American Foulbrood—Old and Always New Challenge
Abstract
Simple Summary
Abstract
1. Introduction
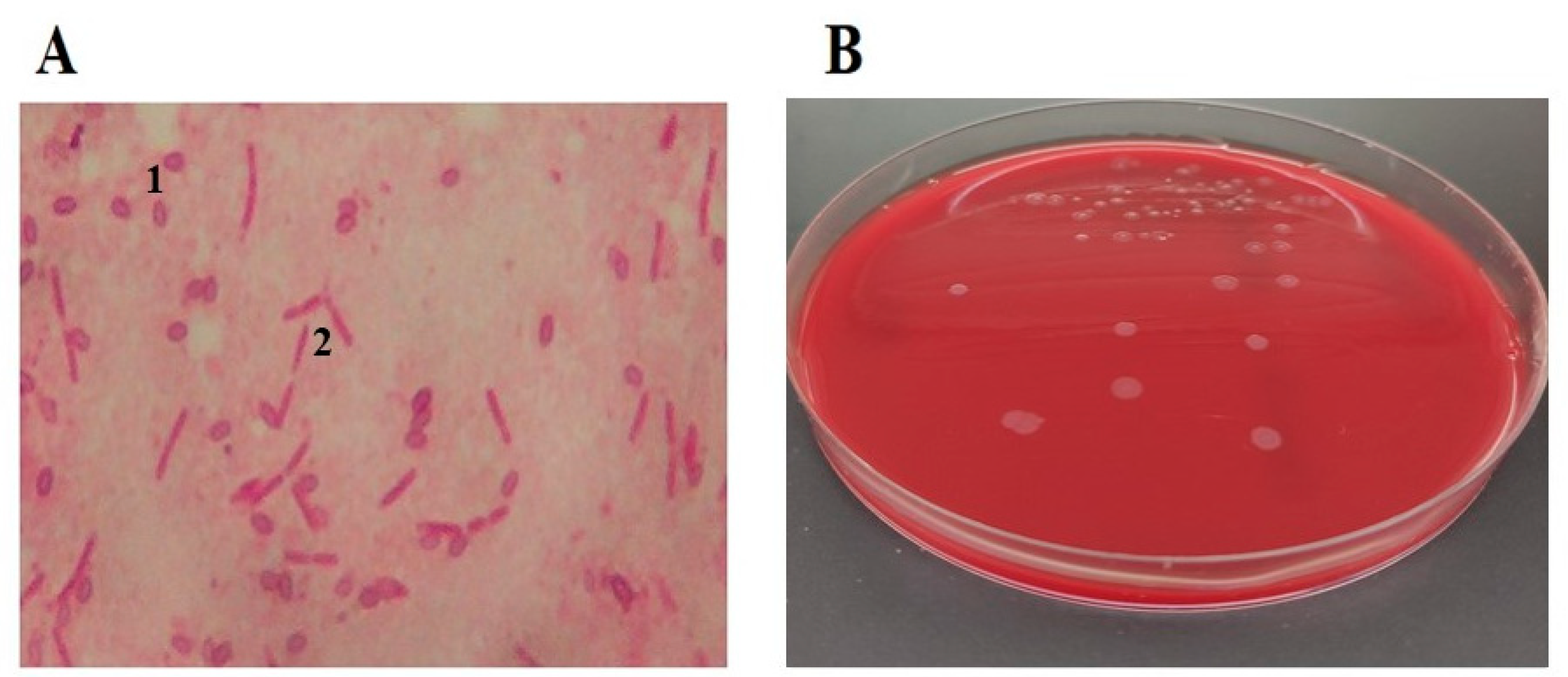
2. Etiology of the Causative Agent of the Disease
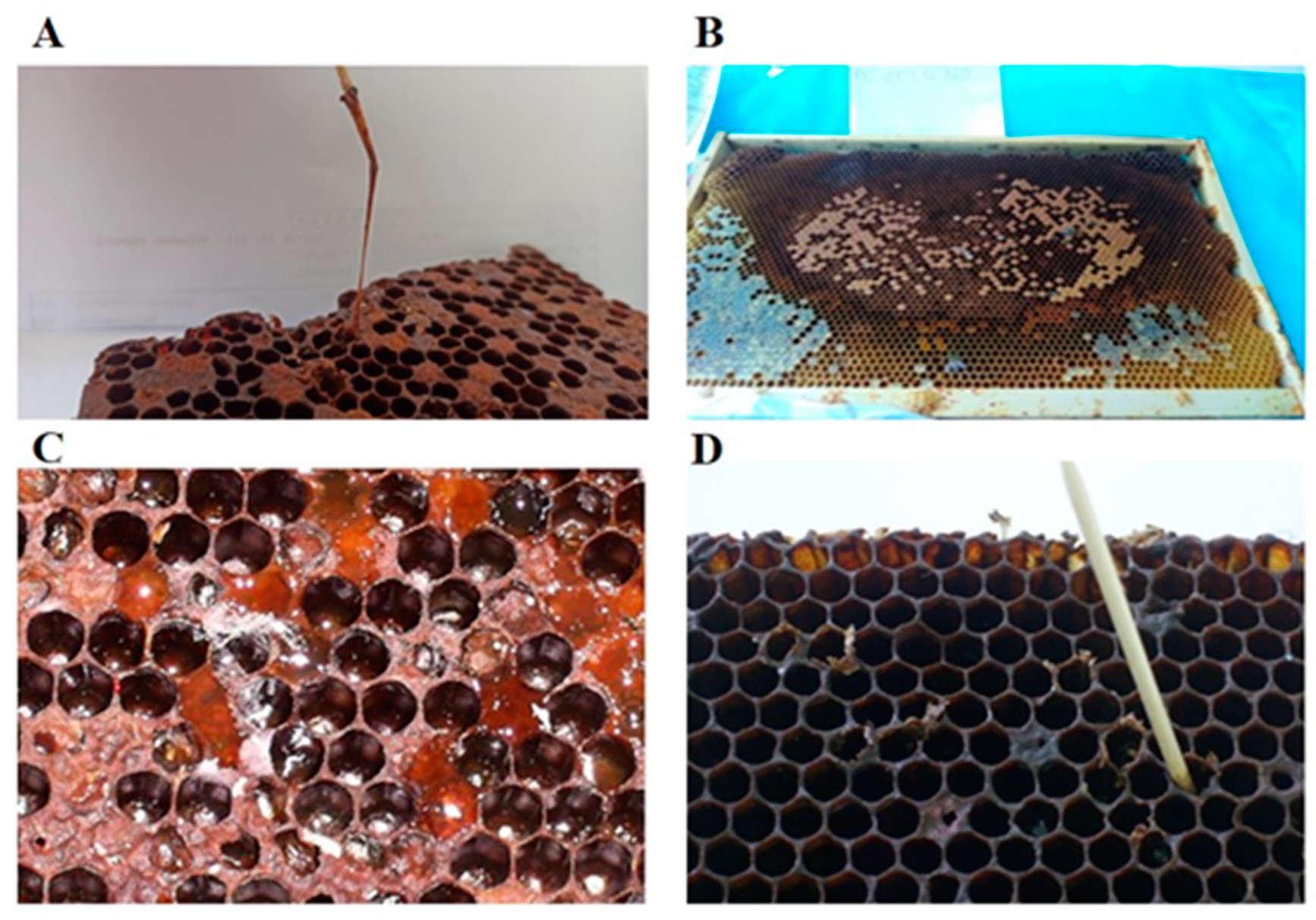
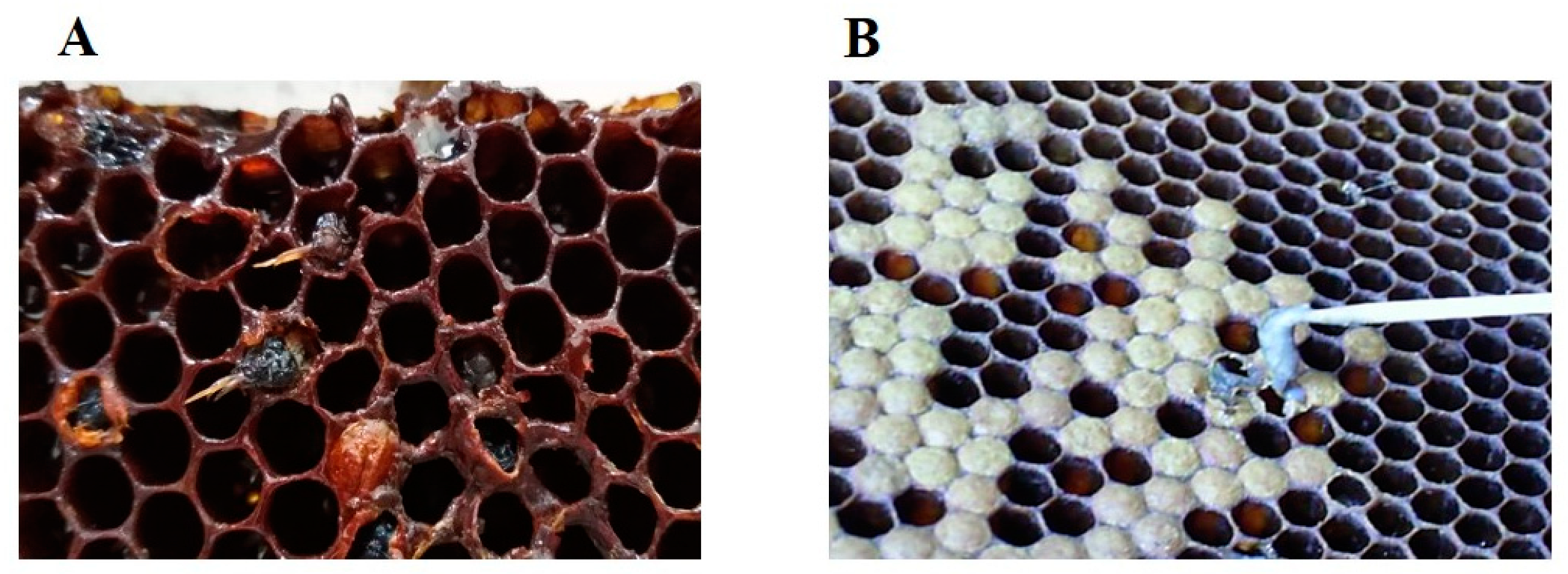
3. Clinical Signs of the Disease
4. Control of the Disease
5. Differential Diagnostics of the Disease
6. Prevention of the Disease
7. Good Beekeeping Practice (GAP+GHP)—A Reminder for Beekeepers
- Meeting the legal requirements for beekeeping
- Beekeeping in adequate areas and appropriate terrains
- Beekeeping is done in adequate hives that are disinfected prior to the bees’ settling in
- Using a sterilized comb foundation
- A laboratory investigation of comb foundation for the presence of P. larvae endospores
- Record of work on the apiary (beekeeper’s diary)
- Beekeeping using strong colonies with high-quality food and good arrangement
- Providing adequate bee nests with good ventilation
- Using young, fertile queens in beekeeping
