IPM Strategy to Control EFB in Apis mellifera: Oxytetracycline Treatment Combined with Partial Shook Swarm and Queen Caging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Preparation of the Sucrose Solution
2.2. Assessing Consumption of Sucrose Solution
2.3. Assessing Colony Strength
2.4. Inspection of the Beehives
2.5. Detecting Clinical and Subclinical Relapses of EFB
2.6. Measuring the Residues of OTC in the Honey
3. Results
3.1. Consumption of the Sucrose Solution
3.2. Colony Strength
3.3. Side Effects of the IPM: Partial Shook Swarm + Queen Caging
3.4. Clinical and Subclinical EFB Relapses
3.4.1. Clinical EFB Relapses
3.4.2. Subclinical EFB Relapses
3.5. Honey Yield
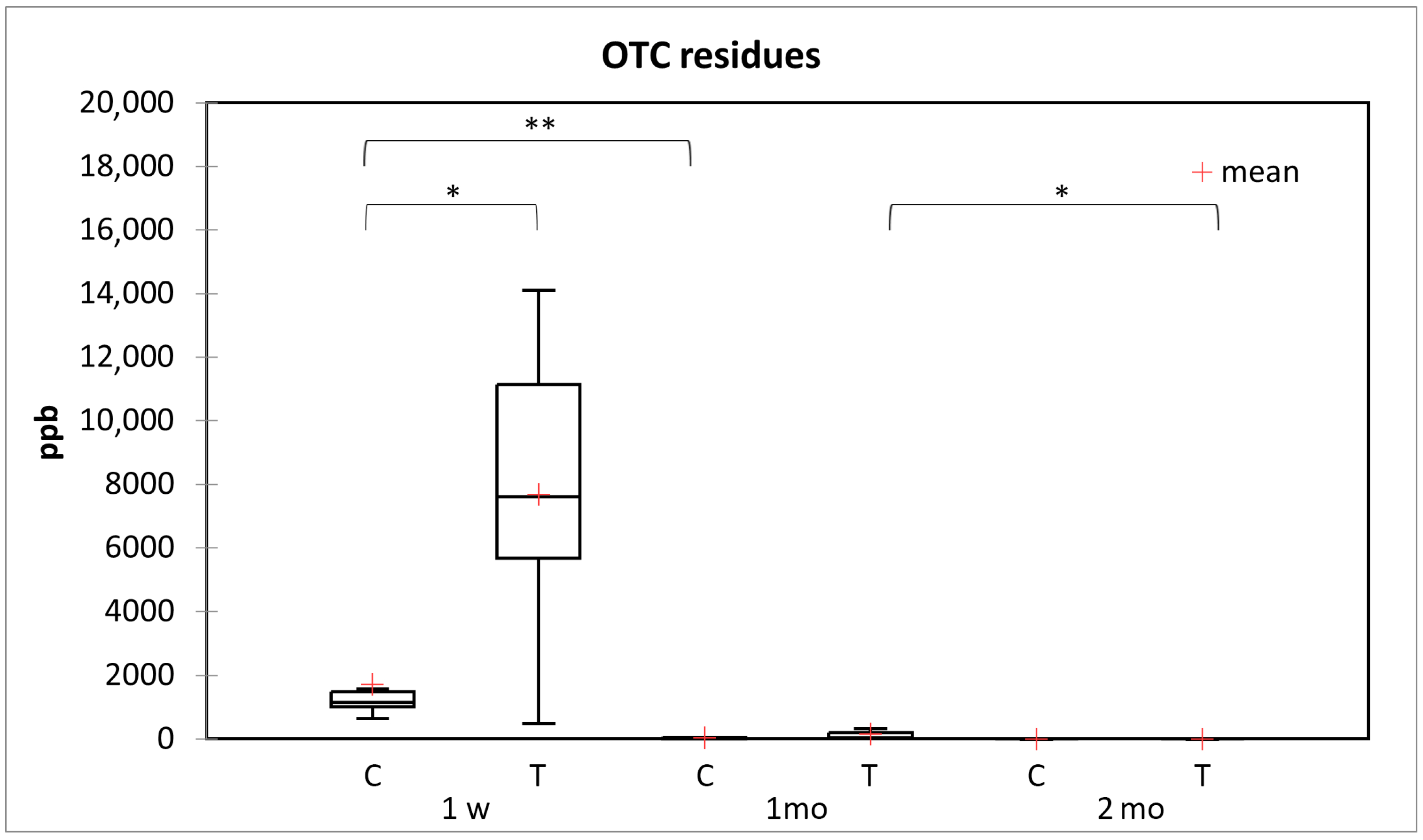
3.6. Amount of OTC Residues in the Honey Samples
4. Discussion
4.1. Consumption of the Administered Sucrose Solution
4.2. Colony Strength
4.3. Side Effects of the IPM: Partial Shook Swarm + Queen Caging
4.4. Relapses of EFB
4.5. Honey Yield
4.6. Residues of OTC in the Produced Honey
- –
- The antibiotic should be administered in a medicated solution, preferably in jar cups of Petri dishes enriched with a piece of net to avoid the contamination of the beehive as well as the drowning of the honey bees. The practice also enhances the exact administration of the active ingredient, as well as the control of the consumption and the separation of the excess infected sucrose solution.
