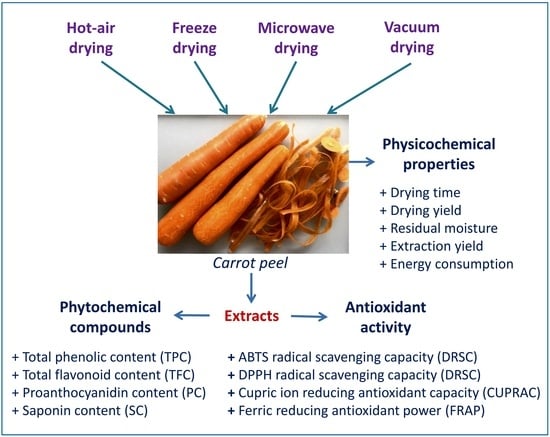
Influence of Various Drying Conditions on Phytochemical Compounds and Antioxidant Activity of Carrot Peel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Dried Carrot Peel
2.3. Preparation of Methanol Extract from Dried Carrot Peel
2.4. Determination of Phytochemical Compounds of Carrot Peel Extracts
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Proanthocyanidin Content (PC)
2.4.4. Saponin Content (SC)
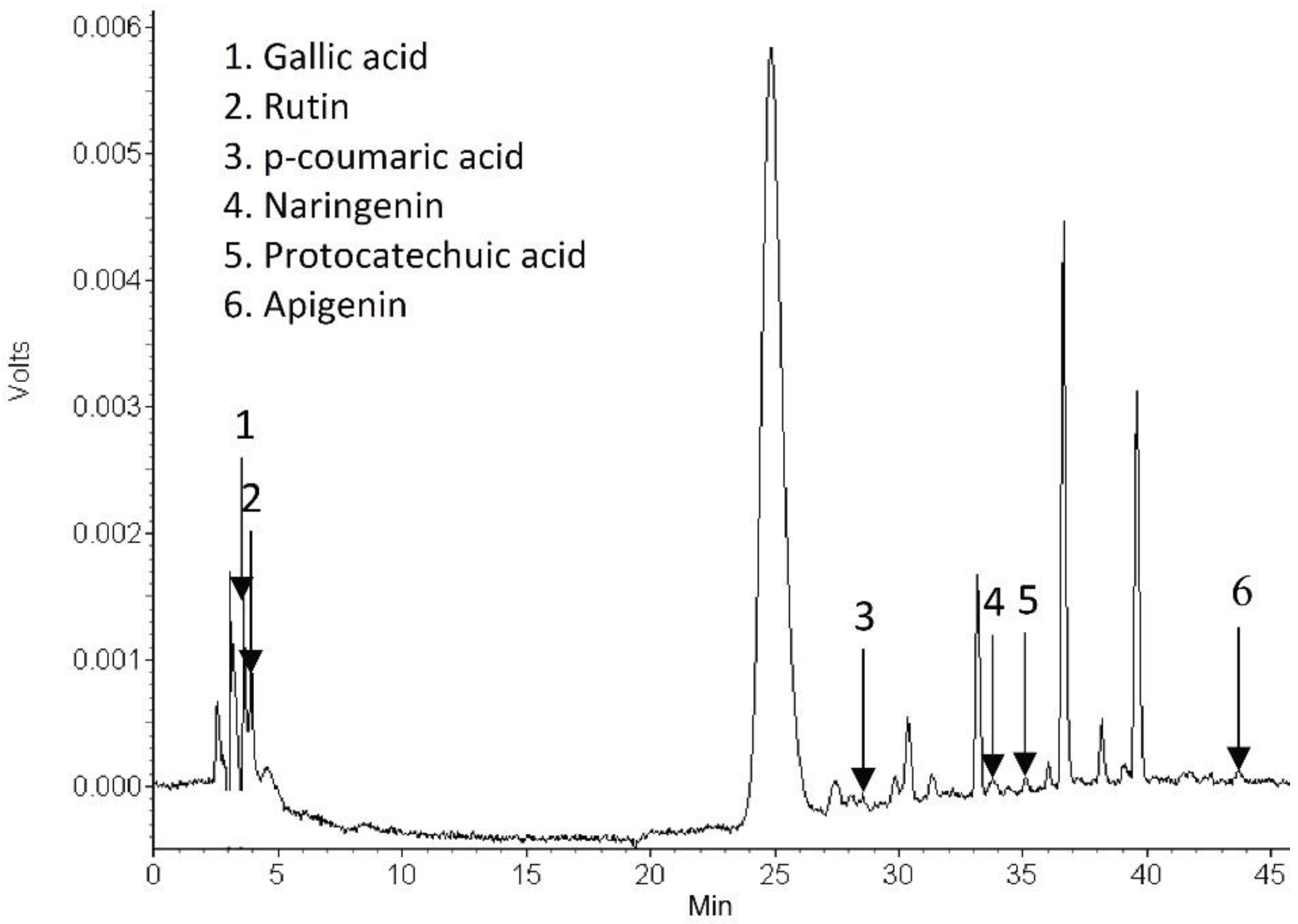
2.4.5. Identification of Phytochemical Compounds in the Dried Carrot Peel
2.5. Determination of Antioxidant Activity of Carrot Peel Extracts
2.5.1. ABTS Radical Scavenging Capacity (ARSC)
2.5.2. DPPH Radical Scavenging Capacity (DRSC)
2.5.3. Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
2.5.4. Ferric Reducing Antioxidant Power (FRAP)
2.6. Statistical Analysis
3. Results
3.1. Effect of Drying Conditions on Physicochemical Properties of Carrot Peel
3.2. Effect of Drying Conditions on Phytochemical Compounds of Carrot Peel
3.2.1. Total Phenolic Content (TPC)
3.2.2. Total Flavonoid Content (TFC)
3.2.3. Proanthocyanidin Content (PC)
3.2.4. Saponin Content (SC)
3.2.5. Phytochemical Compounds in the Dried Carrot Peel
3.3. Effect of Drying Conditions on Antioxidant Activity of Carrot Peel
3.3.1. ABTS Radical Scavenging Capacity (ARSC)
3.3.2. DPPH Radical Scavenging Capacity (DRSC)
3.3.3. Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
3.3.4. Ferric Reducing Antioxidant Power (FRAP)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niamnuy, C.; Charoenchaitrakool, M.; Mayachiew, P.; Devahastin, S. Bioactive compounds and bioactivities of Centella asiatica (L.) urban prepared by different drying methods and conditions. Dry. Technol. 2013, 31, 2007–2015. [Google Scholar] [CrossRef]
- Potisate, Y.; Phoungchandang, S.; Kerr, W.L. The effects of predrying treatments and different drying methods on phytochemical compound retention and drying characteristics of Moringa leaves (Moringa oleifera Lam.). Dry. Technol. 2014, 32, 1970–1985. [Google Scholar] [CrossRef]
- Roknul, A.S.M.; Zhang, M.; Mujumdar, A.S.; Wang, Y. A comparative study of four drying methods on drying time and quality characteristics of stem lettuce slices (Lactuca sativa L.). Dry. Technol. 2014, 32, 657–666. [Google Scholar] [CrossRef]
