Impact of Routine Management Procedures on the Welfare of Suckling Piglets
Abstract
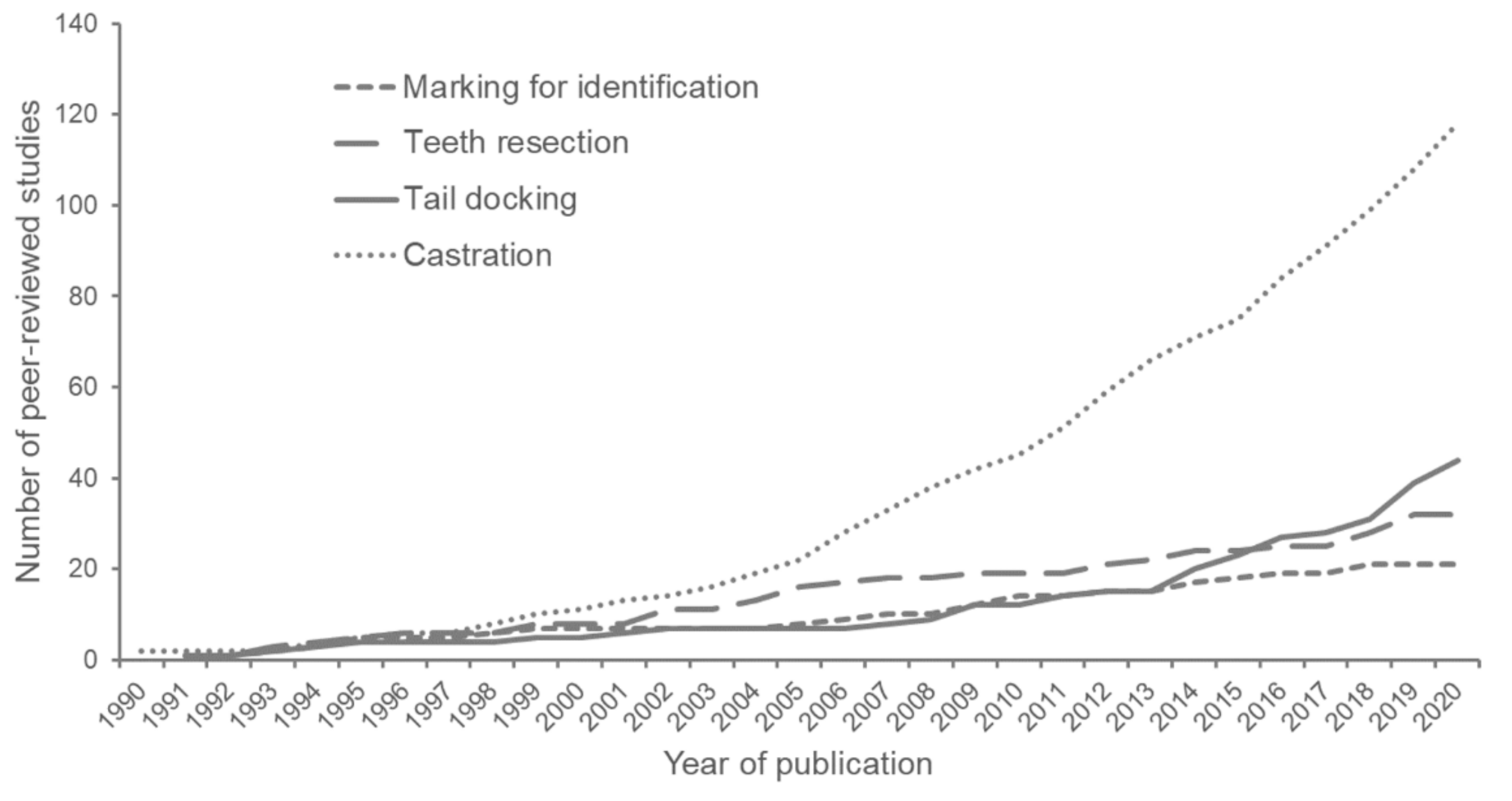
1. Introduction
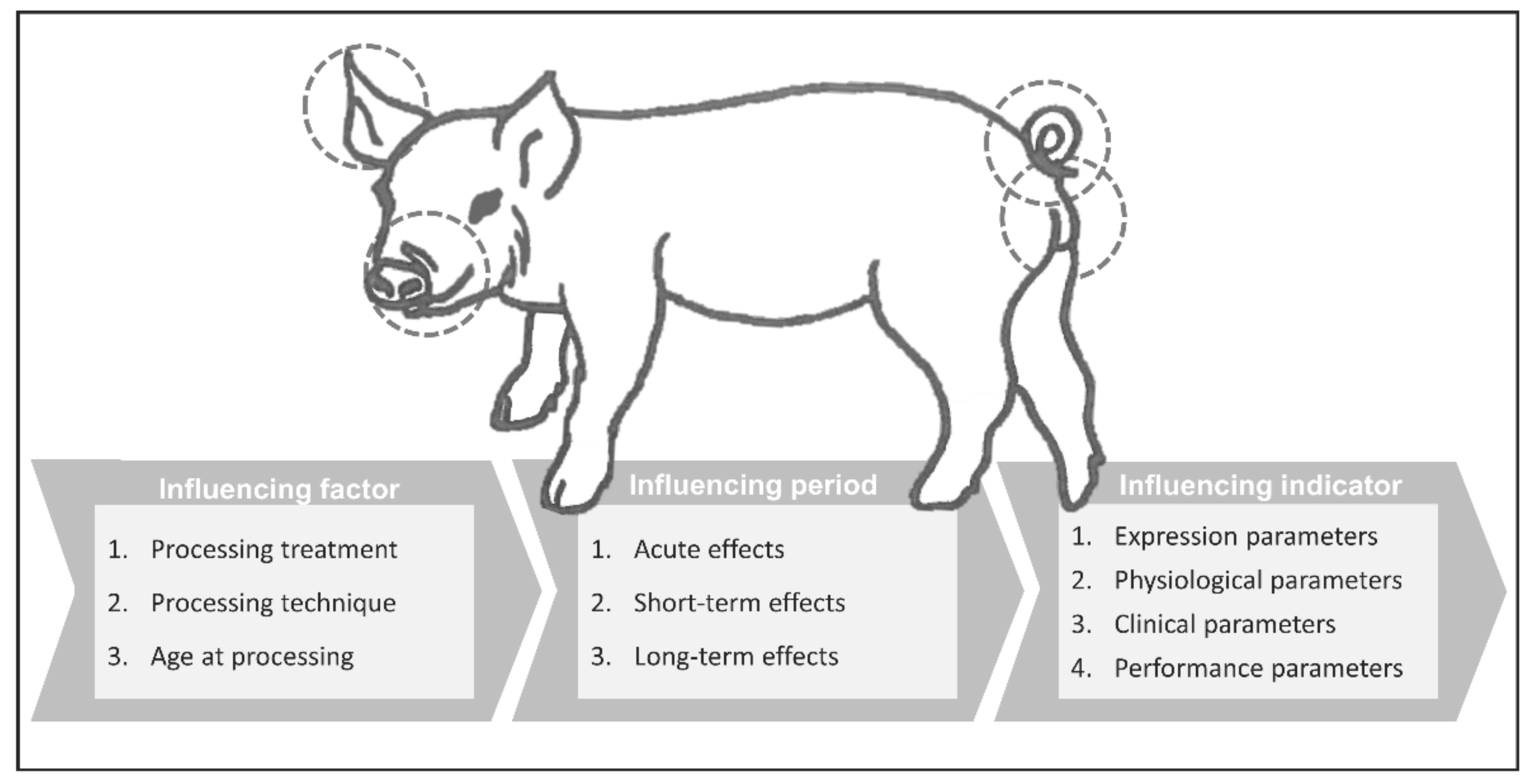
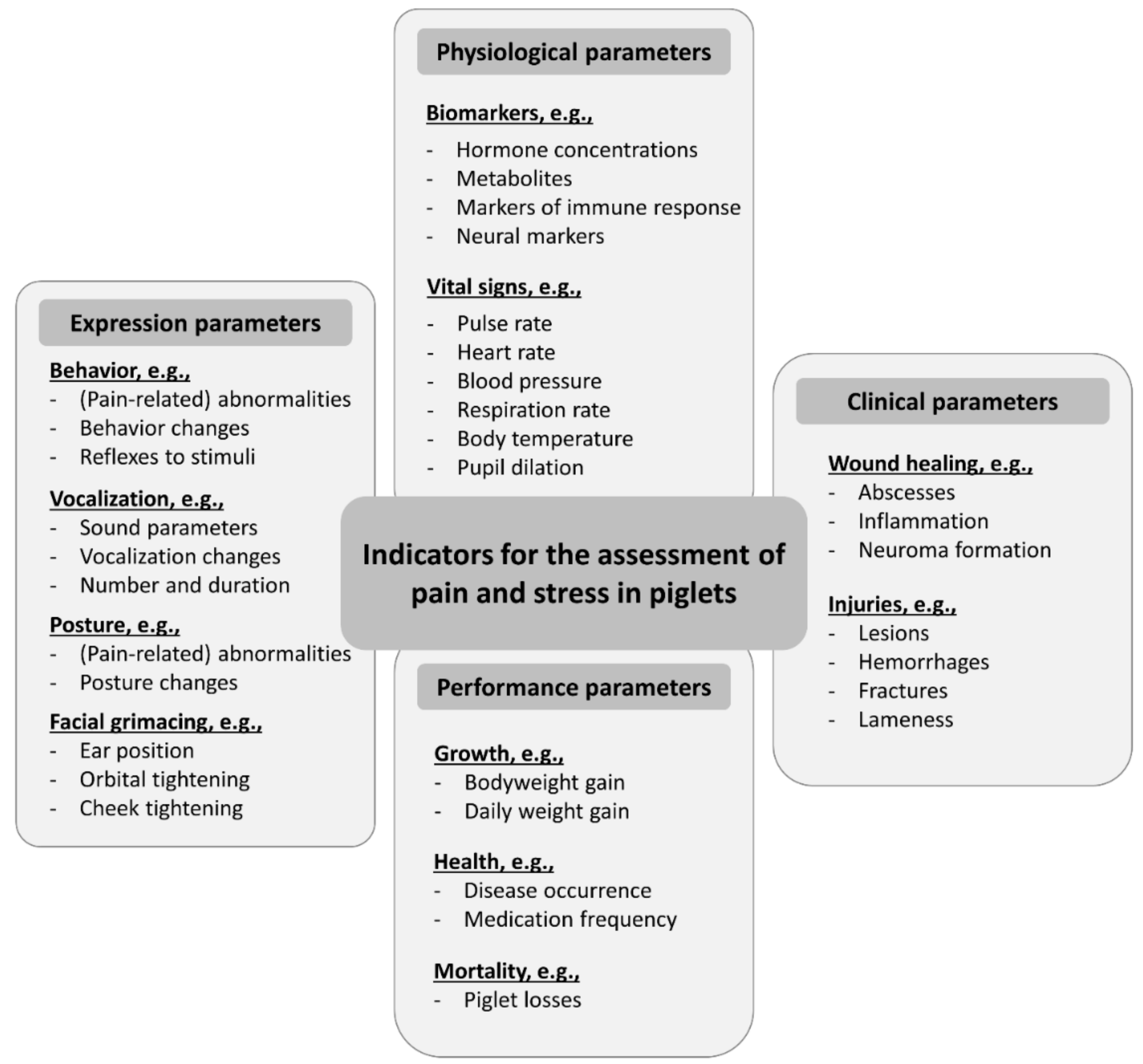
2. Materials and Methods
3. Marking for Identification
3.1. Effects of Marking for Identification in Comparison to Sham Handling
3.2. Effects of Alternative Identification Techniques
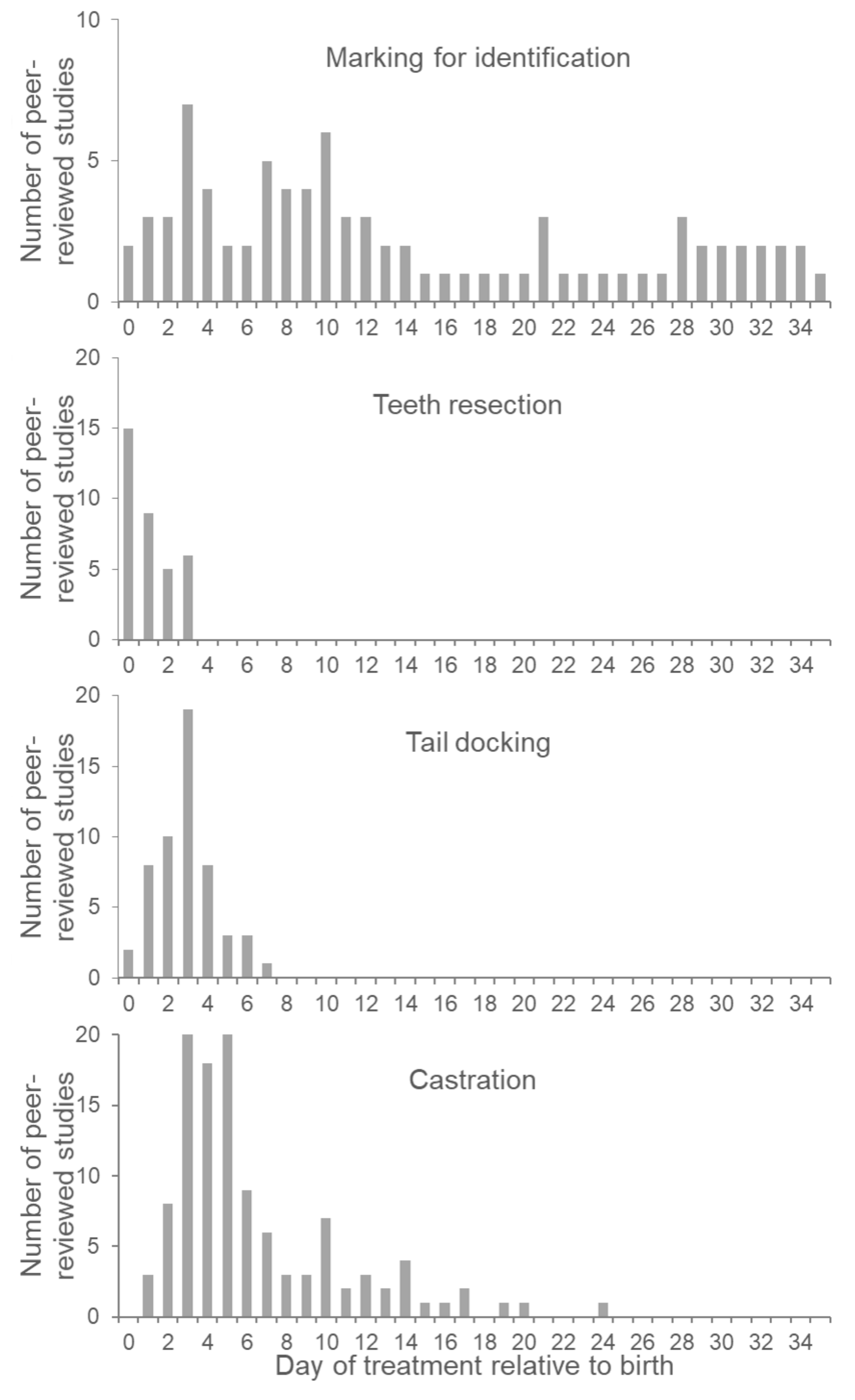
3.3. Effect of Piglet Age at Identification
3.4. Summary of Identification Effects and Practical Implications
4. Teeth Resection
4.1. Effects of Teeth Resection in Comparison to Sham Handling
4.2. Effects of Alternative Teeth Resection Techniques
4.3. Effect of Piglet Age at Teeth Resection
4.4. Summary of Teeth Resection Effects and Practical Implications
5. Tail Docking
5.1. Effects of Tail Docking in Comparison to Sham Handling
5.2. Effects of Alternative Tail Docking Techniques
5.3. Effect of Piglet Age at Tail Docking
5.4. Summary of Tail Docking Effects and Practical Implications
6. Castration
6.1. Effects of Castration in Comparison to Sham Handling
6.2. Effects of Alternative Castration Techniques
6.3. Effect of Piglet Age at Castration
6.4. Summary of Castration Effects and Practical Implications
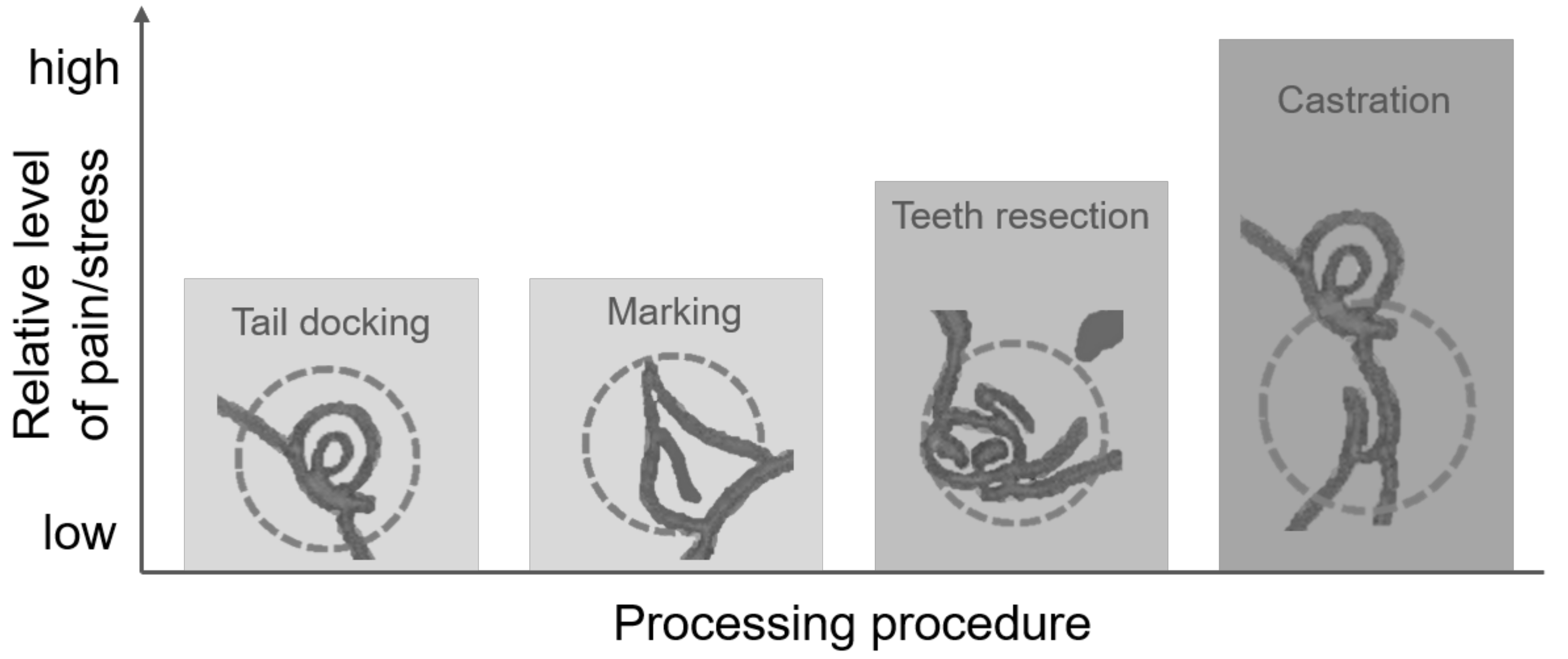
7. Interactions between and Comparison of Procedures
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Informed Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menegatti, L.; Silva, K.C.; Baggio, R.A.; Silva, A.S.; Paiano, D.; Zotti, M.L. Postnatal teeth procedures affect the weight gain and welfare of piglets. Rev. MVZ Córdoba 2018, 23, 6429–6437. [Google Scholar] [CrossRef]
- Numberger, J.; Ritzmann, M.; Übel, N.; Eddicks, M.; Reese, S.; Zöls, S. Ear tagging in piglets: The cortisol response with and without analgesia in comparison with castration and tail docking. Animal 2016, 10, 1864–1870. [Google Scholar] [CrossRef]
- Torrey, S.; Devillers, N.; Lessard, M.; Farmer, C.; Widowski, T. Effect of age on the behavioral and physiological responses of piglets to tail docking and ear notching. J. Anim. Sci. 2009, 87, 1778–1786. [Google Scholar] [CrossRef]
