.; Fernández-Arévalo, A.; Gómez, S.A.; Tebar, S.; Muñoz, C.; Cairó, J.; Gállego, M. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: The example of North-Eastern and Pyrenean areas of Spain. Prev. Vet. Med. 2018, 162, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tabbabi, A. Review of Leishmaniasis in the Middle East and North Africa. Afr Health Sci. 2019, 19, 1329–1337. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Canine leishmaniosis in South America. Parasit. Vectors. 2009, 8, 1–8. [Google Scholar] [CrossRef]
- Pereira, M.A.; Santos, R.; Oliveira, R.; Costa, L.; Prata, A.; Gonçalves, V.; Roquette, M.; Vala, H.; Santos-Gomes, G. Prognostic factors and life expectancy in canine leishmaniosis. Vet. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.F.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Rodríguez-Cortés, A.; Carrillo, E.; Martorell, S.; Todolí, F. Compartmentalized immune response in leishmaniasis: Changing patterns throughout the disease. PLoS ONE 2016, 11, e0155224. [Google Scholar] [CrossRef]
- Strauss-Ayali, D.; Baneth, G.; Shor, S.; Okano, F.; Jaffe, C.L. Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs. Int. J. Parasitol. 2005, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-Corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; Marí-Martorell, D.; Montserrat-Sangrà, S.; Ordeix, L.; Baneth, G.; Solano-Gallego, L. Leishmania infantum-specific IFN-γ production in stimulated blood from dogs with clinical leishmaniosis at diagnosis and during treatment. Vet. Parasitol. 2017, 248, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Stebeck, C.E.; Beecroft, R.P.; Singh, B.N.; Jardim, A.; Olafson, R.W.; Tuckey, C.; Prenevost, K.D.; Pearson, T.W. Kinetoplastid membrane protein-11 (KMP-11) is differentially expressed during the life cycle of African trypanosomes and is found in a wide variety of kinetoplastid parasites. Mol. Biochem. Parasitol. 1995, 71, 1–13. [Google Scholar] [CrossRef]
- Angel, M.; Jose, F.; Perez, M.; Soto, M.; Carlos, M.; Carlos, L. Calcium-induced conformational changes in Leishmania infantum kinetoplastid membrane protein-11. J. Biol. Inorg. Chem. 2001, 6, 107–117. [Google Scholar] [CrossRef]
- Matos, D.C.S.; Faccioli, L.A.P.; Cysne-Finkelstein, L.; Mello De Luca, P.; Corte-Real, S.; Armôa, G.R.G.; Blanco Lemes, E.M.; Decote-Ricardo, D.; Mendonça, S.C.F. Kinetoplastid membrane protein-11 is present in promastigotes and amastigotes of Leishmania amazonensis and its surface expression increases during metacyclogenesis. Memórias Inst. Oswaldo Cruz 2010, 105, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Berberich, C.; Machado, G.; Morales, G.; Carrillo, G.; Jimenez-Ruiz, A.; Alonso, C. The expression of the Leishmania infantum KMP-11 protein is developmentally regulated and stage specific. Biochim. Biophys. Acta 1998, 1442, 230–237. [Google Scholar] [CrossRef]
- Russo, D.M.; Turco, S.J.; Burns, J.M.; Reed, S.G. Stimulation of human T lymphocytes by Leishmania lipophosphoglycan-associated proteins. J. Immunol. 1992, 148, 202–207. [Google Scholar]
- Jardim, A.; Funk, V.; Capriolit, R.M.; Olafson, R.W. Isolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein-11, a major immunoreactive membrane glycoprotein. Biochem. J. 1995, 313, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Crusat, M.; Nieto, J.; Chicharro, C.; del Carmen Thomas, M.; Martínez, E.; Valladares, B.; Cañavate, C.; Requena, J.M.; López, M.C.; et al. Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine 2008, 26, 1902–1911. [Google Scholar] [CrossRef][Green Version]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Checa, R.; Montoya, A.; Hernandez, L.; Dado, D.; Galvez, R. Current situation of Leishmania infantum infection in shelter dogs in northern Spain. Parasit. Vectors 2012, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic challenges in the era of canine Leishmania infantum vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Di Filippo, L.; Ordeix, L.; Planellas, M.; Roura, X.; Altet, L.; Martínez-Orellana, P.; Montserrat, S. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasit. Vectors 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Villanueva-Saz, S.; Carbonell, M.; Trotta, M.; Furlanello, T.; Natale, A. Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan®, ID Screen® and Leishmania 96®), a rapid test (Speed Leish K®) and an in-house IFAT. Parasit. Vectors 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Esteve, L.O.; Saz, S.V.; Hosein, S.; Solano-Gallego, L. Histopathological findings and detection of toll-like receptor 2 in cutaneous lesions of canine leishmaniosis. Vet. Parasitol. 2015, 209, 157–163. [Google Scholar] [CrossRef]
- Riera, C.; Valladares, J.E.; Gállego, M.; Aisa, M.J.; Castillejo, S.; Fisa, R.; Ribas, N.; Carrió, J.; Alberola, J.; Arboix, M. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet. Parasitol. 1999, 84, 33–47. [Google Scholar] [CrossRef]
- Cecílio, P.; Pérez-Cabezas, B.; Fernández, L.; Moreno, J.; Carrillo, E.; Requena, J.M.; Fichera, E.; Reed, S.G.; Coler, R.N.; Kamhawi, S.; et al. Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005951. [Google Scholar] [CrossRef]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against Leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef]
- Goldring, J.P.D. Measuring protein concentration with bbsorbance, Lowry, Bradford coomassie blue, or the Smith bicinchoninic acid assay before electrophoresis. Methods Mol. Biol. 2019, 1855, 31–39. [Google Scholar]
- Solano-Gallego, L.; Montserrrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-specific production of IFN-γ and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasit. Vectors 2016, 9, 317. [Google Scholar] [CrossRef]
- Ramírez, L.; de Moura, L.D.; Mateus, N.L.F.; de Moraes, M.H.; do Nascimento, L.F.M.; de Jesus Melo, N.; Taketa, L.B.; Catecati, T.; Huete, S.G.; Penichet, K.; et al. Improving the serodiagnosis of canine Leishmania infantum infection in geographical areas of Brazil with different disease prevalence. Parasite Epidemiol. Control 2020, 8, e00126. [Google Scholar] [CrossRef]
- Freitas, Y.B.N.; Sousa, C.; Magalhaes, J.M.E.; Sousa, M.L.R.; D’Escoffier, L.N.; Valle, T.Z.; Goncalves, T.C.M.; Gil-Santana, H.R.; Kazimoto, T.A.; Amora, S.S.A. Natural infection by Trypanosoma cruzi in triatomines and seropositivity for Chagas disease of dogs in rural areas of Rio Grande do Norte, Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.L.; Xavier, S.C.; Gerhardt, M.; Silva, M.F.; Lima, V.S.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Pará State, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013, 191, 363–366. [Google Scholar] [CrossRef]
- Thomas, M.C.; Garcia-Perez, J.L.; Alonso, C.; Lopez, M.C. Molecular characterization of KMP11 from Trypanosoma cruzi: A cytoskeleton-associated protein regulated at the translational level. DNA Cell Biol. 2000, 19, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; Del Real, G.; Ruitenberg, J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, E.; Van Der Kaaij, S.Y.; Slappendel, R.; Fragio, C.; Ruitenberg, E.J.; Bernadina, W.; Rutten, V.P.M.G. Detection of canine cytokine gene expression by reverse transcription- polymerase chain reaction. Vet. Immunol. Immunopathol. 1999, 69, 121–126. [Google Scholar] [CrossRef]
