Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria
Abstract
1. Introduction
2. Diversity of Kunu Drinks in Nigeria
2.1. Kunu-Zaki
2.2. Kunu-Gyada
2.3. Kunu-Tsamiya
2.4. Kunu-Akamu
2.5. Kunu-Aya
3. Nutritional and Probiotic Properties of Kunu Drinks
4. Factors That Affect Kunu Production
4.1. Effects of pH on Kunu Production
4.2. Effects of Flavonoids and Additives on Kunu Production
5. Microbial Dynamics and Preservation of Kunu Drink
5.1. Pre-Fermentation in Kunu Production
5.2. Post-Fermentation in Kunu Production
5.3. Effects of Fermentation on Kunu Production
5.4. Malting in Kunu Production
5.5. Effects of Malting on Kunu Production
5.6. Preservation and Shelf-Life Extension
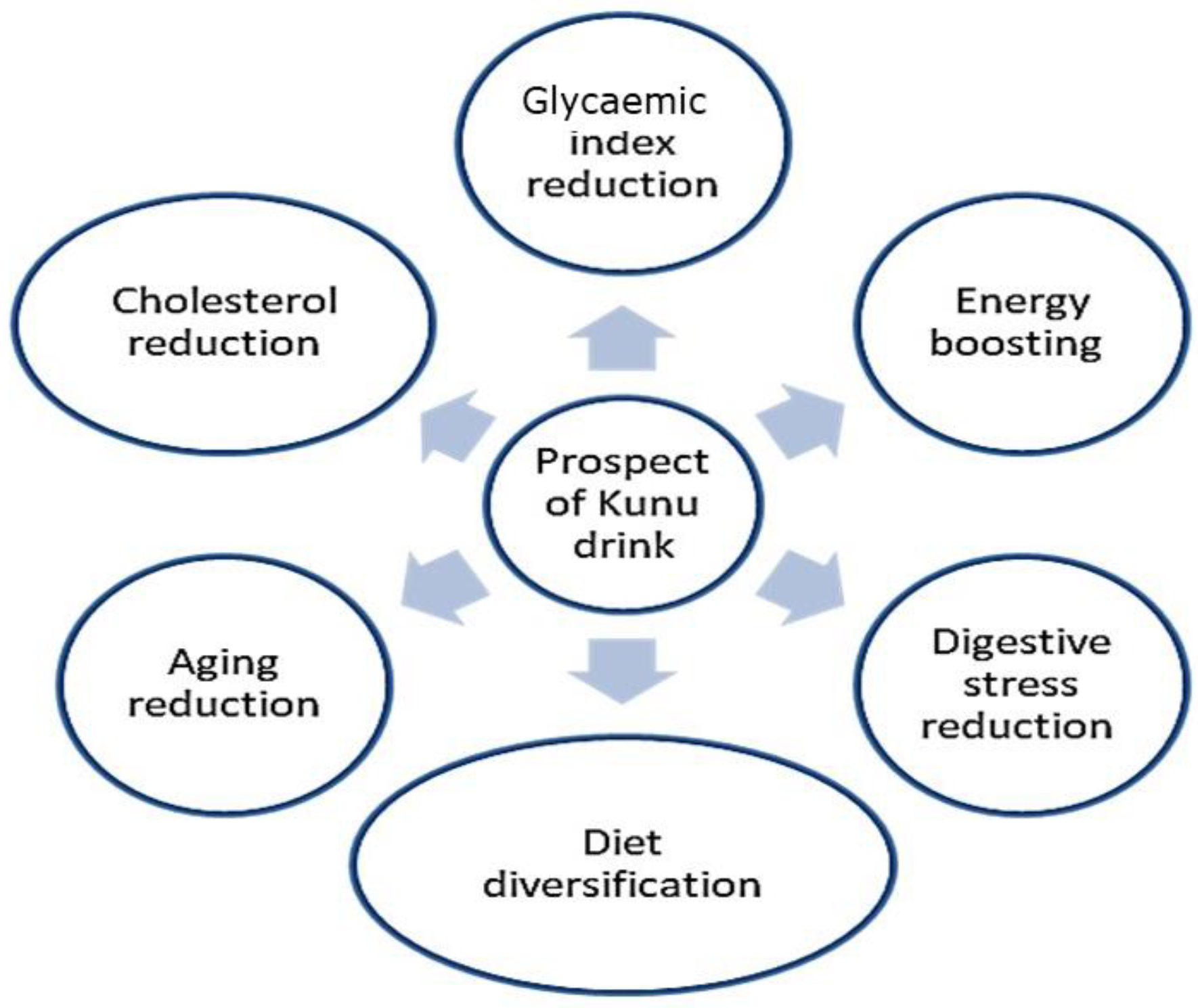
6. Prospects of Kunu Drinks
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amusa, N.A.; Odunbaku, O.A. Microbiological and nutritional quality of hawked kunun (a sorghum based non-alcoholic beverage) widely consumed in Nigeria. Pak. J. Nutr. 2009, 8, 20–25. [Google Scholar] [CrossRef]
- Gaffa, T.; Jideani, I.A.; Nkama, I. Traditional production, consumption and storage of Kunu—A non-alcoholic cereal beverage. Plant Foods Hum. Nutr. 2002, 57, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Ayeni, K.I.; Ezeokoli, O.T.; Sulyok, M.; van Wyk, D.A.B.; Oyedele, O.A.; Akinyemi, O.M.; Chibuzor-Onyema, I.E.; Adeleke, R.A.; Nwangburuka, C.C.; et al. High-throughput sequence analyses of bacterial communities and multi-mycotoxin profiling during processing of different formulations of kunu, a traditional fermented beverage. Front. Microbiol. 2019, 9, 3282. [Google Scholar] [CrossRef] [PubMed]
- Izah, S.C.; Inyang, I.R.; Angaye, T.C.N.; Okowa, I.P. A review of heavy metal concentration and potential health implications of beverages consumed in Nigeria. Toxics 2017, 5, 1. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.I.; Misihairabgwi, J.M.; Somorin, Y.M.; Chibuzor-Onyema, I.E.; Oyedele, O.A.; Abia, W.A.; Sulyok, M.; Shephard, G.S.; Krska, R. Traditionally processed beverages in Africa: A review of the mycotoxin occurrence patterns and exposure assessment. Comp. Rev. Food Sci. Food Saf. 2017, 17, 334–351. [Google Scholar] [CrossRef]
- Amadou, I.; Gbadamosi, O.S.; Le, G.W. Millet-based traditional processed foods and beverages—A review. Cereal Foods World 2011, 56, 115–121. [Google Scholar] [CrossRef]
- Tafere, G. A review on traditional fermented beverages of Ethiopia. J. Nat. Sci. Res. 2015, 5, 94–102. [Google Scholar]
- Kubo, R.; Funakawa, S.; Araki, S.; Kitabatake, N. Production of indigenous alcoholic beverages in a rural village of Cameroon. J. Inst. Brew. 2014, 120, 133–141. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.C.; Pandiella, S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Aduba, C.C.; Igbokwe, V.C.; Sam, C.E.; Ukwuru, M.U. Traditional and artisanal beverages in Nigeria: Microbial diversity and safety issues. Beverages 2020, 6, 53. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Abia, W.A.; Ogara, I.M.; Sulyok, M.; Warth, B.; Krska, R. Fate of mycotoxins in 2 popular traditional cereal-based beverages (kunu-zaki and pito) from rural Nigeria. LWT Food Sci. Technol. 2015, 60, 137–141. [Google Scholar] [CrossRef]
- Mu Ashekele, H.; Embashu, W.; Cheikhyoussef, A. Indigenous knowledge system best practice from Namibia: The case of Oshikundu processing methods. Trends Appl. Sci. Res. 2012, 7, 913–921. [Google Scholar]
- Aka, S.; Konan, G.; Fokou, G.; Dje Koffi, M.; Bonfoh, B. Review on African traditional cereal beverages. Am. J. Res. Commun. 2014, 2, 103–153. [Google Scholar]
- Fadahunsi, I.F.; Ogunbanwo, S.T.; Fawole, A.O. Microbiological and nutritional assessment of burukutu and pito (indigenously fermented alcoholic beverages in West Africa) during storage. Nat. Sci. 2013, 11, 98–103. [Google Scholar]
- Egwaikhide, I.A.; Malu, P.S.; Lawal, U.; Adelagun, R.O.; Andrew, C. Physico-chemical and microbiological analysis of fermented cow milk (nono) consumed within Kaduna Town, north-western Nigeria. Food Sci. Qual. Manag. 2014, 29, 44–48. [Google Scholar]
- Onuoha, O.G.; Haruna, U.S.; Yelmi, B.M.; Samuel, E.; Uhiara, N.S.; Ngwu, P.C. Storage study on color retention in zobo concentrates by increasing concentration of ginger (Zingiber officinale). Afr. J. Food Sci. 2014, 8, 292–296. [Google Scholar] [CrossRef]
- Gaffa, T.; Jideani, I.A.; Nkama, I. Nutritional composition of different types of Kunu produced in Bauchi and Gombe states of Nigeria. Int. J. Food Prop. 2002, 5, 351–357. [Google Scholar] [CrossRef]
- Adeyemi, I.A.; Umar, S. Effect of methods of manufacture on the quality characteristics of Kunu zaki a millet based beverage. Nig. Food J. 1999, 12, 34–40. [Google Scholar]
- Nduka, S.O.; Ezeokeke, T.C.; Onyeneke, E.N. Nutritional and microbiological quality of kunun-zaki beverage produced in Owerri muncipal. J. Agric. Food Sci. 2018, 16, 49–64. [Google Scholar] [CrossRef]
- Igwe, C.U.; Ibegbulem, C.O.; Nwaogu, L.A.; Ujowundu, C.O.; Okwu, G.N. Calcium, Zinc and Phytate Interrelationships in Four Lesser-Known African Seeds Processed into Food Condiments. J. Adv. Chem. 2013, 4, 288–294. [Google Scholar] [CrossRef]
- Gaffa, T. Improving Traditional Kunu Production and its Storage Stability. Ph.D. Thesis, Biological Science Programme, Abubarkar Tafawa Balewa University of Bauchi, Bauchi, Nigeria, 2000. [Google Scholar]
