Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods for Metagenomic Analysis
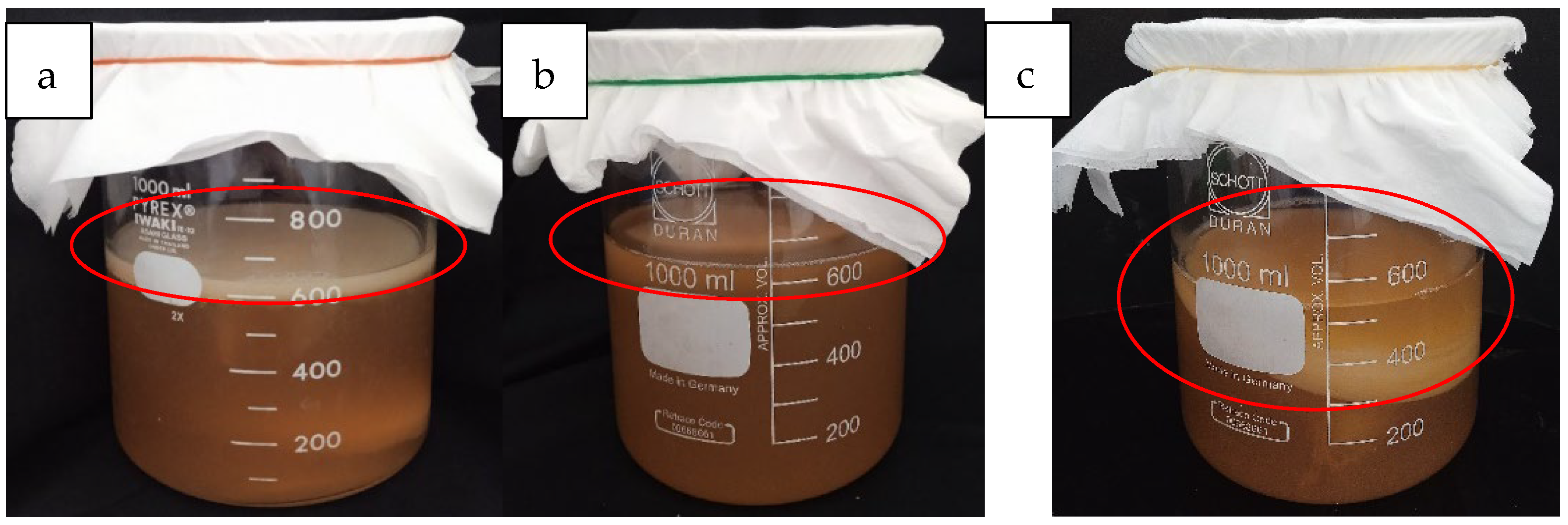
2.1.1. Preparation of Sea Grape Kombucha
2.1.2. Sea Grape Kombucha pH Value Analysis
2.1.3. Sea Grape Kombucha Sugar Content Test
2.1.4. Metagenome DNA Extraction
2.1.5. Sequencing of Bacterial 16S rRNA Genes from Kombucha and Bioinformatics Analysis
2.1.6. Initial 16S rRNA Data Processing and ASV Construction