- Santos-Gomes, G.M.; Rosa, R.; Leandro, C.; Cortes, S.; Romão, P.; Silveira, H. Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. Vet. Immunol. Immunopathol. 2002, 88, 21–30. [Google Scholar] [CrossRef]
- Do Nascimento, P.R.P.; Martins, D.R.A.; Monteiro, G.R.G.; Queiroz, P.V.; Freire-Neto, F.P.; Queiroz, J.W.; Morais Lima, Á.L.; Jeronimo, S.M.B. Association of pro-inflammatory cytokines and Iron Regulatory Protein 2 (IRP2) with Leishmania burden in canine visceral leishmaniasis. PLoS ONE 2013, 8, e73873. [Google Scholar] [CrossRef]
- de Almeida Leal, G.G.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; Carneiro, C.M.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Francisco, A.F.; Cardoso, J.M.; Mathias, F.A.S.; et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 2014, 205, 472–482. [Google Scholar] [CrossRef]
- Basu, R.; Bhaumik, S.; Basu, J.M.; Naskar, K.; De, T.; Roy, S. Kinetoplastid, membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: Evidence for mixed Th1-and Th2-like responses in visceral leishmaniasis. J. Immunol. 2005, 174, 7160–7171. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.P.; Passos, S.; Dutra, W.O.; Soto, M.; Alonso, C.; Gollob, K.J.; Carvalho, E.M.; Ribeiro De Jesus, A. Effect of LACK and KMP11 on IFN-γ production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patients. Scand. J. Immunol. 2005, 61, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Sundar, S. Whole blood assay and visceral leishmaniasis: Challenges and promises. Immunobiology 2014, 219, 323–328. [Google Scholar] [CrossRef] [PubMed]
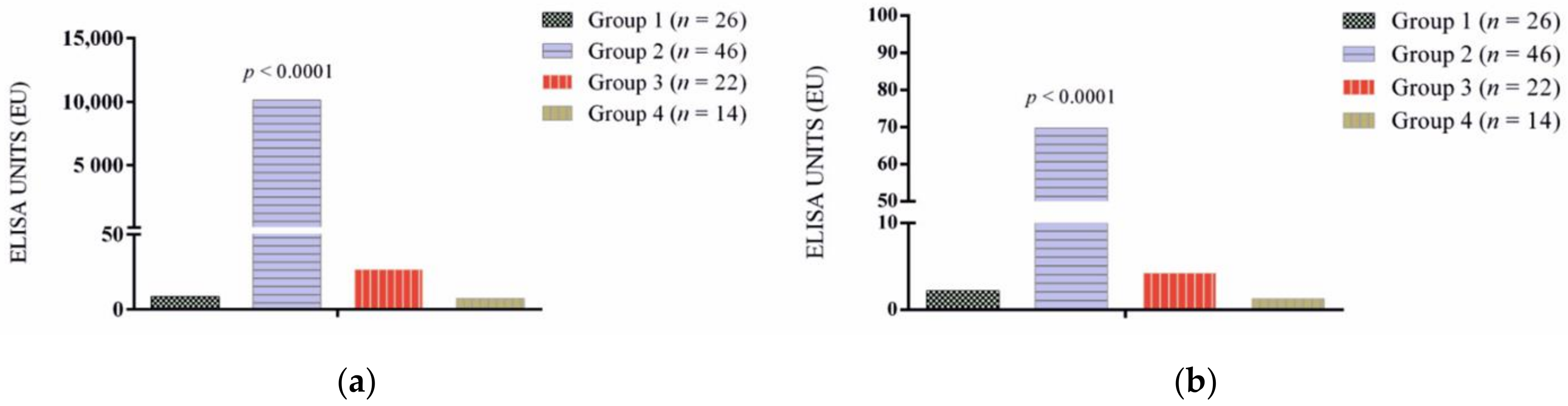
| Total Dogs (n = 108) | Positive Antibody Response | |
|---|---|---|
| L. infantum N (%) | rKMP11 N (%) | |
| Group 1 (n = 26) | 0 (0) | 0 (0) |
| Group 2 (n = 46) | ||
| Stage I (n = 5) Stage IIa (n = 22) Stage IIb (n = 7) Stage III (n = 8) Stage IV (n = 4) | 1 (3.1) 22 (47.8) 7 (15.2) 8 (17.3) 4 (8.6) | 0 (0) 12 (26.0) 6 (13.0) 5 (10.8) 3 (6.5) |
| Group 3 (n = 22) | 4 (18.1) | 0 (0) |
| Group 4 (n = 14) | 0 (0) | 0 (0) |
| Total | 46 (42.5) | 26 (24) |
| Total Dogs (n = 69) | IFNγ Response | |
|---|---|---|
| LSA N (%) | rKMP11 N (%) | |
| Group 1 (n = 25) | 0 (0) | 0 (0) |
| Group 2 (n = 15) | ||
| Stage I (n = 6) Stage IIa (n = 6) Stage IIb (n = 2) Stage III (n = 1) | 2 (13.3) 2 (13.3) 1 (6.6) 0 (0) | 2 (13.3) 0 (0) 0 (0) 0 (0) |
| Group 3 (n = 16) | 12 (75) | 0 (0) |
| Group 4 (n = 13) | 7 (53.8) | 0 (0) |
| Total | 24 (34.7) | 2 (2.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Orellana, P.; González, N.; Baldassarre, A.; Álvarez-Fernández, A.; Ordeix, L.; Paradies, P.; Soto, M.; Solano-Gallego, L. Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein. Vet. Sci. 2022, 9, 116. https://doi.org/10.3390/vetsci9030116
Martínez-Orellana P, González N, Baldassarre A, Álvarez-Fernández A, Ordeix L, Paradies P, Soto M, Solano-Gallego L. Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein. Veterinary Sciences. 2022; 9(3):116. https://doi.org/10.3390/vetsci9030116
Chicago/Turabian StyleMartínez-Orellana, Pamela, Noemí González, Antonella Baldassarre, Alejandra Álvarez-Fernández, Laura Ordeix, Paola Paradies, Manuel Soto, and Laia Solano-Gallego. 2022. "Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein" Veterinary Sciences 9, no. 3: 116. https://doi.org/10.3390/vetsci9030116
APA StyleMartínez-Orellana, P., González, N., Baldassarre, A., Álvarez-Fernández, A., Ordeix, L., Paradies, P., Soto, M., & Solano-Gallego, L. (2022). Humoral Responses and Ex Vivo IFN-γ Production after Canine Whole Blood Stimulation with Leishmania infantum Antigen or KMP11 Recombinant Protein. Veterinary Sciences, 9(3), 116. https://doi.org/10.3390/vetsci9030116