- Ofudje, E.; Okon, U.; Oduleye, O.; Williams, O. Proximate, mineral contents and microbial analysis of kunu-zaki (a non-alcoholic local beverage) in Ogun State, Nigeria. J. Adv. Biol. Biotechnol. 2016, 7, 26670. [Google Scholar] [CrossRef]
- Amusa, N.A.; Ashaye, O.A. Effect of Processing on Nutritional, Microbiological and Sensory Properties of Kunun-Zaki (A Sorghum Based Non-Alcoholic Beverage) Widely Consumed in Nigeria. Pak. J. Nutr. 2009, 8, 288–292. [Google Scholar] [CrossRef][Green Version]
- Onyimba, A.I.; Itelima, J.U.; Job, M.O.; Ogbonna, A.I.; Ode, C.O. Production of sorghum based kunun zaki using selected starter cultures. Int. J. Sci. 2017, 3, 57–63. [Google Scholar] [CrossRef][Green Version]
- Agarry, O.O.; Nkama, I.; Akoma, O. Production of Kunun-zaki (A Nigerian fermented cereal beverage) using starter culture. Int. Res. J. Microbiol. 2010, 1, 18–25. [Google Scholar]
- Nkama, I.; Iliyas, A.; Jato, A. Studies on the preparation and nutrient composition of kunun gyada, a traditional Nigerian groundnut-cereal-based weaning food. Food Nutr. Bullet. 1995, 16, 1–4. [Google Scholar] [CrossRef]
- Yunusa, B.M.; Filli, K.B.; Jiddum, F.A. Application of RSM in modelling and numerical optimisation of proximate composition of instant extruded kunun-gyada (porridge) from the blend of sorghum—Groundnut. J. Food Sci. Technol. 2022, 7, 431–441. [Google Scholar] [CrossRef]
- Ularamu, J.J.; Maina, J.F.; Ta’awu, K.G.; Nwankwo, R.N. Production and nutritional evaluation of instantized kunun gyada, a traditional Nigerian beverage from malted sorghum (Sorghum bicolor) and roasted groundnut (Arachis hypogeae) Paste by Extrusion Cooking. IOSR J. Env. Sci. Toxicol. Food Technol. 2017, 11, 32–37. [Google Scholar] [CrossRef]
- Halilu, M.; Bello, Y.M.; Ghazali, H.M. Consumer Preference and Storage Stability Assessment of Drum Dried-Maltodextrin Added Kunun-gyada Powder. EAS J. Nutr. Food Sci. 2022, 4, 1–7. [Google Scholar] [CrossRef]
- Sanni, A.I.; Sefa-Dedeh, S.; Sakyi-Dawson, E.; Asiedu, M. Microbiological evaluation of Ghanaian maize dough co-fermented with cowpea. Int. J. Food Sci. Nutr. 2002, 53, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Teniola, O.D.; Odunfa, S.A. The effects of processing methods on the levels of lysine, methionine and the general acceptability of Ogi processed using starter cultures. Int. J. Food Microbiol. 2001, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Obinna-Echem, C.P. Development of a Nigerian fermented maize food “Akamu” as a functional food. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2014. [Google Scholar]
- Achi, O.K.; Asamudo, N.U. Cereal-Based Fermented Foods of Africa as Functional Foods. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 1527–1558. [Google Scholar]
- Belewu, M.A.; Abodunrin, O.A. Preparation of kunnu from unexploited rich food Source; Tiger-nut (Cyperus esculentus). Pak. J. Nutr. 2008, 7, 109–111. [Google Scholar] [CrossRef]
- Musa, A.A.; Hamza, A. Comparative analysis of locally prepared ‘kunun aya’ (tiger-nut milk) consumed by students of Kaduna state university, Kaduna-Nigeria. Sci. World J. 2013, 8, 13–18. [Google Scholar]
- Shortt, C. The probiotic century: Historical and current perspectives. Trends Food Sci. Technol. 1999, 10, 411–417. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Laino, J.E.; del Valle, J.M.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, F.G.; de Giori, S.G.; Sesma, F. B-group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Victor-Aduloju, A.T.; Anyamene, C.; Ogbu, K.N.; Ishiwu, C.N. Isolation and identification of probiotic Lactobacillus species from traditional drink kunun-zaki fortified with paddy rice and sweet potatoes. Afr. J. Food Sci. 2018, 12, 230–237. [Google Scholar] [CrossRef]
- Jatmiko, Y.D.; Howarth, G.S.; Barton, M.D. Assessment of probiotic properties of lactic acid bacteria isolated from Indonesian naturally fermented milk. In Proceedings of the AIP Conference 1908, 8th International Conference on Global Resource Conservation (ICGRC 2017), Bydgoszcz, Poland, 9–11 May 2018; Paczkowski, T., Polasik, R., Eds.; AIP Publishing: Melville, NY, USA, 2018; pp. 050008-1–050008-14. [Google Scholar] [CrossRef]
- Ayo, H.N.; Okorie, E.I. In vitro probiotics potential of autochthonos lactic acid bacteria and microbiology of kunu made from mixed grains. Brit. Microbiol. Res. J. 2016, 14, 25403. [Google Scholar]
- Franz, C.M.A.P.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef]
- Giraud, E.; Brauman, A.; Kekele, S.; Lelong, B.; Raimbault, M. Isolation and physiological study of an amylolytic strain of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 1991, 36, 379–383. [Google Scholar] [CrossRef]
- Umaru, H.A.; Umaru, I.J.; Aminu, A.; Umaru, K.I. Influence of different processing methods on proximate and anti-nutritional value of tigernuts (Cyperus esculentus L.). GSC Biol. Pharm. Sci. 2018, 3, 29–34. [Google Scholar] [CrossRef]
- Oladele, K.A.; Osundahunsi, F.O.; Adebowale, A.Y. Influence of processing techniques on the nutrients and anti-nutrients of tiger nut (Cyperus esculentus L.). World J. Dairy Food Sci. 2009, 4, 88–93. [Google Scholar]
- Adeniran, H.A.; Adeniyi, D.M.; Taiwo, K.A. Microbiological properties of probioticated kunun zaki drink enriched with cocoa powder. Eur. J. Agric. Food Sci. 2020, 2, 1–9. [Google Scholar]
- Sengev, I.A.; Akpapunam, M.A.; Ingbian, E.K. Physicochemical and sensory properties of instant kunun-zaki flour blends from sorghum and mango mesocarp flours. Nig. Inst. Food Sci. Tech. 2012, 30, 8–16. [Google Scholar] [CrossRef]
