Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnosis
2.3. RR, SpO2, and Various Blood Tests
2.4. Statistical Analysis
3. Results
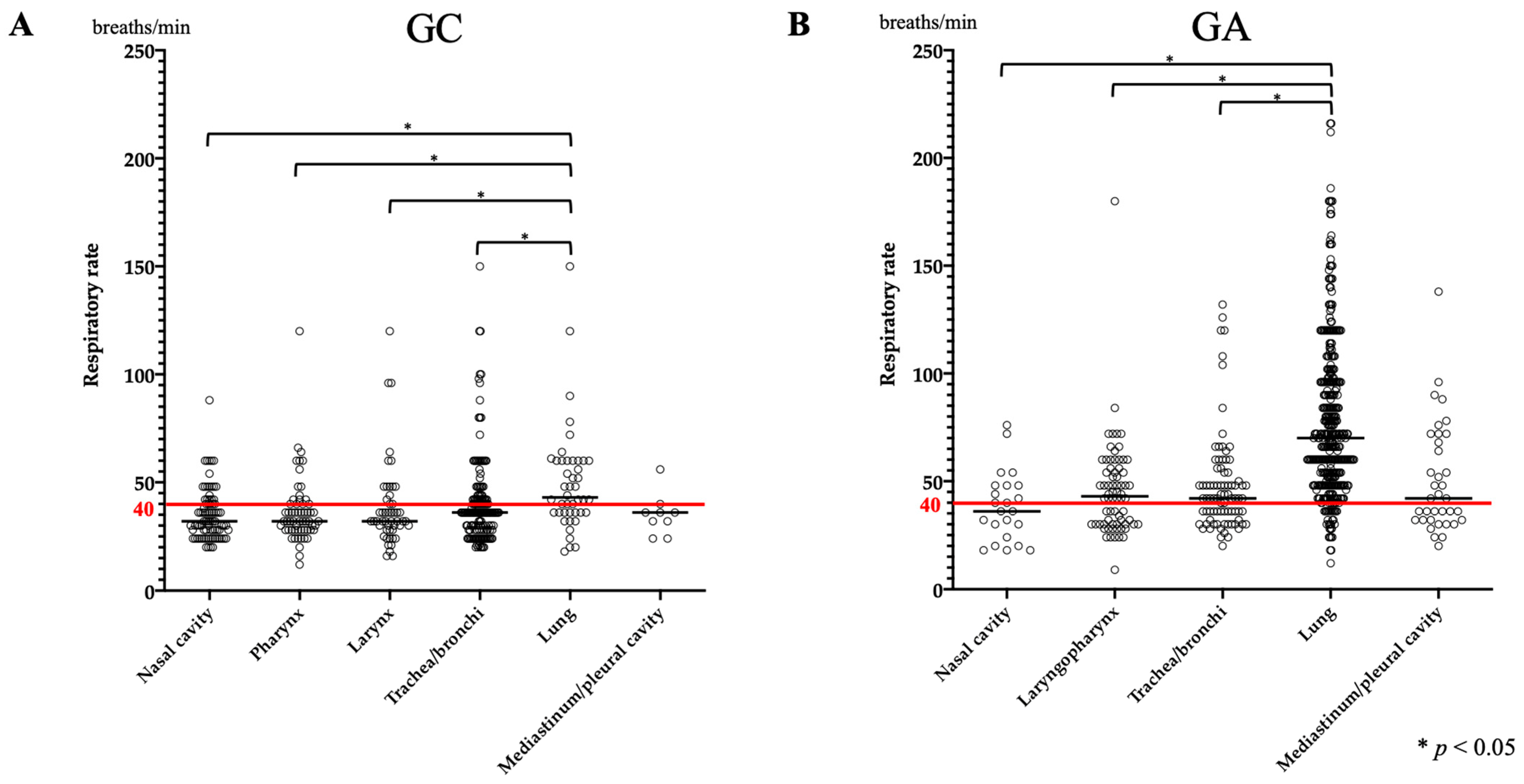
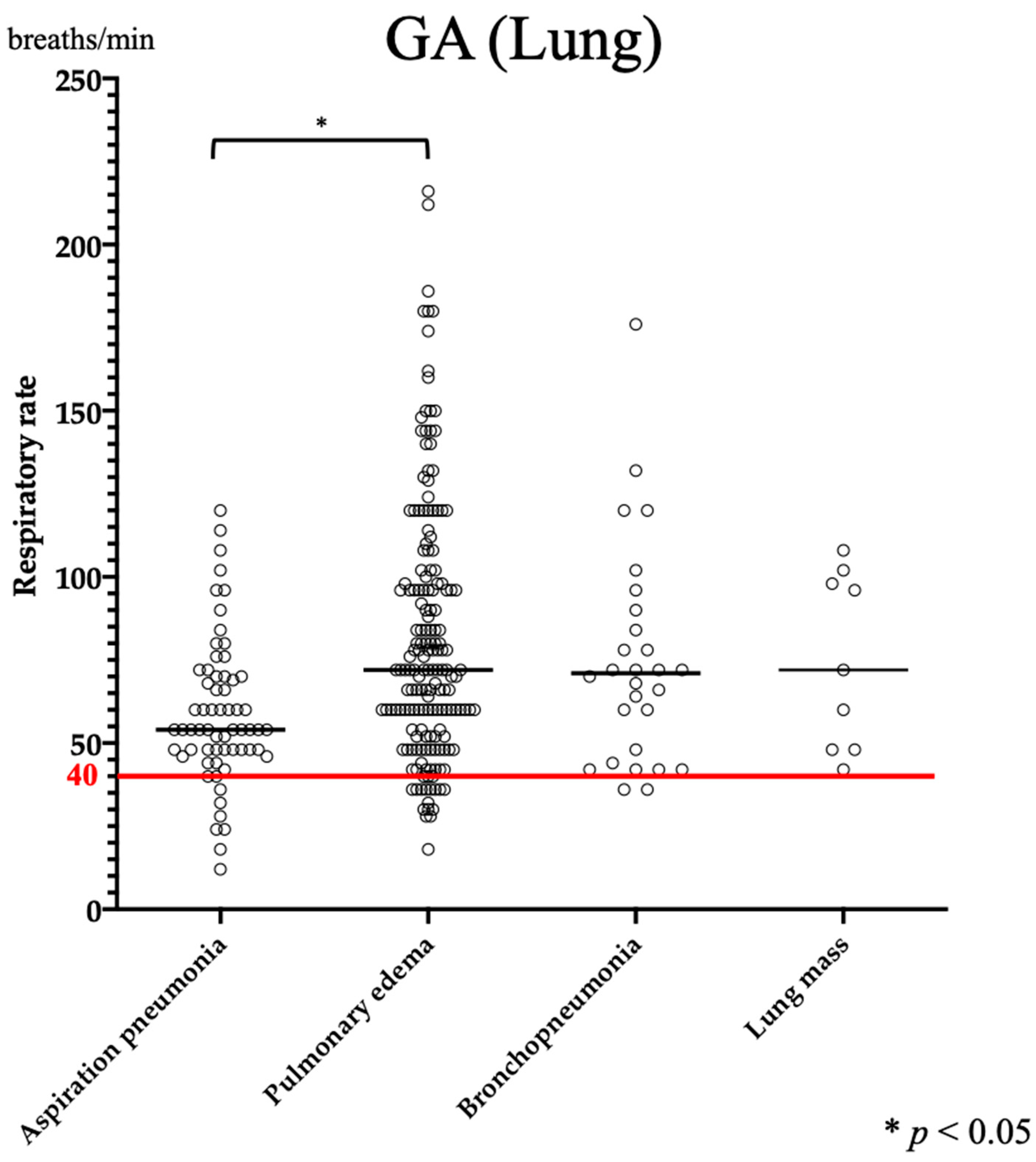
3.1. RR
3.2. SpO2
3.3. CBC
3.4. Blood Gas Analysis
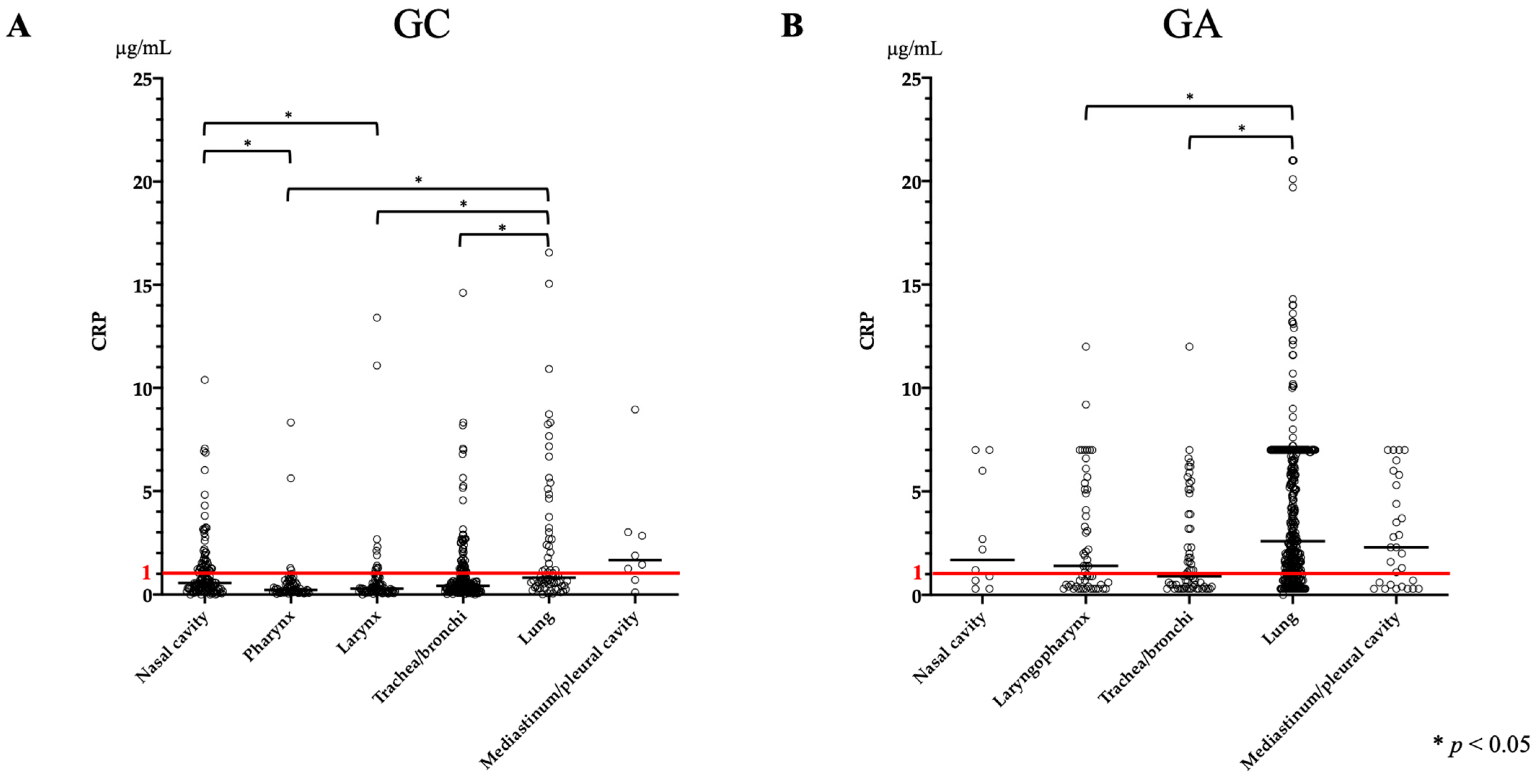
3.5. CRP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domínguez-Ruiz, M.; Reinero, C.R.; Vientos-Plotts, A.; Grobman, M.E.; Silverstein, D.; Gomes, E.; Le Boedec, K. Association between respiratory clinical signs and respiratory localization in dogs and cats with abnormal breathing patterns. Vet. J. 2021, 277, 105761. [Google Scholar] [CrossRef]
- Lee, S.; Tremper, K.K.; Barker, S.J. Effects of anemia on pulse oximetry and continuous mixed venous hemoglobin saturation monitoring in dogs. Anesthesiology 1991, 75, 118–122. [Google Scholar] [CrossRef]
- Jacobson, J.D.; Miller, M.W.; Matthews, N.S.; Hartsfield, S.M.; Knauer, K.W. Evaluation of accuracy of pulse oximetry in dogs. Am. J. Vet. Res. 1992, 53, 537–540. [Google Scholar]
- Proulx, J. Respiratory monitoring: Arterial blood gas analysis, pulse oximetry, and end-tidal carbon dioxide analysis. Clin. Tech Small Anim. Pract. 1999, 14, 227–230. [Google Scholar] [CrossRef]
- Farrell, K.S.; Hopper, K.; Cagle, L.A.; Epstein, S.E. Evaluation of pulse oximetry as a surrogate for PaO(2) in awake dogs breathing room air and anesthetized dogs on mechanical ventilation. J. Vet. Emerg. Crit. Care 2019, 29, 622–629. [Google Scholar] [CrossRef]
- Ralston, A.C.; Webb, R.K.; Runciman, W.B. Potential errors in pulse oximetry. III: Effects of interferences, dyes, dyshaemoglobins and other pigments. Anaesthesia 1991, 46, 291–295. [Google Scholar] [CrossRef]
- Webb, R.K.; Ralston, A.C.; Runciman, W.B. Potential errors in pulse oximetry. II. Effects of changes in saturation and signal quality. Anaesthesia 1991, 46, 207–212. [Google Scholar] [CrossRef]
- Cabanas, A.M.; Fuentes-Guajardo, M.; Latorre, K.; León, D.; Martín-Escudero, P. Skin Pigmentation Influence on Pulse Oximetry Accuracy: A Systematic Review and Bibliometric Analysis. Sensors 2022, 22, 3402. [Google Scholar] [CrossRef]