- Providing water devices in the apiary
- Not merging weak and strong colonies (sick and healthy)
- Regularly checking bee colonies and providing replacement combs
- Bee colonies: compulsory checking (inspection) of bee colonies when bees are foraging
- Implementing proper disinfection during normal work at the apiary
- Undertaking measures for early disease detection (regular monitoring)
- Control of stress factors in beekeeping
- Performing laboratory diagnostics
- Bee colonies infected with the AFB pathogen agent are destroyed by burning and burying any infected bee colony
- Implementing disinfection measures in the apiary in the case of occurrences of diseases
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bertolotti, A.C.; Forsgren, E.; Schäfer, M.O.; Sircoulomb, F.; Gaïani, N. Development and evaluation of a core genome multilocus sequence typing scheme for Paenibacillus larvae, the deadly American foulbrood pathogen of honeybees. Environ. Microbiol. 2021, 23, 5042–5051. Available online: https://hal.archives-ouvertes.fr/hal-03191806/document (accessed on 3 January 2023). [CrossRef]
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, L.; Genersch, E. Molecular pathogenesis of American Foulbrood: How Paenibacillus larvae kills honey bee larvae. Curr. Opin. Insect Sci. 2015, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, J.; Knispel, H.; Hertlein, G.; Fünfhaus, A.; Genersch, E. Biology of Paenibacillus larvae, a deadly pathogen of honey bee larvae. Appl. Microbiol. Biotechnol. 2016, 100, 7387–7395. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.; Al-Ghamdi, M.S.; Ahmed, A.M.; Mohamed, A.S.A.; Shaker, G.H.; Ansari, M.J.; Dorrah, M.A.; Khan, K.A.; Ayaad, T.H. Immune investigation of the honeybee Apis mellifera jemenitica broods: A step toward production of a bee-derived antibiotic against the American foulbrood. Saudi J. Biol. Sci. 2021, 28, 1528–1538. [Google Scholar] [CrossRef]
- Council Directive, 92/65/EEC, Annex A. Off. J. Eur. Union 1992, 268, 58–63.