- –
- In the case where a shook swarm is applied, TSS should be preferred to PSS to prevent the persistence of M. plutonius in the storage combs. Before the shook swarm, healthy colonies must be separated from the infected ones to reduce the risk of cross-contamination. The colonies should not be fed after the shook swarm, in accordance with the principles of cura famis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaggia, F.; Baffoni, L.; Stenico, V.; Alberoni, D.; Buglione, E.; Lilli, A.; Di Gioia, D.; Porrini, C. Microbial investigation on honey bee larvae showing atypical symptoms of European foulbrood. Bull. Insectol. 2015, 68, 321–327. [Google Scholar]
- Lewkowski, O.; Erler, S. Virulence of Melissococcus plutonius and secondary invaders associated with European foulbrood disease of the honey bee. MicrobiologyOpen 2019, 8, e00649. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E. European foulbrood in honey bees. J. Invertebr. Pathol. 2010, 103, S5–S9. [Google Scholar] [CrossRef]
- Rowland, B.W.; Rushton, S.P.; Shirley, M.D.; Brown, M.A.; Budge, G.E. Identifying the climatic drivers of honey bee disease in England and Wales. Sci. Rep. 2021, 11, 21953. [Google Scholar] [CrossRef] [PubMed]
- Grossar, D.; Kilchenmann, V.; Forsgren, E.; Charrière, J.D.; Gauthier, L.; Chapuisat, M.; Dietemann, V. Putative determinants of virulence in Melissococcus plutonius, the bacterial agent causing European foulbrood in honey bees. Virulence 2020, 11, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, S.; Brown, M.A.; Cuthbertson, A.G.S. The Incidence of Honey Bee Pests and Diseases in England and Wales. Pest. Manag. Sci. Former. Pestic. Sci. 2007, 63, 1062–1068. [Google Scholar] [CrossRef]
- Roetschi, A.; Berthoud, H.; Kuhn, R.; Imdorf, A. Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honey bee colonies before and after apiary sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Ory, F.; Duchemin, V.; Kilchenmann, V.; Charrière, J.D.; Dainat, B.; Dietemann, V. Lack of evidence for trans-generational immune priming against the honey bee pathogen Melissococcus plutonius. PLoS ONE 2022, 17, e0268142. [Google Scholar] [CrossRef]
- De León-Door, A.P.; Pérez-Ordóñez, G.; Romo-Chacón, A.; Rios-Velasco, C.; Órnelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Pathogenesis, epidemiology and variants of Melissococcus plutonius (Ex White), the causal agent of European foulbrood. J. Apic. Sci. 2020, 64, 173–188. [Google Scholar] [CrossRef]
- Okamoto, M.; Kumagai, M.; Kanamori, H.; Takamatsu, D. Antimicrobial resistance genes in bacteria isolated from Japanese honey, and their potential for conferring macrolide and lincosamide resistance in the American foulbrood pathogen Paenibacillus larvae. Front. Microbiol. 2021, 12, 667096. [Google Scholar] [CrossRef]