2.1.7. Functional Profiling with PICRUST V3.4
2.2. Methods for Metabolomic Analysis
2.2.1. Preparation of Sea Grapes
2.2.2. Preparation of Sea Grape Infusion
2.2.3. Preparation of Sea Grape Kombucha for Metabolomic Analysis
2.2.4. Sea Grape Kombucha pH Value Test
2.2.5. Sea Grape Kombucha Soluble Solid Content Test
2.2.6. Determination of Vitamin C Content
2.2.7. Determination of Total Tannin Content
2.2.8. Determination of Total Flavonoid Content
2.2.9. Antioxidant Activity
2.2.10. Statistical Analysis
2.2.11. Screening GC-MS/MS
3. Results and Discussion
3.1. Metagenomic and Fermentation Time Analysis
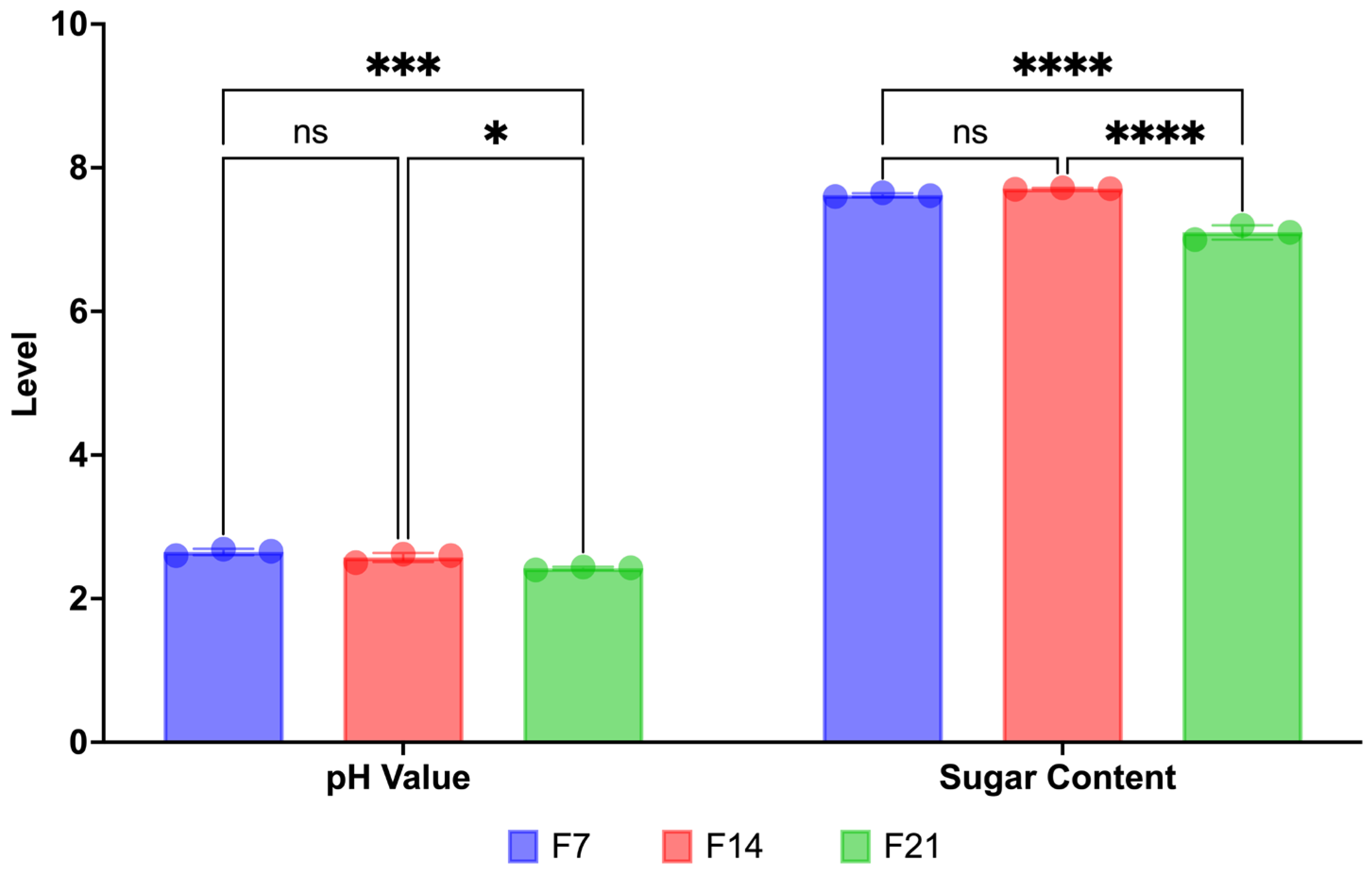
3.1.1. pH Value and Sugar Content of Sea Grape Kombucha
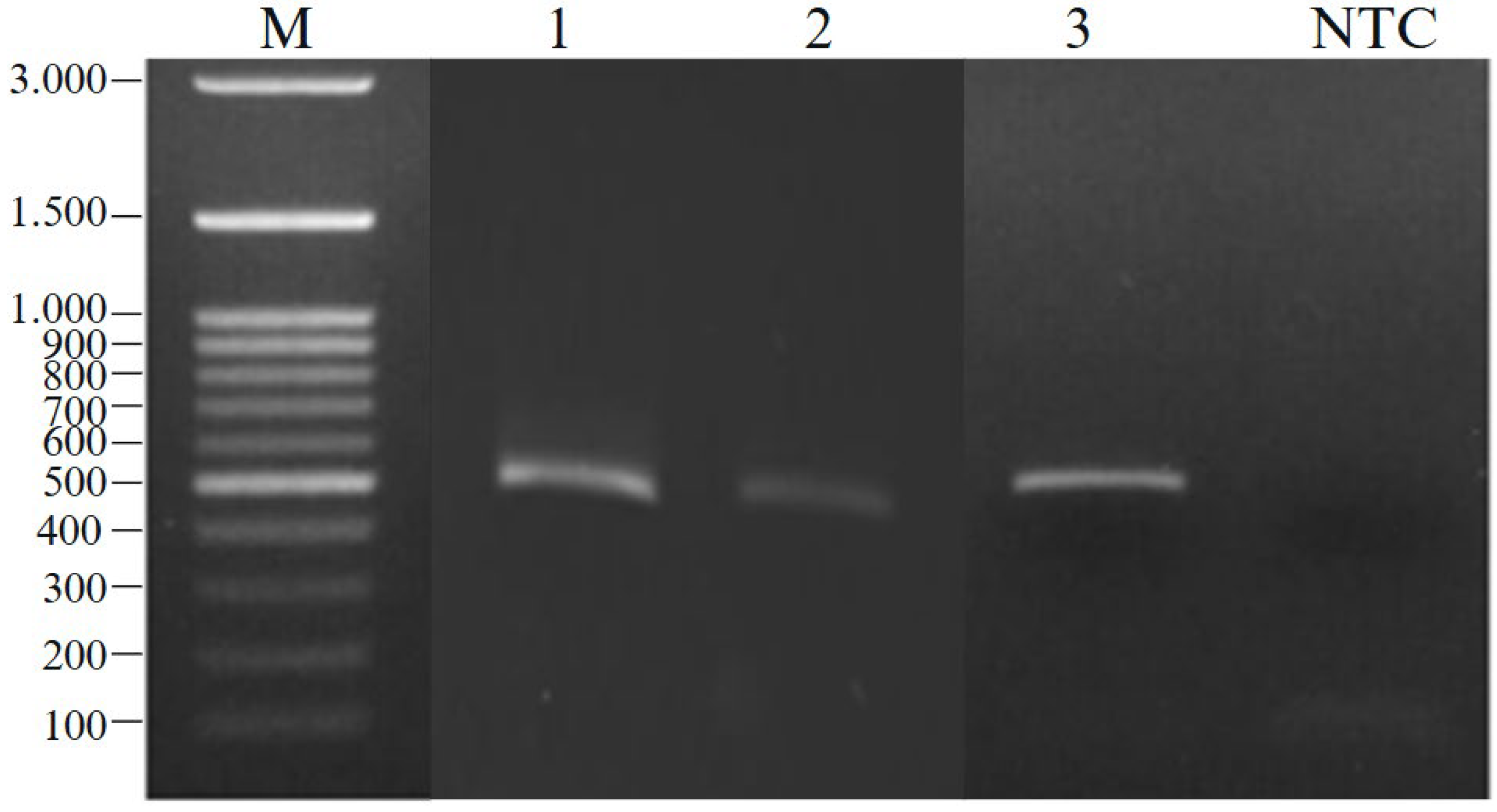
3.1.2. Bacteria’s DNA Metagenome Extraction from Sea Grape Kombucha Fermentation