- Locke, B.; Low, M.; Forsgren, E. An integrated management strategy to prevent outbreaks and eliminate infection pressure of American foulbrood disease in a commercial beekeeping operation. Prev. Vet. Med. 2019, 167, 48–52. [Google Scholar] [CrossRef] [PubMed]
- OIE. Chapter 3.2.2: American Foulbrood of Honey Bees (Infection of Honey Bees with Paenibacillus larvae. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; OIE: Paris, France, 2018; pp. 1–17. [Google Scholar]
- Teixeira, É.W.; Guimarães-Cestaro, L.; Alves, M.L.T.M.F.; Message, D.; Martins, M.F.; da Luz, C.F.P.; Serrão, J.E. Spores of Paenibacillus larvae, Ascosphaera apis, Nosema ceranae and Nosema apis in bee products supervised by the Brazilian Federal Inspection Service. Rev. Bras. Èntomol. 2018, 62, 188–194. [Google Scholar] [CrossRef]
- White, G.F. The Bacteria of the Apiary: With Special Reference to Bee Diseases; Technical Series; USDA, Bureau of Entomology: Washington, DC, USA, 1906; Volume 14, pp. 1–50.
- Genersch, E. Paenibacillus larvae and American Foulbrood—Long since known and still surprising. J. Consum. Prot. Food Saf. 2008, 3, 429–434. [Google Scholar] [CrossRef]
- Lolin, M. Bolesti Pčela; Naučna knjiga: Beograd, Serbia, 1985; p. 12. (In Serbian) [Google Scholar]
- Lindström, A.; Korpela, S.; Fries, I. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J. Invertebr. Pathol. 2008, 99, 82–86. [Google Scholar] [CrossRef]
- Ricchiuti, L.; Rossi, F.; Del Matto, I.; Iannitto, G.; Del Riccio, A.L.; Petrone, D.; Ruberto, G.; Cersini, A.; Di Domenico, M.; Cammà, C. A study in the Abruzzo region on the presence of Paenibacillus larvae spores in honeys indicated underestimation of American foulbrood prevalence in Italy. J. Apic. Res. 2019, 58, 416–419. [Google Scholar] [CrossRef]
- Tomljanović, Z.; Cvitković, D.; Pašić, S.; Volarević, B.; Tlak Gajger, I. Production, practices and attitudes of beekeepers in Croatia. Vet. Arh. 2020, 90, 413–427. [Google Scholar] [CrossRef]
- Noureddine, A.; Nizar, H. Prévalence et répartition de la bactérie Paenibacillus larvae (Agent causal de la Loque américaine) au niveau de quelques ruchers de la région centre d’Algérie. Nat. Technol. 2021, 25, 85–93. [Google Scholar]
- Morrissey, B.J.; Helgason, T.; Poppinga, L.; Fünfhaus, A.; Genersch, E.; Budge, G.E. Biogeography of Paenibacillus larvae, the causative agent of American foulbrood, using a new multilocus sequence typing scheme. Environ. Microbiol. 2015, 17, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of Apoidea as Main Pollinators. Ecological and Economic Impact and Implications for Human Nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Document Selected: A8-0014/2018, report on prospects and challenges for the EU apiculture sector Opinion of the Committee on the Environment, Public Health and Food Safety: 2018. Available online: https://www.europarl.europa.eu/doceo/document/A-8-2018-0014_EN.html (accessed on 3 January 2023).
