Simple Summary
Equine herpesviruses pose a persistent global threat to horse and donkey populations, with nine identified species causing diverse health complications. These pathogens exhibit notable adaptability via specialized glycoprotein networks that mediate cellular and immune evasion. Clinical presentations span from mild respiratory symptoms to fatal neurological conditions, with EHV-1 representing the most dangerous variant. Despite worldwide surveillance efforts documenting cases across five continents, effective interventions remain elusive. Current vaccines provide individual protection but fail to control population-level transmission. Emerging therapeutic research focuses on natural compounds targeting viral replication stages, yet comprehensive disease management requires integrated approaches combining improved diagnostics, enhanced biosecurity measures, and novel treatment strategies.
Abstract
Equine herpesvirus (EHV) infections represent a significant global veterinary and economic challenge affecting both horses and donkeys across all inhabited continents. This narrative review comprehensively examines the nine distinct EHV species (EHV-1 through EHV-9), their taxonomic classification within Alphaherpesvirinae and Gammaherpesvirinae subfamilies, and their diverse host tropism patterns. The complex molecular pathogenesis involves sophisticated viral glycoproteins (gK, gB, gC, gH, gM, gL, gG, gD, gI, gE) that orchestrate cellular invasion, immune evasion, and intercellular transmission. Clinical manifestations vary considerably, ranging from respiratory diseases and reproductive failures to severe neurological disorders, with EHV-1 demonstrating the most severe presentations including myeloencephalopathy. Global distribution analysis reveals widespread circulation across Europe, Asia, Africa, the Americas, and Oceania, with species-specific clinical patterns. Current therapeutic options remain largely supportive, with experimental compounds like berbamine and cepharanthine, celastrol, blebbistatin, and hyperoside showing promise in preclinical studies. Vaccination programs demonstrate limited effectiveness, failing to prevent transmission at population levels despite inducing individual immune responses. The sophisticated immune evasion strategies employed by EHVs, including the “Trojan horse” mechanism utilizing infected leukocytes, highlight the complexity of host–pathogen interactions and underscore the urgent need for innovative prevention and treatment strategies.
Keywords:
equine herpesvirus; donkey; horses; pathogenesis; glycoproteins; vaccination; global epidemiology 1. Introduction
Equids, comprising horses (Equus caballus) and donkeys (Equus asinus), represent economically and culturally significant livestock species with diverse applications across global agricultural systems [1,2]. Donkeys serve as essential working animals in developing regions while also contributing to specialized markets through donkey milk production, which is valued for its nutritional properties and therapeutic applications in human medicine [3,4,5,6,7,8]. Additionally, donkey meat consumption occurs in certain regions [9,10,11,12], and the production of ejiao—a traditional Chinese medicine derived from donkey hide gelatin—has created substantial economic demand [13,14,15]. Horses maintain their historical importance in agriculture as working animals while simultaneously supporting multi-billion-dollar industries including racing, breeding, and recreational activities [1,16,17]. This intimate and extensive human–equid relationship, characterized by close contact during husbandry, training, and recreational activities, necessitates comprehensive understanding of equine diseases that may impact both animal welfare and human health [18,19,20].
Among the infectious diseases affecting equids, equine herpesvirus (EHV) infections represent a significant veterinary and economic concern due to their widespread distribution, diverse clinical manifestations, and potential for causing substantial morbidity and mortality in affected populations (Figure 1) [21,22,23,24,25,26,27]. EHV infection poses a significant therapeutic challenge due to the consistently minimal response to both treatment and vaccination in equines [22,28]. The Equidae family is susceptible to infection by several distinct herpesvirus species, with equine herpesvirus-1 (EHV-1), EHV-2, EHV-3, EHV-4, EHV-5 and EHV-8 being the most clinically relevant pathogens [29,30,31,32,33,34,35,36]. EHV infections are economically important because they cause respiratory disease, abortion storms, reproductive failure, neonatal mortality, and neurological disease known as equine herpesvirus myeloencephalopathy (EHM) [21,37]. The clinical manifestations of EHV infections vary considerably depending on the viral strain, host factors including age and immune status, and environmental stressors [38,39,40,41,42]. The economic impact of EHV infections extends beyond direct treatment costs to include losses from reduced productivity, quarantine measures, event cancellations, and trade restrictions, particularly affecting the racing and breeding industries. Current diagnostic approaches have evolved significantly with the advent of molecular techniques, allowing for rapid and accurate identification of viral nucleic acids and strain differentiation. However, challenges remain in distinguishing between active infection and latent carriage, particularly in the context of disease outbreaks and movement regulations [43,44]. Treatment options remain largely supportive, emphasizing the critical importance of prevention through vaccination programs and management practices [45].
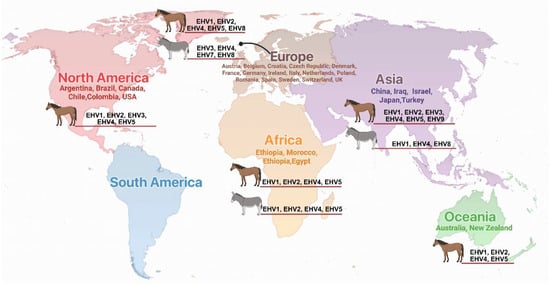
Figure 1.
Global distribution of equine herpes virus infections in horses and donkeys. Countries with documented EHV infections are listed by continent with corresponding viral types (EHV-1 through EHV-9) identified in each region. Continental regions are color-coded for clarity.
This review provides a comprehensive overview of equine herpesvirus infections in horses and donkeys, examining the viral etiology, clinical presentations, diagnostic approaches, treatment strategies, and prevention measures. Understanding the complexities of EHV infections is crucial for developing effective control programs that protect both equine populations and the economic interests dependent upon these valuable animals.
2. The Classification of EHVs and Their Associated Glycoproteins
The EHVs constitute a taxonomically diverse group comprising nine distinct viral species, each characterized by specific phylogenetic relationships and host tropism patterns (Table 1). Within the Alphaherpesvirinae subfamily, several EHV species are classified under the genus Varicellovirus, including EHV-1, EHV-3, EHV-4, EHV-6, EHV-8, and EHV-9 [46]. In contrast, EHV-2, EHV-5 and EHV-7 belong to the Gammaherpesvirinae subfamily, genus Percavirus [47,48]. Host specificity varies among these viral species, with EHV-1, EHV-4, EHV-5, and EHV-8 demonstrating broad equid tropism affecting both horses and donkeys, while EHV-2 similarly infects horses and donkeys, and EHV-7 exhibits preferential infection of donkeys with secondary horse susceptibility, and EHV-3 and EHV-9 display restricted host specificity limited to horses.

Table 1.
Classification of EHVs.
The molecular pathogenesis of equine herpesvirus (EHV) infections involves a sophisticated and complex array of viral glycoproteins that collectively orchestrate the infection process (Table 2) [68]. Each glycoprotein is encoded by specific open reading frames (ORFs) within the viral genome and serves distinct yet interconnected functional roles in mediating viral–host interactions [46]. These proteins work synergistically to establish successful infection, facilitate viral replication, and ensure efficient transmission between host cells. The comprehensive glycoprotein repertoire encompasses several key components, each possessing highly specialized functions that contribute to the overall pathogenic strategy.
Glycoprotein gK (ORF6) serves as a pivotal orchestrator of host cell invasion, functioning as the primary mediator that initiates the infection cascade [69]. Furthermore, this protein facilitates critical viral replication processes within the host cell nucleus and subsequently mediates intercellular viral transmission through complex molecular interactions with cellular receptors. In addition to gK, glycoprotein gB (ORF33) functions as a multifunctional protein that is absolutely essential for successful cellular invasion [70,71]. This protein not only enables efficient viral dissemination between neighboring host cells but also facilitates antigenic epitope presentation, thereby playing a dual role in both infection propagation and immune system evasion strategies. Complementing these invasion mechanisms, glycoprotein gC (ORF16) contributes significantly to host cell invasion processes through its specialized binding domains [72,73]. Moreover, this protein enhances epitope recognition capabilities and serves as a crucial determinant of viral virulence factors. The synergistic action of gC with other glycoproteins amplifies the overall infectivity of the viral particle. Similarly, glycoprotein gH (ORF39) is specifically required for efficient host cell invasion and works in concert with other envelope proteins to facilitate membrane fusion processes [74]. Concurrently, glycoprotein gM (ORF52) assumes a critical role in facilitating viral transmission between neighboring host cells, particularly during the later stages of infection when cell-to-cell spread becomes predominant [74]. The glycoprotein network extends further to include gL (ORF62), which actively participates in cellular invasion processes through its interaction with cellular membrane components [75]. Additionally, glycoprotein gG (ORF70) assumes responsibility for epitope presentation and sophisticated immune system modulation, enabling the virus to evade host immune surveillance mechanisms effectively [76].
Furthermore, glycoprotein gD (ORF72) demonstrates remarkable versatility by mediating both host cell invasion and epitope presentation functions [77]. This dual functionality makes gD a particularly important target for therapeutic interventions. The EHV-1 and EHV-4 gDs interact with equine major histocompatibility complex I (MHC-I) to initiate entry into equine cells [78,79]. Finally, the coordinated action of glycoproteins gI (ORF73) and gE (ORF74) collectively facilitates intercellular viral transmission through specialized cell-to-cell spread mechanisms that bypass extracellular immune recognition.
These glycoproteins, operating as an integrated molecular network, collectively constitute the fundamental framework underlying viral pathogenesis. Consequently, they are essential mediators of host–pathogen interactions and serve as primary modulators of immune response mechanisms. The intricate interplay between these proteins determines the overall success of viral infection and influences disease outcomes in affected hosts.

Table 2.
Major EHV glycoproteins and their functions.
Table 2.
Major EHV glycoproteins and their functions.
| Gene Sequence | Encoded Protein | Main Functions | References |
|---|---|---|---|
| ORF6 | gK | Invasion of host cells, viral replication, viral transmission between host cells | [69] |
| ORF33 | gB | Invasion of host cells, transmission of viruses between host cells, epitopes | [70,71,72,73,80,81] |
| ORF16 | gC | Invasion of host cells, epitopes, viral virulence | [71,72,73] |
| ORF39 | gH | Invasion of host cells | [60,74] |
| ORF52 | gM | Virus transmission between host cells | [74,82,83] |
| ORF62 | gL | Invasion of host cells | [75] |
| ORF70 | gG | Epitopes Immune modulation | [76,84,85,86] |
| ORF72 | gD | Invasion of host cells, epitopes | [52,71,87] |
| ORF73 | gI | Virus transmission between host cells | [88,89,90,91] |
| ORF74 | gE | Virus transmission between host cells |
3. Global Distribution and Clinical Manifestations of Herpes Virus Infections in Donkeys and Horses
Herpes virus infections represent a significant health concern in equids worldwide, affecting both horses and donkeys across all inhabited continents. The documented cases span from Europe to Oceania, demonstrating the global distribution and diverse clinical presentations of these viral pathogens (Table 3). This comprehensive analysis presents the geographic distribution patterns and clinical manifestations of various EHV types based on reported cases from multiple countries.
Europe demonstrates the highest diversity of reported herpes virus infections, with cases documented across 15 countries. The most frequently reported virus types include EHV-1, EHV-2, EHV-4, and EHV-5, with notable clinical presentations ranging from respiratory diseases to neurological manifestations. Countries such as Germany, Italy, and the Netherlands have reported multiple virus types, indicating either higher surveillance capacity or increased viral circulation [57,92,93]. The Asian continent demonstrates significant herpes virus activity, particularly in China, where multiple virus types (EHV-1, EHV-4, and EHV-8) have been identified in both horses and donkeys. Notably, EHV-8 infections in donkeys have been associated with severe clinical outcomes including respiratory disease, abortion, and neurological disorders [61,62,94]. Japan shows a unique pattern with reports of EHV-3, EHV-4, and EHV-9 in horses [63,95]. While in Korea, cases of EHV2 and EHV5 in horses have been reported [96].
African countries, particularly Ethiopia and Morocco, show a concerning pattern of herpes virus infections affecting both horses and donkeys. Ethiopia reports a diverse range of virus types (EHV-1, EHV-2, EHV-4, EHV-5) with clinical manifestations including abortion, respiratory distress, and neurological signs [34,97,98,99]. The North and South American continents demonstrate varied patterns of infection, with reports extending from Canada to Chile, while Oceania contributes additional cases from Australia and New Zealand, completing the global distribution pattern.
Among the various virus types, EHV-1 demonstrates the most severe clinical presentations globally, consistently associated with myeloencephalopathy, viremia, and nasal discharge across multiple continents [100]. Neurological manifestations are particularly prominent in reports from France, Italy, Spain, Chile, and the USA [49,101,102,103,104,105,106]. In contrast, EHV-4 primarily causes respiratory tract infections, with documented cases showing pyrexia, nasal discharge, mandibular lymphadenopathy, and increased lung sounds. This pattern remains consistent across Europe, Asia, and Africa [59,107]. EHV-2 and EHV-5 infections show particular association with respiratory diseases and, notably, squamous cell carcinoma in horses. EHV-2/5 DNA has been detected in horses with head-neck, ocular, penile, and vulvar squamous cell carcinoma, suggesting potential oncogenic properties [108,109]. In addition, while EHV-2/5 DNA has been detected in equine squamous cell carcinoma cases, causality remains unproven. Future studies should employ experimental inoculation models in immunocompromised horses with careful control for confounding factors including UV exposure, genetic predisposition, and co-infections with equine papillomaviruses to definitively establish the oncogenic potential of these gammaherpesviruses. EHV-8 shows a distinctive pattern of causing abortion in pregnant mares and has been associated with respiratory disease and neurological disorders in donkeys, particularly in Asian populations [31,57].
The data reveals distinct susceptibility patterns between horses and donkeys. While horses are susceptible to the full spectrum of EHV types (EHV-1 through EHV-9), donkeys show particular susceptibility to EHV-1, EHV-4, EHV5, EHV7 and EHV-8, AHV types. Regarding asinine herpesviruses (AHV), AHV-1, AHV-5, and AHV-7 appear to be donkey-specific with AHV-1&5 causing severe interstitial pleuropneumonia and pulmonary fibrosis [47,92]. The majority of diagnoses were confirmed through PCR-based methods, demonstrating the reliability of molecular diagnostics for herpes virus identification. Some studies employed ELISA-based serological testing, particularly in African countries, while postmortem pathological findings provided crucial diagnostic information in severe cases [92,110]. This global overview demonstrates the widespread distribution and significant clinical impact of herpes virus infections in equids. The diversity of clinical presentations, from mild respiratory signs to severe neurological manifestations and reproductive failures, underscores the importance of continued surveillance and research into these viral pathogens. The geographic distribution patterns suggest both endemic circulation and possible cross-border transmission, highlighting the need for coordinated international monitoring and control strategies. The documented evidence across five continents confirms that herpes virus infections in equids constitute a global veterinary health challenge requiring sustained scientific attention and collaborative international response efforts.

Table 3.
Global distribution of equine herpes virus and their clinical signs in donkeys and horses.
Table 3.