- Fredriksen, B.; i Furnols, M.F.; Lundström, K.; Migdal, W.; Prunier, A.; Tuyttens, F.A.M.; Bonneau, M. Practice on castration of piglets in Europe. Animal 2009, 3, 1480–1487. [Google Scholar] [CrossRef]
- Leslie, E.; Hernández-Jover, M.; Newman, R.; Holyoake, P. Assessment of acute pain experienced by piglets from ear tagging, ear notching and intraperitoneal injectable transponders. Appl. Anim. Behav. Sci. 2010, 127, 86–95. [Google Scholar] [CrossRef]
- Dzikamunhenga, R.S.; Anthony, R.; Coetzee, J.; Gould, S.; Johnson, A.; Karriker, L.; McKean, J.; Millman, S.T.; Niekamp, S.R.; O’Connor, A.M. Pain management in the neonatal piglet during routine management procedures. Part 1: A systematic review of randomized and non-randomized intervention studies. Anim. Health Res. Rev. 2014, 15, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Noonan, G.J.; Rand, J.S.; Priest, J.; Ainscow, J.; Blackshaw, J.K. Behavioural observations of piglets undergoing tail docking, teeth clipping and ear notching. Appl. Anim. Behav. Sci. 1994, 39, 203–213. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Lay, D.C.; McMunn, K.A.; Cheng, H.W.; Pajor, E.A.; Marchant-Forde, R.M. Postnatal piglet husbandry practices and well-being: The effects of alternative techniques delivered in combination. J. Anim. Sci. 2014, 92, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.N. Auswirkungen von Ohrmarken einziehen im Vergleich zu Kastration und Schwanzkupieren und Etablierung einer Verhaltensmethodik zur Beurteilung kastrationsbedingter Schmerzen beim Saugferkel. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2014. [Google Scholar]
- Cordeiro, A.F.d.S.; Nääs, I.d.A.; Baracho, M.d.S.; Jacob, F.G.; de Moura, D.J. The use of vocalization signals to estimate the level of pain in piglets. Eng. Agric. 2018, 38, 486–490. [Google Scholar] [CrossRef]
- Lomax, S.; Hall, E.; Oehlers, L.; White, P. Topical vapocoolant spray reduces nociceptive response to ear notching in neonatal piglets. Vet. Anaesth. Analg. 2018, 45, 366–373. [Google Scholar] [CrossRef]