- Sui, Y.; Yang, J.; Ye, Q.; Li, H.; Wang, H. Infrared, convective, and sequential infrared and convective drying of wine grape pomace. Dry. Technol. 2014, 32, 686–694. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Çam, M.; Hayta, M.; Doğan, M.; Kayacier, A. Bioactive and physicochemical properties of persimmon as affected by drying methods. Dry. Technol. 2014, 32, 258–267. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vuong, Q.V.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Effects of different drying methods on bioactive compound yield and antioxidant capacity of Phyllanthus amarus. Dry. Technol. 2015, 33, 1006–1017. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Nguyen, V.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Bioactive compound yield and antioxidant capacity of Helicteres hirsuta Lour. stem as affected by various solvents and drying methods. J. Food Proc. Preserv. 2016, 41, 1–9. [Google Scholar]
- Sadowska, U.; Kopeć, A.; Kourimska, L.; Zarubova, L.; Kloucek, P. The effect of drying methods on the concentration of compounds in sage and thyme. J. Food Proc. Preserv. 2017, 41, e13286. [Google Scholar] [CrossRef]
- Shitanda, D.; Wanjala, N.V. Effect of different drying methods on the quality of jute (Corchorus olitorius L.). Dry. Technol. 2006, 24, 95–98. [Google Scholar] [CrossRef]
- Wojdylo, A.; Figiel, A.; Oszmianski, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Rabeta, M.S.; Vithyia, M. Effect of different drying methods on the antioxidant properties of Vitex negundo Linn. tea. Int. Food Res. J. 2013, 20, 3171–3176. [Google Scholar]
- Nguyen, V.T.; Pham, N.M.Q.; Vuong, Q.V.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Phytochemical retention and antioxidant capacity of Xao tam phan (Paramignya trimera) root as prepared by different drying methods. Dry. Technol. 2016, 34, 324–334. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Dry. Technol. 2017, 1141–1151. [Google Scholar] [CrossRef]
- Jin, G.Y.; Zhang, M.; Fang, Z.X.; Cui, Z.W.; Song, C.F. Numerical study on spout elevation of a gas-particle spout fluidized bed in microwave-vacuum dryer. J. Food Eng. 2014, 143, 8–16. [Google Scholar] [CrossRef]
- Swasdisevi, T.; Devahastin, S.; Sa-Adchom, P.; Soponronnarit, S. Mathematical modeling of combined far-infrared and vacuum drying banana slice. J. Food Eng. 2009, 92, 100–106. [Google Scholar] [CrossRef]
- Nimmol, C.; Devahastin, S.; Swasdisevi, T.; Soponronnarit, S. Drying of banana slices using combined low-pressure superheated steam and far-infrared radiation. J. Food Eng. 2007, 81, 624–633. [Google Scholar] [CrossRef]
