Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material and Chemicals
2.2. Extraction Process and Fermentation Medium
2.3. Microorganism and Inoculum Preparation
2.4. Fermentation and Distillation Conditions
2.5. Analytical Methods
2.6. Sensory Analysis
3. Results
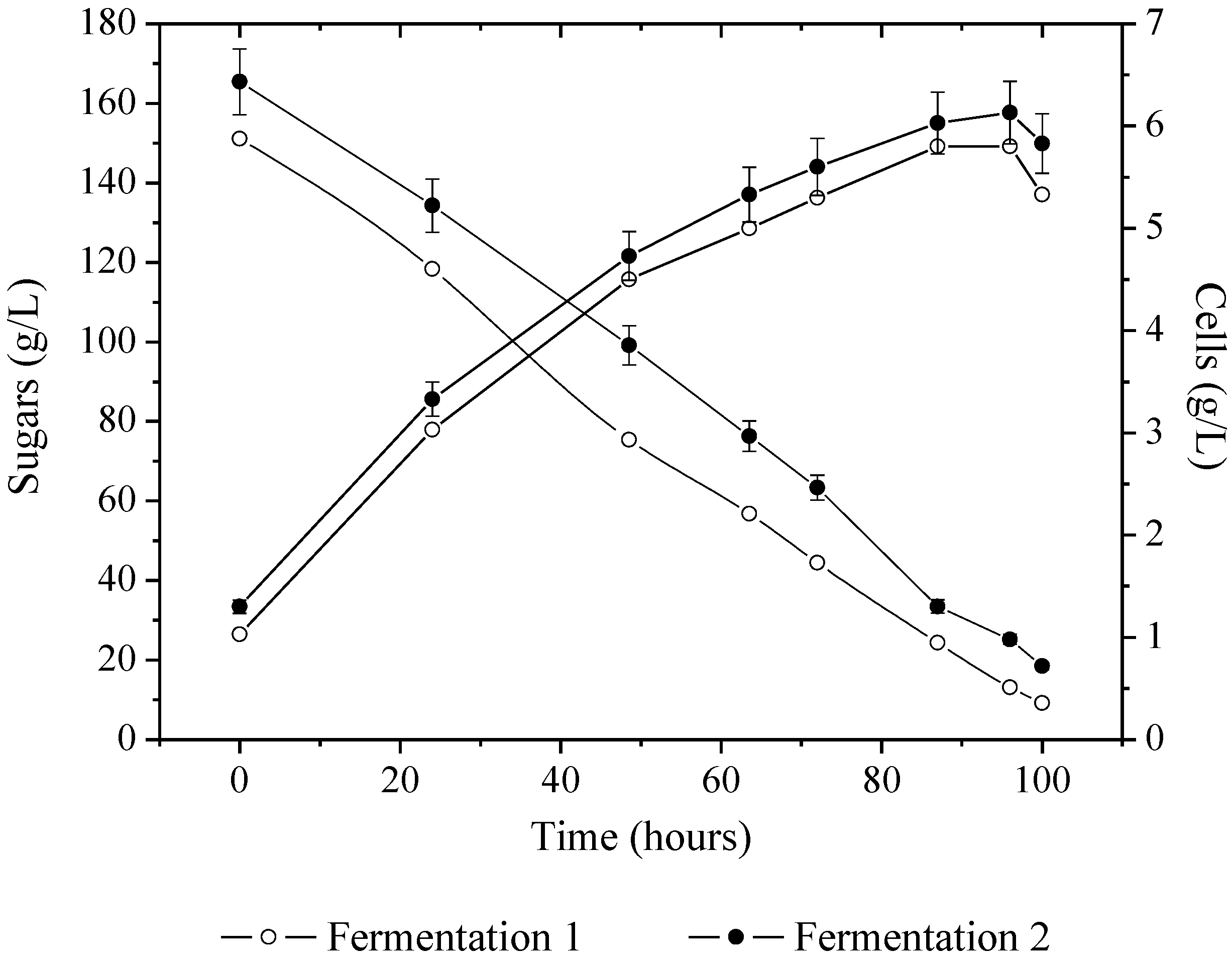
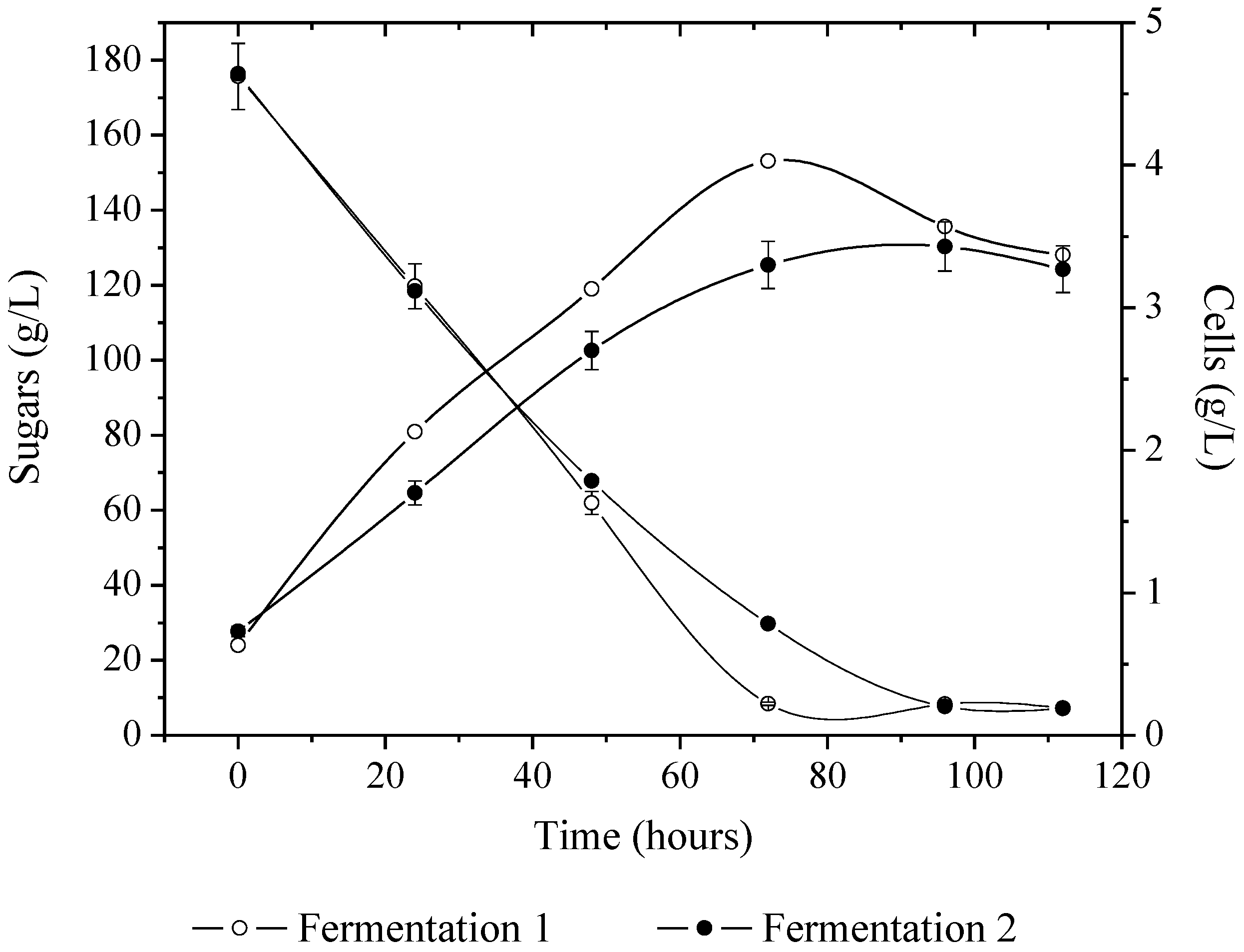
3.1. Beverage Production
3.2. Volatile Composition of Fermented and Spirit Beverages
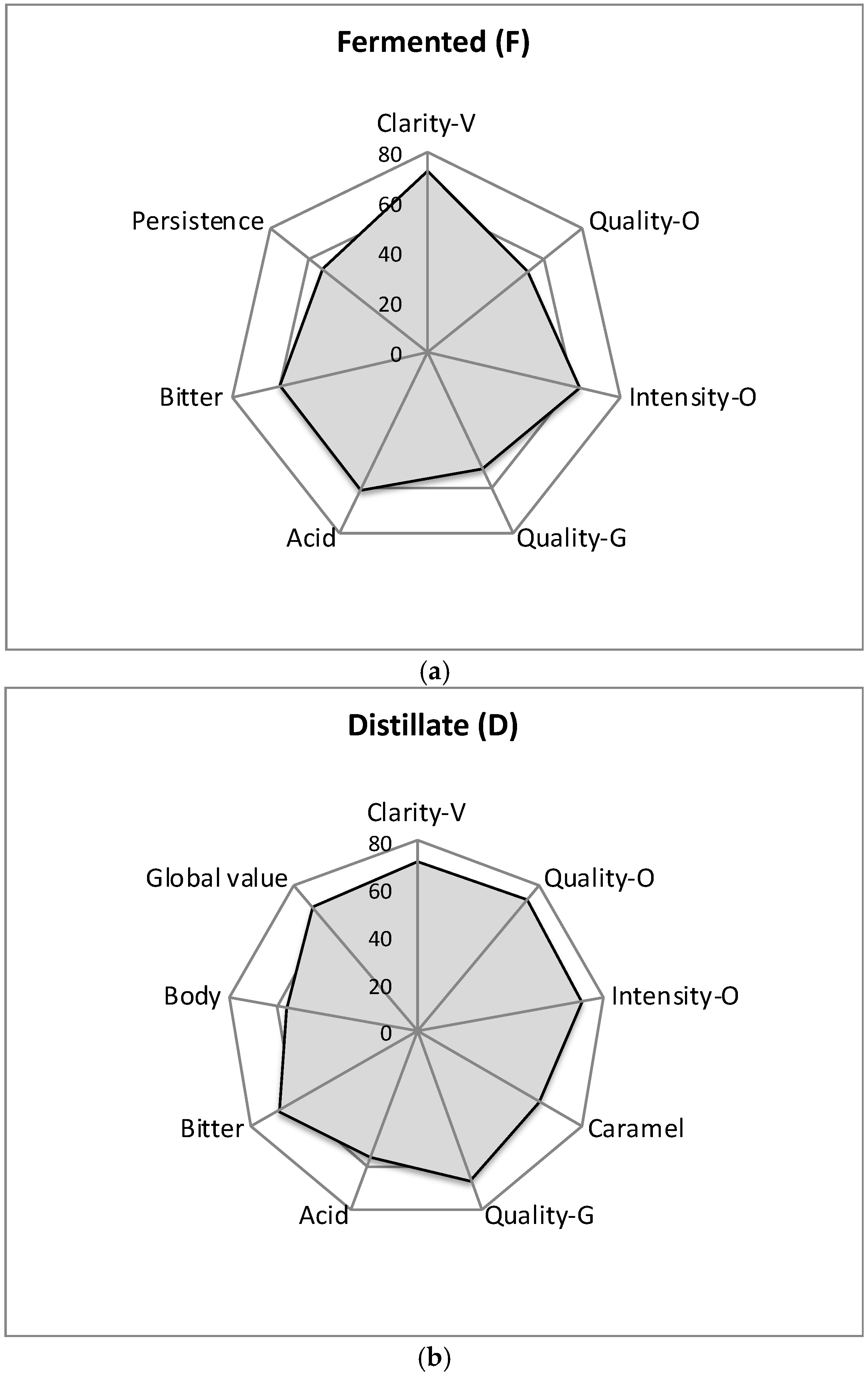
3.3. Sensory Analysis of Fermented and Spirit Beverages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition and application of coffee and its industrial residues. Food Bioprocess. Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carb. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Sampaio, A.; Dragone, G.; Vilanova, M.; Oliveira, J.M.; Teixeira, J.A.; Mussatto, S.I. Production, chemical characterization, and sensory profile of novel spirit elaborated from spent coffee ground. LWT Food Sci. Technol. 2013, 54, 557–563. [Google Scholar] [CrossRef]