- O’Connor, A.; Anthony, R.; Bergamasco, L.; Coetzee, J.; Gould, S.; Johnson, A.K.; Karriker, L.A.; Marchant-Forde, J.N.; Martineau, G.S.; McKean, J.; et al. Pain management in the neonatal piglet during routine management procedures. Part 2: Grading the quality of evidence and the strength of recommendations. Anim. Health Res. Rev. 2014, 15, 39–62. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Lay, D.C.; McMunn, K.A.; Cheng, H.W.; Pajor, E.A.; Marchant-Forde, R.M. Postnatal piglet husbandry practices and well-being: The effects of alternative techniques delivered separately. J. Anim. Sci. 2009, 87, 1479–1492. [Google Scholar] [CrossRef]
- Brady, S.E.; Reese, D.E. Proper Way to Ear Notch Pigs. 2008. Available online: http://extensionpublications.unl.edu/assets/html/g1880/build/g1880.htm (accessed on 23 May 2020).
- Nakamura, S.; Sakaoka, A.; Ikuno, E.; Asou, R.; Shimizu, D.; Hagiwara, H. Optimal implantation site of transponders for identification of experimental swine. Exp. Anim. 2019, 68, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Babot, D.; Hernández-Jover, M.; Caja, G.; Santamarina, C.; Ghirardi, J.J. Comparison of visual and electronic identification devices in pigs: On-farm performances. J. Anim. Sci. 2006, 84, 2575–2581. [Google Scholar] [CrossRef]
- Caja, G.; Hernández-Jover, M.; Conill, C.; Garín, D.; Alabern, X.; Farriol, B.; Ghirardi, J. Use of ear tags and injectable transponders for the identification and traceability of pigs from birth to the end of the slaughter line. J. Anim. Sci. 2005, 83, 2215–2224. [Google Scholar] [CrossRef]
- Gosálvez, L.F.; Santamarina, C.; Averós, X.; Hernández-Jover, M.; Caja, G.; Babot, D. Traceability of extensively produced Iberian pigs using visual and electronic identification devices from farm to slaughter. J. Anim. Sci. 2007, 85, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Prola, L.; Perona, G.; Tursi, M.; Mussa, P.P. Use of injectable transponders for the identification and traceability of pigs. Ital. J. Anim. Sci. 2010, 9, e35. [Google Scholar] [CrossRef]
- Schembri, N.; Sithole, F.; Toribio, J.A.; Hernández-Jover, M.; Holyoake, P.K. Lifetime traceability of weaner pigs in concrete-based and deep-litter production systems in Australia. J. Anim. Sci. 2007, 85, 3123–3130. [Google Scholar] [CrossRef]
- Stärk, K.D.; Morris, R.S.; Pfeiffer, D.U. Comparison of electronic and visual identification systems in pigs. Livest. Prod. Sci. 1998, 53, 143–152. [Google Scholar] [CrossRef]