- Irizarry, R.; Reiss, A. Arterial and venous blood gases: Indications, interpretations, and clinical applications. Compend. Contin. Educ. Vet. 2009, 31, E1–72009, E1–7; quiz E7. [Google Scholar] [PubMed]
- Rieser, T.M. Arterial and venous blood gas analyses. Top Companion Anim. Med. 2013, 28, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ilkiw, J.E.; Rose, R.J.; Martin, I.C. A comparison of simultaneously collected arterial, mixed venous, jugular venous and cephalic venous blood samples in the assessment of blood-gas and acid-base status in the dog. J. Vet. Intern. Med. 1991, 5, 294–298. [Google Scholar] [CrossRef]
- Tamura, J.; Itami, T.; Ishizuka, T.; Fukui, S.; Miyoshi, K.; Sano, T.; Yamashita, K. Central venous blood gas and acid-base status in conscious dogs and cats. J. Vet. Med. Sci. 2015, 77, 865–869. [Google Scholar] [CrossRef]
- Barger, A.M. The complete blood cell count: A powerful diagnostic tool. Vet. Clin. N. Am. Small. Anim. Pract. 2003, 33, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takahashi, M.; Ohno, K.; Koshino, A.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-reactive protein concentration in dogs with various diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef]
- Malin, K.; Witkowska-Piłaszewicz, O. C-Reactive Protein as a Diagnostic Marker in Dogs: A Review. Animals 2022, 12, 2888. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, S.J.; Laurila, H.P.; Lilja-Maula, L.I.; Melamies, M.A.; Rantala, M.; Rajamäki, M.M. Serum C-reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J. Vet. Intern. Med. 2014, 28, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Rodrigues, N.; Giraud, L.; Bolen, G.; Fastrès, A.; Clercx, C.; Boysen, S.; Billen, F.; Gommeren, K. Comparison of lung ultrasound, chest radiographs, C-reactive protein, and clinical findings in dogs treated for aspiration pneumonia. J. Vet. Intern. Med. 2022, 36, 743–752. [Google Scholar] [CrossRef]
- Sherman, R.; Karagiannis, M. Aspiration Pneumonia in the Dog: A Review. Top Companion Anim. Med. 2017, 32, 1–7. [Google Scholar] [CrossRef]
- Dear, J.D. Bacterial Pneumonia in Dogs and Cats: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 447–465. [Google Scholar] [CrossRef]