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT Food Sci. Technol. 2015, 62, 1043–1052. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave–assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Machado, E.M.S. Extraction of Valuable Compounds from Spent Coffee Grounds and Its Use in the Preparation of Fermented and Distilled Beverages. Ph.D. Dissertation, University of Minho, Braga, Portugal, March 2018. [Google Scholar]
- Plutowska, B.; Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principle and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Machado, E.M.S. Reaproveitamento de Resíduos da Indústria do Café Como Matéria-Prima para a Produção de Etanol. Master’s Dissertation, Universidade do Minho, Braga, Portugal, December 2009. [Google Scholar]
- Dreywood, R. Qualitative test for carbohydrate material. Ind. Eng. Chem. Anal. 1946, 18, 499. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- International Organization for Standardization. Norme ISO 8589—Sensory Analysis: General Guidance for the Design of Test Rooms; International Organization for Standardization: Geneva, Switzerland, 1988. [Google Scholar]
- International Organization for Standardization. Norme ISO 11035—Sensory Analysis: Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- Dravnieks, A.; Bock, F.C.; Powers, J.J.; Tibbetts, M.; Ford, M. Comparison of odours directly and through profiling. Chem. Senses 1978, 2, 191–225. [Google Scholar] [CrossRef]
- Dragone, G.; Mussato, S.I.; Oliveira, J.; Teixeira, J.A. Characterization of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee fermentation and flavor. An intricate and delicate relationship. Food Chem. 2015, 15, 182–191. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.; Andrade Lara, J.M.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Steger, C.L.C.; Lambrechts, M.G. The selection of yeast strains for the production of premium quality South African brandy base products. J. Ind. Microbiol. Biotechnol. 2000, 24, 431–440. [Google Scholar] [CrossRef]
- Apostolopoulou, A.A.; Flouros, A.I.; Demertzis, P.G.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control 2005, 16, 157–164. [Google Scholar] [CrossRef]