Global distribution of equine herpes virus and their clinical signs in donkeys and horses.
| Europe | ||||
|---|---|---|---|---|
| Virus Type | Species | Reported Clinical Signs | Country | Reference |
| EHV2/5 | Horses | Herpesvirus DNA (EHV2 and EHV5) was detected in horses with head-neck, ocular, penile, and vulvar squamous cell carcinoma respectively. | Austria | [108,111] |
| EHV1 | Horses | Respiratory disorders, abortion and neonatal foal death | Belgium | [112] |
| EHV1/4 | Horses | Abortion | Bulgaria | [113] |
| EHV-1 | Horses | Abortion and neonatal foal death | Croatia | [114] |
| EHV-2 | Horses | Keratitis and keratoconjunctivitis | Czech Republic | [115] |
| EHV4 | Horses | Pyrexia, nasal discharge, mandibular lymphadenopathy and increased lung sound upon auscultation | Denmark | [59] |
| EHV1 | Horses | Myeloencephalopathy and fever | France | [102,116] |
| EHV4 | Horses | Respiratory diseases (PCR diagnosis) | Germany | [117] |
| EHV1 | Horses | Immune suppression, abortion and respiratory diseases | Germany | [118,119] |
| EHV1/4 | Horses | Rhinopneumonitis and abortion | Germany | [120] |
| EHV2 | Horses | Keratoconjunctivitis | Germany | [121] |
| EHV-8 | Horses | Abortion in pregnant mares | Ireland | [31] |
| AHV-1&5 | Donkeys | Interstitial pleuropneumonia and pulmonary fibrosis (Postmortem findings) | Italy | [92] |
| AHV7&5 | Donkeys | Respiratory distress (increased respiratory rate, nostril flaring, nasal discharge and pyrexia) | Italy | [47] |
| EHV3 | donkeys | Ulcerative stomatitis | Italy | [30] |
| EHV-1 | Horses | Myeloencephalopathy, Viremia, with nasal discharge (PCR diagnosis) | Italy | [49] |
| EHV1,4/5 | Horses | Abortion and neurological disorders. | Italy | [122] |
| EHV-8 | Donkeys | Respiratory disease, abortion and neurological disorders. | Netherlands | [57] |
| EHV1/4 | Horses | Acute respiratory disease, abortion and neurological signs (using PCR) | Netherlands | [123,124,125] |
| EHV1 | Horses | Abortion, neonatal foal death | Poland | [126,127] |
| EHV-4 | Donkeys | Upper respiratory tract infection, abortion and neurological signs | Romania | [107] |
| EHV-1 | Horses | Myeloencephalopathy and Lymphopenia | Spain | [128,129] |
| EHV2 | Horses | Immunosuppression and upper respiratory tract infection | Sweden | [130] |
| EHV5 | Horses | Equine Multinodular Pulmonary Fibrosis with Leukocytosis, hyperfibrinogenemia and hypoxemia. Thoracic radiographs showed pneumonia with a multifocal nodular pattern | Sweden | [131,132] |
| EHV-1 | Horses | Abortion and myeloencephalopathy | Switzerland | [133] |
| EHV-1/4 | Horses | Rhinopneumonitis, abortions, paresis and neonatal foal deaths. | UK | [134,135] |
| Asia | ||||
| EHV-1 | Donkeys | Respiratory distress, abortion and death of young foal | China | [50] |
| EHV-1 | Donkeys | Abortion and neurological signs | China | [136] |
| EHV-1 | Horses | Respiratory distress and abortion | China | [137,138] |
| EHV-1&4 | Donkeys | Abortion and respiratory signs | China | [58] |
| EHV-8 | Donkeys | Respiratory disease, abortion and neurological disorders. | China | [139,140,141] |
| Israel | ||||
| EHV2,4,5 | Horses | Respiratory disease (increased respiratory rate, nasal dis-charge and pyrexia) | China | [142] |
| EHV1 | Donkeys and horses | Respiratory disease (increased respiratory rate, nasal dis-charge and pyrexia) | Iraq | [143] |
| EHV1/4 | Horses | Respiratory diseases and fever | Israel | [144] |
| EHV 3 | Horses | Equine coital exanthema followed formation of papules, pustules, ulcers and scabs on the progenital skin | Japan | [95] |
| EHV 4 | Horses | Nasal discharge, mucosal inflammation of upper respiratory tract and enlargement of mandibular lymph node. | Japan | [145] |
| EHV 9 | Horses | Fever and respiratory distress | Japan | [63] |
| EHV-1 | Horses | Neurological disorders | Japan | [146] |
| EHV4 | Horses | equine rhinopneumonitis | Japan | [147] |
| EHV-1&4 | Donkey and horses | Abortion and respiratory signs | Turkey | [148,149] |
| EHV5 | Horses | Respiratory disease (increased respiratory rate, nasal discharge and pyrexia) | Turkey | [48] |
| Africa | ||||
| EHV-1 | Donkeys | Abortion and neurological signs | Ethiopia | [150] |
| EHV-1&4 | Donkeys and horses | Abortion and respiratory signs | Morocco, Ethiopia | [98,151,152] |
| EHV1,2&5 | Donkeys and horses | Respiratory distress (increased respiratory rate, nostril flaring, nasal discharge and pyrexia) | Ethiopia | [99] |
| EHV-2/5 | Donkeys and horses | Respiratory distress (increased respiratory rate, nostril flaring, nasal discharge and pyrexia) | Ethiopia | [65] |
| EHV-5 | Donkeys | Abortion, respiratory distress and neurological signs | Ethiopia | [47,97] |
| EHV1/4 | Horses | Respiratory diseases and neurological signs (ELISA diagnose) | Morocco | [110] |
| EHV4 | Horses | Abortion | Egypt | [153] |
| North and South America | ||||
| EHV1 | Horses | Abortions, perinatal foal mortality, and myeloencephalopathy | Argentina | [154,155] |
| EHV2 | Horses | Immunosuppression in foals, upper respiratory tract disease, conjunctivitis, general weakness | Argentina | [156] |
| EHV2 | Horse | Respiratory distress (increased respiratory rate, nostril flaring, nasal discharge and pyrexia) | Brazil | [64] |
| EHV1/4 | Horses | Abortion and respiratory distress | Brazil | [157,158] |
| EHV1 | Horses | Myeloencephalopathy and nasal discharge | Canada | [159] |
| EHV-1 | Horses | Myeloencephalopathy, Viremia, with nasal discharge (PCR diagnosis) | Chile | [101] |
| EHV-3 | Horses | Equine coital rash (ECE) (PCR diagnosis) | Chile | [53] |
| EHV1/4 | Horses | Abortion and respiratory distress (Indirect ELISA and PCR) | Colombia | [160] |
| EHV3 | Horses | Equine coital exanthema | Columbia | [161] |
| EHV-5 | Horses | Facial lymphohistiocytic interface dermatitis | USA | [109] |
| EHV5 | Horses | Equine multinodular pulmonary fibrosis and lymphoma | USA | [162,163] |
| EHV1 | Horses | Myeloencephalopathy, Viremia, with nasal discharge | USA | [164,165,166] |
| Oceania | ||||
| EHV1 | Horses | Idiopathic hemorrhagic cystitis | Australia | [167,168] |
| EHV1,2,4/5 | Horses | Respiratory distress (increased respiratory rate, nostril flaring, nasal discharge and pyrexia) | Australia | [169,170,171] |
| EHV2, EHV5 | Horses | Respiratory disease (increased respiratory rate, nasal discharge and pyrexia) | Australia, New Zealand | [172,173,174] |
4. The Pathogenesis of and Immune Response to EHVs
The pathogenesis of equine herpesviruses represents a complex interplay between viral invasion strategies and host immune responses that ultimately determines disease outcome. EHV initiates infection in the upper respiratory tract by exploiting innate immune mechanisms, particularly co-opting antimicrobial equine β-defensins (eBDs) to enhance binding and infection of primary respiratory epithelial cells (ERECs). This process is facilitated by viral glycoprotein gM, which stabilizes the virion envelope and confers resistance to eBD-mediated permeabilization [175]. Despite this enhanced entry mechanism, EHV replication in the respiratory epithelium remains inherently limited, representing a strategic viral mechanism to control local innate immune responses while facilitating establishment of latency in trigeminal ganglion neurons and modulating leukocyte recruitment [176,177,178,179].
Following respiratory tract infection, viral shedding continues for approximately seven days, accompanied by virus migration to local lymph nodes, establishment of leukocyte-associated viremia, and subsequent dissemination to multiple organ systems [180,181,182]. Studies utilizing EHV-8 in murine models have revealed multi-organ tropism with viral DNA detection in lungs, liver, brain, and intestinal tissues [62]. The pulmonary system appears particularly susceptible, with EHV-8 demonstrating preferential replication in lung tissue and triggering significant elevation of pro-inflammatory cytokines including IL-1β, IL-6, IL-8, and IFN-α, indicating the virus’s capacity to induce localized inflammatory responses [62,183].
A validated “old mare model” using horses > 18 years old to experimentally induce equine herpesvirus myeloencephalopathy (EHM) demonstrated that all 9 old mares developed neurological disease compared to only 1 of 9 young horses [184]. Protection from EHM was associated with early, robust type I interferon responses and TH-1 cellular immunity (including increased IFN-α, IL-1β, IFN-γ, and CXCL10), while horses that developed EHM showed delayed interferon responses, higher regulatory cytokines (IL-10, TGF-β), and elevated IgG3/5 antibodies indicative of a TH-2-biased immune response. The EHM horses also exhibited significantly higher cell-associated viremia but reduced nasal viral shedding compared to protected animals, suggesting that the failure to mount timely innate immune responses at the respiratory tract enables systemic viral dissemination and neurological disease [184].
4.1. Innate Immune Responses and Viral Modulation
The host immune response to EHV infection involves activation of multiple signaling pathways and inflammatory mediators. Transcriptomic analysis has revealed that EHV-8 modulates host immune responses through TNF pathway activation, involving key targets such as TNFR1, NF-κB p65, and MAP3K8, along with inflammatory signaling pathways including TNF and NF-κB signaling [61]. The type I interferon system serves as a critical antiviral defense mechanism, with EHV-1 infection upregulating IFN-α in ERECs and mucosal explants [185]. However, viral modulation of this response occurs in a strain-specific manner, with neurovirulent strains demonstrating sensitivity to IFN restriction while non-neurovirulent strains have evolved effective anti-IFN mechanisms to prioritize upper respiratory tract replication [185].
EHV-1 infection triggers a broader innate immune response characterized by upregulation of Toll-like receptors (TLR-3, TLR-9), inflammatory cytokines (IL-1, TNF-α, IL-6), and key chemokines including IL-8, MCP-1/CCL2, and CCL5 to recruit target immune cells such as CD172a+ monocytes and T lymphocytes to infection sites [186,187]. Neurovirulent strains typically induce more robust recruitment of these cellular populations compared to non-neurovirulent strains [188]. Additional systemic markers of infection include elevated serum amyloid A levels in EHV-1-infected horses [189], reflecting the broader inflammatory response associated with viral pathogenesis.
4.2. Viral Immune Evasion Strategies
To counteract immune cell recruitment and recognition, EHV-1 employs sophisticated immunomodulatory strategies through specific viral proteins. The viral chemokine-binding protein (vCKBP) gG binds and neutralizes chemokines like IL-8, effectively dampening neutrophil and monocyte migration [190,191]. Deletion studies of gG demonstrate enhanced inflammatory responses and increased immune cell migration to infected sites [86,186]. Additionally, glycoprotein gp2 exhibits similar chemokine-binding activity, while the early protein pUL56 performs dual immunoevasive functions by downregulating cell surface MHC I to evade cytotoxic T lymphocyte lysis and modulating chemokine expression to control leukocyte recruitment [192].
4.3. Leukocyte-Associated Viremia and Cell-to-Cell Spread
EHV-1 utilizes a sophisticated “Trojan horse” strategy, exploiting infected leukocytes to breach basement membranes and enter systemic circulation [178,179]. This immune evasion mechanism involves restricted expression of late viral glycoproteins (gC, gD) in infected leukocytes, protecting them from immune recognition despite the presence of neutralizing antibodies [193,194]. Viral replication remains highly restricted in primary carrier cells, particularly CD172a+ monocytic cells, with non-neurovirulent strains specifically delaying replication through epigenetic mechanisms involving silencing of viral gene expression by histone deacetylases (HDACs) [195,196]. Entry into these cells occurs inefficiently through sialic acid-containing receptors and integrin αvβ3 co-receptors, triggering entry via cholesterol-dependent, phagocytosis-like endocytic pathways [197].
T lymphocytes, particularly CD4+ cells, serve as important vehicles for viremia through distinct mechanisms. While complete viral replication cycles occur in these cells, glycoproteins aggregate at cell surfaces and virion assembly becomes impaired, preventing antibody recognition. Infectious virus transmission occurs efficiently through virological synapses upon contact with target cells, representing a form of cell-to-cell spread that effectively evades humoral immunity [188].
The adhesion of infected CD172a+ cells to endothelial cells is mediated by upregulated integrins (αVβ3, α4β1, αLβ2) and enhanced by pro-inflammatory cytokines in the local microenvironment [198]. Cell-to-cell contact subsequently activates viral replication in leukocytes and facilitates virion transfer to endothelial cells.
4.4. Endothelial Cell Infection and Interferon Suppression
Upon endothelial cell infection, EHV-1 actively suppresses host IFN responses to enable efficient replication. The virus disrupts IFN pathways at multiple levels by downregulating TLR3/4 and key interferon regulatory factors (IRF-3, IRF-7, IRF-9), inhibiting IRF-3 nuclear translocation, and degrading signaling components like tyrosine kinase 2 (TYK2) to suppress STAT1/STAT2 phosphorylation [199,200,201,202]. This comprehensive suppression of antiviral signaling, coupled with induction of pro-inflammatory states, promotes viral spread within endothelial tissues and contributes to the pathogenesis of clinical disease manifestations including abortion and myeloencephalopathy.
4.5. Pathophysiological Mechanisms of Major Disease Manifestations
4.5.1. Abortion Pathophysiology
EHV-1-induced abortion represents a devastating reproductive complication characterized by complex vascular pathology at the uteroplacental interface. Following establishment of cell-associated viremia, virus-laden peripheral blood mononuclear cells (PBMCs), particularly CD172a+ monocytes and T lymphocytes, adhere to and transfer virus to endothelial cells lining the small arterioles of the pregnant uterus [198,203,204]. This adhesion is facilitated by upregulation of cellular adhesion molecules, including integrins αVβ3, α4β1, and αLβ2, on infected leukocytes and their corresponding receptors on endothelial cells—a process enhanced by pro-inflammatory cytokines in the local microenvironment [198,203].
EHV-1 infection of CD172a+ monocytic cells induces cellular changes that promote their adhesion to endothelial cells through a three- to five-fold increase in adhesion capacity [198]. Both cell-to-cell contact and secretion of soluble factors by endothelial cells, such as tumor necrosis factor-alpha (TNF-α), activate EHV-1 replication in CD172a+ cells, facilitating direct transfer of cytoplasmic viral material to adjacent endothelial cells [198] (Laval et al., 2015). This cell-associated transmission mechanism allows the virus to bypass antibody-mediated immune responses, explaining why vaccination alone cannot prevent viremia [203].
Upon endothelial cell infection and subsequent viral replication, widespread vasculitis develops, particularly affecting the small vascular networks supplying the glandular endometrium at the base of microcotyledons [205,206]. Endothelial cell necrosis triggers release of procoagulant factors, including tissue factor (thromboplastin), initiating intravascular thrombosis and ischemic necrosis of cotyledonary and intercotyledonary tissues [204,207]. This thrombo-ischemic cascade causes rapid progressive placental–endometrial separation immediately preceding fetal expulsion, with the fetus dying of anoxia as oxygen and nutrient supply is abruptly terminated [204,205,208].
Notably, widespread vascular endothelial damage may induce abortion even before detectable transplacental viral transmission to the fetus occurs [206,209]. The severity of abortion correlates with viral strain virulence, magnitude and duration of viremia, and hormonal influences on the uterine immune microenvironment during late pregnancy [210]. Additionally, endometrial resident lymphocytes may directly transfer virus to uterine endothelium, potentially explaining sporadic individual abortions or those occurring weeks to months after apparent resolution of viremia [182].
4.5.2. Myeloencephalopathy Pathophysiology
Equine herpesvirus myeloencephalopathy (EHM) represents the most devastating neurological manifestation of EHV-1 infection, characterized by diffuse multifocal hemorrhagic myeloencephalopathy secondary to central nervous system (CNS) vasculitis and thrombosis [210,211]. The pathogenic sequence mirrors abortion pathophysiology but targets CNS vasculature specifically. Following establishment of cell-associated viremia, infected leukocytes transport virus to CNS endothelial cells lining small arteries and arterioles throughout the brain, brainstem, and spinal cord [203].
The neuropathogenic D752 variant of EHV-1, characterized by a single nucleotide polymorphism in the DNA polymerase gene (ORF30), demonstrates 10- to 100-fold higher viremia levels, longer persistence, and preferential CD4+ T lymphocyte tropism compared to non-neuropathogenic N752 strains, facilitating more efficient CNS invasion [210,212,213]. This SNP at position 2254 of ORF30 results in an amino acid variation (N752/D752) that significantly influences neuropathogenic potential [214,215]. However, recent surveillance data demonstrates that the N752 genotype has become the predominant variant detected not only in respiratory disease (87.5%) and abortion (80%) cases, but also in EHM cases (74.3%), challenging earlier assumptions about genotype-disease associations [216]. Upon close contact or adhesion between virus-infected PBMCs and CNS endothelial cells, cell-to-cell viral transmission occurs, bypassing antibody-mediated immune responses [203]. Infected endothelial cells undergo lytic infection, triggering vasculitis of CNS arterioles. This vasculitis is followed by intravascular thrombosis and focal infarction, ultimately resulting in ischemic degeneration manifesting as areas of malacia (tissue softening) in both white and gray matter [210,211].
Histopathologic examination reveals discrete lesions comprising vasculitis with endothelial cell damage and perivascular cuffing, thrombus formation, hemorrhage, and in advanced cases, extensive malacial regions distributed throughout the brain and spinal cord [217]. Critically, there is no evidence of direct neuronal invasion by the virus; instead, neurological deficits result entirely from ischemic damage secondary to vascular thrombosis [210,218].