- Barbieri, S.; Minero, M.; Barattiero, D.; Cantàfora, A.F.; Crimella, C. Recognised-by-law versus other identification systems in pigs: Piglets discomfort evaluation and performance testing. Ital. J. Anim. Sci. 2012, 11, e35. [Google Scholar] [CrossRef]
- Bergqvist, A.-S.; Forsberg, F.; Eliasson, C.; Wallenbeck, A. Individual identification of pigs during rearing and at slaughter using microchips. Livest. Sci. 2015, 180, 233–236. [Google Scholar] [CrossRef]
- Lambooij, E.; Langeveld, N.G.; Lammers, G.H.; Huiskes, J.H. Electronic identification with injectable transponders in pig production: Results of a field trail on commercial farms and slaughterhouses concerning injectability and retrievability. Vet. Q. 1995, 17, 118–123. [Google Scholar] [CrossRef]
- Merlot, E.; Mounier, A.M.; Prunier, A. Endocrine response of gilts to various common stressors: A comparison of indicators and methods of analysis. Physiol. Behav. 2011, 102, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lammers, G.H.; Langeveld, N.G.; Lambooij, E.; Gruys, E. Effects of injecting electronic transponders into the auricle of pigs. Vet. Rec. 1995, 136, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Bovey, K.E.; Widowski, T.M.; Dewey, C.E.; Devillers, N.; Farmer, C.; Lessard, M.; Torrey, S. The effect of birth weight and age at tail docking and ear notching on the behavioral and physiological responses of piglets. J. Anim. Sci. 2014, 92, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Moya, S.L.; Boyle, L.A.; Lynch, P.B.; Arkins, S. Influence of teeth resection on the skin temperature and acute phase response in newborn piglets. Anim. Welf. 2006, 15, 291–297. [Google Scholar]
- Lewis, E.; Boyle, L.A.; Brophy, P.; O’Doherty, J.V.; Lynch, P.B. The effect of two piglet teeth resection procedures on the welfare of sows in farrowing crates. Part 2. Appl. Anim. Behav. Sci. 2005, 90, 251–264. [Google Scholar] [CrossRef]
- Holyoake, P.K.; Broek, D.J.; Callinan, A.P.L. The effects of reducing the length of canine teeth in sucking pigs by clipping or grinding. Aust. Vet. J. 2004, 82, 574–576. [Google Scholar] [CrossRef]
- Meyer, E.; Gschwender, F.; Müller, S. Untersuchungen zum Zahnschleifen von Saugferkeln. 2017. Available online: https://www.landwirtschaft.sachsen.de/download/MeyerZaehneschleifen_Fachinfo.pdf (accessed on 31 May 2020).