- Dragone, G.; Mussato, S.I.; Vilanova, M.; Oliveira, J.; Teixeira, J.A.; Silva, J. Obtenção e caracterização de bebida destilada a partir da fermentação do soro de queijo. Braz. J. Food Technol. 2009, 120–124. Available online: http://bjft.ital.sp.gov.br/edicao_especial.php (accessed on 16 December 2018).
- Silva, M.L.; Malcata, F.X.; de Revel, G. Volatile contents of grape marcs in Portugal. J. Food Comp. Anal. 1996, 9, 72–80. [Google Scholar] [CrossRef]
- Cortés, S.; Gil, M.L.; Fernández, E. Volatile composition of traditional and industrial orujo spirits. Food Control 2005, 16, 383–388. [Google Scholar] [CrossRef]
- Fundira, M.; Blom, M.; Pretorius, I.; van Rensburg, P. Selection of yeast starter culture strains for the production of marula fruit wines and distillates. J. Agric. Food Chem. 2002, 50, 1535–1542. [Google Scholar] [CrossRef]
- Falqué, E.; Fernandez, E.; Dubourdieu, D. Differentiation of white wines by their aromatic index. Talanta 2001, 54, 271–281. [Google Scholar] [CrossRef]
- EUR–Lex. Available online: http://eur-lex.europa.eu/ (accessed on 15 March 2018).