Cerebrospinal fluid analysis typically reveals xanthochromia, elevated protein concentrations (100–500 mg/dL), and increased albumin quotient, reflecting vasculitis and protein leakage, though white blood cell counts usually remain normal [217]. The acute onset and rapid progression of clinical signs (within 24–48 h) reflect the fulminant nature of CNS vascular damage, with ataxia, paresis, urinary incontinence, and in severe cases, recumbency representing the multifocal distribution of ischemic spinal cord lesions [133,218]. Studies investigating EHV-1-induced encephalomyelitis have demonstrated that intranasal infection with highly neurovirulent strains results in fulminant neurological disease characterized by high viral brain titers, severe encephalitis, and extensive monocyte and CD8+ T cell infiltration. Brain tissue analysis reveals upregulation of chemokines CCL2, CCL3, CCL4, CCL5, CXCL2, CXCL9, and CXCL10, with more virulent strains showing higher levels correlating with increased inflammatory cell numbers [182,219].
4.5.3. Equine Multinodular Pulmonary Fibrosis Pathophysiology
Equine multinodular pulmonary fibrosis (EMPF) represents a progressive fibrosing interstitial lung disease strongly associated with gammaherpesvirus EHV-5 infection, though the exact pathophysiological mechanisms remain incompletely understood [220,221]. Unlike EHV-1-mediated diseases that follow acute viremic dissemination, EMPF appears to develop during latent or persistent EHV-5 infection through mechanisms involving chronic viral stimulation of fibrotic pathways [222,223]. Affected horses demonstrate loss of functional pulmonary parenchyma due to extensive nodular interstitial fibrosis characterized by multiple well-demarcated nodular regions of fibrosis with mixed inflammatory cell infiltration [132,221].
Experimental inoculation studies have confirmed that EHV-5 isolated from spontaneous EMPF cases can induce pulmonary fibrosis when inoculated into clinically normal horses, providing strong evidence for viral causation [223]. This landmark study represents the first demonstration that a gammaherpesvirus can induce lung fibrosis in its natural host species without additional known lung injury. The pathogenic process involves viral infection of alveolar epithelial cells and pulmonary lymphocytes, with subsequent cell-to-cell viral spread within alveolar tissues [222,223].
Critically, the disease involves massive induction and accumulation of myofibroblasts—α-smooth muscle actin-positive cells that produce excessive extracellular matrix proteins, particularly collagen [223]. These myofibroblasts appear within alveolar walls in mildly affected regions, with numbers and density increasing proportionally with fibrosis severity, ultimately becoming embedded within interstitial collagen deposits and beneath epithelial cells lining airspaces [223].
The mechanism by which EHV-5 triggers fibrosis likely involves complex virus–host interactions during latency, with viral replication combined with host-specific predisposing factors eventually triggering fibrotic cascades [222,224]. Recent experimental ex vivo and in vitro studies have shown that infected equine T and B lymphocytes could act as lifelong latency reservoirs for the virus and play a role in the development of disease [224]. Interestingly, not all EHV-5 strains appear pathogenic, with nucleic acid sequence analysis revealing that not all amplicons from inoculated horses matched the original inoculation strains, suggesting that specific viral genomic variations confer fibrogenic properties [223]. The disease parallels human idiopathic pulmonary fibrosis associated with Epstein–Barr virus (another gammaherpesvirus), suggesting shared pathogenic mechanisms across species [224].
Clinical presentation includes pyrexia, progressive weight loss, tachypnea, dyspnea, hypoxemia, neutrophilia, and hyperfibrinogenemia, with thoracic imaging revealing characteristic diffuse nodular interstitial patterns [132,221,225]. The prognosis remains poor, with most affected horses succumbing to progressive respiratory failure despite treatment attempts [226].
5. Treatment of EHV Infection
Currently, no effective treatment has been established for EHV infection [22]. Despite numerous attempts to repurpose antiviral agents commonly used for other herpesvirus infections, most conventional antivirals have demonstrated limited or no clinical efficacy against EHV, particularly in cases of neurological disease.
5.1. Conventional Antivirals with Limited Efficacy
Acyclovir and its prodrug valacyclovir, which are highly effective against human alphaherpesviruses, have shown disappointing results in treating EHV-1 and EHV-4 infections. While these nucleoside analogs inhibit viral DNA polymerase in vitro, clinical studies have failed to demonstrate significant therapeutic benefit in horses with EHV-1 myeloencephalopathy or respiratory disease. The limited efficacy may be attributed to several factors, including poor oral bioavailability in horses, inadequate tissue penetration to sites of viral replication (particularly the central nervous system), and potential differences in viral thymidine kinase activity compared to human herpesviruses. Notably, acyclovir treatment has shown some effectiveness in terminating EHV-3 excretion within 8 days after onset in stallions with equine coital exanthema (ECE), a venereally-transmitted mucocutaneous disease characterized by papules, vesicles, pustules and ulcers on the external genital organs [55,227,228,229]. However, this specific application to EHV-3 does not translate to broader efficacy against the more clinically significant EHV-1 and EHV-4 infections.
5.2. Immunomodulators and Their Uncertain Impact
Various immunomodulatory approaches have been explored for EHV infection management, including the use of inactivated Parapoxvirus ovis (iPPVO)-based immunomodulators in the United States [22,230]. The theoretical rationale for immunomodulation includes enhancement of innate immune responses through stimulation of interferon production and potential reduction of viral shedding [230,231]. One randomized controlled study demonstrated that iPPVO treatment reduced clinical signs and EHV-1 viral shedding in an experimental challenge model [22]. However, a comprehensive systematic review of pharmacologic interventions for EHV-1 concluded that most studies reported either no benefit or minimal efficacy of immunomodulatory treatments, with minimal or limited benefit as either prophylactic or post-exposure treatment in mitigating EHV-1-associated disease outcomes [230]. The 2024 ACVIM consensus statement affirmed that there is no evidence that pharmacologic treatments given after the onset of clinical signs of EHV-1 infection prevent or affect the development or course of EHM [232]. Furthermore, iPPVO (Zylexis, Zoetis, USA) has been discontinued and is no longer available in the United States [233]. The risk–benefit profile of immunostimulation during active herpesvirus infection remains controversial, as excessive immune activation could potentially exacerbate endothelial damage and thrombotic complications characteristic of severe EHV-1 disease.
5.3. Emerging Therapeutic Candidates
Despite the limitations of conventional approaches, recent research has identified several compounds with promising antiviral activity against herpes virus infections in in vitro studies and murine models [234]. These emerging therapeutic candidates demonstrate diverse mechanisms of action and represent potential avenues for future clinical development.
Among the most promising compounds are bioactive alkaloids, particularly berbamine (BBM), which has demonstrated significant therapeutic potential against EHV-1. In vitro studies revealed that BBM effectively suppresses multiple stages of the viral life cycle, including viral entry into host cells, viral DNA replication, and virion release. Subsequent in vivo studies in murine models confirmed that BBM treatment significantly reduced EHV-1-induced pathological damage in brain and lung tissues while decreasing animal mortality rates [234]. Similarly, cepharanthine, a natural bisbenzylisoquinoline alkaloid extracted from Stephania cepharantha Hayata, has shown dose-dependent inhibitory effects against EHV-8 infection in NBL-6 and RK-13 cell lines. Mechanistic analysis revealed that cepharanthine reduces EHV-8-induced oxidative stress through activation of AMPK and Nrf2/HO-1 signaling pathways, with in vivo studies demonstrating significant amelioration of lung pathology and reduced oxidative stress in EHV-8-infected mice [235]. Consistently, another study found that celastrol prevents EHV-8 infection in vitro by activating the Nrf2/HO-1 signaling pathway and relieving oxidative stress [236].
Complementing these alkaloid-based approaches, blebbistatin represents a novel therapeutic strategy targeting cellular mechanisms essential for viral infection. Myosin II ATPase activity is crucial for EHV infection as it drives the cytoskeletal rearrangements, membrane dynamics, and intracellular transport processes required for successful viral cellular entry, trafficking to replication sites, and egress from infected cells. Studies demonstrated that blebbistatin exhibited dose-dependent inhibition of EHV-8 infection in RK-13 and MDBK cell lines by disrupting viral entry through myosin II ATPase modulation, with in vivo studies confirming significant reduction in viral replication and amelioration of pulmonary pathology in infected mice [139].
The therapeutic landscape is further enriched by compounds targeting host antioxidant and immunomodulatory pathways. Hyperoside, isolated from Rhododendron brachycarpum G. Don, exhibits potent antiviral activity against EHV-8 in multiple cell lines, including RK-13 (rabbit kidney cells), MDBK (Madin-Darby bovine kidney), and NBL-6 (E. Derm cells). Mechanistically, hyperoside activates the JNK/Nrf2/Keap1 pathway, inducing heme oxygenase-1 expression, reducing oxidative stress, and triggering antiviral interferon responses, with in vivo studies confirming significant reduction in pulmonary pathology in EHV-8-infected mice [237]. This heme oxygenase-1 pathway is also targeted by cobalt protoporphyrin, which has demonstrated significant inhibition of EHV-8 replication in susceptible cell lines and murine models through HO-1-mediated type I interferon responses [238].
These findings collectively suggest that targeting multiple viral replication stages, cellular entry mechanisms, and host antioxidant pathways may provide effective therapeutic strategies against EHV infections. The diverse mechanisms of action demonstrated by these emerging compounds offer potential for combination therapies that could enhance antiviral efficacy while minimizing the risk of viral resistance development. However, it is important to note that most of these promising candidates have only been evaluated in cell culture systems and murine models, primarily against EHV-8 rather than the clinically more significant EHV-1 and EHV-4. Translation of these findings to equine clinical applications will require rigorous evaluation of safety, pharmacokinetics, and clinical efficacy in the target species. Until such evidence becomes available, EHV management continues to rely primarily on biosecurity measures, vaccination programs, and supportive care rather than specific antiviral therapy.
6. Efficacy and Limitations of Current EHV Vaccination Strategies
6.1. Fundamental Properties of Available EHV Vaccines
EHV vaccines encompass diverse platforms with distinct immunological properties, mechanisms of action, and efficacy profiles [239]. Understanding these fundamental differences is essential for informed vaccination strategies and development of improved vaccines.
6.1.1. Inactivated (Killed) Vaccines
Inactivated EHV vaccines represent the most widely used vaccine platform globally and contain chemically or physically inactivated whole virus particles combined with adjuvants to enhance immunogenicity [240,241,242]. These vaccines induce primarily humoral (antibody-mediated) immune responses, generating virus-neutralizing antibodies and EHV-specific IgG responses, particularly IgG1 and IgG4/7 isotypes [243]. The high-antigen-load inactivated EHV-1 vaccines licensed for abortion prevention demonstrate superior performance compared to respiratory-only formulations, producing higher antibody titers and some evidence of cellular immune responses [244,245,246].
Inactivated vaccines offer excellent safety profiles with no risk of reversion to virulence or shedding of vaccine virus, making them particularly suitable for pregnant mares and immunocompromised animals [211]. They provide consistent, reproducible immune responses and demonstrate relative stability during storage. For abortion prevention specifically, inactivated vaccines administered during months 3, 5, 7, and 9 of gestation have demonstrated measurable efficacy in reducing abortion storms in pregnant mare populations [247]. Research has demonstrated that intramuscular administration of inactivated EHV-1/4 vaccines successfully induces robust mucosal antibody responses at upper respiratory tract entry sites, confirming that systemic vaccination generates localized immunity at sites where viral infection initially establishes [248]. The vaccination protocol primarily stimulates protective IgG4/7 antibodies both systemically and mucosally, with serum antibody levels remaining elevated throughout extended study periods, and mucosal antibodies demonstrating consistent increases following repeated vaccine doses [248].
However, these vaccines stimulate only humoral immunity without robust cell-mediated (Th1-type) responses, potentially providing insufficient protection against respiratory infection and viral replication [245]. The immunity generated is relatively short-lived, lasting approximately 3–6 months, which necessitates frequent revaccination schedules [211,247]. Despite their widespread use, inactivated vaccines do not prevent infection or significantly reduce viral shedding, allowing vaccinated horses to harbor and transmit virus to susceptible animals, thereby perpetuating transmission chains within populations [211,242]. Individual animals maintain susceptibility to infection despite adherence to established vaccination protocols [247].
Clinical observations have revealed complex relationships between vaccination status and disease progression patterns. Paradoxically, appropriately vaccinated horses demonstrated extended hospitalization periods (15.5 days) compared to inadequately vaccinated animals (12.5 days), suggesting that vaccination modulates disease trajectory and recovery patterns rather than providing complete protection against severe disease onset [104]. Despite these extended hospitalization requirements, appropriately vaccinated horses demonstrated superior immune responses, maintaining elevated lymphocyte counts within 24 h of admission and throughout the entire hospitalization period. This preservation of critical immune function during acute disease phases indicates that vaccination favorably modulates immune responses, potentially resulting in more robust yet prolonged immune-mediated recovery processes that necessitate extended veterinary monitoring [104].
6.1.2. Modified-Live Virus (MLV) Vaccines
Modified-live vaccines utilize attenuated EHV-1 strains engineered through targeted gene deletions to reduce virulence while maintaining immunogenicity [246]. The commercially available MLV vaccines, including Rhinomune in the United States and Prevaccinol in Europe, are based on the RacH strain with deletions in open reading frames ORF1, ORF2, and ORF67 [211,242,244]. These vaccines more closely mimic natural infection compared to inactivated vaccines, thereby stimulating both humoral and cell-mediated immunity, including cytotoxic T lymphocyte (CTL) responses that are critical for clearing infected cells [244,245,246].
MLV vaccines induce significantly lower nasal virus shedding following challenge infection compared to inactivated vaccines, potentially reducing viral transmission at population levels [245]. They generate balanced immune responses characterized by both antibody production and Th1-biased cellular immunity, evidenced by lower IgG(T)/IgGa and IgG(T)/IgGb ratios that suggest a cytotoxic immune response bias [245,246]. Notably, experimental deletion mutants such as Ab4ΔORF2 have demonstrated the ability to completely prevent viral replication in the upper respiratory tract, eliminate nasal shedding, and prevent cell-associated viremia in fully protected horses, representing a significant advancement over current commercial products [249].
Despite these immunological advantages, MLV vaccines carry theoretical risks of reversion to virulence, vaccine virus shedding, or establishment of latency, although such events have not been documented with current commercial products [211]. These vaccines typically generate lower virus-neutralizing antibody titers compared to inactivated vaccines, which may concern practitioners accustomed to relying on serological markers as indicators of protection [245,246]. Some individual animals may exhibit stronger vaccine reactions, including localized swelling at injection sites or transient fever. Importantly, current MLV vaccines are licensed only for respiratory disease prevention and are not approved for use in pregnant mares due to theoretical abortion risks, thus limiting their application in breeding populations where abortion prevention is a primary concern [211].
Recent research has revealed an additional concern regarding vaccination frequency. Frequent EHV vaccination with inactivated commercial vaccines at short intervals of 60–90 days can result in declining antibody values and cellular immunity despite additional vaccine boosts, a phenomenon termed “adverse immunity to EHV vaccination” [245]. This finding has important implications for vaccination protocols, particularly in high-risk situations where frequent boosting might seem intuitively beneficial but may actually compromise protective immunity.
6.1.3. Recombinant Vector Vaccines
Recombinant vaccines utilize heterologous viral vectors, particularly canarypox virus or modified vaccinia Ankara, that are engineered to express specific EHV glycoproteins while retaining the ability to replicate in avian cells but not in mammalian cells [211]. This platform combines the safety advantages of inactivated vaccines with the enhanced cellular immunity induction characteristic of MLV vaccines. These vaccines offer excellent safety profiles because the vector cannot replicate in horses, thereby eliminating concerns about reversion to virulence or establishment of latency. They successfully induce both humoral and cell-mediated immune responses, including strong interferon-gamma production and CTL activation. Additionally, the vaccines can be engineered to express multiple antigens simultaneously or combined with other vaccines, such as concurrent administration of recombinant canarypox equine influenza vaccine with inactivated EHV vaccine. However, recombinant vaccines remain largely experimental platforms with limited commercial availability for EHV. A significant concern is that vector-specific immunity may develop upon repeated administration, potentially reducing the efficacy of boost responses over time. Furthermore, the manufacturing complexity and associated costs substantially exceed those of traditional vaccine platforms, limiting their widespread adoption.
6.1.4. DNA and Subunit Vaccines
DNA vaccines consist of plasmids encoding specific EHV antigens, typically glycoproteins gB, gC, or gD, that are delivered intramuscularly to induce immune responses through in vivo antigen expression. Subunit vaccines, in contrast, contain purified viral proteins that directly stimulate the immune system. Both platforms offer precise antigen selection and excellent safety profiles. However, these vaccine types have demonstrated variable and often poor immunogenicity in horses, requiring sophisticated adjuvant systems or novel delivery mechanisms to enhance immune responses [211]. Consequently, neither DNA nor subunit vaccines have achieved widespread commercial success for EHV prevention, remaining primarily in the experimental and research domains.