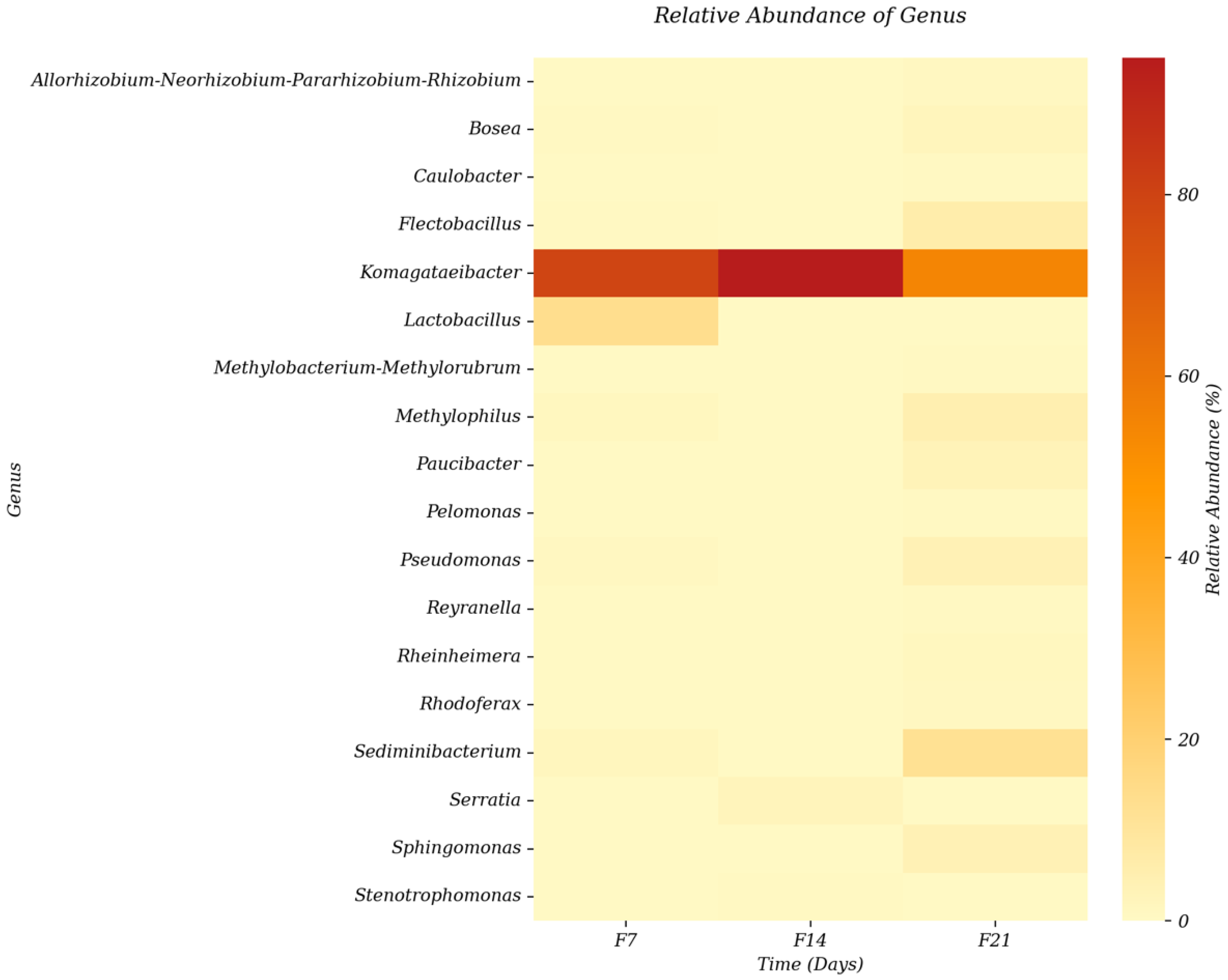
3.1.3. Relative Abundance of Genus from Sea Grape Kombucha
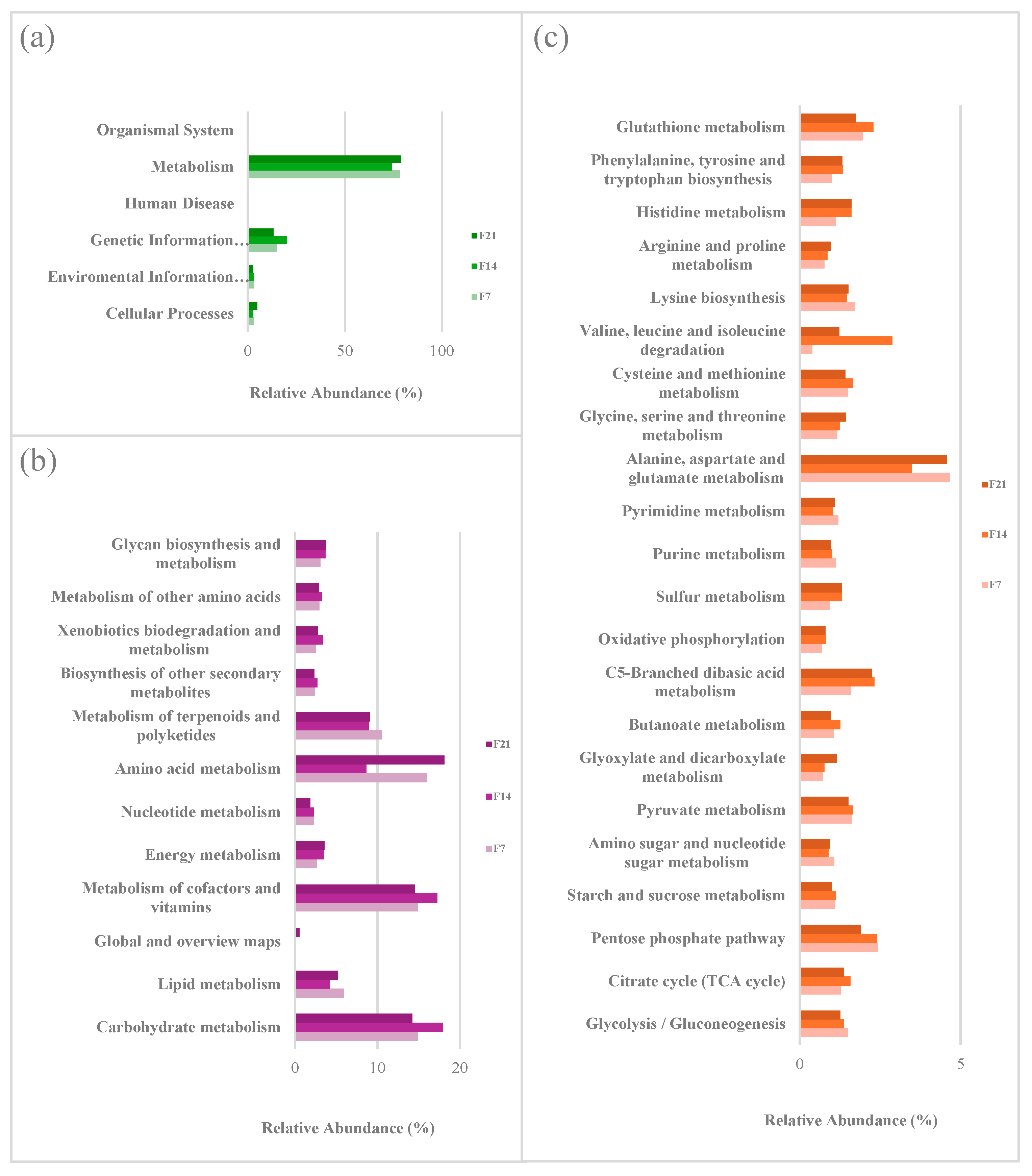
3.1.4. Functional Annotation of Taxa in Sea Grape Kombucha
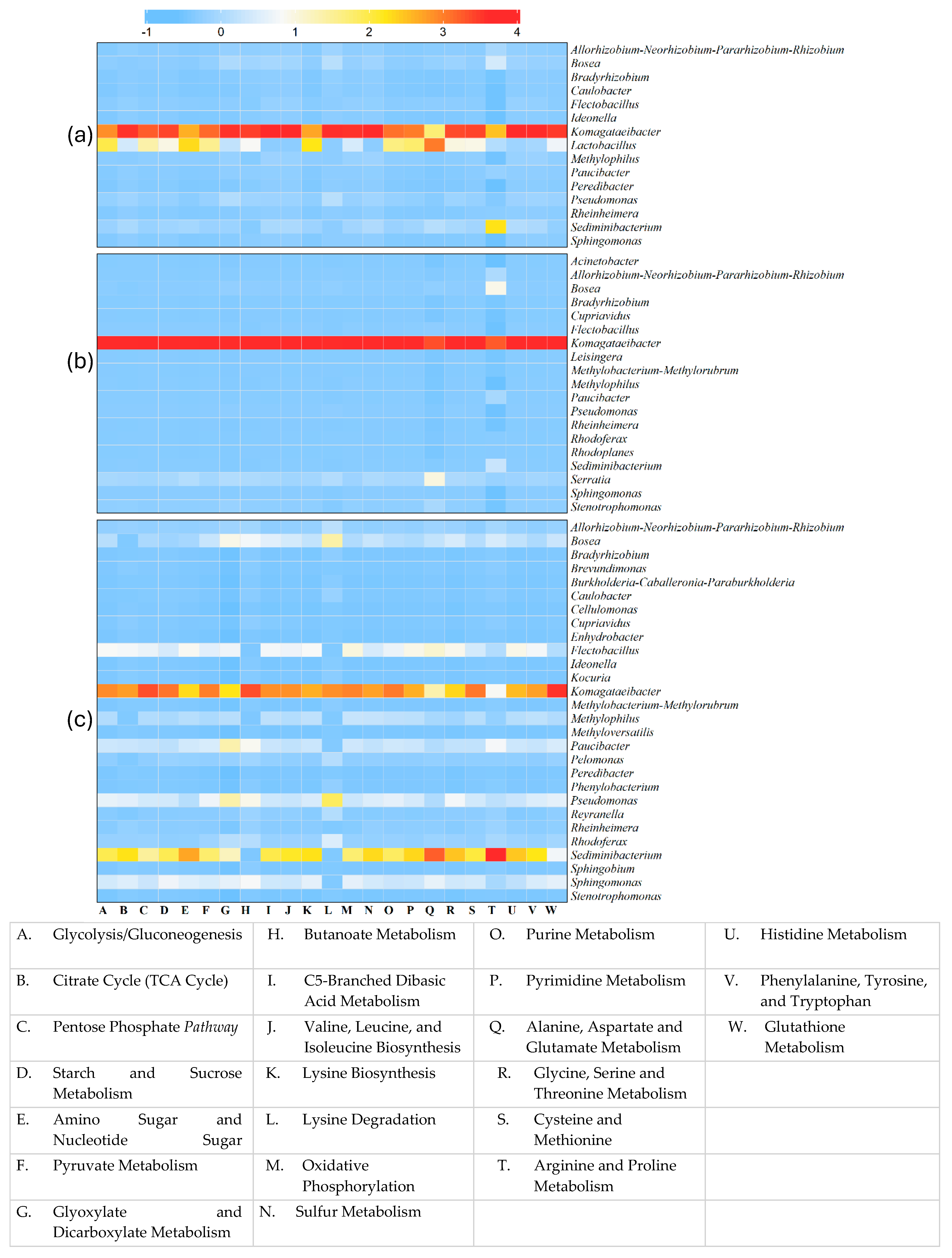
3.1.5. Correlation Analysis Between Metabolic Pathways and Bacterial Communities in Sea Grape Kombucha
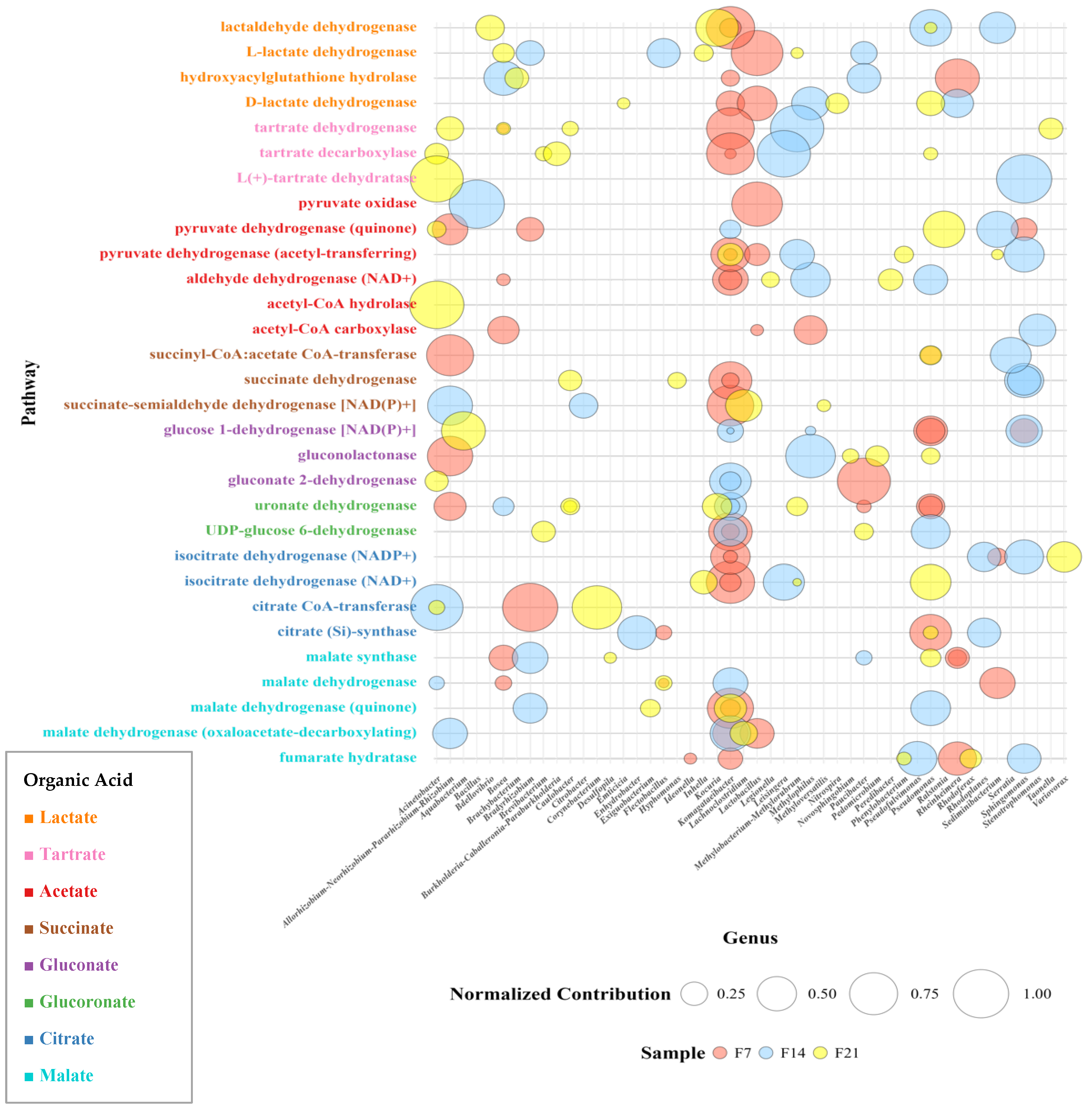