- Turek, I.A. The Effect of Microwave Extraction on the Chemical Composition and the Antioxidant Properties of the Coffee Residue. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, August 2010. [Google Scholar]
- Marais, I. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Mygdalia, A.S.; Natskoulis, P. Characterization and safety evaluation of the traditional Greek fruit distillate “Mouro” by flavor compounds and mineral analysis. Food Chem. 2004, 86, 625–636. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melús, M.A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef]
- Fujimaki, M.; Tsugita, T.; Kurata, T. Fractionation and identification of volatile acids and phenols in the steam distillate of rice bran. J. Agric. Biol. Chem. 1977, 41, 1721–1725. [Google Scholar] [CrossRef]
- Mayer, F.; Czerny, M.; Grosch, W. Sensory study of the character impact aroma compounds of a coffee beverage. Eur. Food Res. Technol. 2000, 211, 272–276. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits—A review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]
- Mangas, J.; Rodríguez, R.; Moreno, J.; Blanco, D. Changes in the major volatile compounds of cider distillates during maturation. LWT Food Sci. Technol. 1996, 29, 357–364. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Chaichia, M.; Ghasemzadeh-Mohammadia, V.; Hashemib, M.; Mohammadia, A. Furanic compounds and furfural in different coffee products by headspace liquid-phase micro-extraction followed by gas chromatography–mass spectrometry: Survey and effect of brewing procedures. Food Addit. Contam. Part B 2015, 8, 73–80. [Google Scholar] [CrossRef]
- Czerny, M.; Mayer, F.; Grosch, W. Sensory study on the character impact odorants of roasted Arabica coffee. J. Agric. Food Chem. 1999, 47, 695–699. [Google Scholar] [CrossRef]
- Kıvançlı, J.; Elmacı, Y. Characterization of Turkish-Style Boiled Coffee Aroma by Gas Chromatography and Mass Spectrometry and Descriptive Analysis Techniques. Int. J. Food Prop. 2016, 19, 1681–1686. [Google Scholar] [CrossRef]
| Compound | F1 | F2 | D1 | D2 | ||||
|---|---|---|---|---|---|---|---|---|
| C/(mg/L) | SD | C/(mg/L) | SD | C/(mg/L) | SD | C/(mg/L) | SD | |
| acetaldehyde | 339.6 | 22.2 | 192.5 | 2.1 | 6.3 | 0.5 | 19.6 | 1.0 |
| ethyl acetate | 73.5 | 1.7 | 72.1 | 11.4 | 7.8 | 1.3 | 18.7 | 2.6 |
| methanol | 30.1 | 1.3 | 44.1 | 1.6 | 14.0 | 1.3 | 7.6 | 1.0 |
| 1-propanol | 15.5 | 0.5 | 17.1 | 0.9 | 23.2 | 1.5 | 35.7 | 1.2 |
| 2-methyl-1-propanol | 152.1 | 1.4 | 231.7 | 6.3 | 49.4 | 2.2 | 222.0 | 6.6 |
| 2-methyl-1-butanol | 40.9 | 0.4 | 36.0 | 0.7 | 31.5 | 0.3 | 137.1 | 4.0 |
| 3-methyl-1-butanol | 106.8 | 2.0 | 97.1 | 2.5 | 191.1 | 4.2 | 633.2 | 19.2 |
| 2-phenylethanol | 34.7 | 4.6 | 38.2 | 4.4 | 35.6 | 3.8 | 21.5 | 2.1 |
| Compound | F1 | F2 | D1 | D2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LRI | C/(µg/L) | SD | C/(µg/L) | SD | C/(µg/L) | SD | C/(µg/L) | SD | |