6.2. Vaccine Efficacy and Population-Level Limitations
Despite providing measurable individual immunological benefits, current EHV vaccines demonstrate fundamental limitations in preventing infection and controlling disease transmission at population levels. A comprehensive meta-analysis examining vaccination efficacy against EHV-1 revealed that vaccination generally results in only slight improvements in clinical and virological outcomes, although these improvements did not reach statistical significance in many parameters [28]. The analysis of 36 randomized controlled trials showed that vaccinated horses experienced a reduced relative risk of pyrexia (0.468, 95% confidence interval: 0.318–0.688), but this finding was accompanied by severe between-trial heterogeneity and significant publication bias, limiting the strength of conclusions.
These findings reflect the inherent challenge that EHV vaccines, regardless of platform, induce incomplete immunity that may protect against severe disease manifestations but fails to prevent infection or significantly reduce viral shedding [211,216]. Vaccinated horses continue to develop cell-associated viremia and can transmit virus to susceptible animals, thereby perpetuating outbreak chains within populations [133,203]. The short duration of vaccine-induced immunity, lasting approximately 3–6 months, combined with the ubiquity of latently infected horses capable of viral reactivation, creates persistent reinfection pressure that overwhelms vaccine-induced protection [210,211].
Most critically, an analysis of twelve EHV-1 outbreak studies revealed no statistically significant difference between the basic reproduction number (R0) in non-vaccinated herds and the reproduction number (Rv) in fully vaccinated herds (p = 0.15) [216]. More significantly, Rv in herds where all horses were vaccinated remained substantially above 1, indicating that vaccination alone was insufficient to prevent sustained viral transmission during natural outbreaks [250]. These findings demonstrate that while vaccination provides individual benefits regarding disease severity and immune responses, it exhibits limited effectiveness as a standalone population-level intervention for EHV-1 transmission control, a conclusion supported by multiple independent studies [251,252,253,254].
In outbreak situations characterized by high viral loads and the presence of “superspreader” horses that shed large quantities of virus, even the immunity of vaccinated horses can become overwhelmed [255]. Recent outbreak investigations have identified horses that shed substantially more virus than others despite showing few or no clinical signs, presenting a significant challenge to outbreak control regardless of vaccination status [103]. While vaccines can reduce the severity of clinical disease and may decrease nasal viral shedding to some degree, they do not prevent the establishment of viremia or eliminate the potential for horses to transmit infection to other animals [28,216].
The emergence of the novel H752 genotype further complicates vaccination strategies and outbreak management. This variant, first identified in Europe and subsequently detected in North American outbreaks, does not fit into the previously recognized D752 or N752 genotype classifications and can result in negative EHV-1 test results when diagnostic laboratories rely exclusively on genotype-specific assays [255]. This diagnostic challenge emphasizes the need for multi-gene molecular approaches rather than relying solely on single nucleotide polymorphism detection at the ORF30 position.
Consequently, effective EHV control requires integrated, multi-faceted approaches that combine vaccination for individual disease mitigation with rigorous biosecurity measures. These measures must include movement restrictions, strict isolation protocols for incoming horses (minimum 21 days), environmental disinfection, twice-daily temperature monitoring to enable early outbreak detection, and minimizing stress-induced viral reactivation through appropriate management practices [43,116,211]. Vaccination alone cannot ensure population-level protection, and unrealistic expectations of vaccine performance may inadvertently compromise outbreak control efforts by creating false confidence in protection [211,216]. The equine industry and governing bodies have increasingly recognized these limitations, implementing more stringent biosecurity protocols at competition venues and requiring documentation of vaccination status as part of comprehensive disease management strategies [43,216].
Future vaccine development should target conserved epitopes in glycoproteins B, D, and H/L complex that are functionally constrained across EHV-1/-4/-8. Efficacy evaluation must occur in high-transmission settings such as racing stables and donkey milk farms to determine if next-generation vaccines can achieve the population-level transmission control that current vaccines fail to provide.
7. Conclusions and Future Research Directions
Equine herpesvirus infections constitute a multifaceted global health challenge that significantly impacts equine welfare and industry economics worldwide. The taxonomic diversity of nine EHV species, coupled with their sophisticated molecular pathogenesis mechanisms and complex glycoprotein networks, creates substantial obstacles for effective disease control. While global surveillance has documented widespread distribution and diverse clinical manifestations across all continents, current therapeutic and preventive approaches remain inadequate. The limited efficacy of existing vaccines and absence of effective treatments highlight critical gaps in our disease management capabilities. The virus’s sophisticated immune evasion strategies, particularly the exploitation of infected leukocytes for systemic dissemination, demonstrate the evolutionary adaptation of these pathogens to their equine hosts. Comprehensive integration of advanced molecular diagnostics, innovative therapeutic development, and enhanced biosecurity measures is essential for developing effective control programs that protect both individual animal health and broader industry interests.
Future research should prioritize development of next-generation vaccines targeting conserved viral epitopes across multiple EHV species, potentially utilizing mRNA or viral vector platforms to enhance mucosal immunity. Advanced therapeutic discovery programs should focus on a combination of antiviral strategies targeting multiple viral replication stages and host cellular pathways simultaneously. Comprehensive genomic surveillance networks are needed to monitor viral evolution, emergence of new variants, and cross-species transmission events. Investigation of host genetic factors influencing susceptibility and disease severity could identify biomarkers for personalized prevention strategies. Integration of One Health approaches examining human–animal–environmental interfaces will be crucial for understanding transmission dynamics. Additionally, development of rapid point-of-care diagnostic tools and artificial intelligence-based prediction models for outbreak management represents critical technological advances needed for effective disease control in modern equine populations.
Author Contributions
M.Z.K., C.W., W.L.: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing—original draft, Visualization, Writing—review & editing. Y.J., X.F., Y.L., W.L., C.W., M.Z.K.: Resources, Data curation, Software, Writing—review & editing. C.W., W.L.: Project administration, Funding acquisition and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key R&D Program of China (grant numbers 2023YFD1302004; 2022YFD1600103), Liaocheng Municipal Bureau of Science and Technology, High-talented Foreign Expert Introduction Program (GDWZ202401), The Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team (grant no. SDAIT-27), The Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology (grant no. 3193308), Research on Donkey Pregnancy Improvement (grant no. K20LC0901) and Liaocheng University scientific research fund (grant no. 318052025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Conflicts of Interest
The authors have declared no conflicting interests.
References
- Rzekęć, A.; Vial, C.; Bigot, G. Green assets of equines in the European context of the ecological transition of agriculture. Animals 2020, 10, 106. [Google Scholar] [CrossRef]
- Khan, M.Z.; Chen, W.; Wang, X.; Liang, H.; Wei, L.; Huang, B.; Kou, X.; Liu, X.; Zhang, Z.; Chai, W. A review of genetic resources and trends of omics applications in donkey research: Focus on China. Front. Vet. Sci. 2024, 11, 1366128. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Q.; Khan, M.Z.; Wang, M.; Xiang, F.; Zhang, X.; Kou, X.; Li, S.; Wang, C.; Li, Y. Proteomic profiling of donkey milk exosomes highlights bioactive proteins with immune-related functions. Int. J. Mol. Sci. 2025, 26, 2892. [Google Scholar] [CrossRef]
- Huimin, C.; Peng, W.; Man, C.; Hong, L.; Yuxin, Z.; Zhiyu, P.; Jiajun, P. Donkey milk supplementation alleviates renal fibrosis of chronic kidney disease by enhancing anti-inflammatory ability. J. Dairy Sci. 2025, 108, 1198–1210. [Google Scholar] [CrossRef]
- Sami, M.; Azizi, S.; Kheirandish, R.; Ebrahimnejad, H.; Alizadeh, S. Protective effects of donkey milk on ethanol-induced gastric ulcer in rat. Vet. Med. Sci. 2025, 11, e70156. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, L.; Chen, X.; Zhu, H.; Wei, J.; Zhu, M.; Khan, M.Z.; Wang, C.; Zhang, Z. Nutritional composition and biological activities of donkey milk: A narrative review. Foods 2025, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.; Li, M.; Liu, W.; Liu, Y.; Wang, M.; Wang, C.; Khan, M.Z.; Zhu, M.; Wang, C.; et al. Non-bovine milk as functional foods with focus on their antioxidant and anti-inflammatory bioactivities. Antioxidants 2025, 14, 801. [Google Scholar] [CrossRef]
- Khan, M.Z.; Chen, W.; Li, M.; Ren, W.; Huang, B.; Kou, X.; Ullah, Q.; Wei, L.; Wang, T.; Khan, A.; et al. Is there sufficient evidence to support the health benefits of including donkey milk in the diet? Front. Nutr. 2024, 11, 1404998. [Google Scholar] [CrossRef] [PubMed]
- Aroua, M.; Fehri, N.E.; Ben Said, S.; Quattrone, A.; Agradi, S.; Brecchia, G.; Balzaretti, C.M.; Mahouachi, M.; Castrica, M. The use of horse and donkey meat to enhance the quality of the traditional meat product (kaddid): Analysis of physicochemical traits. Foods 2024, 13, 2974. [Google Scholar] [CrossRef]
- Aroua, M.; Haj Koubaier, H.; Rekik, C.; Fatica, A.; Ben Said, S.; Malek, A.; Mahouachi, M.; Salimei, E. Comparative study of carcass characteristics and meat quality of local Mediterranean donkey breeds. Foods 2024, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, H.; Wang, X.; Wang, T.; Ma, Q.; Khan, M.Z.; Zhu, M.; Wang, C.; Liu, W.; Chai, W. Data-independent acquisition method for in-depth proteomic screening of donkey meat. Agriculture 2024, 14, 2102. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, X.; Liu, S.; Sun, M.; Man, L.; Zhu, M.; Liu, G.; Zahoor Khan, M.; Wang, C.; Li, M. Characterization and discrimination of volatile compounds of donkey and horse meat based on gas chromatography–ion mobility spectrometry. Foods 2025, 14, 1203. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidau, C.; Stefan, L.M.; Lazea-Stoyanova, A.; Berechet, M.D.; Iosageanu, A.; Matei, E.; Jankauskaitė, V.; Predescu, C.; Valeika, V.; et al. Donkey gelatin and keratin nanofibers loaded with antioxidant agents for wound healing dressings. Gels 2024, 10, 391. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Liang, H.; Zahoor Khan, M.; Ren, W.; Huang, B.; Chen, Y.; Xing, S.; Zhan, Y.; Wang, C. Comprehensive transcriptomic analysis unveils the interplay of mRNA and LncRNA expression in shaping collagen organization and skin development in Dezhou donkeys. Front. Genet. 2024, 15, 1335591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, W.; Peng, Y.; Khan, M.Z.; Liang, H.; Zhang, Y.; Wang, L.; Wang, C.; Zhan, Y. Elucidating the role of transcriptomic networks and DNA methylation in collagen deposition of Dezhou donkey skin. Animals 2024, 14, 1222. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Y. China’s equine industries in a transitional economy: Development, trends, challenges, and opportunities. Sustainability 2020, 12, 5135. [Google Scholar] [CrossRef]
- Armstrong, A.A.; Kayser, J.P.; Gardner, J.G. The beneficial effects of equine events on the local economy. J. Equine Vet. Sci. 2011, 31, 288–289. [Google Scholar] [CrossRef]
- Khan, M.Z.; Li, Y.; Zhu, M.; Li, M.; Wang, T.; Zhang, Z.; Liu, W.; Ma, Q.; Wang, C. Advances in donkey disease surveillance and microbiome characterization in China. Microorganisms 2025, 13, 749. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Ma, H.; Akhtar, M.F.; Tan, Y.; Wang, T.; Liu, W.; Khan, A.; Khan, M.Z.; Wang, C. An overview of infectious and non-infectious causes of pregnancy losses in equine. Animals 2024, 14, 1961. [Google Scholar] [CrossRef]
- Wilkes, R.P.; Kattoor, J. Herpesviridae. In Veterinary Microbiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 496–521. [Google Scholar]
- Afify, A.F.; Hassanien, R.T.; El Naggar, R.F.; Rohaim, M.A.; Munir, M. Unmasking the ongoing challenge of equid herpesvirus-1 (EHV-1): A comprehensive review. Microb. Pathog. 2024, 193, 106755. [Google Scholar] [CrossRef]
- Goehring, L.; Dorman, D.C.; Osterrieder, K.; Burgess, B.A.; Dougherty, K.; Gross, P.; Neinast, C.; Pusterla, N.; Soboll-Hussey, G.; Lunn, D.P. Pharmacologic interventions for the treatment of equine herpesvirus-1 in domesticated horses: A systematic review. J. Vet. Intern. Med. 2024, 38, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Martins-Bessa, A.; McLean, A.K. Advances in donkey and mule research. Animals 2024, 14, 2238. [Google Scholar] [CrossRef]
- Lebrasseur, O.; More, K.D.; Orlando, L. Equine herpesvirus 4 infected domestic horses associated with Sintashta spoke-wheeled chariots around 4,000 years ago. Virus Evol. 2024, 10, vead087. [Google Scholar] [CrossRef]
- Mahmoud, H.Y.; Fouad, S.S.; Amin, Y.A. Review of two viral agents of economic importance to the equine industry (equine herpesvirus-1, and equine arteritis virus). Equine Vet. Educ. 2023, 35, 92–102. [Google Scholar] [CrossRef]
- Pusterla, N.; Hussey, G.S.; Goehring, L.S. Equine herpesvirus-1 myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 2022, 38, 339–362. [Google Scholar] [CrossRef]
- Câmara, R.J.; Bueno, B.L.; Resende, C.F.; Balasuriya, U.B.; Sakamoto, S.M.; Reis, J.K. Viral diseases that affect donkeys and mules. Animals 2020, 10, 2203. [Google Scholar] [CrossRef]