- Hackner, S.G. Panting. In Textbook of Respiratory Disease in Dogs and Cats; WB Saunders: Orlando, FL, USA, 2004; pp. 46–48. [Google Scholar]
- Hoareau, G.L.; Jourdan, G.; Mellema, M.; Verwaerde, P. Evaluation of arterial blood gases and arterial blood pressures in brachycephalic dogs. J. Vet. Intern. Med. 2012, 26, 897–904. [Google Scholar] [CrossRef]
- Sharp, C.R.; Rozanski, E.A. Physical examination of the respiratory system. Top Companion Anim. Med. 2013, 28, 79–85. [Google Scholar] [CrossRef]
- Arulpagasam, S.; Lux, C.; Odunayo, A.; Biskup, J.; Sun, X. Evaluation of Pulse Oximetry in Healthy Brachycephalic Dogs. J. Am. Anim. Hosp. Assoc. 2018, 54, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.R.; Hawkins, E.C.; Hurley, K.J.; Monce, K. Frequency of pulmonary mineralization and hypoxemia in 21 dogs with pituitary-dependent hyperadrenocorticism. J. Vet. Intern. Med. 2000, 14, 151–156. [Google Scholar] [PubMed]
- Reinero, C. Interstitial lung diseases in dogs and cats part I: The idiopathic interstitial pneumonias. Vet. J. 2019, 243, 48–54. [Google Scholar] [CrossRef]
- Aroch, I.; Klement, E.; Segev, G. Clinical, biochemical, and hematological characteristics, disease prevalence, and prognosis of dogs presenting with neutrophil cytoplasmic toxicity. J. Vet. Intern. Med. 2005, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Stockham, S.L.; Keeton, K.S.; Szladovits, B. Clinical assessment of leukocytosis: Distinguishing leukocytoses caused by inflammatory, glucocorticoid, physiologic, and leukemic disorders or conditions. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 1335–1357. [Google Scholar] [CrossRef] [PubMed]
- Similowski, T.; Agustí, A.; MacNee, W.; Schönhofer, B. The potential impact of anaemia of chronic disease in COPD. Eur. Respir. J. 2006, 27, 390–396. [Google Scholar] [CrossRef]
- Nitsche, E.K. Erythrocytosis in dogs and cats: Diagnosis and management. Compend. Contin. Educ. Pract. Vet. 2004, 26, 104–118. [Google Scholar]
- Heikkilä, H.P.; Lappalainen, A.K.; Day, M.J.; Clercx, C.; Rajamäki, M.M. Clinical, bronchoscopic, histopathologic, diagnostic imaging, and arterial oxygenation findings in West Highland White Terriers with idiopathic pulmonary fibrosis. J. Vet. Intern. Med. 2011, 25, 433–439. [Google Scholar] [CrossRef]