- Olaoye, O.A.; Ubbor, S.C.; Uduma, E.A. Determination of vitamins, minerals, and microbial loads of fortified nonalcoholic beverage (kunun zaki) produced from millet. Food Sci. Nutr. 2016, 4, 96–102. [Google Scholar] [CrossRef]
- Mohammed, S.F. Effect of different ratios of ginger solution concentrations on the quality of millet-based Kunun-Zaki. MOJ Food Process. Technol. 2018, 6, 469–475. [Google Scholar]
- Elmahmood, A.M.; Doughari, J.H. Microbial quality assessment of kunun-zaki beverage sold in Girei town of Adamawa State, Nigeria. Afr. J. Food Sci. 2007, 1, 11–15. [Google Scholar]
- Ekwem, O.H.; Okolo, B.N. Microorganisms isolated during fermentation of sorghum for production of Akamu (a Nigerian fermented gruel). Microbiol. Res. J. Int. 2017, 21, 1–5. [Google Scholar] [CrossRef]
- Omemu, A.M.; Oyewole, O.B.; Bankole, M.O. Significance of yeasts in the fermentation of maize for ogi production. Food Microbiol. 2007, 24, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, E.; Achi, O.K.; Ijeoma, I.O. Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. Afr. J. Food Sci. Technol. 2010, 1, 021–026. [Google Scholar]
- Elihu, A.; Buba, Z.M.; Naphthali, N.P. Quality assessment of the ‘Kunun-zaki’soft drink based on bacteria and fungi content. Afr. Schol. J. Agric. Agric. Technol. 2021, 22, 1–10. [Google Scholar]
- Terna, G.; Ayo, J. Innovations in the traditional kunun-zaki production process. Pak. J. Nutr. 2002, 1, 202–205. [Google Scholar]
- Ekanem, J.O.; Mensah, B.J.; Marcus, N.S.; Ukpe, B.A. Microbial Quality and Proximate Composition of Kunu Drinks Produced and Sold in Ikot Ekpene Metropolis, Akwa Ibom State, Nigeria. J. Appl. Sci. Environ. Manag. 2018, 22, 1713–1718. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Dauda, A.O.; Adebisi, T.T.; Alonge, C.D. Physico-chemical, microbial and sensory properties of kunu zaki beverage sweetened with black velvet tamarind (Dialium guineense). Croat. J. Food Sci. Technol. 2017, 9, 46–56. [Google Scholar] [CrossRef]
- Braide, W.; Adeleye, S.A.; Ukagwu, N.; Lugbe, P.B. Chemical properties and microbiological profile of kunu zaki, a non-alcoholic beverage. Biomed. J. Sci. Tech. Res. 2018, 4, 3731–3735. [Google Scholar]
- Oluwajoba, S.O.; Akinyosoye, F.A.; Oyetayo, O.V. Comparative sensory and proximate evaluation of spontaneously fermenting kunu-zaki made from germinated and ungerminated composite cereal grains. Food Sci. Nutr. 2013, 1, 336–349. [Google Scholar] [CrossRef]
- Akinhanmi, F.T.; Arogundade, A.L.; Tiamiyi, O.M.; Oloruntoba, E. Protein fractions of legumes and cereals consumed in Nigeria. Int. J. Agric. Sci. Environ. Technol. 2008, 7, 54–62. [Google Scholar]
- Obadina, A.O.; Oyewole, O.B.; Awojobi, T.M. Effect of steeping time of milled grains on the quality of Kunnu-Zaki (A Nigerian beverage). Afr. J. Food Sci. Res. 2019, 7, 1–4. [Google Scholar]
- Ojokoh, A.O.; Fayemi, O.E.; Ocloo, F.C.K.; Nwokolo, F.I. Effect of fermentation on proximate composition, physicochemical and microbial characteristics of pearl pearl millet (Pennisetum glaucum (L.) R. Br.) and Acha (Digitaria exilis (Kippist) Stapf) flour blends. J. Agric. Biotechnol. Sustain. Dev. 2015, 7, 1–8. [Google Scholar]
- Afify, A.M.R.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Bioavailability of Iron, Zinc, Phytate and Phytase Activity during Soaking and Germination of white Sorghum Varieties. PLoS ONE 2015, 6, e255512. [Google Scholar] [CrossRef]
- Williana, N.M.; Babasola, A.O.; Cajethan, O.E.; Fapohunda, S.O. Role of organic spices in the preservation of traditionally fermented kunun-zaki. Microbiol. Biotechnol. Lett. 2021, 49, 192–200. [Google Scholar] [CrossRef]
- Fapohunda, S.O.; Adewale, A. Microbial Load and Keeping Quality of Kunu under Various Preservative Regimes. J. Nutr. Food Sci. 2012, 2, 141. [Google Scholar] [CrossRef]
- Annunziata, A.; Vecchio, R. Functional foods development in the European market: A consumer perspective. J. Funct. Foods 2011, 3, 223–228. [Google Scholar] [CrossRef]
- Adelekan, A.O.; Alamu, A.E.; Arisa, N.U.; Adebayo, Y.O.; Dosa, A.S. Nutritional, microbiological and sensory characteristics of malted soy kunu zaki: An improved traditional beverage. Adv. Microbiol. 2013, 3, 389–397. [Google Scholar] [CrossRef]
- Orishagbemi, C.O.; Bushiratu, A.; Laisi, I.R.; Igbatigbi, J. Organic preservation and shelf–life evaluation of liquid kunu zaki food drink, with extract of West African black pepper (Piper guineense). Int. J. Agric. Res. Food Prod. 2020, 5, 1–18. [Google Scholar]
- Dwivedi, S.; Prajapati, P.; Vyas, N.; Malviya, S.; Kharia, A. A review on food preservation: Methods, harmful effects and better alternatives. Asian J. Pharm. Pharmacol. 2017, 3, 193–199. [Google Scholar]
- Mirza, S.K.; Asema, U.K.; Kasim, S.S. To study the harmful effects of food preservatives on human health. J. Med. Chem. Drug Discov. 2017, 2, 610–616. [Google Scholar]
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496. [Google Scholar]
- Benkeblia, N.; Lanzotti, V. Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Foods 2007, 1, 193–201. [Google Scholar]
- Bruna, G.L.; Thais, A.C.; Lgia, A.C. Food additives and their health effects: A review on preservative sodium benzoate. Afr. J. Biotechnol. 2018, 17, 306–310. [Google Scholar] [CrossRef]
- Sharif, Z.I.M.; Mustapha, F.A.; Jai, J.; Yusof, N.M.; Zaki, N.A.M. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bullet. 2017, 19, 145–153. [Google Scholar] [CrossRef]