- Masood, F.; Thebeau, J.M.; Cloet, A.; Kozii, I.V.; Zabrodski, M.W.; Biganski, S.; Liang, J.; Guarna, M.M.; Simko, E.; Ruzzini, A.; et al. Evaluating approved and alternative treatments against an oxytetracycline-resistant bacterium responsible for European foulbrood disease in honey bees. Sci. Rep. 2002, 12, 5906. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Zhang, X.; Liu, Y.; Chen, H.; Zheng, H.; Zhai, Y.; Zheng, H. The honey bee gut resistome and its role in antibiotic resistance dissemination. Integr. Zool. 2023, 18, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.D.; Tell, L.A.; Davis, J.L.; Baynes, R.E.; Zhoumeng, L.; Maunsell, F.P.; Riviere, J.E.; Jaberi-Douraki, M.; Martin, K.L.; Davidson, G. Honey bee medicine for veterinarians and guidance for avoiding violative chemical residues in honey. J. Am. Vet. Med. Assoc. 2021, 259, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cao, L.; Huang, S.; Neumann, P.; Hu, F. Current Status of the Beekeeping Industry in China. In Asian Beekeeping in the 21st Century; Chantawannakul, P., Williams, G., Neumann, P., Eds.; Springer: Singapore, 2018; Chapter 6; pp. 129–158. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ganssi, S.; Albanese, G.; Letizia, F.; Testa, B.; Tedino, C.; Petrarca, S.; Mutinelli, F.; Mazzeo, A.; De Cristofaro, A. Antimicrobial Activity from Putative Probiotic Lactic Acid Bacteria for the Biological Control of American and European Foulbrood Diseases. Vet. Sci. 2022, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Veterinary Medicinal Products. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32001L0082 (accessed on 27 October 2023).
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32019R0006 (accessed on 27 October 2023).
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010R0037 (accessed on 27 October 2023).
- Commission Implementing Regulation (EU) 2018/470 of 21 March 2018 on Detailed Rules on the Maximum Residue Limit to Be Considered for Control Purposes for Foodstuffs Derived from Animals Which Have Been Treated in the EU under Article 11 of Directive 2001/82/EC. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32018R0470 (accessed on 27 October 2023).
- Authorised Bee Products: Situation in Europe. Centrally Authorised Veterinary Medicinal Products. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/724720/1.4.1.7_QA76_Bee_products_in_EU_0.40.pdf (accessed on 19 October 2023).
- Shendy, A.H.; Al-Ghobashy, M.A.; Gad Alla, S.A.; Lotfy, H.M. Development and validation of a modified QuEChERS protocol coupled to LC–MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem. 2016, 190, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Buzia, O.D.; Ploscutanu, G.; Elisei, A.M. Tetracyclines residues in honey. Rev. Chim. 2019, 70, 1544–1550. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Popiela, E.; Owczarek, B.; Hodyra-Stefaniak, K.; Świtała-Jeleń, K.; Łodej, N.; Kula, D.; Neuberg, J.; Migdał, P.; Bagin’ska, N.; et al. Phages in therapy and prophylaxis of american foulbrood–recent implications from practical applications. Front. Microbiol. 2020, 11, 1913. [Google Scholar] [CrossRef]
- European Commission RASFF Window. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 18 July 2023).
- Olatoye, I.O.; Ehinmowo, A.A. Oxytetracycline residues in edible tissues of cattle slaughtered in Akure, Nigeria. Niger. Vet. J. 2010, 31, 93–102. [Google Scholar] [CrossRef]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Topp, E. The scourge of antibiotic resistance: The important role of the environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef]
- FAO Integrated Pest Management. Available online: http://www.fao.org/agriculture/crops/core-themes/theme/pests/ipm/en/ (accessed on 3 July 2023).