- Holopainen, S.; Laurila, H.P.; Lappalainen, A.K.; Rajamäki, M.M.; Viitanen, S.J. Polycythemia in dogs with chronic hypoxic pulmonary disease. J. Vet. Intern. Med. 2022, 36, 1202–1210. [Google Scholar] [CrossRef]
- Johnson, R.A. A Quick Reference on Respiratory Alkalosis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A. A Quick Reference on Respiratory Acidosis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 185–189. [Google Scholar]
- De Morais, H.S.A.; DiBartola, S.P. Ventilatory and Metabolic Compensation in Dogs with Acid-Base Disturbances. J. Vet. Emerg. Crit. Care 1991, 1, 39–49. [Google Scholar] [CrossRef]
- Viitanen, S.J.; Lappalainen, A.K.; Christensen, M.B.; Sankari, S.; Rajamäki, M.M. The Utility of Acute-Phase Proteins in the Assessment of Treatment Response in Dogs with Bacterial Pneumonia. J. Vet. Intern. Med. 2017, 31, 124–133. [Google Scholar] [CrossRef]
| Anatomical Sites and Final Diagnosis | Number of Dogs in GC (%) | Number of Dogs in GA (%) |
|---|---|---|
| Total | 704 | 682 |
| Nasal cavity | 146 (20.7%) | 26 (3.7%) |
| Non-infectious rhinitis | 56 (8.0%) | 0 |
| Neoplasia | 47 (6.7%) | 1 (0.1%) |
| Foreign body | 21 (2.9%) | 0 |
| Rhinitis secondary to dental disease | 16 (2.3%) | 0 |
| Fungal rhinitis | 5 (0.7%) | 0 |
| Rhinitis (cause unknown) * | 0 | 22 (3.2%) |
| Other diseases | 1 (0.1%) | 3 (0.4%) |
| Laryngopharynx † | 195 (27.7%) | 96 (14.1%) |
| BOAS | 98 (13.9%) | 2 (0.3%) |
| Laryngopharyngitis | 16 (2.3%) | 45 (6.6%) |
| Upper airway obstruction (cause unknown) | 0 | 35 (5.1%) |
| Laryngeal paralysis | 28 (4.0%) | 5 (0.8%) |
| Laryngeal collapse | 13 (1.8%) | 0 |
| Other diseases | 40 (5.7%) | 9 (1.3%) |
| Trachea/bronchi | 277 (39.3%) | 100 (14.7%) |
| Tracheobronchial collapse | 135 (19.2%) | 34 (5.0%) |
| Chronic bronchitis | 120 (17.0%) | 0 |
| Bronchitis | 0 | 53 (7.8%) |
| Bronchiectasis | 10 (1.4%) | 1 (0.1%) |
| Infectious bronchitis | 7 (1.0%) | 12 (1.8%) |
| Other diseases | 5 (0.7%) | 0 |
| Lung | 77 (10.9%) | 418 (61.3%) |
| Cardiogenic pulmonary edema | 0 | 172 (25.2%) |
| Pneumonia (cause unknown) * | 0 | 83 (12.2%) |