- Naumovski, N. Bioactive composition of plants and plant foods. In Plant Bioactive Compounds for Pancreatic Cancer Prevention and Treatment; Scarlett, C.J., Vuong, Q.V., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 81–115. [Google Scholar]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Doughari, J.H. Phytochemicals—A global perspective of their role in nutrition and health. In Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents; Rao, V., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 1–32. [Google Scholar]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Scarlett, C.J. Mass proportion, bioactive compounds and antioxidant capacity of carrot peel as affected by various solvents. Technologies 2016, 4, 36. [Google Scholar] [CrossRef]
- Nguyen, V.T. Potential, uses and future perspectives of agricultural wastes. In Recovering Bioactive Compounds from Agricultural Wastes; Nguyen, V.T., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2017; pp. 1–32. [Google Scholar]
- Xu, Y.Y.; Zhang, M.; Mujumdar, A.S.; Duan, X.; Jin-Cai, S. A two-stage vacuum freeze and convective air drying method for strawberries. Dry. Technol. 2006, 24, 1019–1023. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Nguyen, V.T.; Sakoff, J.A.; Scarlett, C.J. Physicochemical, antioxidant and cytotoxic properties of Xao tam phan (Paramignya trimera) root extract and its fractions. Chem. Biodivers. 2017, 14, e1600396. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate composition, total phenolic content, and antioxidant activity of Seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef] [PubMed]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Sakoff, J.A.; Scarlett, C.J. Physicochemical properties, antioxidant and cytotoxic activities of crude extracts and fractions from Phyllanthus amarus. Medicines 2017, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Ibtissem, H.S.; Fatma, Z.R.; Iness, B.R.; Soumaya, B.; Ferid, L.; Brahim, M. Total phenolics, flavonoids, and antioxidant activity of Sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioproc. Technol. 2013, 6, 806–817. [Google Scholar]
| Drying Method | Drying Time (h) | Drying Yield (g DS/100 g FS) | Residual Moisture (g water/100 g DS) | Extraction Yield (g DE/100 g DS) | Energy Consumption (kWh) |
|---|---|---|---|---|---|
| HAD50 | 25.0 | 9.60 ± 0.11 d | 6.86 ± 0.10 a | 35.13 ± 1.35 c | 30.0 |
| HAD100 | 5.5 | 11.78 ± 0.34 ab | 5.22 ± 0.22 b | 36.92 ± 2.24 bc | 6.6 |
| VCD50 | 21.0 | 9.94 ± 0.01 d | 7.00 ± 0.02 a | 42.11 ± 2.96 ab | 16.8 |
| VCD100 | 5.0 | 12.75 ± 0.70 a | 3.13 ± 0.46 c | 44.80 ± 2.24 a | 4.0 |
| MWD600 | 0.15 | 11.26 ± 0.45 bc | 5.02 ± 0.31 b | 42.11 ± 0.82 ab | 0.09 |