| ethyl butyrate | 995 | 10.3 | 0.8 | 3.9 | 0.8 | tr | - | tr | - |
| ethyl 2-methylbutyrate | 1052 | - | - | - | - | 17.0 | 2.4 | 33.7 | 3.9 |
| ethyl 3-methylbutyrate | 1070 | - | - | - | - | 6.5 | 2.8 | 28.6 | 3.6 |
| 3-methylbutyl acetate | 1119 | 38.1 | 1.9 | 33.8 | 2.8 | - | - | 35.2 | 3.6 |
| ethyl hexanoate | 1229 | 42.9 | 1.9 | 20.2 | 1.6 | 57.9 | 1.3 | 156.9 | 10.1 |
| 1-pentanol | 1239 | 21.0 | 1.2 | 16.9 | 0.7 | 19.6 | 2.4 | 37.9 | 1.7 |
| 2-methylpyrazine | 1255 | - | - | - | - | 13.8 | 0.6 | 13.0 | 0.8 |
| 2,6-dimethylpyrazine | 1318 | - | - | - | - | 36.5 | 3.9 | 42.9 | 2.0 |
| 2-ethylpyrazine | 1324 | - | - | - | - | 6.6 | 1.2 | 7.2 | 0.3 |
| 2,3-dimethylpyrazine | 1334 | - | - | - | - | 8.3 | 2.1 | 4.7 | 0.1 |
| ethyl lactate | 1335 | 21.6 | 0.3 | 103.1 | 9.4 | 54.1 | 3.6 | 45.9 | 5.6 |
| 1-hexanol | 1344 | 23.9 | 0.9 | 19.3 | 2.6 | 10.5 | 1.5 | 44.1 | 4.5 |
| ethyl octanoate | 1429 | 13.2 | 1.5 | - | - | 239.4 | 11.1 | 698.0 | 88.7 |
| furan linalool oxide, trans- | 1434 | - | - | - | - | tr | - | 4.8 | 0.6 |
| 1-heptanol | 1448 | 4.2 | 0.8 | 4.0 | 0.5 | 1.3 | 0.6 | 11.7 | 0.5 |
| furfural | 1457 | 58.4 | 3.0 | 54.7 | 3.2 | 3054.9 | 187.5 | 2853.8 | 262.5 |
| 2-ethyl-1-hexanol | 1483 | 11.6 | 1.6 | 15.7 | 1.2 | - | - | 6.0 | 0.5 |
| benzaldehyde | 1511 | 7.2 | 6.3 | 11.1 | 0.9 | 80.3 | 6.1 | 315.3 | 21.8 |
| furfuryl acetate | 1532 | 9.1 | 1.3 | 5.2 | 0.4 | - | - | tr | - |
| linalool | 1542 | - | - | - | - | tr | - | 22.3 | 0.9 |
| propanoic acid | 1545 | 6.1 | 0.7 | 6.4 | 0.8 | - | - | tr | - |
| 5-methylfurfural | 1564 | 5.4 | 0.7 | 4.7 | 0.9 | 732.1 | 36.0 | 474.3 | 41.9 |
| 2-methylpropanoic acid | 1574 | 277.5 | 32.9 | 364.2 | 19.5 | 122.5 | 7.9 | 182.5 | 22.0 |
| ethyl decanoate | 1632 | - | - | - | - | 17.8 | 1.9 | 324.1 | 47.6 |
| 2-furanmethanol | 1653 | 308.4 | 42.3 | 329.5 | 9.8 | 156.0 | 9.8 | 89.3 | 14.6 |
| diethyl succinate | 1668 | 16.7 | 1.8 | 19.9 | 1.7 | 146.8 | 9.9 | 722.8 | 76.0 |
| 2-methylbutyric + 3-methylbutyric acids | 1675 | 187.0 | 8.1 | 175.0 | 6.7 | 534.6 | 34.4 | 564.3 | 76.4 |
| γ-caprolactone | 1685 | 17.5 | 1.4 | 18.5 | 0.7 | 9.0 | 0.5 | 3.3 | 0.8 |
| methionol | 1705 | 26.0 | 2.0 | 19.3 | 0.5 | - | - | - | - |
| citronellol | 1759 | 8.1 | 0.6 | 2.4 | 0.2 | - | - | 12.8 | 1.3 |
| ethyl phenylacetate | 1774 | 3.1 | 0.9 | 7.1 | 1.0 | 8.4 | 2.0 | 16.3 | 2.4 |
| nerol | 1790 | 2.7 | 0.5 | 1.0 | 0.1 | - | - | tr | - |
| 2-phenylethyl acetate | 1801 | - | - | - | - | 104.7 | 5.6 | 126.4 | 14.3 |
| β-damascenone | 1804 | - | - | - | - | - | - | tr | - |
| hexanoic acid | 1850 | 239.2 | 23.1 | 203.0 | 2.7 | 453.0 | 23.6 | 425.4 | 32.9 |
| guaiacol | 1851 | 31.5 | 3.1 | 33.5 | 1.4 | 138.8 | 6.2 | 89.9 | 9.8 |
| benzyl alcohol | 1862 | 9.7 | 0.1 | 19.2 | 1.8 | 7.0 | 0.4 | 4.1 | 0.2 |
| γ-nonalactone | 2009 | 67.0 | 2.8 | 62.0 | 3.3 | 168.7 | 9.6 | 106.2 | 11.1 |