- Ellert, P. Zahnverletzungen durch das Abschleifen von Zähnen bei Saugferkeln-Untersuchung eines neu entwickelten Schleifkopfes im Vergleich zur herkömmlichen Methode. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hanover, Germany, 2017. [Google Scholar]
- Weary, D.M.; Fraser, D. Partial tooth-clipping of suckling pigs: Effects on neonatal competition and facial injuries. Appl. Anim. Behav. Sci. 1999, 65, 21–27. [Google Scholar] [CrossRef]
- Hessling-Zeinen, U. The opening of the pulp cavities by the routine grinding of the incisors and canines of newborn piglets. Praktische Tierarzt 2014, 95, 1143–1150. [Google Scholar]
- Brookes, J.B.; Lean, I.J. Teeth Clipping in Piglets. Proc. Br. Soc. Anim. Prod. 1993, 1993, 75. [Google Scholar] [CrossRef]
- Hay, M.; Rue, J.; Sansac, C.; Brunel, G.; Prunier, A. Long-term detrimental effects of tooth clipping or grinding in piglets: A histological approach. Anim. Welf. 2004, 13, 27–32. [Google Scholar]
- Zhou, B.; Yang, X.J.; Zhao, R.Q.; Huang, R.H.; Wang, Y.H.; Wang, S.T.; Yin, C.P.; Shen, Q.; Wang, L.Y.; Schinckel, A.P. Effects of tail docking and teeth clipping on the physiological responses, wounds, behavior, growth, and backfat depth of pigs. J. Anim. Sci. 2013, 91, 4908–4916. [Google Scholar] [CrossRef]
- Fu, L.-L.; Zhou, B.; Li, H.-Z.; Liang, T.-T.; Chu, Q.-P.; Schinckel, A.P.; Li, Y.; Xu, F.-L. Effects of tail docking and/or teeth clipping on behavior, lesions, and physiological indicators of sows and their piglets. Anim. Sci. J. 2019, 90, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Meunier-Salaün, M.-C.; Bataille, G.; Rugraff, Y.; Prunier, A. Influence of tail docking and tooth resection on behavior and performance of piglets. J. Anim. Sci. 2002, 80, 371. [Google Scholar]
- Sinclair, A.R.L.; Tallet, C.; Renouard, A.; Brunton, P.J.; D’Eath, R.B.; Sandercock, D.A.; Prunier, A. Behaviour of isolated piglets before and after tooth clipping, grinding or shamgrinding. In Proceedings of the 53rd Congress of the International Society for Applied Ethology (ISAE), Bergen, Norway, 5–9 August 2019. [Google Scholar]
- Boyle, L.A.; Boyle, R.M.; Lynch, P.B. Effect of tooth clipping on piglet behaviour. Proc. Br. Soc. Anim. Sci. 2002, 2002, 226. [Google Scholar] [CrossRef]
- Bates, R.O.; Hoge, M.D.; Edwards, D.B.; Straw, B.E. The influence of canine teeth clipping on nursing and nursery pig performance. J. Swine Health Prod. 2002, 11, 75–79. [Google Scholar]
- Brown, J.M.; Edwards, S.A.; Smith, W.J.; Thompson, E.; Duncan, J. Welfare and production implications of teeth clipping and iron injection of piglets in outdoor systems in Scotland. Prev. Vet. Med. 1996, 27, 95–105. [Google Scholar] [CrossRef]
- Robert, S.; Thompson, B.K.; Fraser, D. Selective tooth clipping in the management of low-birth-weight piglets. Can. J. Anim. Sci. 1995, 75, 285–289. [Google Scholar] [CrossRef]
- Fraser, D.; Thompson, B.K. Armed sibling rivalry among suckling piglets. Behav. Ecol. Sociobiol. 1991, 29, 9–15. [Google Scholar] [CrossRef]
- Gallois, M.; Le Cozler, Y.; Prunier, A. Influence of tooth resection in piglets on welfare and performance. Prev. Vet. Med. 2005, 69, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Lundeheim, N. Facial lesions in piglets with intact or grinded teeth. Acta Vet. Scand. 2012, 54, 23. [Google Scholar] [CrossRef]
- van Beirendonck, S.; Driessen, B.; Verbeke, G.; Permentier, L.; van de Perre, V.; Geers, R. Improving survival, growth rate, and animal welfare in piglets by avoiding teeth shortening and tail docking. J. Vet. Behav. 2012, 7, 88–93. [Google Scholar] [CrossRef]
- Sinclair, A.R.L.; Renouard, A.; Tallet, C.; Sandercock, D.A.; Prunier, A. Piglets exhibit no overt behavioural indicators of pain in the short or long term following tooth resection. In Proceedings of the ESLAV-ECLAM Annual Scientific Meeting on Animal Welfare, Lyon, France, 15–18 November 2016; pp. 141–143. [Google Scholar]
- Hutter, S.; Heinritzi, K.; Reich, E.; Ehret, W. Auswirkungen verschiedener Methoden der Zahnresektion beim Saugferkel. Tierarztl Prax. Ausg. G Grosstiere Nutztiere 1993, 21, 417–428. [Google Scholar]
- Lewis, E.; Boyle, L.A.; Lynch, P.B.; Brophy, P.; O’Doherty, J.V. The effect of two teeth resection procedures on the welfare of piglets in farrowing crates. Part 1. Appl. Anim. Behav. Sci. 2005, 90, 233–249. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, A.M.; Hay, M. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J. Anim. Sci. 2005, 83, 216–222. [Google Scholar] [CrossRef]
- Sinclair, A.R.L.; D’Eath, R.B.; Brunton, P.J.; Prunier, A.; Sandercock, D.A. Traumatic tooth shortening in commercial piglets: Effects on gross tooth pathology and mRNA markers of inflammation and pain. In Proceedings of the International Association for the Study of Pain (IASP) 17th World Congress on Pain, Boston, MA, USA, 12–15 September 2018. [Google Scholar]
- Kober, J.A.; Thacker, B.J. Comparing piglet performance: Day 1 versus day 2 processing. Compend. Contin. Educ. Pract. Vet. 1999, 21, S149–S151. [Google Scholar]
- Brandt, P.; Hakansson, F.; Jensen, T.; Nielsen, M.B.F.; Lahrmann, H.P.; Hansen, C.F.; Forkman, B. Effect of pen design on tail biting and tail-directed behaviour of finishing pigs with intact tails. Animal 2020, 14, 1034–1042. [Google Scholar] [CrossRef]
- Nalon, E.; De Briyne, N. Efforts to Ban the Routine Tail Docking of Pigs and to Give Pigs Enrichment Materials via EU Law: Where do We Stand a Quarter of a Century on? Animals 2019, 9, 132. [Google Scholar] [CrossRef]