- Sharma, S. Food preservatives and their harmful effects. Int. J. Scient. Res Pub. 2015, 5, 1–2. [Google Scholar]
- Anal, A.K.; Perpetuini, G.; Petchkongkaew, A.; Tan, R.; Avallone, S.; Tofalo, R.; Waché, Y. Food safety risks in traditional fermented food from South-East Asia. Food Cont. 2019, 109, 106922. [Google Scholar] [CrossRef]
- Abulude, F.O.; Ogunkoya, M.O.; Oni, V.A. Mineral composition, shelf life, and sensory attributes of fortified ‘Kunuzaki’ beverage. Acta Sci. Pol. Technol. Aliment. 2006, 5, 155–162. [Google Scholar]
- Owuama, I.C. Brewing beer with sorghum. J. Inst. Brew. 1998, 105, 23–24. [Google Scholar] [CrossRef]
- Ingbian, E.K.; Agwu, O. Effect of different steeping regimes on the microbiological, chemical and steep water characteristic of selected maize grain varieties. J. Food Process. Preserv. 2010, 34, 426–439. [Google Scholar] [CrossRef]
- Osungbaro, T.O. Physical and nutritive properties of fermented cereal foods. Afr. J. Food Sci. 2009, 3, 23–27. [Google Scholar]
- Teniola, O.D.; Holzapfel, W.H.; Odunfa, S.A. Comparative assessment of fermentation techniques useful in the processing of ogi. World J. Microbiol. Biotechnol. 2005, 21, 39–43. [Google Scholar] [CrossRef]
- Chibuzor-Onyema, I.E.; Ezeokoli, O.T.; Sulyok, M.; Notununu, I.; Petchkongkaew, A.; Elliott, C.T.; Ezekiel, C.N. Metataxonomic analysis of bacterial communities and mycotoxin reduction during processing of three millet varieties into ogi, a fermented cereal beverage. Food Res. Int. 2021, 143, 110241. [Google Scholar] [CrossRef]
- Inyang, C.U.; Dabot, Y.A. Storability and potability of pasteurized-sterilized “kunun-zaki”: A fermented sorghum beverage. J. Food Process. Preserv. 1997, 21, 1–7. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Narbad, A. Molecular characterization of lactic acid bacteria and in situ amylase expression during traditional fermentation of cereal foods. Food Microbiol. 2012, 3, 254–262. [Google Scholar] [CrossRef]
- Oyeyiola, G.P. Fermentation of millet to produce kamu a Nigerian starch-cake food. World J. Microbiol. Biotechnol. 1991, 7, 196–201. [Google Scholar] [CrossRef]
- Efiuvwevwere, B.J.O.; Akona, O. The microbiology of ‘kunun-zaki’, a cereal beverage from northern Nigeria, during the fermentation (production) process. World J. Microbiol. Biotechnol. 1995, 11, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Annan, N.; Poll, L.; Sefa-Dedeh, S.; Plahar, W.; Jakobsen, M. Volatile compounds produced by Lactobacillus fermentum, Saccharomyces cerevisiae and Candida krusei in single starter culture fermentations of Ghanaian maize dough. J. Appl. Microbiol. 2003, 94, 462–474. [Google Scholar] [CrossRef]
- Gaffa, T.; Gaffa, A.T. Microbial succession during ‘kunun-zaki’ production with sorghum (Sorghum biocolor) grains. World J. Microbiol. Biotechnol. 2004, 20, 449–453. [Google Scholar] [CrossRef]
- Osuntogun, B.; Aboaba, O.O. Microbiological and Physio-chemical Evaluation of some Non-alcoholic beverages. Pak. J. Nutr. 2004, 3, 188–192. [Google Scholar] [CrossRef]
- Ikpoh, I.S.; Lennox, J.A.; Ekpo, L.A.; Agbo, B.E.; Henshaw, E.E.; Udoekong, N.S. Microbial quality assessment of kunu beverage locally prepared and hawked in Calabar, Cross river state, Nigeria. Glob. J. Biodivers. Sci. Manag. 2013, 3, 58–61. [Google Scholar]
- Aboh, M.I.; Oladosu, P. Microbiological assessment of kunu-zaki marketed in Abuja municipal area council (AMAC) in the federal capital territory (FCT), Nigeria. Afr. J. Microbiol. Res. 2014, 8, 1633–1637. [Google Scholar] [CrossRef]
- Russell, A.D. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Akharaiyi, F.C.; Omoya, F.O. Effect of processing methods on the microbiological quality of liquid pap ogi prepared from maize. Trends Appl. Sci. Res. 2008, 3, 330–334. [Google Scholar] [CrossRef][Green Version]
- Wolfe, B.E.; Dutton, R.J. Fermented foods as experimentally tractable microbial ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.L.; Tu, Z.C.; Wang, X.L. High-throughput sequencing of microbial community diversity and dynamics during douche fermentation. PLoS ONE 2016, 11, e0168166. [Google Scholar] [CrossRef] [PubMed]
- Giraffa, G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ijabadeniyi, A.O. Microorganisms associated with ogi traditionally produced from three varieties of maize. Res. J. Microbiol. 2007, 2, 247–253. [Google Scholar] [CrossRef][Green Version]
- Van der Meulen, R.; Scheirlinck, I.; Van Schoor, A.; Huys, G.; Vancanneyt, M.; Vandamme, P.; De Vuyst, L. Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Sanni, A.I.; Adesulu, A.T. Microbiological and physicochemical changes during fermentation of maize for masa production. Afr. J. Microbiol. Res. 2013, 7, 4355–4362. [Google Scholar]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Rapid differentiation among Lactobacillus, Pediococcus and Weissella species from some Nigerian indigenous fermented foods. LWT- Food Sci. Technol. 2017, 77, 39–44. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Tourlomousis, P.; Gasson, M.J.; Narbad, A. Analysis of bacterial communities of traditional fermented West African cereal foods using culture independent methods. Int. J. Food Microbiol. 2011, 145, 205–210. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef]
- Barrangou, R.; Azcarate-Peril, M.A.; Duong, T.; Conners, S.B.; Kelly, R.M.; Klaenhammer, T.R. Global analysis of carbohydrate utilization by Lactobacillus acidophilus using cDNA microarrays. Proc. Natl. Acad. Sci. USA 2006, 103, 3816–3821. [Google Scholar] [CrossRef]