| Aspiration pneumonia | 19 (2.7%) | 66 (9.7%) |
| Bronchopneumonia | 6 (0.9%) | 29 (4.2%) |
| Non-cardiogenic pulmonary edema | 1 (0.1%) | 28 (4.1%) |
| Lung masses | 25 (3.6%) | 10 (1.5%) |
| Interstitial lung disease | 18 (2.5%) | 2 (0.3%) |
| Other diseases | 8 (1.1%) | 28 (4.1%) |
| Mediastinum/pleural Cavity | 9 (1.4%) | 42 (6.2%) |
| Pericardial effusion | 0 | 11 (1.6%) |
| Pleural effusion | 3 (0.5%) | 6 (0.8%) |
| Anterior mediastinum tumor | 4 (0.6%) | 2 (0.3%) |
| Pulmonary hypertension | 0 | 5 (0.7%) |
| Other diseases | 2 (0.3%) | 19 (2.8%) |
| Median Value (Range) | |||||||
|---|---|---|---|---|---|---|---|
| GC | Total | Nasal Cavity | Pharynx | Larynx | Trachea/Bronchi | Lung | Mediastinum/Pleural Cavity |
| Respiratory rate (/min) | 36 (12–150) | 32 (20–88) | 32 (12–120) | 32 (16–120) | 36 (20–150) | 43 (18–150) | 36 (24–56) |
| SpO2 (%) | 98 (80–100) | 99 (93–100) | 98 (90–100) | 98 (83–100) | 98 (90–100) | 97 (80–100) | 98 (95–100) |
| CBC | |||||||
| Red blood cell (/μL) | 6.79 × 106 (2.70–10.09) | 6.97 × 106 (2.99–9.67) | 6.64 × 106 (3.62–8.67) | 6.72 × 106 (2.70–8.72) | 6.85 × 106 (4.60–10.09) | 6.34 × 106 (4.54–9.83) | 6.62 × 106 (3.34–7.98) |
| Hematocrit (%) | 46.9 (21.9–64.0) | 47.5 (23.1–62.8) | 47.7 (26–61.7) | 47.1 (33.5–59.5) | 46.9 (32.0–60.9) | 45.2 (29.7–64.0) | 44.9 (21.9–54.5) |
| Hemoglobin (g/dL) | 15.8 (6.8–70.2) | 16.2 (7.3–70.2) | 15.9 (8.6–21.3) | 16.2 (10.9–21.0) | 15.8 (9.8–23.4) | 15.1 (9.5–21.6) | 15.3 (6.8–18.1) |
| White blood cell (/μL) | 11,650 (2400–101,100) | 12,200 (4800–52,000) | 10,800 (4400–25,400) | 9600 (4400–34,600) | 11,300 (2400–47,500) | 15,050 (3800–101,100) | 17,150 (10,700–34,600) |
| Segmented neutrophil (/μL) | 11,822 (1154–92,507) | 10,875 (1154–46,810) | 9792 (3476–23,368) | 8298 (3276–32,752) | 11,700 (2204–43,225) | 15,096 (5976–92,507) | 12,060 (11,822–32,752) |
| Platelet (/μL) | 423 × 103 (9–1430) | 415 × 103 (41.4–1195) | 423 × 103 (64.2–810) | 442 × 103 (88–902) | 408 × 103 (9–1430) | 453 × 103 (34–1280) | 452 × 103 (290–720) |
| Blood gas analysis | |||||||
| pH | 7.42 (7.17–7.55) | 7.43 (7.35–7.55) | 7.40 (7.17–7.51) | 7.42 (7.17–7.54) | 7.42 (7.26–7.54) | 7.42 (7.33–7.54) | 7.46 (7.36–7.50) |