- Mosca, M.; Bubnic, J.; Giannetti, L.; Fortugno, L.; Pietropaoli, M.; Manara, V.; Bonerba, E.; Formato, G. Adoption of Partial Shook Swarm in the Integrated Control of American and European Foulbrood of Honey Bee (Apis mellifera L.). Agriculture 2023, 13, 363. [Google Scholar] [CrossRef]
- OIE. European Foulbrood of Honey Bees (Infection of Honey Bees with Melissococcus plutonius). In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees); World Organization for Animal Health: Paris, France, 2018; Volume 1, Chapter 3.2.3; pp. 736–743. [Google Scholar]
- Delaplane, K.S.; van der Steen, J.; Guzman-Novoa, E. Standard Methods for Estimating Strength Parameters of Apis mellifera Colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Mosca, M.; Giannetti, L.; Franco, A.; Iurescia, M.; Milito, M.; Pietropaoli, M.; Leto, A.; Di Ruggiero, C.; Mezher, Z.; Palazzetti, M.; et al. Impact of Oxytetracycline on Colonies: Preliminary Results on Residues and Antibiotic Resistance. J. Apic. Sci. 2022, 66, 159–170. [Google Scholar] [CrossRef]
- Giannetti, L.; Longo, F.; Buiarelli, F.; Russo, V.; Neri, B. Tetracycline residues in royal jelly and honey by liquid chromatography tandem mass spectrometry: Validation study according to Commission Decision 2002/657/EC. Anal. Bioanal. Chem. 2010, 398, 1017–1023. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Data Analysis and Statistical Solution for Microsoft Excel; ADDINSOFT: Paris, France, 2023. [Google Scholar]
- Hepburn, H.R.; Reece, S.L.; Neumann, P.; Moritz, R.F.A.; Radloff, S.E. Absconding in honey bees (Apis mellifera) in relation to queen status and mode of worker reproduction. Insectes Sociaux 1999, 46, 323–326. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Goodman, R.D.; Hornitzky, M.A.Z.; Chandler, D. The effect on American foulbrood of standard oxytetracycline hydrochloride treatments for the control of European foulbrood of honey bees (Apis mellifera). Aust. J. Agric. Res. 1989, 40, 691–697. [Google Scholar] [CrossRef]
- Raymann, K.; Shaffer, Z.; Moran, N.A. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honey bees. PLoS Biol. 2017, 15, e2001861. [Google Scholar] [CrossRef] [PubMed]
- Liberti, J.; Engel, P. The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera gastrointestinal microbiota and lactic acid bacteria for honey bee protection—A review. Cells 2001, 10, 701. [Google Scholar] [CrossRef]
- Skirkevicius, A. First symptoms of queen loss in a honey bee colony (Apis mellifera). Apidologie 2004, 35, 565–573. [Google Scholar] [CrossRef][Green Version]
- Pietropaoli, M.; Formato, G. Acaricide efficacy and honey bee toxicity of three new formic acid-based products to control Varroa destructor. J. Apic. Sci. 2018, 58, 824–830. [Google Scholar] [CrossRef]
- Hornitzky, M. Honey bee diseases. In The Australian and New Zealand Standard Diagnostic Procedure (ANZSDP) for Honey Bee Diseases; Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2010. Available online: https://www.agriculture.gov.au/agriculture-land/animal/health/laboratories/procedures/anzsdp/honey-bee-diseases#daff-page-main (accessed on 19 October 2023).
- Milbrath, M.O.G.; Fowler, P.D.; Abban, S.K.; Lopez, D.; Evans, J.D. Validation of diagnostic methods for European foulbrood on commercial honey bee colonies in the United States. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Ricchiuti, L.; Petrollini, E.; Annunziata, L.; D’Aloise, A.; Leonardi, D.; Pomilio, F. Contamination of honey by oxytetracycline from pig manure. Vet. Ital. 2019, 55, 123–129. [Google Scholar] [PubMed]