- Smith, M.R.; Mueller, N.D.; Springmann, M.; Sulser, T.B.; Garibaldi, L.A.; Gerber, J.; Wiebe, K.; Myers, S.S. Pollinator Deficits, Food Consumption, and Consequences for Human Health: A Modeling Study. Environ. Health Perspect. 2022, 130, 127003. [Google Scholar] [CrossRef]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef]
- Yue, D.; Nordhoff, M.; Wieler, L.H.; Genersch, E. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environ. Microbiol. 2008, 10, 1612–1620. [Google Scholar] [CrossRef]
- Hasemann, L. How long can spores of American Foulbrood live? Am. Bee J. 1961, 101, 298–299. [Google Scholar]
- Hristov, Y.V.; Le Roux, J.J.; Allsopp, M.H.; Wossler, T.C. Identity and distribution of American foulbrood (Paenibacillus larvae) in South Africa. J. Apic. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Beims, H.; Bunk, B.; Erler, S.; Mohr, K.I.; Spröer, C.; Pradella, S.; Günther, G.; Rohde, M.; von der Ohe, W.; Steinert, M. Discovery of Paenibacillus larvae ERIC V: Phenotypic and genomic comparison to genotypes ERIC I-IV reveal different inventories of virulence factors which correlate with epidemiological prevalences of American Foulbrood. Int. J. Med. Microbiol. 2020, 310, 151394. [Google Scholar] [CrossRef] [PubMed]
- Jończyk-Matysiak, E.; Popiela, E.; Owczarek, B.; Hodyra-Stefaniak, K.; Świtała-Jeleń, K.; Łodej, N.; Kula, D.; Neuberg, J.; Migdał, P.; Bagińska, N.; et al. Phages in Therapy and Prophylaxis of American Foulbrood—Recent Implications from Practical Applications. Front. Microbiol. 2020, 11, 1913. [Google Scholar] [CrossRef]
- Neuendorf, S.; Hedtke, K.; Tangen, G.; Genersch, E. Biochemical characterization of different genotypes of Paenibacillus larvae subsp. larvae, a honey bee bacterial pathogen. Microbiology 2004, 150, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative Correlation between Individual-Insect-Level Virulence and Colony-Level Virulence of Paenibacillus larvae, the Etiological Agent of American Foulbrood of Honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, D.C.; Alippi, A.M.; Brown, M.; Evans, J.D.; Feldlaufer, M.; Gregorc, A.; Hornitzky, M.; Pernal, S.F.; Schuch, D.M.T.; Titĕra, D.; et al. Under the microscope. Diagnosis of american foulbrood disease in honeybees: A synthesis and proposed analytical protocols. Lett. Appl. Microbiol. 2006, 43, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A.; Korpela, S.; Fries, I. Horizontal transmission of Paenibacillus larvae spores between honey bee (Apis mellifera) colonies through robbing. Apidologie 2008, 39, 515–521. [Google Scholar] [CrossRef]
- Woodrow, A.W. Susceptibility of honey bee larvae to American foulbrood. Gleanings Bee Cult. 1941, 69, 148–151. [Google Scholar]