| PCO2 (mmHg) | 38 (18–72) | 35.5 (19–52) | 41 (19–72) | 38 (22–72) | 37 (18–68) | 39 (27–48) | 35 (30–47) |
| HCO3 (mmol/L) | 24.2 (14.4–40.0) | 23.5 (15.9–35.9) | 25.1 (15.2–36.4) | 23.6 (18.4–40.0) | 24.2 (14.4–38.7) | 25.2 (16.0–33.5) | 23.9 (21.3–26.8) |
| BE (mmol/L) | −0.4 (−11.4–14.1) | −1.1 (−8–13.2) | 0.3 (−7.8–11.8) | −0.4 (−7.2–13.2) | −0.6 (−11.4–14.1) | 0.8 (−9.1–10.6) | 0.3 (−2.5–3.3) |
| CRP (μg/mL) | 0.47 (0–16.56) | 0.57 (0–10.39) | 0.22 (0.04–8.33) | 0.29 (0–13.4) | 0.43 (0–14.61) | 0.82 (0.02–16.56) | 1.67 (0.11–8.96) |
| GA | Total | Nasal Cavity | Laryngopharynx | Trachea/Bronchi | Lung | Mediastinum/Pleural Cavity | |
| Respiratory rate (/min) | 60 (9–216) | 36 (9–216) | 43 (9–72) | 42 (20–132) | 70 (12–216) | 42 (20–138) | |
| SpO2 (%) | 93 (60–100) | 96 (60–100) | 97 (75–100) | 96 (71–100) | 91 (60–100) | 95 (80–100) | |
| CBC | |||||||
| Red blood cell (/μL) | 6.92 × 106 (2.62–11.65) | 6.98 × 106 (3.09–10.68) | 6.99 × 106 (3.38–9.46) | 7.03 × 106 (3.90–10.35) | 6.92 × 106 (2.62–11.65) | 6.38 × 106 (3.01–10.57) | |
| Hematocrit (%) | 45.6 (16.8–73.7) | 45.4 (21.7–73.5) | 47.1 (26.4–65.0) | 46.3 (24.3–64.0) | 45.6 (16.8–73.7) | 42.1 (22.6–67.3) | |
| Hemoglobin (g/dL) | 15.6 (5.7–25.7) | 15.6 (7.3–24.4) | 16.2 (8.8–22.8) | 15.7 (8.1–21.4) | 15.6 (5.7–25.7) | 14.8 (7.5–22.8) | |
| White blood cell (/μL) | 15,300 (840–73,000) | 18,150 (5900–42,400) | 12,100 (840–56,500) | 12,650 (4100–44,500) | 16,400 (1200–60,000) | 16,350 (7400–73,000) | |
| Segmented neutrophil (/μL) | 15,747 (516–68,620) | 19,888 (4968–24,055) | 14,233 (2408–50,850) | 11,340 (3034–32,930) | 16,544 (516–54,126) | 13,689 (6992–68,620) | |
| Platelet (/μL) | 436 × 103 (0–1330) | 514 × 103 (216–716) | 383 × 103 (115–947) | 404 × 103 (115–857) | 455 × 103 (0–1330) | 342 × 103 (51–887) | |
| Blood gas analysis (Vein) | |||||||
| pH | 7.35 (6.80–7.59) | 7.41 (7.29–7.49) | 7.33 (7.13–7.47) | 7.38 (7.13–7.50) | 7.34 (6.80–7.59) | 7.35 (6.95–7.56) | |
| PCO2 (mmHg) | 44 (20–139) | 30 (28–31) | 44 (26–72) | 43 (27–90) | 44 (21–139) | 41 (20–59) | |
| HCO3 (mmol/L) | 22.6 (8.3–42.5) | 19.3 (13.5–22.1) | 23.2 (13.7–29.5) | 24.7 (16.7–31.2) | 23.7 (12.1–42.5) | 23.4 (8.3–31.3) | |