| MWD1200 | 0.08 | 10.76 ± 0.62 bcd | 4.91 ± 0.12 b | 27.60 ± 1.12 d | 0.1 |
| FD | 70.0 | 10.23 ± 0.23 cd | 6.91 ± 0.28 a | 31.72 ± 3.80 cd | 245.0 |
| Drying Method | TPC (mg GAE/g DS) | TFC (mg RE/g DS) | PC (mg CE/g DS) | SC (mg EE/g DS) |
|---|---|---|---|---|
| HAD50 | 4.80 ± 0.32 cd | 10.81 ± 0.67 c | 1.88 ± 0.16 d | 213.03 ± 21.12 d |
| HAD100 | 2.74 ± 0.26 e | 11.94 ± 0.52 c | 2.06 ± 0.20 d | 222.88 ± 10.94 d |
| VCD50 | 3.77 ± 0.07 de | 12.33 ± 0.73 c | 2.37 ± 0.10 cd | 262.11 ± 12.16 bc |
| VCD100 | 3.11 ± 0.22 de | 13.16 ± 0.92 c | 2.27 ± 0.18 cd | 237.33 ± 1.83 cd |
| MWD600 | 12.54 ± 0.67 b | 20.29 ± 1.36 b | 4.59 ± 0.11 b | 291.23 ± 20.29 b |
| MWD1200 | 23.49 ± 1.73 a | 28.09 ± 2.56 a | 6.89 ± 0.35 a | 353.87 ± 7.55 a |
| FD | 6.00 ± 0.06 c | 17.89 ± 0.32 b | 2.67 ± 0.11 c | 283.58 ± 6.26 b |
| Drying Method | ARSC (mg TE/g DS) | DRSC (mg TE/g DS) | CUPRAC (mg TE/g DS) | FRAP (mg TE/g DS) |
|---|---|---|---|---|
| HAD50 | nd | 17.99 ± 4.26 c | 4.73 ± 0.36 cd | 16.20 ± 0.94 cd |
| HAD100 | nd | nd | 1.88 ± 0.16 e | 9.56 ± 0.33 e |
| VCD50 | nd | 3.51 ± 0.24 d | 2.87 ± 0.04 e | 12.28 ± 1.06 de |
| VCD100 | nd | 1.16 ± 0.04 d | 2.36 ± 0.15 e | 10.77 ± 1.01 de |
| MWD600 | 85.70 ± 3.71 b | 48.33 ± 1.63 b | 12.06 ± 0.49 b | 48.07 ± 2.19 b |
| MWD1200 | 166.35 ± 5.11 a | 97.41 ± 6.90 a | 24.03 ± 3.08 a | 95.19 ± 4.55 a |
| FD | 23.81 ± 5.74 c | 23.74 ± 1.33 c | 6.48 ± 0.16 c | 21.67 ± 1.18 c |
| Correlations * (R2) | ARSC | DRSC | CUPRAC | FRAP |
|---|---|---|---|---|
| TPC | 0.99 | 0.98 | 1.00 | 1.00 |
| TFC | 0.95 | 0.92 | 0.92 | 0.90 |
| PC | 0.98 | 0.93 | 0.96 | 0.98 |
| SC | 0.84 | 0.79 | 0.82 | 0.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.; Le, M.D. Influence of Various Drying Conditions on Phytochemical Compounds and Antioxidant Activity of Carrot Peel. Beverages 2018, 4, 80. https://doi.org/10.3390/beverages4040080
Nguyen VT, Le MD. Influence of Various Drying Conditions on Phytochemical Compounds and Antioxidant Activity of Carrot Peel. Beverages. 2018; 4(4):80. https://doi.org/10.3390/beverages4040080
Chicago/Turabian StyleNguyen, Van Tang, and Minh Duong Le. 2018. "Influence of Various Drying Conditions on Phytochemical Compounds and Antioxidant Activity of Carrot Peel" Beverages 4, no. 4: 80. https://doi.org/10.3390/beverages4040080
APA StyleNguyen, V. T., & Le, M. D. (2018). Influence of Various Drying Conditions on Phytochemical Compounds and Antioxidant Activity of Carrot Peel. Beverages, 4(4), 80. https://doi.org/10.3390/beverages4040080