- Osterrieder, K.; Dorman, D.C.; Burgess, B.A.; Goehring, L.S.; Gross, P.; Neinast, C.; Pusterla, N.; Hussey, G.S.; Lunn, D.P. Vaccination for the prevention of equine herpesvirus-1 disease in domesticated horses: A systematic review and meta-analysis. J. Vet. Intern. Med. 2024, 38, 1858–1871. [Google Scholar] [CrossRef]
- Wang, T.; Hu, L.; Wang, Y.; Liu, W.; Liu, G.; Zhu, M.; Zhang, W.; Wang, C.; Ren, H.; Li, L. Identification of equine herpesvirus 8 in donkey abortion: A case report. Virol. J. 2022, 19, 10. [Google Scholar] [CrossRef]
- Martella, V.; Lanave, G.; Camero, M.; Larocca, V.; Lorusso, E.; Catella, C.; Capozza, P.; Tempesta, M.; Buonavoglia, C. Identification of a novel α-herpesvirus associated with ulcerative stomatitis in donkeys. Emerg. Infect. Dis. 2020, 26, 3044. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Suarez, N.M.; Kerr, K.; Hector, R.; Moloney-Quinn, L.; Arkins, S.; Davison, A.J.; Cullinane, A. Equid herpesvirus 8: Complete genome sequence and association with abortion in mares. PLoS ONE 2018, 13, e0192301. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Wilkie, G.S.; Russell, C.A.; Compston, L.; Grafham, D.; Clissold, L.; McLay, K.; Medcalf, L.; Newton, R.; Davison, A.J.; et al. Genetic diversity of equine herpesvirus 1 isolated from neurological, abortigenic and respiratory disease outbreaks. Transbound. Emerg. Dis. 2018, 65, 817–832. [Google Scholar] [CrossRef]
- Azab, W.; Bedair, S.; Abdelgawad, A.; Eschke, K.; Farag, G.K.; Abdel-Raheim, A.; Greenwood, A.D.; Osterrieder, N.; Ali, A.A.H. Detection of equid herpesviruses among different Arabian horse populations in Egypt. Vet. Med. Sci. 2019, 5, 361–371. [Google Scholar] [CrossRef]
- Negussie, H.; Gizaw, D.; Tesfaw, L.; Li, Y.; Oguma, K.; Sentsui, H.; Tessema, T.S.; Nauwynck, H.J. Detection of equine herpesvirus (EHV)-1,-2,-4 and-5 in ethiopian equids with and without respiratory problems and genetic characterization of EHV-2 and EHV-5 strains. Transbound. Emerg. Dis. 2017, 64, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Stefanetti, V.; Danzetta, M.L.; Timoney, P.J. Gammaherpesvirus infections in equids: A review. Vet. Med. Res. Rep. 2015, 6, 91–101. [Google Scholar] [CrossRef]
- Reed, S.M.; Toribio, R.E. Equine herpesvirus 1 and 4. Vet. Clin. N. Am. Equine Pract. 2004, 20, 631–642. [Google Scholar] [CrossRef]
- Barrandeguy, M.E.; Carossino, M. Infectious diseases in donkeys and mules: An overview and update. J. Equine Vet. Sci. 2018, 65, 98–105. [Google Scholar] [CrossRef]
- Hussey, G.S.; Giessler, K.S. Contribution of the immune response to the pathogenesis of equine herpesvirus-1 (EHV-1): Are there immune correlates that predict increased risk or protection from EHV-1 myeloencephalopathy? Vet. J. 2022, 282, 105827. [Google Scholar] [CrossRef] [PubMed]
- Van Crombrugge, E.; Vanbeylen, E.; Van Cleemput, J.; Van den Broeck, W.; Laval, K.; Nauwynck, H. Bacterial toxins from Staphylococcus aureus and Bordetella bronchiseptica predispose the horse’s respiratory tract to equine herpesvirus type 1 infection. Viruses 2022, 14, 149. [Google Scholar] [CrossRef]
- Klouth, E.; Zablotski, Y.; Petersen, J.L.; de Bruijn, M.; Gröndahl, G.; Müller, S.; Goehring, L.S. Epidemiological aspects of equid herpesvirus-associated myeloencephalopathy (EHM) outbreaks. Viruses 2022, 14, 2576. [Google Scholar] [CrossRef] [PubMed]
- Saklou, N.T.; Burgess, B.A.; Ashton, L.V.; Morley, P.S.; Goehring, L.S. Environmental persistence of equid herpesvirus type-1. Equine Vet. J. 2021, 53, 349–355. [Google Scholar] [CrossRef]
- Dayaram, A.; Seeber, P.A.; Greenwood, A.D. Environmental detection and potential transmission of equine herpesviruses. Pathogens 2021, 10, 423. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carvelli, A.; Nielsen, S.S.; Paillot, R.; Broglia, A.; Kohnle, L. Clinical impact, diagnosis and control of Equine Herpesvirus-1 infection in Europe. EFSA J. 2022, 20, e07230. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales, R.J.L.; Gortázar, C.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Infection with Equine Herpesvirus-1. EFSA J. 2022, 20, e07036. [Google Scholar]
- Khusro, A.; Aarti, C.; Rivas-Caceres, R.R.; Barbabosa-Pliego, A. Equine herpesvirus-I infection in horses: Recent updates on its pathogenicity, vaccination, and preventive management strategies. J. Equine Vet. Sci. 2020, 87, 102923. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, X.; Wang, X. The genomic characterization of equid alphaherpesviruses: Structure, function, and genetic similarity. Vet. Sci. 2025, 12, 228. [Google Scholar] [CrossRef]
- Mira, F.; Canuti, M.; Di Bella, S.; Puleio, R.; Lavazza, A.; Lelli, D.; Vicari, D.; Purpari, G.; Cannella, V.; Chiaramonte, G.; et al. Detection and molecular characterization of two gammaherpesviruses from Pantesco breed donkeys during an outbreak of mild respiratory disease. Viruses 2021, 13, 1527. [Google Scholar] [CrossRef] [PubMed]
- Akkutay, A.Z.; Osterrieder, N.; Damiani, A.; Tischer, B.K.; Borchers, K.; Alkan, F. Prevalence of equine gammaherpesviruses on breeding farms in Turkey and development of a TaqMan MGB real-time PCR to detect equine herpesvirus 5 (EHV-5). Arch. Virol. 2014, 159, 2989–2995. [Google Scholar] [CrossRef]
- Perkins, G.A.; Wagner, B.; Rollins, A.; Sfraga, H.; Pearson, E.; Cercone, M. Serum and mucosal antibody testing to detect viral exposure in contact horses during an equine herpesvirus myeloencephalopathy outbreak. Am. J. Vet. Res. 2025, 1, 1–9. [Google Scholar] [CrossRef]
- Ruan, L.; Li, L.; Yang, R.; You, A.; Khan, M.Z.; Yu, Y.; Chen, L.; Li, Y.; Liu, G.; Wang, C.; et al. Equine herpesvirus-1 induced respiratory disease in Dezhou donkey foals: Case study from China, 2024. Vet. Sci. 2025, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Musoles-Cuenca, B.; Padilla-Blanco, M.; Vitale, V.; Lorenzo-Bermejo, T.; de la Cuesta-Torrado, M.; Ballester, B.; Maiques, E.; Rubio-Guerri, C.; Velloso Alvarez, A. First molecular evidence of equine herpesvirus type 1 (EHV-1) in ocular swabs of clinically affected horses. Viruses 2025, 17, 862. [Google Scholar] [CrossRef] [PubMed]
- Azab, W.; Zajic, L.; Osterrieder, N. The role of glycoprotein H of equine herpesviruses 1 and 4 (EHV-1 and EHV-4) in cellular host range and integrin binding. Vet. Res. 2012, 43, 61. [Google Scholar] [CrossRef]
- Troncoso, I.; Calvanese, R.; Saravia, F.; Muñoz-Leal, S.; Zegpi, N.A.; Ortega, R. First molecular detection of Equine Herpesvirus type 3 (EHV-3) in Chile. Vet. Med. Sci. 2023, 9, 717–720. [Google Scholar] [CrossRef]
- Thorsteinsdóttir, L.; Guðmundsson, G.Ö.; Jensson, H.; Torsteinsdóttir, S.; Svansson, V. Isolation of equid alphaherpesvirus 3 from a horse in Iceland with equine coital exanthema. Acta Vet. Scand. 2021, 63, 6. [Google Scholar] [CrossRef]
- Toishi, Y.; Tsunoda, N.; Kirisawa, R. Period of excretion of equine herpesvirus 3 (EHV-3) from a stallion before showing clinical signs of equine coital exanthema and the effect of acyclovir treatment on the duration of EHV-3 excretion. J. Vet. Med. Sci. 2020, 82, 1299–1305. [Google Scholar] [CrossRef]
- Kirisawa, R.; Toishi, Y.; Akamatsu, A.; Soejima, K.; Miyashita, T.; Tsunoda, N. Isolation of equine herpesvirus 3 (EHV-3) from equine coital exanthema of two stallions and sero-epidemiology of EHV-3 infection in Japan. J. Vet. Med. Sci. 2017, 79, 636–643. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, K.; van der Zaag, E.; Buter, R.; van den Wollenberg, L.; van Oldruitenborgh-Oosterbaan, M.S. Asinine herpesvirus-3 (equine herpesvirus-8)-associated neurological disease in a donkey. Vet. Rec. Case Rep. 2017, 5, e000498. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, X.; Liu, W. Detection of equine herpesvirus antibodies in large-scale donkey farms in Liaocheng area. Vet. Med. Sci. 2024, 10, e70016. [Google Scholar] [CrossRef]
- Ryt-Hansen, P.; Johansen, V.K.; Cuicani, M.M.; Larsen, L.E.; Hansen, S. Outbreak of equine herpesvirus 4 (EHV-4) in Denmark: Tracing patient zero and viral characterization. BMC Vet. Res. 2024, 20, 287. [Google Scholar] [CrossRef]
- Azab, W.; Osterrieder, N. Glycoproteins D of equine herpesvirus type 1 (EHV-1) and EHV-4 determine cellular tropism independently of integrins. J. Virol. 2012, 86, 2031–2044. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, D.; Si, W.; Zhang, Y.; Khan, M.Z.; Zhao, X.; Liu, W. Transcriptomic and proteomic profiling of rabbit kidney cells infected with equine herpesvirus 8. Viruses 2025, 17, 647. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xu, D.; Si, W.; Zhang, Y.; Khan, M.Z.; Zhao, X.; Liu, W. Characterization and pathogenicity of equine herpesvirus type 8 using in-vitro and in-vivo models. Vet. Sci. 2025, 12, 367. [Google Scholar] [CrossRef]
- Taniguchi, A.; Fukushi, H.; Matsumura, T.; Yanai, T.; Masegi, T.; Hirai, K. Pathogenicity of a new neurotropic equine herpesvirus 9 (gazelle herpesvirus 1) in horses. J. Vet. Med. Sci. 2000, 62, 215–218. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Campos, A.C.; Cicolo, S.; De Oliveira, C.M.; Molina, C.V.; Navas-Suárez, P.E.; Poltronieri dos Santos, T.; da Silveira, V.B.; Barbosa, C.M.; Baccarin, R.Y.A.; Durigon, E.L.; et al. Potential outbreak by herpesvirus in equines: Detection, clinical, and genetic analysis of equid gammaherpesvirus 2 (EHV-2). Braz. J. Microbiol. 2023, 54, 1137–1143. [Google Scholar] [CrossRef]
- Wondimagegnehu, K.; Leta, S.; Amenu, K.; Negussie, H. Molecular detection and assessment of the epidemiological risk factors associated with equine herpesvirus 2 and 5 in working equids in central Ethiopia. Vet. Med. Sci. 2022, 8, 2396–2403. [Google Scholar] [CrossRef]
- James, K.; Chappell, D.E.; Craig, B.; Pariseau, C.; Wright, C.; van Harreveld, P.; Barnum, S.; Pusterla, N. Investigation of selected prevalence factors associated with EHV-2 and/or EHV-5 infection in horses with acute onset of fever and respiratory signs. Viruses 2025, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- LeCuyer, T.E.; Rink, A.; Bradway, D.S.; Evermann, J.F.; Nicola, A.V.; Baszler, T.; Haldorson, G.J. Abortion in a Mediterranean miniature donkey (Equus asinus) associated with a gammaherpesvirus similar to Equid herpesvirus 7. J. Vet. Diagn. Investig. 2015, 27, 749–753. [Google Scholar] [CrossRef]
- Osterrieder, N.; Van de Walle, G.R. Pathogenic potential of equine alphaherpesviruses: The importance of the mononuclear cell compartment in disease outcome. Vet. Microbiol. 2010, 143, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.; Osterrieder, N. Equine herpesvirus type 1 (EHV-1) glycoprotein K is required for efficient cell-to-cell spread and virus egress. Virology 2004, 329, 18–32. [Google Scholar] [CrossRef]
- Stasiak, K.; Dunowska, M.; Trewick, S.; Rola, J. Genetic variation in the glycoprotein b sequence of equid herpesvirus 5 among horses of various breeds at polish national studs. Pathogens 2021, 10, 322. [Google Scholar] [CrossRef]
- Hussey, G.S. Key determinants in the pathogenesis of equine herpesvirus 1 and 4 infections. Vet. Pathol. 2019, 56, 656–659. [Google Scholar] [CrossRef]
- Mahmoud, H.Y.; Andoh, K.; Hattori, S.; Terada, Y.; Noguchi, K.; Shimoda, H.; Maeda, K. Characterization of glycoproteins in equine herpesvirus-1. J. Vet. Med. Sci. 2013, 75, 1317–1321. [Google Scholar] [CrossRef]
- Azab, W.; Tsujimura, K.; Maeda, K.; Kobayashi, K.; Mohamed, Y.M.; Kato, K.; Matsumura, T.; Akashi, H. Glycoprotein C of equine herpesvirus 4 plays a role in viral binding to cell surface heparan sulfate. Virus Res. 2010, 151, 1–9. [Google Scholar] [CrossRef]
- Azab, W.; Lehmann, M.J.; Osterrieder, N. Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses. J. Virol. 2013, 87, 5937–5948. [Google Scholar] [CrossRef]
- Jambunathan, N.; Clark, C.M.; Musarrat, F.; Chouljenko, V.N.; Rudd, J.; Kousoulas, K.G. Two sides to every story: Herpes simplex type-1 viral glycoproteins gB, gD, gH/gL, gK, and cellular receptors function as key players in membrane fusion. Viruses 2021, 13, 1849. [Google Scholar]
- Losinno, A.; Vissani, M.A.; Sanchez, D.; Damiani, A.M. Equid herpesvirus type 3 infection produces membrane-associated and secreted forms of glycoprotein G that are not required for efficient cell-to-cell spread of the virus in vitro. Arch. Virol. 2023, 168, 122. [Google Scholar] [CrossRef]
- Kremling, V.; Loll, B.; Pach, S.; Dahmani, I.; Weise, C.; Wolber, G.; Chiantia, S.; Wahl, M.C.; Osterrieder, N.; Azab, W. Crystal structures of glycoprotein D of equine alphaherpesviruses reveal potential binding sites to the entry receptor MHC-I. Front. Microbiol. 2023, 14, 1197120. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Hasebe, R.; Makino, Y.; Suzuki, T.; Fukushi, H.; Okamoto, M.; Matsuda, K.; Taniyama, H.; Sawa, H.; Kimura, T. Equine major histocompatibility complex class I molecules act as entry receptors that bind to equine herpesvirus-1 glycoprotein D. Genes Cells 2011, 16, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kim, E.; Igarashi, M.; Ito, K.; Hasebe, R.; Fukushi, H.; Sawa, H.; Kimura, T. Single amino acid residue in the A2 domain of major histocompatibility complex class I is involved in the efficiency of equine herpesvirus-1 entry. J. Biol. Chem. 2011, 286, 39370–39378. [Google Scholar] [CrossRef] [PubMed]
- Spiesschaert, B.; Osterrieder, N.; Azab, W. Comparative analysis of glycoprotein B (gB) of equine herpesvirus type 1 and type 4 (EHV-1 and EHV-4) in cellular tropism and cell-to-cell transmission. Viruses 2015, 7, 522–542. [Google Scholar]
- Diallo, I.S.; Hewitson, G.; Wright, L.L.; Kelly, M.A.; Rodwell, B.J.; Corney, B.G. Multiplex real-time PCR for the detection and differentiation of equid herpesvirus 1 (EHV-1) and equid herpesvirus 4 (EHV-4). Vet. Microbiol. 2007, 123, 93–103. [Google Scholar] [CrossRef]
- Seyboldt, C.; Granzow, H.; Osterrieder, N. Equine herpesvirus 1 (EHV-1) glycoprotein M: Effect of deletions of transmembrane domains. Virology 2000, 278, 477–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ziegler, C.; Just, F.T.; Lischewski, A.; Elbers, K.; Neubauer, A. A glycoprotein M-deleted equid herpesvirus 4 is severely impaired in virus egress and cell-to-cell spread. J. Gen. Virol. 2005, 86, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Azab, W.; Osterrieder, N. Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)—Masters of co-evolution and a constant threat to equids and beyond. Vet. Microbiol. 2013, 167, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Thormann, N.; Van de Walle, G.R.; Azab, W.; Osterrieder, N. The role of secreted glycoprotein G of equine herpesvirus type 1 and type 4 (EHV-1 and EHV-4) in immune modulation and virulence. Virus Res. 2012, 169, 203–211. [Google Scholar] [CrossRef]