- Adams, S.J.; Fussell, R.J.; Dickinson, M.; Wilkins, S.; Sharman, M. Study of the depletion of lincomycin residues in honey extracted from treated honey bee (Apis mellifera L.) colonies and the effect of the shook swarm procedure. Anal. Chim. Acta 2009, 637, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Waite, R.J.; Brown, M.A.; Thompson, H.M.; Bew, M.H. Controlling European foulbrood with the shook swarm method and oxytetracycline in the UK. Apidologie 2003, 34, 569–575. [Google Scholar] [CrossRef][Green Version]
| Number of Frames Covered by Adult Honey Bees | Number of Frames Covered by Brood | |||
|---|---|---|---|---|
| Untreated (Control) Group | OTC-Treated Group | Untreated (Control) Group | OTC-Treated Group | |
| Pre | 8.4 ± 1.8 | 8.7 ± 1.5 | 5.8 ± 1.2 | 6.2 ± 1.3 |
| 1w | 4.1 ± 2.1 | 3.8 ± 1.9 | 1.3 ± 1.0 | 1.0 ± 1.0 |
| 1mo | 5.0 ± 2.8 | 5.5 ± 1.8 | 4.2 ± 2.7 | 3.8 ± 1.6 |
| 2mo | 8.6 ± 1.3 | 6.1 ± 2.1 | 6.2 ± 0.8 | 4.8 ± 1.4 |
| Pre | 1w | 1mo | 2mo | |||||
|---|---|---|---|---|---|---|---|---|
| Bees | Brood | Bees | Brood | Bees | Brood | Bees | Brood | |
| Pre | – | – | p < 0.003 | p < 0.002 | p < 0.024 | Not significant | p < 0.011 | p < 0.026 |
| – | – | p < 0.003 ‡ | p < 0.003 ‡ | p < 0.007 ‡ | p < 0.007 ‡ | p < 0.011 ‡ | p < 0.026 ‡ | |
| 1w | p < 0.003 | p < 0.002 | – | – | p < 0.011 | p < 0.007 | Not significant | – |
| p< 0.003 ‡ | p < 0.003 ‡ | – | – | p < 0.034 ‡ | p < 0.007 ‡ | Not significant ‡ | – | |
| 1mo | p < 0.024 | Not significant | p < 0.011 | p < 0.007 | – | – | – | Not significant |
| p < 0.007 ‡ | p < 0.007 ‡ | p < 0.034 ‡ | p < 0.007 ‡ | – | – | – | Not significant ‡ | |
| 2mo | p < 0.011 | p < 0.026 | – | – | Not significant | Not significant | – | – |
| p < 0.011 ‡ | p < 0.026 ‡ | – | – | Not significant ‡ | Not significant ‡ | – | – | |
| Side Effects of the Queen Caging | Group | Colony | Total |
|---|---|---|---|
| Queen loss | OTC-treated group | 6/13 (46.2%) | 13/25 (52%) |
| Untreated group | 7/12 (58.3%) | ||
| Absconding | OTC-treated group | 2/13 (15.4%) | 2/25 (8%) |
| Untreated group | 0/12 (0%) | ||
| Drone mother colony | OTC-treated group | 0/13 (0%) | 1/25 (4%) |
| Untreated group | 1/12 (8.3%) |
| EFB Clinical Relapses | 4 Months | 7 Months |
|---|---|---|
| OTC-treated group | 0/13 (0%) | 0/13 (0%) |
| Untreated Group | 2/12 (17%) | 0/12 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, M.; Gyorffy, A.; Pietropaoli, M.; Giannetti, L.; Cersini, A.; Fortugno, L.; Formato, G. IPM Strategy to Control EFB in Apis mellifera: Oxytetracycline Treatment Combined with Partial Shook Swarm and Queen Caging. Vet. Sci. 2024, 11, 28. https://doi.org/10.3390/vetsci11010028
Mosca M, Gyorffy A, Pietropaoli M, Giannetti L, Cersini A, Fortugno L, Formato G. IPM Strategy to Control EFB in Apis mellifera: Oxytetracycline Treatment Combined with Partial Shook Swarm and Queen Caging. Veterinary Sciences. 2024; 11(1):28. https://doi.org/10.3390/vetsci11010028
Chicago/Turabian StyleMosca, Michela, Andrea Gyorffy, Marco Pietropaoli, Luigi Giannetti, Antonella Cersini, Luca Fortugno, and Giovanni Formato. 2024. "IPM Strategy to Control EFB in Apis mellifera: Oxytetracycline Treatment Combined with Partial Shook Swarm and Queen Caging" Veterinary Sciences 11, no. 1: 28. https://doi.org/10.3390/vetsci11010028
APA StyleMosca, M., Gyorffy, A., Pietropaoli, M., Giannetti, L., Cersini, A., Fortugno, L., & Formato, G. (2024). IPM Strategy to Control EFB in Apis mellifera: Oxytetracycline Treatment Combined with Partial Shook Swarm and Queen Caging. Veterinary Sciences, 11(1), 28. https://doi.org/10.3390/vetsci11010028