- Sandercock, D.A.; Barnett, M.W.; Coe, J.E.; Downing, A.C.; Nirmal, A.J.; Di Giminiani, P.; Edwards, S.A.; Freeman, T.C. Transcriptomics Analysis of Porcine Caudal Dorsal Root Ganglia in Tail Amputated Pigs Shows Long-Term Effects on Many Pain-Associated Genes. Front. Vet. Sci. 2019, 6, 314. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Davis, B.L.; McGlone, J.J. The effect of local or general anesthesia on the physiology and behavior of tail docked pigs. Animal 2011, 5, 1237–1246. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Tucker, C.B. The long and short of it: A review of tail docking in farm animals. Appl. Anim. Behav. Sci. 2011, 135, 179–191. [Google Scholar] [CrossRef]
- EFSA. The risks associated with tail biting in pigs and possible means to reduce the need for tail docking considering the different housing and husbandry systems-Scientific Opinion of the Panel on Animal Health and Welfare. EFSA J. 2007, 5, 611. [Google Scholar] [CrossRef]
- Wallgren, T.; Larsen, A.; Lundeheim, N.; Westin, R.; Gunnarsson, S. Implication and impact of straw provision on behaviour, lesions and pen hygiene on commercial farms rearing undocked pigs. Appl. Anim. Behav. Sci. 2019, 210, 26–37. [Google Scholar] [CrossRef]
- Marcet-Rius, M.; Fàbrega, E.; Cozzi, A.; Bienboire-Frosini, C.; Descout, E.; Velarde, A.; Pageat, P. Are Tail and Ear Movements Indicators of Emotions in Tail-Docked Pigs in Response to Environmental Enrichment? Animals 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Bryer, P.J.; Krebs, N.; McGlone, J.J. Tail docking in pigs: Acute physiological and behavioural responses. Animal 2008, 2, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Herskin, M.S.; Di Giminiani, P.; Thodberg, K. Effects of administration of a local anaesthetic and/or an NSAID and of docking length on the behaviour of piglets during 5 h after tail docking. Res. Vet. Sci. 2016, 108, 60–67. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Turner, P.V. Use of meloxicam, buprenorphine, and Maxilene® to assess a multimodal approach for piglet pain management, part 2: Tail-docking. Anim. Welf. 2019, 28, 499–510. [Google Scholar] [CrossRef]
- Tallet, C.; Rakotomahandry, M.; Herlemont, S.; Prunier, A. Evidence of Pain, Stress, and Fear of Humans During Tail Docking and the Next Four Weeks in Piglets (Sus scrofa domesticus). Front. Vet. Sci. 2019, 6, 462. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Brierley, V.L.M.H.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The Assessment of Facial Expressions in Piglets Undergoing Tail Docking and Castration: Toward the Development of the Piglet Grimace Scale. Front. Vet. Sci. 2016, 3, 100. [Google Scholar] [CrossRef]
- Vitali, M.; Santacroce, E.; Correa, F.; Salvarani, C.; Maramotti, F.P.; Padalino, B.; Trevisi, P. On-Farm Welfare Assessment Protocol for Suckling Piglets: A Pilot Study. Animals 2020, 10, 1016. [Google Scholar] [CrossRef]
- Yun, J.; Ollila, A.; Valros, A.; Larenza-Menzies, P.; Heinonen, M.; Oliviero, C.; Peltoniemi, O. Behavioural alterations in piglets after surgical castration: Effects of analgesia and anaesthesia. Res. Vet. Sci. 2019, 125, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, G.; Scollo, A.; Gottardo, F.; Stefani, A.L.; Schiavon, E.; Capello, K.; Marangon, S.; Bonfanti, L. The effect of tail docking on the welfare of pigs housed under challenging conditions. Livest. Sci. 2015, 173, 78–86. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Bryer, P.J.; Krebs, N.; McGlone, J.J. The effect of method of tail docking on tail-biting behaviour and welfare of pigs. Anim. Welf. 2009, 18, 561–570. [Google Scholar]
- Sandercock, D.A.; Gibson, I.F.; Rutherford, K.M.D.; Donald, R.D.; Lawrence, A.B.; Brash, H.M.; Scott, E.M.; Nolan, A.M. The impact of prenatal stress on basal nociception and evoked responses to tail-docking and inflammatory challenge in juvenile pigs. Physiol. Behav. 2011, 104, 728–737. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Edwards, S.A.; Malcolm, E.M.; Leach, M.C.; Herskin, M.S.; Sandercock, D.A. Characterization of short- and long-term mechanical sensitisation following surgical tail amputation in pigs. Sci. Rep. 2017, 7, 4827. [Google Scholar] [CrossRef]
- Simonsen, H.B.; Klinken, L.; Bindseil, E. Histopathology of intact and docked pigtails. Br. Vet. J. 1991, 147, 407–412. [Google Scholar] [CrossRef]
- Simonsen, H.B. Effect of Early Rearing Environment and Tail Docking on Later Behaviour and Production in Fattening Pigs. Acta Agric. Scand. 1995, 45, 139–144. [Google Scholar] [CrossRef]
- Lecchi, C.; Zamarian, V.; Gini, C.; Avanzini, C.; Polloni, A.; Rota Nodari, S.; Ceciliani, F. Salivary microRNAs are potential biomarkers for the accurate and precise identification of inflammatory response after tail docking and castration in piglets. J. Anim. Sci. 2020, 98, skaa153. [Google Scholar] [CrossRef]
- Herskin, M.S.; Thodberg, K.; Jensen, H.E. Effects of tail docking and docking length on neuroanatomical changes in healed tail tips of pigs. Animal 2015, 9, 677–681. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Welfare aspects of the castration of piglets. EFSA J. 2004, 91, 1–18. [Google Scholar]
- Rault, J.-L.; Lay, D.C.; Marchant-Forde, J.N. Castration induced pain in pigs and other livestock. Appl. Anim. Behav. Sci. 2011, 135, 214–225. [Google Scholar] [CrossRef]
- Hay, M.; Vulin, A.; Génin, S.; Sales, P.; Prunier, A. Assessment of pain induced by castration in piglets: Behavioral and physiological responses over the subsequent 5 days. Appl. Anim. Behav. Sci. 2003, 82, 201–218. [Google Scholar] [CrossRef]
- Prunier, A.; Bonneau, M.; von Borell, E.H.; Cinotti, S.; Gunn, M.; Fredriksen, B.; Giersing, M.; Morton, D.B.; Tuyttens, F.A.; Velarde, A. A review of the welfare consequences of surgical castration in piglets and the evaluation of non-surgical methods. Anim. Welf. 2006, 15, 277–289. [Google Scholar]
- Jäggin, N.; Gerber, S.; Schatzmann, U. General anaesthesia, analgesia and pain associated with the castration of newborn piglets. Acta Vet. Scand. 2006, 48, S12. [Google Scholar] [CrossRef]