- Akinrele, I.A. Fermentation studies on maize during the preparation of a traditional African starch cake food. J. Sci. Food Agric. 1970, 21, 619–625. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Lioger, D.; Leenhardt, F.; Demigne, C.; Remesy, C. Sourdough fermentation of wheat fractions rich in fibres before their use in processed food. J. Sci. Food Agric. 2007, 87, 1368–1373. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Lindegaard, P.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrition 2010, 2, 449–473. [Google Scholar] [CrossRef]
- Reale, A.; Mannina, L.; Tremonte, P.; Sobolev, A.P.; Succi, M.; Sorrentino, E.; Coppola, R. Phytate degradation by lactic acid bacteria and yeast during the whole meal dough fermentation: A 31P NMR study. J. Agric. Food Chem. 2004, 52, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C. Fermented cereal-based products: Nutritional aspects, possible impact on gut microbiota and health implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Soro-Yao, A.A.; Brou, K.; Amani, G.; Thonart, P.; Djè, K.M. The use of lactic acid bacteria starter cultures during the processing of fermented cereal-based foods in West Africa: A review. Trop. Life Sci. Res. 2014, 25, 81. [Google Scholar]
- Mugocha, P.T.; Taylor, J.R.N.; Bester, B.H. Fermentation of a composite finger millet-dairy beverage. World J. Microbiol. Biotechnol. 2000, 16, 341–344. [Google Scholar] [CrossRef]
- Gotcheva, V.; Pandiella, S.S.; Angelov, A.; Roshkova, Z.; Webb, C. Monitoring the fermentation of the traditional Bulgarian beverage boza. Int. J. Food Sci. Technol. 2001, 36, 129–134. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Well. 2019, 8, 203–243. [Google Scholar]
- Erkmen, O.; Bozouḡlu, T.F. Fermented cereal and grain products. In Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of attributes of cereals by germination and fermentation: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Akoma, O.; Agarry, O.O.; Nkama, I. Influence of thermal enzymatic hydrolysis of cereal starch on the physico-chemical quality of kunun-zaki (A fermented non-alcoholic cereal beverage). Int. J. Appl. Biol. Pharmaceut. Technol. 2010, 3, 821–829. [Google Scholar]
- Okpara, A.N.; Okolo, B.N.; Ugwuanyi, J.O. Antimicrobial activities of lactic acid bacteria isolated from akamu and kunun-zaki (cereal based non-alcoholic beverages) in Nigeria. Afr. J. Biotechnol. 2014, 13, 2977–2984. [Google Scholar] [CrossRef]
- Aderinola, T.; Oluwamukomi, M. Effect of sodium benzoate on the quality and sensory properties of kunun-zaki supplemented with groundnut. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 426–431. [Google Scholar]
- Amadou, I. Millet based fermented beverages processing. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 433–472. [Google Scholar]
- Mohammed, A.M.; Emmanuel, O.A. Hydrolytic enzyme levels in malted cereals. Adv. Biochem. 2014, 2, 76–79. [Google Scholar]
- Awodi, P.S.; Ella, A.B.; Asaar, G.B.; Tivkaa, T.J. Effect of malted cereals on the sugar content of sorghum gruel. Int. J. Curr. Res. Biosci. Plant Biol. 2018, 5, 43–48. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51–57. [Google Scholar]
- Mani, A. Food Preservation by Fermentation and Fermented food products. Int. J. Acad. Res. Dev. 2018, 1, 51–57. [Google Scholar]
- Kambabazi, M.R.; Hitayezu, E.; Mukandahiro, Y.; Vasanthakaalam, H. Assessment of microbiological changes during production of malted and fermented finger millet flour. RWA J. Agric. Sci. 2019, 1, 31–35. [Google Scholar]
- Kalpana, P.; Sushima, W.; Krishnapura, S. Bio accessible Mineral Content of Malted Finger Millet (Eleusine coracana), Wheat (Triticum aestivum), and Barley (Hordeum vulgare). J. Agric. Food Chem. 2010, 58, 8100–8103. [Google Scholar]
- Muyanja, C.; Namugumya, B.S. Traditional processing, microbiological, physiochemical and sensory characteristics of Kwete, a Ugandan fermented maize based beverage. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1046–1059. [Google Scholar] [CrossRef]
- Livingstone, A.S.; Sandhu, J.; Malleshi, N. Microbiological evaluation of malted wheat, chickpea, and weaning food based on them. J. Prop. Ped. 1992, 38, 74–77. [Google Scholar] [CrossRef]
- Badau, M.H. Microorganisms associated with pearl millet cultivars at various malting stages. Int. J. Food Saf. 2006, 8, 66–72. [Google Scholar]
- Shankar, I.; Usha, A. Assessment of the microbiological quality of koozh, a fermented millet beverage. Afr. J. Microbiol. Res. 2014, 8, 308–312. [Google Scholar] [CrossRef]
- Inyang, C.U.; Zakari, U.M. Effect of germination and fermentation of pearl millet on proximate, chemical and sensory properties of instant fura—A Nigerian cereal food. Pak. J. Nutr. 2008, 7, 9–12. [Google Scholar] [CrossRef]
- Bello, F.A.; Inyang, U.E.; Umoh, A.P. Effect of alkaline steeping on the nutritional, antinutritional and functional properties of malted millet (Pennisetum glaucum) flour. Int. J. Innov. Food Nutr. Sust. Agric. 2017, 5, 17–23. [Google Scholar]
- Uvere, P.O.; Amazikwu, U.C. Processing and evaluation of instant kunun zaki from millet-cowpea malt and millet-soybean malt. Afr. J. Food Sci. 2011, 5, 761–768. [Google Scholar]
- Ahmed, A.I.; Abdalla, A.A.; Tinay, A.E. Effect of traditional processing on chemical composition and mineral content of two cultivars of pearl millet (Pennisetum glaucum). J. Appl. Sci. 2009, 5, 2271–2276. [Google Scholar]