- Stephan, J.G.; de Miranda, J.R.; Forsgren, E. American foulbrood in a honeybee colony: Spore-symptom relationship and feedbacks between disease and colony development. BMC Ecol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Poppinga, L.; Janesch, B.; Fünfhaus, A.; Sekot, G.; Garcia-Gonzalez, E.; Hertlein, G.; Hedtke, K.; Schäffer, C.; Genersch, E. Identification and Functional Analysis of the S-Layer Protein SplA of Paenibacillus larvae, the Causative Agent of American Foulbrood of Honey Bees. PLoS Pathog. 2012, 8, e1002716. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Poppinga, L.; Fünfhaus, A.; Hertlein, G.; Hedtke, K.; Jakubowska, A.; Genersch, E. Paenibacillus larvae Chitin-Degrading Protein PlCBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees. PLoS Pathog. 2014, 10, e1004284. [Google Scholar] [CrossRef]
- Fünfhaus, A.; Poppinga, L.; Genersch, E. Identification and characterization of two novel toxins expressed by the lethal honey bee pathogen Paenibacillus larvae, the causative agent of American foulbrood. Environ. Microbiol. 2013, 15, 2951–2965. [Google Scholar] [CrossRef]
- Krska, D.; Ravulapalli, R.; Fieldhouse, R.J.; Lugo, M.R.; Merrill, A.R. C3larvin Toxin, an ADP-ribosyltransferase fromPaenibacillus larvae. J. Biol. Chem. 2015, 290, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, J.; Fünfhaus, A.; Genersch, E. The Buzz about ADP-Ribosylation Toxins from Paenibacillus larvae, the Causative Agent of American Foulbrood in Honey Bees. Toxins 2021, 13, 151. [Google Scholar] [CrossRef]
- Turner, M.; Tremblay, O.; Heney, K.A.; Lugo, M.R.; Ebeling, J.; Genersch, E.; Merrill, A.R. Characterization of C3larvinA, a novel RhoA-targeting ADP-ribosyltransferase toxin produced by the honey bee pathogen, Paenibacillus larvae. Biosci. Rep. 2020, 40, BSR20193405. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, J.A.; Oliver, D.O. Microorganisms in honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 2001, 32, 199–214. [Google Scholar] [CrossRef]
- Nedić, N.; Matović, K. Other Pests of Bees. In No Bees, No Life; Bee Books: Žirovnica, Slovenija, 2017; pp. 63–69. Available online: https://adeleinslovenia,com/tag/bee-books/ (accessed on 3 January 2023).
- Formato, G.; Smulders, F.J. Risk management in primary apicultural production. Part 1: Bee health and disease prevention and associated best practices. Veter-Q. 2011, 31, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Rivera Gomis, J.; Bubnic, J.; Ribarits, A.; Moosbeckhofer, R.; Alber, O.; Kozmus, P.; Formato, G.; Muz, M.N. Good farming practices in apiculture. Rev. Sci. Tech. L’oie 2019, 38, 879–890. [Google Scholar] [CrossRef]
- Somerville, D.; Annand, N.; New South, W. Healthy Bees: Managing Pests, Diseases and Other Disorders of the Honey Bee. (Sydney, NSW?): Tocal College, NSW DPI (AgGuide: A Practical Handbook). 2016. Available online: http://ezproxy.nb.rs:2059/login.aspx?direct=true&db=e000xww&AN=1580990&site=eds-live (accessed on 3 January 2023).