- Schmid, S.M.; Leubner, C.D.; Köster, L.N.; Steinhoff-Wagner, J. Status quo-Erhebung zum betriebsindividuellen Management der Kastration von Saugferkeln in Deutschland. Züchtungskunde 2020, 92, 355–372. [Google Scholar]
- White, R.G.; DeShazer, J.A.; Tressler, C.J.; Borcher, G.M.; Davey, S.; Waninge, A.; Parkhurst, A.M.; Milanuk, M.J.; Clemens, E.T. Vocalization and physiological response of pigs during castration with or without a local anesthetic. J. Anim. Sci. 1995, 73, 381–386. [Google Scholar] [CrossRef]
- Weary, D.M.; Braithwaite, L.A.; Fraser, D. Vocal response to pain in piglets. Appl. Anim. Behav. Sci. 1998, 56, 161–172. [Google Scholar] [CrossRef]
- Taylor, A.A.; Weary, D.M. Vocal responses of piglets to castration: Identifying procedural sources of pain. Appl. Anim. Behav. Sci. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; Coetzee, J.F. The physiological and behavioral response of pigs castrated with and without anesthesia or analgesia. J. Anim. Sci. 2012, 90, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Horn, T.; Thielebein, J.; Knubel, B.; von Borell, E. Analysis of pain-related vocalization in young pigs. J. Sound Vib. 2003, 266, 687–698. [Google Scholar] [CrossRef]
- Taylor, A.A.; Weary, D.M.; Lessard, M.; Braithwaite, L. Behavioural responses of piglets to castration: The effect of piglet age. Appl. Anim. Behav. Sci. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Puppe, B.; Schön, P.C.; Tuchscherer, A.; Manteuffel, G. Castration-induced vocalisation in domestic piglets, Sus scrofa: Complex and specific alterations of the vocal quality. Appl. Anim. Behav. Sci. 2005, 95, 67–78. [Google Scholar] [CrossRef]
- Schön, P.C.; Puppe, B.; Tuchscherer, A.; Manteuffel, G. Veränderungen der Vokalisation während der Kastration beim Hausschwein weisen auf Schmerzempfindung hin. Züchtungskunde 2006, 78, 44–54. [Google Scholar]
- Leidig, M.S.; Hertrampf, B.; Failing, K.; Schumann, A.; Reiner, G. Pain and discomfort in male piglets during surgical castration with and without local anaesthesia as determined by vocalisation and defence behaviour. Appl. Anim. Behav. Sci. 2009, 116, 174–178. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; McGlone, J.J. Physiology and behavior of pigs before and after castration: Effects of two topical anesthetics. Animal 2010, 4, 2071–2079. [Google Scholar] [CrossRef]
- Kluivers-Poodt, M.; Houx, B.B.; Robben, S.R.M.; Koop, G.; Lambooij, E.; Hellebrekers, L.J. Effects of a local anaesthetic and NSAID in castration of piglets, on the acute pain responses, growth and mortality. Animal 2012, 6, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Backus, B.L.; Brooks, T.A.; McGlone, J.J. The effect of needle-free administration of local anesthetic on the behavior and physiology of castrated pigs. J. Vet. Behav. 2017, 21, 71–76. [Google Scholar] [CrossRef]
- Keita, A.; Pagot, E.; Prunier, A.; Guidarini, C. Pre-emptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet. Anaesth. Analg. 2010, 37, 367–374. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Hunniford, M.; Lawlis, P.; Leach, M.; Turner, P.V. Development of a Piglet Grimace Scale to Evaluate Piglet Pain Using Facial Expressions Following Castration and Tail Docking: A Pilot Study. Front. Vet. Sci. 2017, 4, 51. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Turner, P.V. Efficacy of buprenorphine for management of surgical castration pain in piglets. BMC Vet. Res. 2018, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, A.V.; Turner, P.V. Use of Meloxicam or Ketoprofen for Piglet Pain Control Following Surgical Castration. Front. Vet. Sci. 2018, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Wemelsfelder, F.; van Putten, G. Behavior as a Possible Indicator for Pain in Piglets: Report B-260; Instituut voor Veeteeltkundig Onderzoek ‘Schoonoord’: Zeist, The Netherlands, 1985. [Google Scholar]
- Carroll, J.A.; Berg, E.L.; Strauch, T.A.; Roberts, M.P.; Kattesh, H.G. Hormonal profiles, behavioral responses, and short-term growth performance after castration of pigs at three, six, nine, or twelve days of age. J. Anim. Sci. 2006, 84, 1271–1278. [Google Scholar] [CrossRef]
- Llamas Moya, S.; Boyle, L.A.; Lynch, P.B.; Arkins, S. Effect of surgical castration on the behavioural and acute phase responses of 5-day-old piglets. Appl. Anim. Behav. Sci. 2008, 111, 133–145. [Google Scholar] [CrossRef]
- Kluivers-Poodt, M.; Zonderland, J.J.; Verbraak, J.; Lambooij, E.; Hellebrekers, L.J. Pain behaviour after castration of piglets; effect of pain relief with lidocaine and/or meloxicam. Animal 2013, 7, 1158–1162. [Google Scholar] [CrossRef]
- Langhoff, R.; Zöls, S.; Barz, A.; Palzer, A.; Ritzmann, M.; Heinritzi, K. Investigation about the use of analgesics for the reduction of castration-induced pain in suckling piglets. Berl. Munch. Tierarztl. Wochenschr. 2009, 122, 325–332. [Google Scholar] [CrossRef]
- Lackner, A. Untersuchungen zur Schmerzhaftigkeit und der Wundheilung bei der Kastration männlicher Ferkel zu unterschiedlichen Kastrationszeitpunkten. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2003. [Google Scholar]
- Byrd, C.J.; Radcliffe, J.S.; Craig, B.A.; Eicher, S.D.; Lay, D.C. Measuring piglet castration pain using linear and non-linear measures of heart rate variability. Anim. Welf. 2020, 29, 257–269. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, L.; Le Neindre, P.; Leterrier, C.; Mormède, P.; Paulmier, V.; Prunet, P.; Terlouw, C.; Guatteo, R. Identifying and monitoring pain in farm animals: A review. Animal 2013, 7, 998–1010. [Google Scholar] [CrossRef]
- Davis, K.; Seddon, Y.M.; Creutzinger, K.; Bouvier, M.; Brown, J. An investigation into the use of sucrose to reduce castration pain in piglets. Can. J. Anim. Sci. 2017, 97, 439–447. [Google Scholar] [CrossRef][Green Version]
- Mühlbauer, I.C. Untersuchungen zur Belastung bei der Kastration von Saugferkeln unter CO2-Narkose. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2009. [Google Scholar]