- Sehgal, S.; Kawatra, A. In vitro protein and starch digestibility of pearl millet (Pennisetum gluacum L.) as affected by processing techniques. Food/Nahr. 2001, 45, 25–27. [Google Scholar]
- Hassan, A.B.; Ahmed, I.A.M.; Osman, N.M.; Eltayeb, M.M.; Osman, G.A.; Babiker, E.E. Effect of processing treatments followed by fermentation on protein content and digestibil-ity of pearl millet (Pennisetum typhoideum) cultivars. Pak. J. Nutr. 2006, 5, 86–89. [Google Scholar]
- Jaybhaye, R.V.; Pardeshi, I.L.; Vengaiah, P.C.; Srivastav, P.P. Processing and technology for millet based food products: A review. J. Ready Eat Food 2014, 1, 32–48. [Google Scholar]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Akoma, O.; Jiya, E.A.; Akumka, D.D.; Mshelia, E. Influence of malting on the nutritional characteristics of kunun-zaki. Afr. J. Biotechnol. 2006, 5, 996–1000. [Google Scholar]
- Choudhury, M.; Das, P.; Baroova, B. Nutritional evaluation of popped and malted indigenous millet of Assam. J. Food Sci. Technol. 2011, 48, 706–711. [Google Scholar] [CrossRef]
- Hooda, S.; Jood, S. Effect of soaking and germination on nutrient and antinutrient contents of fenugreek (Trigonella foenum graecum L.). J. Food Biochem. 2003, 27, 165–176. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.A.; van den Broeck, H.C.; Brouns, F.J.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Comp. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahaman, S.M.; Elmaki, H.B.; Idris, W.H.; Hassan, A.B.; Babiker, E.E.; El Tinay, A.H. Antinutritional factor content and hydrochloric acid extractability of minerals in pearl millet cultivars as affected by germination. Int. J. Food Sci. Nutr. 2007, 58, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Anjum, F.M.; Amir, R.M.; Khan, M.R.; Hussain, S.; Javed, M.S. An overview of anti-nutritional factors in cereal grains with special reference to wheat-A review. Pak. J. Food Sci. 2010, 20, 54–61. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Badau, M.H. Sensory and microbial evaluation of pearl millet kunu-zaki saccharified with malt from some pearl millet and sorghum cultivars. Agro Sci. 2007, 6, 116–127. [Google Scholar] [CrossRef]
- Mbachu, A.E.; Etok, C.A.; Agu, K.C.; Okafor, O.I.; Awah, N.S.; Chidi-Onuorah, L.C.; Ekwueme, V.C.; Okpala, J.; Ogbue, M.O.; Ikele, M.O. Microbial quality of kunu drink sold in Calabar, Cross River state, Nigeria. J. Glob. Biosci. 2014, 3, 511–515. [Google Scholar]
- Adegoke, G.O.; Odeyemi, A.O.; Hussien, O. Ikheorah Control of Ochratoxin A (OTA) in Kunuzaki (a Non-alcoholic Beverage) using Daniellin (TM). Afr. J. Agric. 2007, 2, 200–202. [Google Scholar]
- Orutugu, L.A.; Izah, S.C.; Aseibai, E.R. Microbiological quality of kunu drink sold in some major markets of Yenagoa metropolis, Nigeria. Cont. J. Biomed. Sci. 2015, 9, 9–16. [Google Scholar] [CrossRef]
- Umaru, G.A.; Tukur, I.S.; Akensire, U.A.; Adamu, Z.; Bello, O.A.; Shawulu, A.H.B.; Audu, M. Microflora of Kunun-Zaki and Sobo drinks in relation to public health in Jalingo Metropolis, North-Eastern Nigeria. Int. J. Food Res. 2014, 1, 16–21. [Google Scholar]
- Bukar, A.; Uba, A.; Oyeyi, T.I. Occurrence of some entropathogenic bacteria in some minimally and fully processed read –to-eat foods in Kano metropolis. Nig. Afr. J. Food Sci. 2010, 4, 32–36. [Google Scholar]
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Olayanju, A. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Cont. 2019, 110, 106963. [Google Scholar] [CrossRef]
- Halm, M.; Lillie, A.; Sorensen, A.K.; Jakobsen, M. Microbiological and aromatic characteristics of fermented maize doughs for kenkey production in Ghana. Int. J. Food Microbiol. Biotechnol. 1993, 12, 531–536. [Google Scholar]
- Ananou, S.; Maqueda, M.; Martinez-Bueno, M.; Valdivia, E. Biopreservation, an ecological approach to improve the safety and shelf-life of foods. In Communicating Current Research and Education Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; FORMATEX: Madrid, Spain, 2007. [Google Scholar]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Jeyaram, K.; Sanni, A.I. Probiotic and technological properties of exopolysaccharide producing Lactic acid bacteria isolated from some cereal-based Nigerian indigenous fermented food products. Food Contr. 2018, 92, 225–231. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Jeyaram, K.; Sanni, A.I.; Banwo, K. Production of exopolysaccharide by strains of Lactobacillus plantarum YO175 and OF101 isolated from traditional fermented cereal beverage. PeerJ 2018, 6, e5326. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and In vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT Food Sci. Technol. 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Ojediran, J.O.; Ogunsakin, A.O.; Banwo, K. Extracellular polysaccharide from Weissella confusa OF126: Production, optimization, and characterization. Int. J. Biol. Macromol. 2018, 111, 514–525. [Google Scholar] [CrossRef]
- Nkama, I.; Agarry, O.O.; Akoma, O. Sensory and nutritional quality characteristics of powdered ‘Kunun-zaki’: A Nigerian fermented cereal beverage. Afr. J. Food Sci. 2010, 4, 364–370. [Google Scholar]
- Omowaye-Taiwo, O.A.; Oluwamukomi, M.O. Physical and nutritional composition of instant kunun-zaki powder obtained by three drying methods. FUTA J. Res. Sci. 2015, 11, 114–122. [Google Scholar]