| 4-ethylguaiacol | 2017 | 5.1 | 1.3 | 4.9 | 0.5 | 174.5 | 6.1 | 106.5 | 8.6 |
| nerolidol, trans- | 2034 | - | - | - | - | 86.3 | 9.7 | 138.6 | 21.7 |
| octanoic acid | 2065 | 246.0 | 6.5 | 168.0 | 5.1 | 5614.9 | 207.8 | 4179.0 | 348.5 |
| γ-decalactone | 2122 | 6.9 | 0.1 | 3.7 | 0.1 | 28.7 | 1.0 | 27.8 | 3.4 |
| 4-vinylguaiacol | 2181 | 42.9 | 3.5 | 24.6 | 1.9 | 224.1 | 6.3 | 343.5 | 37.9 |
| γ-undecalactone | 2237 | - | - | - | - | 9.2 | 1.1 | 6.8 | 0.9 |
| decanoic acid | 2279 | 7.1 | 1.6 | 2.1 | 0.5 | 2995.4 | 211.8 | 2535.4 | 193.9 |
| E,E-farnesol | 2344 | - | - | - | - | 54.2 | 9.4 | 133.3 | 15.8 |
| dodecanoic acid | 2492 | - | - | - | - | 39.3 | 4.4 | 27.9 | 3.9 |
| 5-hydroxymethylfurfural | 2494 | - | - | - | - | 26.7 | 1.8 | 80.3 | 9.2 |
| 3-hydroxyl-β-damascone | 2513 | 6.3 | 0.5 | 4.5 | 0.3 | - | - | 4.3 | 0.1 |
| vanillin | 2543 | 12.8 | 2.4 | 11.6 | 1.3 | 25.4 | 2.2 | 48.4 | 5.1 |
| acetovanillone | 2615 | 41.8 | 4.3 | 45.0 | 1.9 | 26.5 | 0.7 | 20.3 | 3.6 |
| tyrosol | 2989 | 18.4 | 5.0 | 16.9 | 2.1 | - | - | - | - |
| Phases | Descriptor | Fermented (F) | Distillated (D) | ||||
|---|---|---|---|---|---|---|---|
| I/% | F/% | GM/% | I/% | F/% | GM/% | ||
| Visual | Clarity | 53 | 100 | 73 | 51 | 100 | 71 |
| Olfactory | Quality | 27 | 100 | 52 | 53 | 100 | 73 |
| Intensity | 40 | 100 | 63 | 51 | 100 | 71 | |
| Toasted | 13 | 20 | 16 | 0 | 0 | 0 | |
| Caramel | 0 | 0 | 0 | 44 | 80 | 60 | |
| Vanilla | 0 | 0 | 0 | 20 | 40 | 28 | |
| Strawberry | 9 | 20 | 13 | 13 | 20 | 16 | |
| Coffee | 0 | 0 | 0 | 20 | 40 | 28 | |
| Apple | 9 | 20 | 13 | 9 | 20 | 13 | |
| Gustatory | Quality | 27 | 100 | 52 | 44 | 100 | 67 |
| Sweet | 13 | 60 | 28 | 18 | 80 | 38 | |
| Salt | 16 | 60 | 31 | 22 | 80 | 42 | |
| Acid | 38 | 100 | 61 | 31 | 100 | 56 | |
| Bitter | 44 | 80 | 60 | 44 | 100 | 67 | |
| Body | 9 | 40 | 19 | 38 | 80 | 55 | |
| Persistence | 36 | 80 | 53 | 36 | 60 | 46 | |
| Astringent | 18 | 60 | 33 | 29 | 60 | 42 | |
| Global Value | 20 | 80 | 40 | 47 | 100 | 68 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, E.; Mussatto, S.I.; Teixeira, J.; Vilanova, M.; Oliveira, J. Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages. Beverages 2018, 4, 105. https://doi.org/10.3390/beverages4040105
Machado E, Mussatto SI, Teixeira J, Vilanova M, Oliveira J. Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages. Beverages. 2018; 4(4):105. https://doi.org/10.3390/beverages4040105
Chicago/Turabian StyleMachado, Ercília, Solange I. Mussatto, José Teixeira, Mar Vilanova, and José Oliveira. 2018. "Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages" Beverages 4, no. 4: 105. https://doi.org/10.3390/beverages4040105
APA StyleMachado, E., Mussatto, S. I., Teixeira, J., Vilanova, M., & Oliveira, J. (2018). Increasing the Sustainability of the Coffee Agro-Industry: Spent Coffee Grounds as a Source of New Beverages. Beverages, 4(4), 105. https://doi.org/10.3390/beverages4040105