- Matović, K.; Žarković, A.; Vidanović, D.; Debeljak, Z.; Vasković, N.; Šekler, M. American foulbrood disease in the southwestern part of Serbia. In Improving Beekeeping in Serbia; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2016; pp. 105–119. (In Serbian) [Google Scholar]
- Forsgren, E.; Laugen, A.T. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie 2014, 45, 10–20. [Google Scholar] [CrossRef]
- Beims, H.; Janke, M.; Von der Ohe, W.; Steinert, M. Rapid identification and genotyping of the honeybee pathogen Paenibacillus larvae by combining culturing and multiplex quantitative PCR. Open Vet. J. 2020, 10, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kušar, D.; Papić, B.; Zajc, U.; Zdovc, I.; Golob, M.; Žvokelj, L.; Knific, T.; Avberšek, J.; Ocepek, M.; Ocepek, M.P. Novel TaqMan PCR Assay for the Quantification of Paenibacillus larvae Spores in Bee-Related Samples. Insects 2021, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.S.; Pernal, S.F.; Noot, D.K.; Melathopoulos, A.P.; Heever, J.P.V.D. Degradation of incurred tylosin to desmycosin—Implications for residue analysis of honey. Anal. Chim. Acta 2007, 586, 304–311. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Sci. World J. 2012, 2012, 930849. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO) of the United Nations, Roma. Good Beekeeping Practices: Practical Manual on How to Identify and Control the Main Diseases of the Honeybee (Apis Mellifera); Food and Agriculture Organization (FAO): Roma, Italy, 2020. [Google Scholar]
- Alippi, A.M. A comparison of laboratory techniques for the detection of significant bacteria of the honey bee, Apis mellifera, in Argentina. J. Apic. Res. 1991, 30, 75–80. [Google Scholar] [CrossRef]
- Peng, Y.-S.; Peng, K.-Y. A study on the possible utilization of immunodiffusion and immunofluorescence techniques as the diagnostic methods for American foulbrood of honeybees (Apis mellifera). J. Invertebr. Pathol. 1979, 33, 284–289. [Google Scholar] [CrossRef]
- Otte, E. Contribution to the laboratory diagnosis of American foulbrood (AFB) of the honey bee with particular reference to the fluorescent antibody technique. Apidologie 1973, 4, 331–339. [Google Scholar] [CrossRef]
- Olsen, P.E.; Grant, G.A.; Nelson, D.L.; Rice, W.A. Detection of American foulbrood disease of the honeybee, using a monoclonal antibody specific to Bacillus larvae in an enzyme-linked immunosorbent assay. Can. J. Microbiol. 1990, 36, 732–735. [Google Scholar] [CrossRef]
- Descamps, T.; De Smet, L.; Stragier, P.; De Vos, P.; de Graaf, D.C. Multiple Locus Variable number of tandem repeat Analysis: A molecular genotyping tool for Paenibacillus larvae. Microb. Biotechnol. 2016, 9, 772–781. [Google Scholar] [CrossRef]
- Bozdeveci, A.; Akpınar, R.; Karaoğlu, A. Isolation, characterization, and comparative genomic analysis of vB_PlaP_SV21, new bacteriophage of Paenibacillus larvae. Virus Res. 2021, 305, 198571. [Google Scholar] [CrossRef]
- Erban, T.; Ledvinka, O.; Kamler, M.; Nesvorna, M.; Hortova, B.; Tyl, J.; Titera, D.; Markovic, M.; Hubert, J. Honeybee (Apis mellifera)-associated bacterial community affected by American foulbrood: Detection of Paenibacillus larvae via microbiome analysis. Sci. Rep. 2017, 7, 5084. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.O.; Genersch, E.; Fünfhaus, A.; Poppinga, L.; Formella, N.; Bettin, B.; Karger, A. Rapid identification of differentially virulent genotypes of Paenibacillus larvae, the causative organism of American foulbrood of honey bees, by whole cell MALDI-TOF mass spectrometry. Vet. Microbiol. 2014, 170, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Crudele, S.; Ricchiuti, L.; Ruberto, A.; Rossi, F. Quantitative PCR (qPCR) vs culture-dependent detection to assess honey contamination by Paenibacillus larvae. J. Apic. Res. 2020, 59, 218–222. [Google Scholar] [CrossRef]
- Dobbelare, W.; De Graaf, D.C.; Peeters, J.E.; Jacobs, F.J. Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 2001, 32, 363–370. [Google Scholar] [CrossRef]
- Santos, S.B.; Oliveira, A.; Melo, L.D.R.; Azeredo, J. Identification of the first endolysin Cell Binding Domain (CBD) targeting Paenibacillus larvae. Sci. Rep. 2019, 9, 2568. [Google Scholar] [CrossRef] [PubMed]
- Yones, M.; Ma’Moun, S.A.; Farag, R.M.; El-Raouf, M.A. Hyperspectral application for early diagnosis of American foulbrood disease in the honeybee (Apis mellifera L.) larvae. Egypt. J. Remote Sens. Space Sci. 2019, 22, 271–277. [Google Scholar] [CrossRef]
- Anonymous. OIE Terrestrial Animal Health Code. Apidae. Terrestrial Code Online Access. Section 9. Chapter 9.2. 2022. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 3 January 2023).