- Chaven, U.D.; Chaven, J.K.; Kadan, S.S. Effect of fermentation on soluble proteins and in vitro protein digestibility of sorghum, green gram and sorghum-green gram blends. J. Food Sci. 1988, 53, 1574. [Google Scholar] [CrossRef]
- Cook, P.E. Fermented foods as biotechnological resourses. Food Res. Int. 1994, 27, 309. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Itumoh, M. Chemical Contaminants Associated with Palm Wine from Nigeria Are Potential Food Safety Hazards. Beverages 2017, 3, 16. [Google Scholar] [CrossRef]
- Gedi, M.A.; di Bari, V.; Ibbett, R.; Darwish, R.; Nwaiwu, O.; Umar, Z.; Agarwal, D.; Worrall, R.; Gray, D.; Foster, T. Upcycling and valorisation of food waste. In Routledge Handbook of Food Waste; Reynolds, C., Soma, T., Spring, C., Lazell, J., Eds.; Routledge Taylor and Francis Publishers: Oxford, UK, 2020; 516p. [Google Scholar]
- Nout, M.J.R. Rich nutrition from the poorest cereal fermentations in Africa and Asian. Food Microbiol. 2009, 26, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Abah, C.R.; Ishiwu, C.N.; Obiegbuna, J.E.; Oladejo, A.A. Nutritional Composition, Functional Properties and Food Applications of Millet Grains. Asian Food Sci J. 2020, 14, 9–19. [Google Scholar] [CrossRef]
- Alejo, A.O.; Ilesanmi, F.F.; Ishola, D.T.; Afolabi, A.A.; Oyelakin, M.O. Sensory, Shelf-Life and Nutritional Evaluation of Kunu (Nigeria Non-Alcoholic Beverage) Produced from Different Grains. Int. J. Res. Stud. Agric. Sci. 2017, 3, 20–25. [Google Scholar]
- Adaramola-Ajibola, K.M.; Osaloni, A.R.; Arijeniwa, O.C. Nutritional Composition, Microbiological Quality and Sensory Properties of Kunu-Zaki Produced from Millet and Tigernut Blend. Asian Food Sci. J. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Kayode, R.M.; Joseph, J.K.; Adegunwa, M.O.; Dauda, A.O.; Akeem, S.A.; Kayode, B.I.; Babayeju, A.A.; Olabanji, S.O. Effects of addition of different spices on the quality attributes of tiger-nut milk (kunun-aya) during storage. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Adair, L.S.; Bentley, M.E.; Flax, V.L.; Jamieson, D.J.; Ellington, S.R.; Tegha, G.; Chasela, C.S.; Kamwendo, D.; et al. Thiamin and Riboflavin in Human Milk: Effects of Lipid-Based Nutrient Supplementation and Stage of Lactation on Vitamer Secretion and Contributions to Total Vitamin Content. PLoS ONE 2016, 11, e0149479. [Google Scholar] [CrossRef]
- Ofoeze, M.A.; Ukpabi, U.J.; Adiele, J.G.; Sanjeet, K. Quality Characteristic of Kunu Produced from Orange Fleshed Sweetpotato for Empowerment of Rural Women in Nigeria. Nig. Agric. J. 2021, 52, 325–330. [Google Scholar]
- Bintsis, T. Lactic Acid Bacteria: Their Applications in Foods. J. Bacteriol. Mycol. 2018, 5, 1065. [Google Scholar]
- Snigdha, M.M.; Mohanty, D.; Mohapatra, S. Applications of Probiotics as a Functional Ingredient in Food and Gut Health. J. Food Nutr. Res. 2019, 7, 213–223. [Google Scholar]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef]
- Oboh, H.; Obahiagbon, F.; Osagie, A.; Omotosho, A. Glycemic response of some local Nigerian drinks in healthy subjects. Nig. J. Nutr. Sci. 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Taiwo, A.A.; Adeoyegun, J.A.; Ijaola, T.A.; Lawal, O. Comparative study of nutritive composition andmicrobial level of Kunun sold in three campuses in Abeokuta. Sky J. Food Sci. 2017, 6, 7–13. [Google Scholar]
- Omakwu, J. The Preservation Effect of Spices in Kunnu-Samiya. Ph.D. Thesis, Ahmadu Bello University, Zaria, Nigeria, 1980. [Google Scholar]
- Telrandhe, U.B.; Uplanchiwar, V. Phyto-Pharmacological Perspective of Cadaba farinosa forsk. Am. J. Phytomed. Clin. Ther. 2013, 1, 1011–1022. [Google Scholar]
- Rahman, M.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, D.A.; Albokhari, E.J.; Yaradua, S.S.; Abba, A. Comparative Analysis of Chloroplast Genomes of Four Medicinal Capparaceae Species: Genome Structures, Phylogenetic Relationships and Adaptive Evolution. Plants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Awonorin, S.O.; Udeozor, L.O. Chemical properties of tiger nut -soy milk extract. IOSR J. Environ. Sci. Toxicol. Food Tech. 2014, 8, 87–98. [Google Scholar]
- Chevalier, A. The Encyclopeadia of Medicinal Plants; Dorling Kingsley Press: London, UK, 1998; pp. 48–51. [Google Scholar]
- Gambo, A.; Da’u, A. Tiger nut (Cyperus esculentus): Composition, products, uses and health benefits-a review. Bayero J. Pure Appl. Sci. 2014, 7, 56–61. [Google Scholar] [CrossRef]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize-A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- De Caluwé, E.; Halamová, K.; Van Damme, P. Tamarindus indica L.—A review of traditional uses, phytochemistry and pharmacology. Afr. Focus 2010, 23, 53–83. [Google Scholar] [CrossRef]
- Bristone, C.; Ariahu, C.C.; Ikya, J.K.; Eke, M.O. Potentials of Nigerian indigenous food products for addressing nutritional needs of persons in internally displaced persons’ camps (I. D. P. Camps). Acad. J. Food Res. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Kankara, S.S.; Ibrahim, M.H.; Mustafa, M.; Go, R. Ethnobotanical survey of medicinal plants used for traditional maternal healthcare in Katsina state, Nigeria. S. Afr. J. Bot. 2015, 97, 165–175. [Google Scholar] [CrossRef]
- Ojo, O.O.; Nadro, M.S.; Tell, I.O. Protection of rats by extracts of some common Nigerian trees against acetaminophen-induced hepatotoxicity. Afr. J. Biotechnol. 2006, 5, 755–760. [Google Scholar]
- Iwu Maurice, M. Handbook of African Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1993; p. 129. [Google Scholar]