- von Einem, J.; Smith, P.M.; Van de Walle, G.R.; O’Callaghan, D.J.; Osterrieder, N. In vitro and in vivo characterization of equine herpesvirus type 1 (EHV-1) mutants devoid of the viral chemokine-binding glycoprotein G (gG). Virology 2007, 362, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.A.; Stanfield, B.A.; Vladimir, N.; Leib, D.A. Intramuscular immunization of mice with the live-attended herpes simplex virus 1 vaccine strain VC2 expressing equine herpesvirus 1 (EHV-1) glycoprotein D generates anti-EHV-1 immune responses in mice. J. Virol. 2017, 91, e02389-16. [Google Scholar] [CrossRef]
- Andoh, K.; Takasugi, M.; Mahmoud, H.Y.; Hattori, S.; Terada, Y.; Noguchi, K.; Shimoda, H.; Bannai, H.; Tsujimura, K.; Matsumura, T.; et al. Identification of a major immunogenic region of equine herpesvirus-1 glycoprotein E and its application to enzyme-linked immunosorbent assay. Vet. Microbiol. 2013, 164, 18–26. [Google Scholar] [CrossRef]
- Tsujimura, K.; Shiose, T.; Yamanaka, T.; Nemoto, M.; Kondo, T.; Matsumura, T. Equine herpesvirus type 1 mutant defective in glycoprotein E gene as candidate vaccine strain. J. Vet. Med. Sci. 2009, 71, 1439–1448. [Google Scholar] [CrossRef][Green Version]
- Tsujimura, K.; Yamanaka, T.; Kondo, T.; Fukushi, H.; Matsumura, T. Pathogenicity and immunogenicity of equine herpesvirus type 1 mutants defective in either gI or gE gene in murine and hamster models. J. Vet. Med. Sci. 2006, 68, 1029–1038. [Google Scholar] [CrossRef]
- Damiani, A.M.; Matsumura, T.; Yokoyama, N.; Mikami, T.; Takahashi, E. A deletion in the gI and gE genes of equine herpesvirus type 4 reduces viral virulence in the natural host and affects virus transmission during cell-to-cell spread. Virus Res. 2000, 67, 189–202. [Google Scholar] [CrossRef]
- Imposimato, I.; Muscatello, L.V.; Ellero, N.; Lelli, D.; Mira, F.; Sarli, G.; Freccero, F. Identification of asinine gamma herpesviruses in a donkey with interstitial pulmonary fibrosis, pleural effusion and thrombocytopenia. J. Equine Vet. Sci. 2024, 134, 105014. [Google Scholar] [CrossRef]
- Osterrieder, N.; Seyboldt, C.; Elbers, K. Deletion of gene 52 encoding glycoprotein M of equine herpesvirus type 1 strain RacH results in increased immunogenicity. Vet. Microbiol. 2001, 81, 219–226. [Google Scholar] [CrossRef]
- Wang, T.; Xi, C.; Yu, Y.; Liu, W.; Akhtar, M.F.; Li, Y.; Wang, C.; Li, L. Characteristics and epidemiological investigation of equid herpesvirus 8 in donkeys in Shandong, China. Arch. Virol. 2023, 168, 99. [Google Scholar] [CrossRef]
- Seki, Y.; Seimiya, Y.M.; Yaegashi, G.; Kumagai, S.I.; Sentsui, H.; Nishimori, T.; Ishihara, R. Occurrence of equine coital exanthema in pastured draft horses and isolation of equine herpesvirus 3 from progenital lesions. J. Vet. Med. Sci. 2004, 66, 1503–1508. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Lee, S.K.; Lee, J.S.; Kwon, O.D.; Kwak, D. Molecular detection and genetic characteristics of equine herpesvirus in Korea. Pathogens 2020, 9, 110. [Google Scholar] [CrossRef]
- Worku, A.; Molla, W.; Kenubih, A.; Negussie, H.; Admassu, B.; Ejo, M.; Dagnaw, G.G.; Bitew, A.B.; Fentahun, T.; Getnet, K.; et al. Molecular detection of equine herpesviruses from field outbreaks in donkeys in northwest Amhara region, Ethiopia. Vet. Med. Int. 2024, 2024, 9928835. [Google Scholar] [CrossRef]
- Worku, A.; Molla, W.; Kenubih, A.; Gizaw, D.; Muluneh, A.; Admassu, B.; Ejo, M.; Dagnaw, G.G.; Bitew, A.B.; Fentahun, T.; et al. Seroprevalence and associated risk factors of equine herpesvirus type-1/-4 in selected districts of Northwest Amhara, Ethiopia. Comp. Immunol. Microbiol. Infect. Dis. 2024, 107, 102155. [Google Scholar] [CrossRef]
- Temesgen, T.; Getachew, Y.; Negussie, H. Molecular identification of equine herpesvirus 1, 2, and 5 in equids with signs of respiratory disease in central Ethiopia. Vet. Med. Res. Rep. 2021, 12, 337–345. [Google Scholar] [CrossRef]
- Soboll-Hussey, G.; Dorman, D.C.; Burgess, B.A.; Goehring, L.; Gross, P.; Neinast, C.; Osterrieder, K.; Pusterla, N.; Lunn, D.P. Relationship between equine herpesvirus-1 viremia and abortion or equine herpesvirus myeloencephalopathy in domesticated horses: A systematic review. J. Vet. Intern. Med. 2024, 38, 1872–1891. [Google Scholar] [CrossRef]
- Durán, M.C.; Suazo, M.; Maturana, A.; Vargas, M.P.; García, A.; Ahumada, C.; Pezoa, A.; Goehring, L.S.; Lara, F. First equine herpes myeloencephalopathy (EHM) outbreak in Chile. Animals 2025, 15, 2344. [Google Scholar] [CrossRef]
- Couroucé, A.; Normand, C.; Tessier, C.; Pomares, R.; Thévenot, J.; Marcillaud-Pitel, C.; Legrand, L.; Pitel, P.H.; Pronost, S.; Lupo, C. Equine herpesvirus-1 outbreak during a show-jumping competition: A clinical and epidemiological study. J. Equine Vet. Sci. 2023, 128, 104869. [Google Scholar] [CrossRef]
- de la Cuesta-Torrado, M.; Velloso Alvarez, A.; Santiago-Llorente, I.; Armengou, L.; Nieto, F.; Ríos, J.; Cruz-López, F.; Jose-Cunilleras, E. Risk factors and long-term outcomes in horses after the 2021 outbreak of equine herpesvirus 1 myeloencephalopathy, Valencia, Spain. J. Vet. Intern. Med. 2025, 39, e70040. [Google Scholar] [CrossRef]
- de la Cuesta-Torrado, M.; Vitale, V.; Velloso Alvarez, A.; Neira-Egea, P.; Diss, C.; Cuervo-Arango, J. The effect of vaccination status on total lymphocyte count in horses affected by equine herpes virus-1 myeloencephalopathy. Animals 2025, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Lawton, K.; Barnum, S.; Ross, K.; Purcell, K. Investigation of an outbreak of equine herpesvirus-1 myeloencephalopathy in a population of aged working equids. Viruses 2024, 16, 1963. [Google Scholar] [CrossRef]
- Pusterla, N.; Dorman, D.C.; Burgess, B.A.; Goehring, L.; Gross, M.; Osterrieder, K.; Soboll, H.G.; Lunn, D.P. Viremia and nasal shedding for the diagnosis of equine herpesvirus-1 infection in domesticated horses. J. Vet. Intern. Med. 2024, 38, 1765–1791. [Google Scholar] [CrossRef]
- Mureşan, A.; Mureşan, C.; Siteavu, M.; Avram, E.; Bochynska, D.; Taulescu, M. An outbreak of equine herpesvirus-4 in an ecological donkey milk farm in Romania. Vaccines 2022, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Miglinci, L.; Reicher, P.; Nell, B.; Koch, M.; Jindra, C.; Brandt, S. Detection of equine papillomaviruses and gamma-herpesviruses in equine squamous cell carcinoma. Pathogens 2023, 12, 179. [Google Scholar] [CrossRef]
- Peters-Kennedy, J.; Löhr, C.V.; Cossic, B.; Glaser, A.L.; Duhamel, G.E. Association of equine gammaherpesvirus-5 with facial lymphohistiocytic interface dermatitis in seven adult horses from the United States. Vet. Pathol. 2023, 60, 888–897. [Google Scholar] [CrossRef]
- El Brini, Z.; Fassi Fihri, O.; Paillot, R.; Lotfi, C.; Amraoui, F.; El Ouadi, H.; Dehhaoui, M.; Colitti, B.; Alyakine, H.; Piro, M. Seroprevalence of equine herpesvirus 1 (EHV-1) and equine herpesvirus 4 (EHV-4) in the Northern Moroccan horse populations. Animals 2021, 11, 2851. [Google Scholar] [CrossRef]
- Rushton, J.O.; Kolodziejek, J.; Tichy, A.; Nowotny, N.; Nell, B. Clinical course of ophthalmic findings and potential influence factors of herpesvirus infections: 18 month follow-up of a closed herd of lipizzaners. PLoS ONE 2013, 8, e79888. [Google Scholar] [CrossRef]
- van der Meulen, K.M.; Nauwynck, H.J.; Buddaert, W.; Pensaert, M.B. Replication of equine herpesvirus type 1 in freshly isolated equine peripheral blood mononuclear cells and changes in susceptibility following mitogen stimulation. J. Gen. Virol. 2000, 81, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Martinov, S.P.; Chenchev, I.; Yordanov, S.; Nordengrahn, A. Equine herpesvirus infections in Bulgaria. Biotechnol. Biotechnol. Equip. 2000, 14, 60–62. [Google Scholar] [CrossRef][Green Version]
- Barbić, L.; Lojkić, I.; Stevanović, V.; Bedeković, T.; Starešina, V.; Lemo, N.; Lojkić, M.; Madić, J. Two outbreaks of neuropathogenic equine herpesvirus type 1 with breed-dependent clinical signs. Vet. Rec. 2012, 170, 227. [Google Scholar] [CrossRef] [PubMed]
- Krisová, Š.; Tóthová, K.; Molinková, D.; Makra, Z.; Zisopoulou, A.M. Prevalence of equine herpesvirus 2 (EHV-2) in equine ocular disease. Acta Vet. Brno 2020, 89, 115–123. [Google Scholar] [CrossRef]
- Pronost, S.; Legrand, L.; Pitel, P.H.; Wegge, B.; Lissens, J.; Freymuth, F.; Richard, E.; Fortier, G. Outbreak of equine herpesvirus myeloencephalopathy in France: A clinical and molecular investigation. Transbound. Emerg. Dis. 2012, 59, 256–263. [Google Scholar] [CrossRef]
- Pavulraj, S.; Eschke, K.; Theisen, J.; Westhoff, S.; Reimers, G.; Andreotti, S.; Osterrieder, N.; Azab, W. Equine herpesvirus type 4 (EHV-4) outbreak in Germany: Virological, serological, and molecular investigations. Pathogens 2021, 10, 810. [Google Scholar] [CrossRef]
- Damiani, A.M.; de Vries, M.; Reimers, G.; Winkler, S.; Osterrieder, N. A severe equine herpesvirus type 1 (EHV-1) abortion outbreak caused by a neuropathogenic strain at a breeding farm in northern Germany. Vet. Microbiol. 2014, 172, 555–562. [Google Scholar] [CrossRef]
- Stierstorfer, B.; Eichhorn, W.; Schmahl, W.; Brandmüller, C.; Kaaden, O.R.; Neubauer, A. Equine herpesvirus type 1 (EHV-1) myeloencephalopathy: A case report. J. Vet. Med. B 2002, 49, 37–41. [Google Scholar] [CrossRef]
- Lang, A.; de Vries, M.; Feineis, S.; Müller, E.; Osterrieder, N.; Damiani, A.M. Development of a peptide ELISA for discrimination between serological responses to equine herpesvirus type 1 and 4. J. Virol. Methods 2013, 193, 667–673. [Google Scholar] [CrossRef]
- Kershaw, O.; Von Oppen, T.; Glitz, F.; Deegen, E.; Ludwig, H.; Borchers, K. Detection of equine herpesvirus type 2 (EHV-2) in horses with keratoconjunctivitis. Virus Res. 2001, 80, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Bietta, A.; Lepri, E.; Casagrande Proietti, P.; Cordioli, P.; Canelli, E.; Stefanetti, V.; Coletti, M.; Timoney, P.J.; Passamonti, F. Role of equine herpesviruses as co-infecting agents in cases of abortion, placental disease and neonatal foal mortality. Vet. Res. Commun. 2013, 37, 311–317. [Google Scholar] [CrossRef]
- van Maanen, K.; van den Wollenberg, L.; de Haan, T.; Frippiat, T. Epidemiology of infectious pathogens in horses with acute respiratory disease, abortion, and neurological signs: Insights gained from the veterinary surveillance system for horses in The Netherlands (SEIN). Vet. Sci. 2025, 12, 567. [Google Scholar] [CrossRef]
- van Maanen, C. Equine herpesvirus 1 and 4 infections: An update. Vet. Q. 2002, 24, 57–78. [Google Scholar] [CrossRef]
- Heldens, J.G.; Kersten, A.J.; Weststrate, M.W.; Van Den Hoven, R. Vaccinology: Duration of immunity induced by an adjuvanted and inactivated equine influenza, tetanus and equine herpesvirus 1 and 4 combination vaccine. Vet. Q. 2001, 23, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, K.; Dunowska, M.; Rola, J. Outbreak of equid herpesvirus 1 abortions at the Arabian stud in Poland. BMC Vet. Res. 2020, 16, 374. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, A.K.; Skarbek, M.; Jackulak, N.A.; Rola, J. Molecular characterisation of equid alphaherpesvirus 1 strains isolated from aborted fetuses in Poland. Virol. J. 2018, 15, 186. [Google Scholar] [CrossRef]
- de la Cuesta-Torrado, M.; Velloso Alvarez, A.; Neira-Egea, P.; Cuervo-Arango, J. Long-term performance of show-jumping horses and relationship with severity of ataxia and complications associated with myeloencephalopathy caused by equine herpes virus-1. J. Vet. Intern. Med. 2024, 38, 1799–1807. [Google Scholar] [CrossRef]
- Vereecke, N.; Carnet, F.; Pronost, S.; Vanschandevijl, K.; Theuns, S.; Nauwynck, H. Genome sequences of equine herpesvirus 1 strains from a European outbreak of neurological disorders linked to a horse gathering in Valencia, Spain, in 2021. Microbiol. Resour. Announc. 2021, 10, e00128-21. [Google Scholar] [CrossRef]
- Nordengrahn, A.; Klingeborn, B.; Lindholm, A.; Merza, M. The use of a neutralizing monoclonal antibody to detect infections of equine herpesvirus type 2 (EHV-2). J. Vet. Diagn. Investig. 2001, 13, 389–393. [Google Scholar] [CrossRef]
- Back, H.; Ullman, K.; Leijon, M.; Söderlund, R.; Penell, J.; Ståhl, K.; Pringle, J.; Valarcher, J.F. Genetic variation and dynamics of infections of equid herpesvirus 5 in individual horses. J. Gen. Virol. 2016, 97, 169–178. [Google Scholar] [CrossRef]
- Back, H.; Kendall, A.; Grandön, R.; Ullman, K.; Treiberg-Berndtsson, L.; Ståhl, K.; Pringle, J. Equine multinodular pulmonary fibrosis in association with asinine herpesvirus type 5 and equine herpesvirus type 5: A case report. Acta Vet. Scand. 2012, 54, 57. [Google Scholar] [CrossRef]
- Walter, J.; Seeh, C.; Fey, K.; Bleul, U.; Osterrieder, N. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Vet. Scand. 2013, 55, 19. [Google Scholar] [CrossRef]
- Smith, D.J.; Hamblin, A.S.; Edington, N. Infection of endothelial cells with Equine herpesvirus-1 (EHV-1) occurs where there is activation of putative adhesion molecules: A mechanism for transfer of virus. Equine Vet. J. 2001, 33, 138–142. [Google Scholar] [CrossRef]
- Tearle, J.P.; Smith, K.C.; Platt, A.J.; Hannant, D.; Davis-Poynter, N.J.; Mumford, J.A. In vitro characterisation of high and low virulence isolates of equine herpesvirus-1 and-4. Res. Vet. Sci. 2003, 75, 83–86. [Google Scholar] [CrossRef]
- Tong, P.; Pan, J.; Dang, Y.; Yang, E.; Jia, C.; Duan, R.; Tian, S.; Palidan, N.; Kuang, L.; Wang, C.; et al. First identification and isolation of equine herpesvirus type 1 in aborted fetal lung tissues of donkeys. Virol. J. 2024, 21, 117. [Google Scholar] [CrossRef]
- Tong, P.; Yang, E.; Liu, B.; Tian, S.; Suo, Y.; Pan, J.; Dang, Y.; Palidan, N.; Jia, C.; Kuang, L.; et al. Identification of neuropathogenic Varicellovirus equidalpha1 as a potential cause of respiratory disease outbreaks among horses in North Xinjiang, China, from 2021–2023. BMC Vet. Res. 2024, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Duan, R.; Palidan, N.; Deng, H.; Duan, L.; Ren, M.; Song, X.; Jia, C.; Tian, S.; Yang, E.; et al. Outbreak of neuropathogenic equid herpesvirus 1 causing abortions in Yili horses of Zhaosu, North Xinjiang, China. BMC Vet. Res. 2022, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, X.; Yu, Y.; Sun, Q.; Li, W.; Li, Y.; Li, S.; Chen, L.; Khan, M.Z.; Wang, C.; et al. Blebbistatin as a novel antiviral agent targeting equid herpesvirus type 8. Front. Vet. Sci. 2024, 11, 1390304. [Google Scholar] [CrossRef]
- Wang, T.; Hu, L.; Liu, M.; Wang, T.; Hu, X.; Li, Y.; Liu, W.; Li, Y.; Wang, Y.; Ren, H.; et al. The emergence of viral encephalitis in donkeys by equid herpesvirus 8 in China. Front. Microbiol. 2022, 13, 840754. [Google Scholar] [CrossRef]