- Shimanuki, H.; Knox, D.A. Diagnosis of Honey Bee Diseases. In Agriculture Handbook; No. 69061; U.S. Department of Agriculture: Washington, DC, USA, 2000. [Google Scholar]
- Bargańska, Ż.; Namiesnik, J.; Ślebioda, M. Determination of antibiotic residues in honey. TrAC Trends Anal. Chem. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Mezher, Z.; Bubnic, J.; Condoleo, R.; Jannoni-Sebastianini, F.; Leto, A.; Proscia, F.; Formato, G. Conducting an international, exploratory survey to sollect data on honey bee disease management and control. Appl. Sci. 2021, 11, 7311. [Google Scholar] [CrossRef]
- Andrade, V.D.M.; Flores, J.L.H.; López, M.A.R.; Hernández, A.C.; Gómez, S.R.; Calvillo, R.P.M.; Martínez, A.G.E.; Pérez, J.C.; Hernández, I.A.; Hidalgo, E.; et al. Evaluation of the presence of Paenibacillus larvae in commercial bee pollen using PCR amplification of the gene for tRNACys. Braz. J. Microbiol. 2019, 50, 471–480. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standard for Honey Codex Stan 12-1981 Council Directive 2001/110/EC; Codex Alimentarius Commission: Rome, Italy, 2001. [Google Scholar]
- Matović, K.; Ćirić, J.; Kaljević, V.; Nedić, N.; Jevtić, G.; Vasković, N.; Baltić, M.Ž. Physicochemical parameters and microbiological status of honey produced in an urban environment in Serbia. Environ. Sci. Pollut. Res. 2018, 25, 14148–14157. [Google Scholar] [CrossRef]
- Dickel, F.; Bos, N.M.P.; Hughes, H.; Martín-Hernández, R.; Higes, M.; Kleiser, A.; Freitak, D. The oral vaccination with Paenibacillus larvae bacterin can decrease susceptibility to American Foulbrood infection in honey bees—A safety and efficacy study. Front. Veter-Sci. 2022, 9, 946237. [Google Scholar] [CrossRef] [PubMed]
- USDA Licenses Honeybee Vaccine to Dalan Animal Health. Available online: https://federallabs.org/flc-highlights/federal-lab-news/usda-licenses-honeybee-vaccine-to-dalan-animal-health (accessed on 3 January 2023).

| Genotype | ERIC I | ERIC II | ERIC III | ERIC IV | ERIC V |
|---|---|---|---|---|---|
| Virulence | Kill larvae within 12 days | Kills larvae within 7 days | Kills larvae within 7 days | Kills even after 3 days | |
| Frequency | Most frequent genotype, found throughout the world | Isolated worldwide, especially in Europe | Not identified in recent decades | Identified in Spain | |
| References | [2,22,29] | [2,22,29] | [2,22,29] | [26] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matović, K.; Žarković, A.; Debeljak, Z.; Vidanović, D.; Vasković, N.; Tešović, B.; Ćirić, J. American Foulbrood—Old and Always New Challenge. Vet. Sci. 2023, 10, 180. https://doi.org/10.3390/vetsci10030180
Matović K, Žarković A, Debeljak Z, Vidanović D, Vasković N, Tešović B, Ćirić J. American Foulbrood—Old and Always New Challenge. Veterinary Sciences. 2023; 10(3):180. https://doi.org/10.3390/vetsci10030180
Chicago/Turabian StyleMatović, Kazimir, Aleksandar Žarković, Zoran Debeljak, Dejan Vidanović, Nikola Vasković, Bojana Tešović, and Jelena Ćirić. 2023. "American Foulbrood—Old and Always New Challenge" Veterinary Sciences 10, no. 3: 180. https://doi.org/10.3390/vetsci10030180
APA StyleMatović, K., Žarković, A., Debeljak, Z., Vidanović, D., Vasković, N., Tešović, B., & Ćirić, J. (2023). American Foulbrood—Old and Always New Challenge. Veterinary Sciences, 10(3), 180. https://doi.org/10.3390/vetsci10030180