- Übel, N.; Zöls, S.; Otten, W.; Sauter-Louis, C.; Heinritzi, K.; Ritzmann, M.; Eddicks, M. Auswirkungen der zeitgleichen Durchführung zootechnischer Eingriffe an Saugferkeln. Tierarztl Prax. Ausg. G Grosstiere Nutztiere 2015, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Rauh, A.; Harlizius, J.; Weiß, C.; Scholz, T.; Schulze-Horsel, T.; Escribano, D.; Ritzmann, M.; Zöls, S. Schmerz- und Stressbestimmung bei der Injektion und Kastration von Saugferkeln unter Lokalanästhesie mit Procain und Lidocain. Tierarztl Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zöls, S.; Ritzmann, M.; Heinritzi, K. Einsatz einer Lokalanästhesie bei der Kastration von Ferkeln. Tierarztl Prax. Ausg. G Grosstiere Nutztiere 2006, 34, 103–106. [Google Scholar] [CrossRef]
- Bonastre, C.; Mitjana, O.; Tejedor, M.T.; Calavia, M.; Yuste, A.G.; Úbeda, J.L.; Falceto, M.V. Acute physiological responses to castration-related pain in piglets: The effect of two local anesthetics with or without meloxicam. Animal 2016, 10, 1474–1481. [Google Scholar] [CrossRef]
- Zankl, A. Untersuchungen zur Wirksamkeit und Gewebeverträglichkeit von Lokalanästhetika bei der Kastration männlicher Saugferkel. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2007. [Google Scholar]
- Lomax, S.; Harris, C.; Windsor, P.A.; White, P.J. Topical anaesthesia reduces sensitivity of castration wounds in neonatal piglets. PLoS ONE 2017, 12, e0187988. [Google Scholar] [CrossRef]
- McGlone, J.J.; Hellman, J.M. Local and general anesthetic effects on behavior and performance of two- and seven-week-old castrated and uncastrated piglets. J. Anim. Sci. 1988, 66, 3049–3058. [Google Scholar] [CrossRef]
- McGlone, J.J.; Nicholson, R.I.; Hellman, J.M.; Herzog, D.N. The development of pain in young pigs associated with castration and attempts to prevent castration-induced behavioral changes. J. Anim. Sci. 1993, 71, 1441–1446. [Google Scholar] [CrossRef]
- Pérez-Pedraza, E.; Mota-Rojas, D.; Ramírez-Necoechea, R.; Guerrero-Legarreta, I.; Martínez-Burnes, J.; Lezama-García, K.; Mora-Medina, P.; Rosas, M.; Martínez, V.; González-Lozano, M. Effect of the number of incisions and use of local anesthesia on the physiological indicators of surgically-castrated piglets. Int. J. Vet. Sci. Med. 2018, 6, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Gasteiner, J. Abschlussbericht SanftKast II: Untersuchungen zu einer Alternativen Methode der Schmerzkontrolle durch Kryoanalgesie und Lokalanästhesie bei der Chirurgischen Kastration von Saugferkeln, Raumberg-Gumpenstein. 2009. Available online: https://raumberg-gumpenstein.at/jdownloads/FODOK/sonstige/fodok_3_7210_abschlussbericht_ferkelkastration_sanftkast.pdf (accessed on 1 January 2022).
- Lessard, M.; Taylor, A.A.; Braithwaite, L.; Weary, D.M. Humoral and cellular immune responses of piglets after castration at different ages. Can. J. Anim. Sci. 2002, 82, 519–526. [Google Scholar] [CrossRef][Green Version]
- Kielly, J.; Dewey, C.E.; Cochran, M. Castration at 3 days of age temporarily slows growth of pigs. J. Swine Health Prod. 1999, 7, 151–153. [Google Scholar]
- Tallet, C.; Linhart, P.; Policht, R.; Hammerschmidt, K.; Šimeček, P.; Kratinova, P.; Špinka, M. Encoding of situations in the vocal repertoire of piglets (Sus scrofa): A comparison of discrete and graded classifications. PLoS ONE 2013, 8, e71841. [Google Scholar] [CrossRef]
- Rauh, A.; Hofmann, K.; Harlizius, J.; Weiß, C.; Numberger, J.; Scholz, T.; Schulze-Horsel, T.; Otten, W.; Ritzmann, M.; Zöls, S. Schmerz- und Stressbestimmung bei der Injektion und Kastration von Saugferkeln unter Lokalanästhesie mit Procain und Lidocain. Tierarztl Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.; Hemsworth, P. Tail Docking of Piglets 1: Stress Response of Piglets to Tail Docking. Animals 2020, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
| Identification Method a | Marking Site(s) | Tools Needed for Marking | Induced Tissue Damage | Readability | Losses | References |
|---|---|---|---|---|---|---|
| Ear tagging | Ear (one) | |||||
| Visual ear tag | Ear tag pliers, visual ear tag | Single punched hole | 66–100% (on farm); 69–100% (at slaughter) | 0–29% (on farm); 3–31% (at slaughter) | [16,17,18,19,20,21] | |
| Electronic ear tag | Ear tag pliers, electronic ear tag | 0–100% (on farm); 45–91% (at slaughter) | 0–45% (on farm); 0–31% (at slaughter) | [16,17,18,20,21,22,23] | ||
| Ear notching | Ear (both) | Ear notching pliers | Multiple notched marks | - | - | - |
| Tattooing | Ear (one) | Tattoo pliers, character dies, ink | Multiple dies punctures, injected ink | 0–56.3% (on farm) 0% (at slaughter) | - | [18,20,22] |
| Microchipping | Auricle base (TAB), perineum (TP), peritoneum (TIP) | Syringe and needle, transponder | Single needle puncture, injected transponder | TAB: 22.5–100% (on farm); 18–98% (at slaughter) TP: 100% (at slaughter) TIP: 69–100% (on farm); 69–100% (at slaughter) b | TAB: 17–73 (on farm); 10% (at slaughter) TIP: 0–2% (on farm) b | [16,17,18,19,21,22,23,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, S.M.; Steinhoff-Wagner, J. Impact of Routine Management Procedures on the Welfare of Suckling Piglets. Vet. Sci. 2022, 9, 32. https://doi.org/10.3390/vetsci9010032
Schmid SM, Steinhoff-Wagner J. Impact of Routine Management Procedures on the Welfare of Suckling Piglets. Veterinary Sciences. 2022; 9(1):32. https://doi.org/10.3390/vetsci9010032
Chicago/Turabian StyleSchmid, Simone M., and Julia Steinhoff-Wagner. 2022. "Impact of Routine Management Procedures on the Welfare of Suckling Piglets" Veterinary Sciences 9, no. 1: 32. https://doi.org/10.3390/vetsci9010032
APA StyleSchmid, S. M., & Steinhoff-Wagner, J. (2022). Impact of Routine Management Procedures on the Welfare of Suckling Piglets. Veterinary Sciences, 9(1), 32. https://doi.org/10.3390/vetsci9010032