- Schvartz, G.; Edery, N.; Moss, L.; Hadad, R.; Steinman, A.; Karniely, S. Equid herpesvirus 8 isolated from an adult donkey in Israel. J. Equine Vet. Sci. 2020, 94, 103247. [Google Scholar] [CrossRef]
- Xie, J.; Tong, P.; Zhang, L.; Ren, M.; Song, X.; Jia, C.; Palidan, N.; Zhang, L.; Kuang, L. First detection and genetic characterization of equid herpesvirus 2, 4, and 5 in China. Arch. Virol. 2021, 166, 1421–1426. [Google Scholar] [CrossRef]
- Al-Ajeeli, K.S. Molecular detection of equine herpes virus-1 in local horses (Equus ferus caballus) and donkeys (Equus asinus). Iraqi J. Vet. Med. 2018, 42, 72–78. [Google Scholar] [CrossRef]
- Aharonson-Raz, K.; Davidson, I.; Porat, Y.; Altory, A.; Klement, E.; Steinman, A. Seroprevalence and rate of infection of equine influenza virus (H3N8 and H7N7) and equine herpesvirus (1 and 4) in the horse population in Israel. J. Equine Vet. Sci. 2014, 34, 828–832. [Google Scholar] [CrossRef]
- Mizukoshi, F.; Maeda, K.; Hamano, M.; Iwata, H.; Matsumura, T.; Kondo, T.; Sugiura, T. IgG antibody subclass response against equine herpesvirus type 4 in horses. Vet. Immunol. Immunopathol. 2002, 88, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, K.; Oyama, T.; Katayama, Y.; Muranaka, M.; Bannai, H.; Nemoto, M.; Yamanaka, T.; Kondo, T.; Kato, M.; Matsumura, T. Prevalence of equine herpesvirus type 1 strains of neuropathogenic genotype in a major breeding area of Japan. J. Vet. Med. Sci. 2011, 73, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kai, K.; Matsumura, T. Genomic diversity among equine herpesvirus-4 field isolates. J. Vet. Med. Sci. 2005, 67, 555–561. [Google Scholar] [CrossRef][Green Version]
- Yildirim, Y.; Yilmaz, V.; Kirmizigul, A.H. Equine herpes virus type 1 (EHV-1) and 4 (EHV-4) infections in horses and donkeys in northeastern Turkey. Iran J. Vet. Res. 2015, 16, 341–346. [Google Scholar][Green Version]
- Ataseven, V.S.; Dağalp, S.B.; Güzel, M.; Başaran, Z.; Tan, M.T.; Geraghty, B. Prevalence of equine herpesvirus-1 and equine herpesvirus-4 infections in equidae species in Turkey as determined by ELISA and multiplex nested PCR. Res. Vet. Sci. 2009, 86, 339–344. [Google Scholar] [CrossRef]
- Negussie, H.; Gizaw, D.; Tessema, T.S.; Nauwynck, H.J. Equine herpesvirus-1 myeloencephalopathy, an emerging threat of working equids in ethiopia. Transbound. Emerg. Dis. 2017, 64, 389–397. [Google Scholar] [CrossRef]
- El Brini, Z.; Cullinane, A.; Garvey, M.; Fassi Fihri, O.; Fellahi, S.; Amraoui, F.; Loutfi, C.; Sebbar, G.; Paillot, R.; Piro, M. First molecular and phylogenetic characterization of equine herpesvirus-1 (EHV-1) and equine herpesvirus-4 (EHV-4) in Morocco. Animals 2025, 15, 102. [Google Scholar] [CrossRef]
- Mekonnen, A.; Eshetu, A.; Gizaw, D. Equine herpesvirus 1 and/or 4 in working equids: Seroprevalence and risk factors in North Shewa Zone, Ethiopia. Ethiop. Vet. J. 2017, 21, 28–39. [Google Scholar] [CrossRef][Green Version]
- Khattab, O.M.; AbdelmegeedHK, M.M.; HamdyME, H.N.; Hamed, A.; Fahmy, H.A.; Ibrahim, E.; Ahmed, E.M. Equine herpes virus 4 (EHV4) investigation in aborted Egyptian mares; molecular detection, isolation, and phylogeny for viral glycoprotein B. Adv. Anim. Vet. Sci. 2022, 10, 1907–1915. [Google Scholar] [CrossRef]
- Tau, R.L.; Marandino, A.E.; Panzera, Y.; Alamos, F.; Vissani, M.A.; Romera, S.A.; Pérez, R.; Maidana, S.S. The complete genome of equid herpesvirus-1 (EHV-1) field isolates from Argentina reveals an interspecific recombinant strain. Virus Genes 2024, 60, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Martín Ocampos, G.P.; Fuentealba, N.A.; Sguazza, G.H.; Jones, L.R.; Cigliano, M.M.; Barbeito, C.G.; Galosi, C.M. Genomic and phylogenetic analysis of Argentinian Equid Herpesvirus 1 strains. Virus Genes 2009, 38, 113–117. [Google Scholar] [CrossRef]
- Craig, M.I.; Barrandeguy, M.E.; Fernández, F.M. Equine herpesvirus 2 (EHV-2) infection in thoroughbred horses in Argentina. BMC Vet. Res. 2005, 1, 9. [Google Scholar] [CrossRef]
- Carvalho, R.; Oliveira, A.M.; Souza, A.M.; Passos, L.M.; Martins, A.S. Prevalence of equine herpesvirus type 1 latency detected by polymerase chain reaction. Arch. Virol. 2000, 145, 1773–1787. [Google Scholar] [CrossRef]
- Carvalho, R.; Passos, L.M.; Martins, A.S. Development of a differential multiplex PCR assay for equine herpesvirus 1 and 4 as a diagnostic tool. J. Vet. Med. B 2000, 47, 351–359. [Google Scholar] [CrossRef]
- Burgess, B.A.; Tokateloff, N.; Manning, S.; Lohmann, K.; Lunn, D.P.; Hussey, S.B.; Morley, P.S. Nasal shedding of equine herpesvirus-1 from horses in an outbreak of equine herpes myeloencephalopathy in Western Canada. J. Vet. Intern. Med. 2012, 26, 384–392. [Google Scholar] [CrossRef]
- Petano-Duque, J.M.; Urueña-Martinez, E.; Cabezas-Callejas, L.L.; Perilla-Amaya, J.; Rueda-García, V.; Rondón-Barragán, I.S.; Lopera-Vásquez, R. Molecular and serological investigation of equine herpesvirus type 1 (EHV-1) and type 4 (EHV-4) in horses in Ibagué, Tolima. Vet. Med. Int. 2025, 2025, 1661949. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Schommer, S.K.; Johnson, P.J.; Ehlers, B.; Turnquist, S.E.; Boucher, M.; Kreeger, J.M. Association of two newly recognized herpesviruses with interstitial pneumonia in donkeys (Equus asinus). J. Vet. Diagn. Investig. 2002, 14, 273–280. [Google Scholar] [CrossRef]
- Bawa, B.; Vander Werf, K.; Beard, L.; Davis, E.; Andrews, G.; Almes, K. Equine multinodular pulmonary fibrosis and lymphoma in a horse associated with equine herpesvirus-5. J. Equine Vet. Sci. 2014, 34, 694–700. [Google Scholar] [CrossRef]
- Vander Werf, K.A.; Davis, E.G.; Janardhan, K.; Bawa, B.; Bolin, S.; Almes, K. Identification of equine herpesvirus 5 in horses with lymphoma. J. Equine Vet. Sci. 2014, 34, 738–741. [Google Scholar] [CrossRef]
- Wilcox, A.; Barnum, S.; Wademan, C.; Corbin, R.; Escobar, E.; Hodzic, E.; Schumacher, S.; Pusterla, N. Frequency of detection of respiratory pathogens in clinically healthy show horses following a multi-county outbreak of equine herpesvirus-1 myeloencephalopathy in California. Pathogens 2022, 11, 1161. [Google Scholar] [CrossRef]
- Breathnach, C.C.; Yeargan, M.R.; Sheoran, A.S.; Allen, G.P. The mucosal humoral immune response of the horse to infective challenge and vaccination with equine herpesvirus-1 antigens. Equine Vet. J. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Friday, P.A.; Scarratt, W.K.; Elvinger, F.; Timoney, P.J.; Bonda, A. Ataxia and paresis with equine herpesvirus type 1 infection in a herd of riding school horses. J. Vet. Intern. Med. 2000, 14, 197–201. [Google Scholar] [CrossRef]
- Easther, R.; Manthorpe, E.; Woolford, L.; Kawarizadeh, A.; Hemmatzadeh, F.; Agne, G.F. Eosinophilic inflammation and equine herpesvirus-1 associated with haemorrhagic cystitis in a horse. J. Equine Vet. Sci. 2022, 119, 104161. [Google Scholar] [CrossRef]
- Studdert, M.J.; Hartley, C.A.; Dynon, K.; Sandy, J.R.; Slocombe, R.R.; Charles, J.A.; Milne, M.E.; Clarke, A.F.; El-Hage, C. Outbreak of equine herpesvirus type 1 myeloencephalitis: New insights from virus identification by PCR and the application of an EHV-1-specific antibody detection ELISA. Vet. Rec. 2003, 153, 417–423. [Google Scholar] [CrossRef]
- Wang, L.; Raidal, S.L.; Pizzirani, A.; Wilcox, G.E. Detection of respiratory herpesviruses in foals and adult horses determined by nested multiplex PCR. Vet. Microbiol. 2007, 121, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Varrasso, A.; Dynon, K.; Ficorilli, N.; Hartley, C.A.; Studdert, M.J.; Drummer, H.E. Identification of equine herpesviruses 1 and 4 by polymerase chain reaction. Aust. Vet. J. 2001, 79, 563–569. [Google Scholar] [CrossRef]
- Ruitenberg, K.M.; Love, D.N.; Gilkerson, J.R.; Wellington, J.E.; Whalley, J.M. Equine herpesvirus 1 (EHV-1) glycoprotein D DNA inoculation in horses with pre-existing EHV-1/EHV-4 antibody. Vet. Microbiol. 2000, 76, 117–127. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, C.; Mekuria, Z.; Dynon, K.; Hartley, C.; McBride, K.; Gilkerson, J. Association of equine herpesvirus 5 with mild respiratory disease in a survey of EHV1,-2,-4 and-5 in 407 Australian horses. Animals 2021, 11, 3418. [Google Scholar] [CrossRef]
- Dunowska, M.; Howe, L.; Hanlon, D.; Stevenson, M. Kinetics of Equid herpesvirus type 2 infections in a group of Thoroughbred foals. Vet. Microbiol. 2011, 152, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Dunowska, M.; Holloway, S.A.; Wilks, C.R.; Meers, J. Genomic variability of equine herpesvirus-5. Arch. Virol. 2000, 145, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, J.; Poelaert, K.C.K.; Laval, K.; Vanderheijden, N.; Dhaenens, M.; Daled, S.; Boyen, F.; Pasmans, F.; Nauwynck, H.J. An alphaherpesvirus exploits antimicrobial β-defensins to initiate respiratory tract infection. J. Virol. 2020, 94, e01676-19. [Google Scholar] [CrossRef] [PubMed]
- Baghi, H.B.; Nauwynck, H.J. Impact of equine herpesvirus type 1 (EHV-1) infection on the migration of monocytic cells through equine nasal mucosa. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 321–329. [Google Scholar] [CrossRef]
- Hussey, G.S.; Ashton, L.V.; Quintana, A.M.; Lunn, D.P.; Goehring, L.S.; Annis, K.; Landolt, G. Innate immune responses of airway epithelial cells to infection with equine herpesvirus-1. Vet. Microbiol. 2014, 170, 28–38. [Google Scholar] [CrossRef]
- Gryspeerdt, A.C.; Vandekerckhove, A.; Garré, B.; Barbé, F.; Van de Walle, G.; Nauwynck, H. Differences in replication kinetics and cell tropism between neurovirulent and non-neurovirulent EHV1 strains during the acute phase of infection in horses. Vet. Microbiol. 2010, 142, 242–253. [Google Scholar] [CrossRef]
- Vandekerckhove, A.P.; Glorieux, S.; Gryspeerdt, A.C.; Steukers, L.; Duchateau, L.; Osterrieder, N.; Van-de-Walle, G.R.; Nauwynck, H.J. Replication kinetics of neurovirulent versus non-neurovirulent equine herpesvirus type 1 strains in equine nasal mucosal explants. J. Gen. Virol. 2010, 91, 2019–2028. [Google Scholar] [CrossRef]
- Holmes, C.M.; Babasyan, S.; Eady, N.; Schnabel, C.L.; Wagner, B. Immune horses rapidly increase antileukoproteinase and lack type I interferon secretion during mucosal innate immune responses against equine herpesvirus type 1. Microbiol. Spectr. 2024, 12, e01092-24. [Google Scholar] [CrossRef]
- Holmes, C.M.; Wagner, B. Characterization of nasal mucosal T cells in horses and their response to equine herpesvirus type 1. Viruses 2024, 16, 1514. [Google Scholar] [CrossRef]
- Laval, K.; Poelaert, K.C.; Van Cleemput, J.; Zhao, J.; Vandekerckhove, A.P.; Gryspeerdt, A.C.; Garré, B.; van-der-Meulen, K.; Baghi, H.B.; Dubale, H.N.; et al. The pathogenesis and immune evasive mechanisms of equine herpesvirus type 1. Front. Microbiol. 2021, 12, 662686. [Google Scholar] [CrossRef]
- Hu, L.; Wang, T.; Ren, H.; Liu, W.; Li, Y.; Wang, C.; Li, L. Characterizing the pathogenesis and immune response of equine herpesvirus 8 infection in lung of mice. Animals 2022, 12, 2495. [Google Scholar] [CrossRef]
- Giessler, K.S.; Goehring, L.S.; Jacob, S.I.; Davis, A.; Esser, M.M.; Lee, Y.; Zarski, L.M.; Weber, P.S.D.; Hussey, G.S. Impact of the host immune response on the development of equine herpesvirus myeloencephalopathy in horses. J. Gen. Virol. 2024, 105, 001987. [Google Scholar] [CrossRef]
- Poelaert, K.C.K.; Van Cleemput, J.; Laval, K.; Favoreel, H.W.; Soboll Hussey, G.; Maes, R.K.; Nauwynck, H.J. Abortigenic but not neurotropic equine Herpes Virus 1 modulates the interferon antiviral defense. Front. Cell. Infect. Microbiol. 2018, 8, 312. [Google Scholar] [CrossRef]
- Poelaert, K.C.K.; Van Cleemput, J.; Laval, K.; Xie, J.; Favoreel, H.W.; Nauwynck, H.J. Equine herpesvirus 1 infection orchestrates the expression of chemokines in equine respiratory epithelial cells. J. Gen. Virol. 2019, 100, 1567–1579. [Google Scholar] [CrossRef]
- Zhao, J.; Poelaert, K.C.K.; Van Cleemput, J.; Nauwynck, H.J. CCL2 and CCL5 driven attraction of CD172a+ monocytic cells during an equine herpesvirus type 1 (EHV-1) infection in equine nasal mucosa and the impact of two migration inhibitors, rosiglitazone (RSG) and quinacrine (QC). Vet. Res. 2017, 48, 14. [Google Scholar] [CrossRef] [PubMed]
- Poelaert, K.C.K.; Van Cleemput, J.; Laval, K.; Favoreel, H.W.; Couck, L.; Van den Broeck, W.; Azab, W.; Nauwynck, H.J. Equine herpesvirus 1 bridles T lymphocytes to reach its target organs. J. Virol. 2019, 93, e02098-18. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Miller, J.; Varnell, S.; Armstrong, W.; Frost, L.; Michon, C.; Lambert, K.; Whitfield, S.; Cowles, B. Investigation of the usefulness of serum amyloid A in characterizing selected disease forms of equine herpesvirus-1 infection. J. Equine Vet. Sci. 2021, 104, 103699. [Google Scholar] [CrossRef]
- Bryant, N.A.; Davis-Poynter, N.; Vanderplasschen, A.; Alcami, A. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J. 2003, 22, 833–846. [Google Scholar] [CrossRef]
- Van de Walle, G.R.; May, M.L.; Sukhumavasi, W.; von Einem, J.; Osterrieder, N. Herpesvirus chemokine-binding glycoprotein G (gG) efficiently inhibits neutrophil chemotaxis in vitro and in vivo. J. Immunol. 2007, 179, 4161–4169. [Google Scholar] [CrossRef]
- Soboll Hussey, G.; Hussey, S.B.; Wagner, B.; Horohov, D.W.; Van de Walle, G.R.; Osterrieder, N.; Goehring, L.S.; Rao, S.; Lunn, D.P. Evaluation of immune responses following infection of ponies with an EHV-1 ORF1/2 deletion mutant. Vet. Res. 2011, 42, 23. [Google Scholar] [CrossRef]
- Gryspeerdt, A.C.; Vandekerckhove, A.P.; Baghi, H.B.; Van de Walle, G.R.; Nauwynck, H.J. Expression of late viral proteins is restricted in nasal mucosal leucocytes but not in epithelial cells during early-stage equine herpes virus-1 infection. Vet. J. 2012, 193, 576–578. [Google Scholar] [CrossRef]
- van der Meulen, K.; Caij, B.; Pensaert, M.; Nauwynck, H. Absence of viral envelope proteins in equine herpesvirus 1-infected blood mononuclear cells during cell-associated viremia. Vet. Microbiol. 2006, 113, 265–273. [Google Scholar] [CrossRef]
- Laval, K.; Favoreel, H.W.; Nauwynck, H.J. Equine herpesvirus type 1 replication is delayed in CD172a+ monocytic cells and controlled by histone deacetylases. J. Gen. Virol. 2015, 96, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Laval, K.; Van Cleemput, J.; Poelaert, K.C.; Brown, I.K.; Nauwynck, H.J. Replication of neurovirulent equine herpesvirus type 1 (EHV-1) in CD172a(+) monocytic cells. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Laval, K.; Favoreel, H.W.; Van Cleemput, J.; Poelaert, K.C.K.; Brown, I.K.; Verhasselt, B.; Nauwynck, H.J. Entry of equid herpesvirus 1 into CD172a+ monocytic cells. J. Gen. Virol. 2016, 97, 733–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laval, K.; Favoreel, H.W.; Poelaert, K.C.; Van Cleemput, J.; Nauwynck, H.J. Equine herpesvirus type 1 enhances viral replication in CD172a+ monocytic cells upon adhesion to endothelial cells. J. Virol. 2015, 89, 10912–10923. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Sarkar, S.; Reedy, S.; Balasuriya, U.B.R.; Horohov, D.W.; Chambers, T.M. Equid herpesvirus 1 targets the sensitization and induction steps to inhibit the type I interferon response in equine endothelial cells. J. Virol. 2019, 93, e01342-19. [Google Scholar] [CrossRef]