- Murthy, H.N.; Yadav, G.G.; Dewir, Y.H.; Ibrahim, A. Phytochemicals and Biological Activity of Desert Date (Balanites aegyptiaca (L.) Delile). Plants 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Istifanus, M.F.; Agbo, E.B. Nutritional and Health Benefits of Acha (Digitaria exilis) in the Human Diet—A Review. Niger. Food J. 2016, 34, 72–78. [Google Scholar]
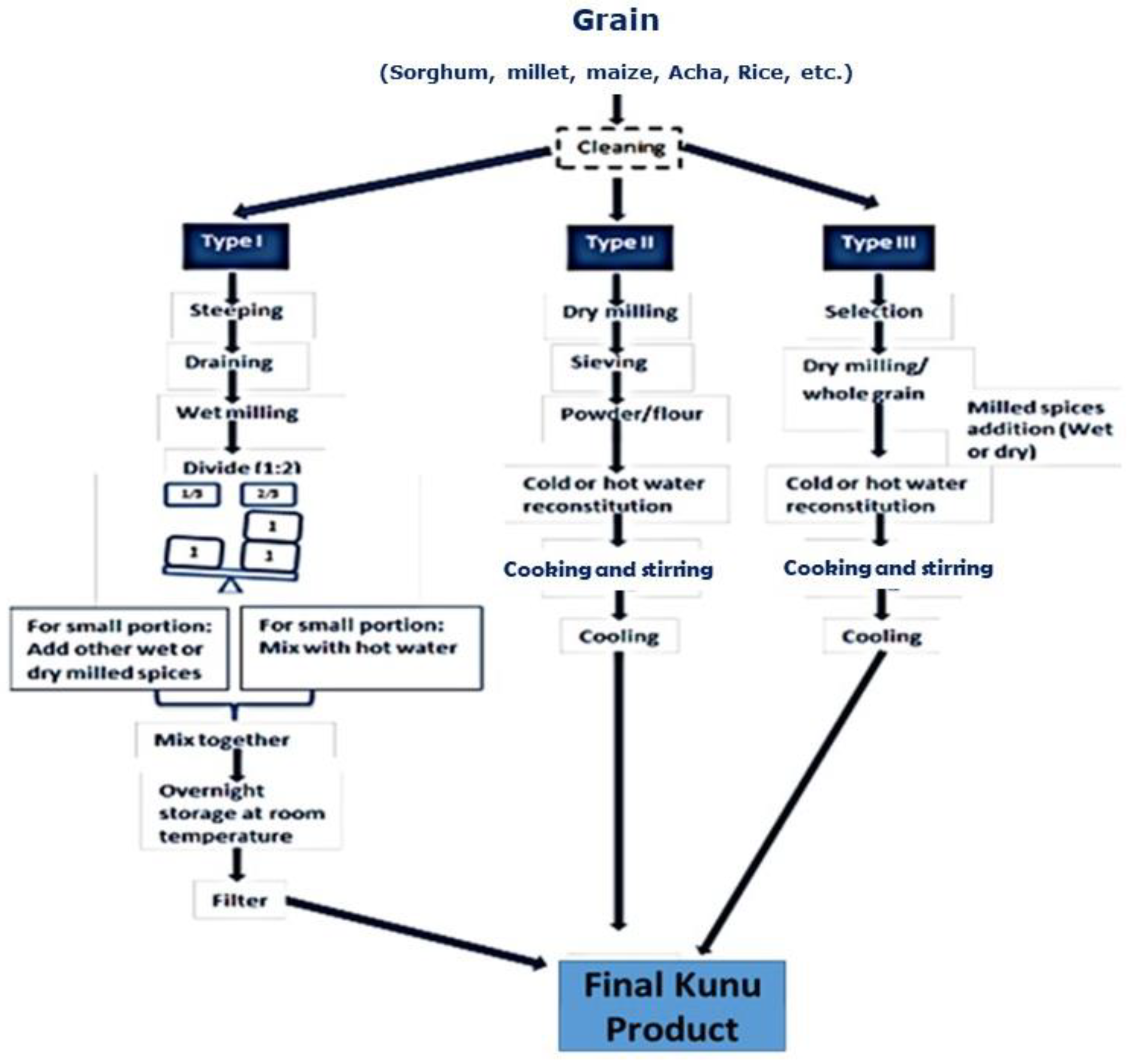

| Kunu Type | Varieties | Used Grains | Sweetener/Additives |
|---|---|---|---|
| Type I | Kunu zaki | Millet, maize or sorghum, or malted rice or sorghum | Ginger, clove, red pepper, sweet potatoes paste or extracts of Cadaba farinosa (dangarafa) |
| Kunu baule | Sorghum or millet | Cadaba farinosa | |
| Kunu jiko | Sorghum, millet, malted cereal | Ginger and roots of certain plants. | |
| Kunu aya | Tiger nut, Sorghum, millet, malted cereal | Ginger, clove, red pepper and roots of certain plants | |
| Type II | Kunu akamu | Maize or millet or sorghum | Ginger and sugar |
| Kunu tsamiya | sorghum or rice | Tsamiya, red pepper and sugar | |
| Kunu bururu | Millet or sorghum | Fresh cow milk, tamari and sugar | |
| Kunu koko | Millet or sorghum | Ginger and pepper | |
| Kunu kanwa | Millet or sorghum | Potash, ginger and pepper | |
| Kunu aduwa | sorghum, millet or maize | Aduwa (Balanites aegyptica) fruit pulp, tamarind and sugar | |
| Type III | Kunu acha | Acha | Tsamiya, milk and sugar |
| Kunu gyada | Millet or sorghum or rice | Ground nut paste, tamarind fruit pulp extract and sugar | |
| Others | Kunu Gayamba | Malted millet | Any available sweetener |
| Kunu Amshau | Maize, sorghum or millet | Any available sweetener |
| Processing Stages/Condition | Nutritional Contribution | References |
|---|---|---|
| Malting | Amino acids, digestible sugars, B-group vitamins, reduced dry matter, starch, anti-nutrients | [58,59] |
| Steeping | Improved flavour and taste | [60] |
| Fermentation | Soluble protein, nitrogen, probiotics | [20,61,62] |
| pH | Probiotics, phytic acids, tannins and other food inhibitors reduction | [33,42] |
| Process Stage | Microbiota | References |
|---|---|---|
| Malting | LAB (Lactobacillus fermentum, Lactobacillus salivarius, Pediococcus sp. Leuconostoc sp. | [130,136] |
| Pre-fermentation (steeping) | Lactobacillus sp., Lactococcus sp., Pediococcus sp., Burkholderia-Caballeronia-Paraburkholderia sp., Saccharomyces sp. Bacillus sp., Enferobacfer aerogenes and E. cloacae | [3,81,85] |
| Post-fermentation | Saccharomyces sp., lactic acid bacteria (LAB) | [3,88,89,90] |
| Kunu Type | Varieties | Health and Nutritional Benefits | References |
|---|---|---|---|
| General | Body tissues development and repair, gallstones prevention heart, breast cancer protective properties with other antioxidant protective properties as millet or sorghum is used | [182] | |
| Type I | Kunu-zaki | Bowels purging and relief of flatulent conditions, enhancement of lactation in nursing mothers. | [3,183] |
| Kunu-baule | Purgative, anthelmintic, anti-syphilitic, emmenagogue, aperients, stimulant, antiscorbutic and antiphlogistic properties | [184,185,186] | |
| Kunu-jiko | NA | NA | |
| Kunu-aya | Prevention of heart attacks, thrombosis and cancer; infants’ teeth and bone development; reduction of body cholesterol and the risk of atherosclerosis | [187,188,189] | |
| Type II | Kunu-akamu | Prevention of rheumatism symptoms, functional improvement of thyroid gland and immune system due to maize fermentation | [190] |
| Kunu-tsamiya | Rich in vitamin C and α-carotene; minerals (particularly P, K, Ca and Mg) with antioxidant, anti-inflammatory, anti-microbial and anti-fungal activity due to tsamiya additive. | [191] | |
| Kunu-bururu | NA | NA | |
| Kunu-koko | Helps in reduction of persistent diarrhoea in young children | [13,192] | |
| Kunu-kanwa | Taste improvement and wound healing enhancement | [193] | |
| Kunu-aduwa | Good for children and people with haemorrhoids, stomach aches, jaundice, yellow fever, syphilis and epilepsy | [194,195,196] | |
| Type III | Kunu-acha | Aids digestion and cardiovascular function, good for diabetics, gluten-free diet, an excellent meal for weight loss | [197] |
| Kunu-gyada | Sustainable alternative with acceptable nutritional profile that could provide mitigation on protein energy malnutrition; tends to improve blood lipid values in general. | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndukwe, J.K.; Aduba, C.C.; Ughamba, K.T.; Chukwu, K.O.; Eze, C.N.; Nwaiwu, O.; Onyeaka, H. Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria. Beverages 2023, 9, 14. https://doi.org/10.3390/beverages9010014
Ndukwe JK, Aduba CC, Ughamba KT, Chukwu KO, Eze CN, Nwaiwu O, Onyeaka H. Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria. Beverages. 2023; 9(1):14. https://doi.org/10.3390/beverages9010014
Chicago/Turabian StyleNdukwe, Johnson K., Claret Chiugo Aduba, Kingsley Tochukwu Ughamba, Kenechi Onyejiaka Chukwu, Chijioke Nwoye Eze, Ogueri Nwaiwu, and Helen Onyeaka. 2023. "Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria" Beverages 9, no. 1: 14. https://doi.org/10.3390/beverages9010014
APA StyleNdukwe, J. K., Aduba, C. C., Ughamba, K. T., Chukwu, K. O., Eze, C. N., Nwaiwu, O., & Onyeaka, H. (2023). Diet Diversification and Priming with Kunu: An Indigenous Probiotic Cereal-Based Non-Alcoholic Beverage in Nigeria. Beverages, 9(1), 14. https://doi.org/10.3390/beverages9010014