| BE (mmol/L) | −2.4 (−21.0–16.9) | −5.5 (−13.1–1.2) | −2.0 (−12.7–4.2) | −0.4 (−9.7–7.0) | −2.1 (−21.0–16.9) | −1.6 (−20.9–7.6) | |
| CRP (μg/mL) | 2.1 (0–21.0) | 2.7 (0.3–7.0) | 1.4 (0.3–12.0) | 0.9 (0.3–12.0) | 2.6 (0–21.0) | 2.3 (0.3–7.0) | |
| Inspection Item | Median Value (Range) | |||
|---|---|---|---|---|
| GC | Interstitial Lung Disease | Lung Mass | Aspiration Pneumonia | Bronchopneumonia |
| Respiratory rate (/min) | 50 (20–150) | 44 (20–72) | 40 (32–120) | 60 (56–61) |
| SpO2 (%) | 91 (83–100) | 98 (90–100) | 96 (80–100) | 97 (90–98) |
| White blood cell (/μL) | 15,600 (8400–50,800) | 14,500 (3800–101,100) | 15,300 (6300–29,700) | 22,400 (14,600–36,000) |
| Segmented neutrophil (/μL) | 16,248 (7189–52,490) | 12,994 (5976–92,507) | 17,436 (10,777–27,027) | 19,712 (12,647–32,400) |
| Blood gas analysis | ||||
| pH | 7.43 (7.38–7.49) | 7.41 (7.34–7.50) | 7.42 (7.33–7.52) | 7.39 (7.35–7.54) |
| PCO2 (mmHg) | 39 (29–46) | 35 (27–41) | 42 (36–48) | 35.5 (31–40) |
| HCO3 (mmol/L) | 26.5 (18.0–32.0) | 22.4 (16.0–27.8) | 26.3 (19.0–33.5) | 22.5 (20.1–26.5) |
| BE (mmol/L) | 2.3 (−6.8–8.0) | −1.8 (−9.1–3.6) | 1.5 (−6.9–10.6) | −2.7 (−5.0–4.0) |
| CRP (μg/mL) | 0.64 (0.02–5.65) | 1.14 (0.05–10.92) | 0.71 (0.20–15.05) | 4.85 (1.19–16.56) |
| GA | Bronchopneumonia | Aspiration Pneumonia | Pulmonary Edema | |
| Respiratory rate (/min) | 60 (9–216) | 36 (9–216) | 43 (9–72) | |
| SpO2 (%) | 90 (67–99) | 91 (67–100) | 92 (64–100) | |
| White blood cell (/μL) | 17,500 (11,100–39,400) | 16,800 (2000–60,000) | 14,600 (1200–60,000) | |
| Segmented neutrophil (/μL) | 15,708 (8769–34,145) | 16,544 (1120–53,400) | 15,327 (516–50,850) | |
| Blood gas analysis (Vein) | ||||
| pH | 7.29 (7.03–7.48) | 7.39 (6.86–7.49) | 7.32 (6.80–7.59) | |
| PCO2 (mmHg) | 46 (29–81) | 38 (26–83) | 47 (21–139) | |
| HCO3 (mmol/L) | 24.05 (18.7–30.8) | 22.1 (12.1–32.0) | 24.0 (13.0–39.2) | |
| BE (mmol/L) | −1.5 (−9.5–4.6) | −2.4 (−18.7–8.0) | −2.15 (−21.0–13.6) | |
| CRP (μg/mL) | 4.0 (0.3–14.3) | 2.6 (0.3–13.1) | 1.6 (0–21.0) | |
| Acid-Base Disorders | Number of Dogs in GC (%) | Number of Dogs in GA (%) |
|---|---|---|
| Total | 269 | 359 |