- Sarkar, S.; Balasuriya, U.B.; Horohov, D.W.; Chambers, T.M. Equine herpesvirus-1 suppresses type-I interferon induction in equine endothelial cells. Vet. Immunol. Immunopathol. 2015, 167, 122–129. [Google Scholar] [CrossRef]
- Sarkar, S.; Balasuriya, U.B.; Horohov, D.W.; Chambers, T.M. Equine herpesvirus-1 infection disrupts interferon regulatory factor-3 (IRF-3) signaling pathways in equine endothelial cells. Vet. Immunol. Immunopathol. 2016, 173, 1–9. [Google Scholar] [CrossRef]
- Sarkar, S.; Balasuriya, U.B.; Horohov, D.W.; Chambers, T.M. The neuropathogenic T953 strain of equine herpesvirus-1 inhibits type-I IFN mediated antiviral activity in equine endothelial cells. Vet. Microbiol. 2016, 183, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.S.; Hussey, G.S.; Ashton, L.V.; Schenkel, A.R.; Lunn, D.P. Infection of central nervous system endothelial cells by cell-associated EHV-1. Vet. Microbiol. 2011, 148, 389–395. [Google Scholar] [CrossRef]
- Smith, K.C.; Whitwell, K.E.; Blunden, A.S.; Bestbier, M.E.; Scase, T.J.; Geraghty, R.J.; Nugent, J.; Davis-Poynter, N.J.; Cardwell, J.M. Equine herpesvirus-1 abortion: Atypical cases with lesions largely or wholly restricted to the placenta. Equine Vet. J. 2004, 36, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C.; Mumford, J.A.; Lakhani, K. A comparison of equid herpesvirus-1 (EHV-1) vascular lesions in the early versus late pregnant equine uterus. J. Comp. Pathol. 1996, 114, 231–247. [Google Scholar] [CrossRef]
- Edington, N.; Smyth, B.; Griffiths, L. The role of endothelial cell infection in the endometrium, placenta and foetus of equid herpesvirus 1 (EHV-1) abortions. J. Comp. Pathol. 1991, 104, 379–387. [Google Scholar] [CrossRef]
- Wilsterman, S.; Soboll-Hussey, G.; Lunn, D.P.; Ashton, L.V.; Callan, R.J.; Hussey, S.B.; Rao, S.; Goehring, L.S. Equine herpesvirus-1 infected peripheral blood mononuclear cell subpopulations during viremia. Vet. Microbiol. 2011, 149, 40–47. [Google Scholar] [CrossRef]
- Hussey, G.S.; Goehring, L.S.; Lunn, D.P.; Hussey, S.B.; Huang, T.; Osterrieder, N.; Powell, C.; Hand, J.; Holz, C.; Slater, J. Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet. Res. 2013, 44, 118. [Google Scholar] [CrossRef]
- Smith, K.C.; Borchers, K. A study of the pathogenesis of equid herpesvirus-1 (EHV-1) abortion by DNA in-situ hybridization. J. Comp. Pathol. 2001, 125, 304–310. [Google Scholar] [CrossRef]
- Allen, G.P. Risk factors for development of neurologic disease after experimental exposure to equine herpesvirus-1 in horses. Am. J. Vet. Res. 2008, 69, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef]
- Allen, G.P.; Breathnach, C.C. Quantification by real-time PCR of the magnitude and duration of leucocyte-associated viraemia in horses infected with neuropathogenic vs. non-neuropathogenic strains of EHV-1. Equine Vet. J. 2006, 38, 252–257. [Google Scholar] [CrossRef]
- Nugent, J.; Birch-Machin, I.; Smith, K.C.; Mumford, J.A.; Swann, Z.; Newton, J.R.; Bowden, R.J.; Allen, G.P.; Davis-Poynter, N. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 2006, 80, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Goodman, L.B.; Tsujimura, K.; Van de Walle, G.R.; Kim, S.G.; Dubovi, E.J.; Osterrieder, N. Investigation of the prevalence of neurologic equine herpes virus type 1 (EHV-1) in a 23-year retrospective analysis (1984–2007). Vet. Microbiol. 2009, 139, 375–378. [Google Scholar] [CrossRef]
- Van de Walle, G.R.; Goupil, R.; Wishon, C.; Damiani, A.; Perkins, G.A.; Osterrieder, N. A single-nucleotide polymorphism in a herpesvirus DNA polymerase is sufficient to cause lethal neurological disease. J. Infect. Dis. 2009, 200, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Barnum, S.; Lawton, K.; Wademan, C.; Corbin, R.; Hodzic, E. Investigation of the EHV-1 genotype (N752, D752, and H752) in swabs collected from equids with respiratory and neurological disease and abortion from the United States (2019–2022). J. Equine Vet. Sci. 2023, 123, 104244. [Google Scholar] [CrossRef]
- Pusterla, N.; Hussey, G.S. Equine herpesvirus 1 myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 2014, 30, 489–506. [Google Scholar] [CrossRef]
- Edington, N.; Bridges, C.G.; Patel, J.R. Endothelial cell infection and thrombosis in paralysis caused by equid herpesvirus-1: Equine stroke. Arch. Virol. 1986, 90, 111–124. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Costa, R.C.; Zanatto, D.A.; Bruhn, F.R.; Mesquita, L.L.; Lara, M.C.; Villalobos, E.M.; Massoco, C.O.; Mori, C.M.; Mori, E.; et al. Equine herpesvirus 1 elicits a strong pro-inflammatory response in the brain of mice. J. Gen. Virol. 2021, 102, 001556. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Magdesian, K.G.; Mapes, S.M.; Zavodovskaya, R.; Kass, P.H. Assessment of quantitative polymerase chain reaction for equine herpesvirus-5 in blood, nasal secretions and bronchoalveolar lavage fluid for the laboratory diagnosis of equine multinodular pulmonary fibrosis. Equine Vet. J. 2017, 49, 34–38. [Google Scholar] [CrossRef]
- Williams, K.J.; Maes, R.; Del Piero, F.; Lim, A.; Wise, A.; Bolin, D.C.; Caswell, J.; Jackson, C.; Robinson, N.E.; Derksen, F.; et al. Equine multinodular pulmonary fibrosis: A newly recognized herpesvirus-associated fibrotic lung disease. Vet. Pathol. 2007, 44, 849–862. [Google Scholar] [CrossRef]
- Van Cleemput, J.; Poelaert, K.C.; Laval, K.; Nauwynck, H.J. Unravelling the first key steps in equine herpesvirus type 5 (EHV5) pathogenesis using ex vivo and in vitro equine models. Vet. Res. 2019, 50, 13. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Robinson, N.E.; Lim, A.; Brandenberger, C.; Maes, R.; Behan, A.; Bolin, S.R. Experimental induction of pulmonary fibrosis in horses with the gammaherpesvirus equine herpesvirus 5. PLoS ONE 2013, 8, e77754. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J. Gammaherpesviruses and pulmonary fibrosis: Evidence from humans, horses, and rodents. Vet. Pathol. 2014, 51, 372–384. [Google Scholar] [CrossRef]
- Wong, D.M.; Belgrave, R.L.; Williams, K.J.; Del Piero, F.; Alcott, C.J.; Bolin, S.R.; Marr, C.M.; Nolen-Walston, R.; Myers, R.K.; Wilkins, P.A. Multinodular pulmonary fibrosis in five horses. J. Am. Vet. Med. Assoc. 2008, 232, 898–905. [Google Scholar] [CrossRef]
- Lauteri, E.; Tortereau, A.; Peyrecave, X.; Pin, D.; Desjardins, I. Equine multinodular pulmonary fibrosis and presumed corticosteroid-induced side effects in a horse. Equine Vet. Educ. 2023, 35, e563–e570. [Google Scholar] [CrossRef]
- Vissani, M.A.; Damiani, A.M.; Barrandeguy, M.E. Equine coital exanthema: New insights on the knowledge and leading perspectives for treatment and prevention. Pathogens 2021, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Vissani, M.A.; Perglione, C.O.; Zabal, O.; Alvarez, G.; Thiry, E.; Barrandeguy, M.; Parreño, V. Topical ganciclovir reduces viral excretion in mares with equine coital exanthema. J. Equine Vet. Sci. 2020, 94, 103199. [Google Scholar] [CrossRef]
- Toishi, Y.; Tsunoda, N.; Kirisawa, R. Occurrence of equine coital exanthema (ECE) in stallions in Japan and effectiveness of treatment with valacyclovir for ECE. J. Vet. Med. Sci. 2017, 79, 632–635. [Google Scholar] [CrossRef][Green Version]
- Hue, E.S.; Richard, E.A.; Fortier, C.I.; Fortier, G.D.; Paillot, R.; Raue, R.; Pronost, S.L. Equine PBMC Cytokines Profile after In Vitro α- and γ-EHV Infection: Efficacy of a Parapoxvirus Ovis Based-Immunomodulator Treatment. Vaccines 2017, 5, 28. [Google Scholar] [CrossRef]
- Lunn, D.P.; Burgess, B.A.; Dorman, D.C.; Goehring, L.S.; Gross, P.; Osterrieder, K.; Pusterla, N.; Soboll Hussey, G. Updated ACVIM Consensus Statement on Equine Herpesvirus-1. J. Vet. Intern. Med. 2024, 38, 1290–1299. [Google Scholar] [CrossRef]
- Ons, E.; Van Brussel, L.; Lane, S.; King, V.; Cullinane, A.; Kenna, R.; Lyons, P.; Hammond, T.A.; Salt, J.; Raue, R. Efficacy of a Parapoxvirus ovis-based immunomodulator against equine herpesvirus type 1 and Streptococcus equi equi infections in horses. Vet. Microbiol. 2014, 173, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R. A Systematic Review of the Immune-Modulators Parapoxvirus ovis and Propionibacterium acnes for the Prevention of Respiratory Disease and Other Infections in the Horse. Vet. Immunol. Immunopathol. 2013, 153, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Ge, L.; Quan, R.; Chen, J.; Hu, Y.; Sa, R.; Liu, J.; Ran, D.; Fu, Q.; et al. Berbamine, a bioactive alkaloid, suppresses equine herpesvirus type 1 in vitro and in vivo. Front. Vet. Sci. 2023, 10, 1163780. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Sun, Y.; Khan, M.Z.; Yu, Y.; Ruan, L.; Chen, L.; Zhao, J.; Jia, J.; Li, Y.; et al. Protective role of cepharanthine against equid herpesvirus type 8 through AMPK and Nrf2/HO-1 pathway activation. Viruses 2024, 16, 1765. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Ruan, L.; Chen, L.; Khan, M.Z.; You, A.; Wang, C.; Li, L.; Ren, H.; Wang, T.; et al. Evaluation of Celastrol antiviral activity against equid alphaherpesvirus type 8 infection. Viruses 2025, 17, 347. [Google Scholar] [CrossRef]
- Wang, T.; Hu, L.; Li, R.; Ren, H.; Li, S.; Sun, Q.; Ding, X.; Li, Y.; Wang, C.; Li, L. Hyperoside inhibits EHV-8 infection via alleviating oxidative stress and IFN production through activating JNK/Keap1/Nrf2/HO-1 signaling pathways. J. Virol. 2024, 98, e00159-24. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, X.; Li, S.; Li, Y.; Zhao, S.; Shen, F.; Wang, C.; Li, Y.; Wang, T. Cobalt protoporphyrin blocks EHV-8 infection via IFN-α/β production. Animals 2023, 13, 2690. [Google Scholar] [CrossRef]
- Attili, A.R.; Colognato, R.; Preziuso, S.; Moriconi, M.; Valentini, S.; Petrini, S.; De Mia, G.M.; Cuteri, V. Evaluation of three different vaccination protocols against EHV1/EHV4 infection in mares: Double blind, randomized clinical trial. Vaccines 2020, 8, 268. [Google Scholar] [CrossRef]
- Warda, F.F.; Ahmed, H.E.; Shafik, N.G.; Mikhael, C.A.; Abd-ElAziz, H.M.; Mohammed, W.A.; Shosha, E.A. Application of equine herpesvirus-1 vaccine inactivated by both formaldehyde and binary ethylenimine in equine. Vet. World 2021, 14, 1815. [Google Scholar] [CrossRef] [PubMed]
- Bannai, H.; Tsujimura, K.; Nemoto, M.; Ohta, M.; Yamanaka, T.; Kokado, H.; Matsumura, T. Epizootiological investigation of equine herpesvirus type 1 infection among Japanese racehorses before and after the replacement of an inactivated vaccine with a modified live vaccine. BMC Vet. Res. 2019, 15, 280. [Google Scholar] [CrossRef]
- Patel, J.R.; Heldens, J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)–epidemiology, disease and immunoprophylaxis: A brief review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef]
- Breathnach, C.C.; Yeargan, M.R.; Timoney, J.F.; Allen, G.P. Detection of equine herpesvirus-specific effector and memory cytotoxic immunity in the equine upper respiratory mucosa. Vet. Immunol. Immunopathol. 2006, 111, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.B.; Loregian, A.; Perkins, G.A.; Nugent, J.; Buckles, E.L.; Mercorelli, B.; Kydd, J.H.; Palù, G.; Smith, K.C.; Osterrieder, N.; et al. A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog. 2007, 3, e160. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.B.; Wagner, B.; Flaminio, M.J.B.F.; Sussman, K.H.; Metzger, S.M.; Holland, R.; Osterrieder, N. Comparison of the efficacy of inactivated combination and modified-live virus vaccines against challenge infection with neuropathogenic equine herpesvirus type 1 (EHV-1). Vaccine 2006, 24, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Hussey, S.B.; Clark, R.; Lunn, K.F.; Breathnach, C.; Soboll, G.; Whalley, J.M.; Lunn, D.P. Detection and quantification of equine herpesvirus-1 viremia and nasal shedding by real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Goodman, L.B.; Wimer, C.; Dubovi, E.J.; Gold, C.; Wagner, B. Immunological correlates of vaccination and infection for equine herpesvirus 1. Clin. Vaccine Immunol. 2012, 19, 235–241. [Google Scholar] [CrossRef]
- Wagner, B.; Schnabel, C.L.; Rollins, A. Increase in virus-specific mucosal antibodies in the upper respiratory tract following intramuscular vaccination of previously exposed horses against equine herpesvirus type-1/4. Vaccines 2025, 13, 290. [Google Scholar] [CrossRef]
- Schnabel, C.L.; Babasyan, S.; Rollins, A.; Freer, H.; Wimer, C.L.; Perkins, G.A.; Raza, F.; Osterrieder, N.; Wagner, B. An equine herpesvirus type 1 (EHV-1) Ab4 open reading frame 2 deletion mutant provides immunity and protection from EHV-1 infection and disease. J. Virol. 2019, 93, e01245-19. [Google Scholar] [CrossRef]
- Houben, R.M.; van Maanen, C.; Newton, J.R.; van den Broek, J.; van Oldruitenborgh-Oosterbaan, M.S.; Heesterbeek, J.A. A model-based approach to evaluate the effect of vaccination of the herd on transmission of equine herpesvirus 1 in naturally occurring outbreaks. Prev. Vet. Med. 2025, 236, 106418. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; De Waure, C.; Timoney, P.J. Efficacy of vaccination against equine herpesvirus type 1 (EHV-1) infection: Systematic review and meta-analysis of randomised controlled challenge trials. Equine Vet. J. 2023, 55, 389–404. [Google Scholar] [CrossRef]
- Spann, K.; Barnum, S.; Pusterla, N. Investigation of the systemic antibody response and antigen detection following intranasal administration of two commercial equine herpesvirus-1 vaccines to adult horses. J. Equine Vet. Sci. 2023, 122, 104229. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Cai, S.; Lu, G.; Zhang, G. Challenges to develop an equine herpesvirus vaccine in China. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
- Yasunaga, S.; Maeda, K.; Matsumura, T.; Kondo, T.; Kai, K. Application of a type-specific enzyme-linked immunosorbent assay for equine herpesvirus types 1 and 4 (EHV-1 and-4) to horse populations inoculated with inactivated EHV-1 vaccine. J. Vet. Med. Sci. 2000, 62, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Barnum, S.; Miller, J.; Varnell, S.; Dallap-Schaer, B.; Aceto, H.; Marlin, D. Investigation of an EHV-1 Outbreak in the United States Caused by a New H752 Genotype. Pathogens 2021, 10, 747. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).