| Acidosis | 43 (16.0%) | 234 (65.2%) |
| Metabolic | 18 (6.7%) | 84 (23.4%) |
| Nasal cavity | 0 | 4 (1.1%) |
| Laryngopharynx | 9 (3.3%) | 7 (2.0%) |
| Trachea/bronchi | 5 (1.9%) | 7 (2.0%) |
| Lung | 4 (1.5%) | 59 (16.3%) |
| Mediastinum/pleural cavity | 0 | 7 (2.0%) |
| Respiratory | 23 (8.6%) | 107 (29.8%) |
| Nasal cavity | 1 (0.4%) | 0 |
| Laryngopharynx | 16 (6.0%) | 8 (2.2%) |
| Trachea/bronchi | 6 (2.2%) | 3 (0.8%) |
| Lung | 0 | 89 (24.8%) |
| Mediastinum/pleural cavity | 0 | 7 (2.0%) |
| Metabolic and respiratory | 2 (0.7%) | 43 (12.0%) |
| Nasal cavity | 0 | 0 |
| Laryngopharynx | 2 (0.7%) | 2 (0.6%) |
| Trachea/bronchi | 0 | 0 |
| Lung | 0 | 39 (10.8%) |
| Mediastinum/pleural cavity | 0 | 2 (0.6%) |
| Alkalosis | 226 (84.0%) | 125 (34.8%) |
| Metabolic | 53 (19.7%) | 25 (7.0%) |
| Nasal cavity | 11 (4.1%) | 0 |
| Laryngopharynx | 17 (6.3%) | 1 (0.3%) |
| Trachea/bronchi | 13 (4.8%) | 2 (0.6%) |
| Lung | 12 (4.5%) | 20 (5.5%) |
| Mediastinum/pleural cavity | 0 | 2 (0.6%) |
| Respiratory | 173 (64.3%) | 100 (27.8%) |
| Nasal cavity | 51 (19.0%) | 4 (1.1%) |
| Laryngopharynx | 49 (18.2%) | 10 (2.8%) |
| Trachea/bronchi | 49 (18.2%) | 13 (3.6%) |
| Lung | 20 (7.4%) | 65 (18.1%) |
| Mediastinum/pleural cavity | 4 (1.5%) | 8 (2.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakazawa, Y.; Ohshima, T.; Kitagawa, M.; Nuruki, T.; Fujiwara-Igarashi, A. Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study. Vet. Sci. 2024, 11, 27. https://doi.org/10.3390/vetsci11010027
Nakazawa Y, Ohshima T, Kitagawa M, Nuruki T, Fujiwara-Igarashi A. Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study. Veterinary Sciences. 2024; 11(1):27. https://doi.org/10.3390/vetsci11010027
Chicago/Turabian StyleNakazawa, Yuta, Takafumi Ohshima, Mami Kitagawa, Takaomi Nuruki, and Aki Fujiwara-Igarashi. 2024. "Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study" Veterinary Sciences 11, no. 1: 27. https://doi.org/10.3390/vetsci11010027
APA StyleNakazawa, Y., Ohshima, T., Kitagawa, M., Nuruki, T., & Fujiwara-Igarashi, A. (2024). Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study. Veterinary Sciences, 11(1), 27. https://doi.org/10.3390/vetsci11010027