3.1.6. Correlation Data Between Organic Acid-Related Enzymatic Pathways and Bacterial Community Composition in Sea Grape Kombucha
3.2. Metabolomic Analysis
3.2.1. Physicochemical Characteristics of Sea Grape Kombucha
3.2.2. pH Value
3.2.3. Soluble Solid Content
3.2.4. Vitamin C Content
3.2.5. Total Tannin Content
3.2.6. Total Flavonoid Content
3.2.7. Antioxidant Activity of Sea Grape Kombucha
3.2.8. Bioactive Compound of Sea Grape Kombucha
Fatty Acids and Their Ester Compounds
Polyhydroxy Alcohol and Polyol
Terpenoid (Monoterpene and Diterpene)
Phytosterol dan Steroid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in Cultivation, Wastewater Treatment Application, Bioactive Components of Caulerpa Lentillifera and Their Biotechnological Applications. PeerJ 2019, 7, e6118. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, D.-Q.; Liang, T.-J.; Li, J.; Zhang, H.-Y.; Liu, A.-H.; Guo, Y.-W.; Mao, S.-C. Bioactive Constituents from the Green Alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Verlaque, M.; Durand, C.; Huisman, J.M.; Boudouresque, C.-F.; Le Parco, Y. On the Identity and Origin of the Mediterranean Invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur. J. Phycol. 2003, 38, 325–339. [Google Scholar] [CrossRef]
- Kuswari, M.; Nurkolis, F.; Mayulu, N.; Ibrahim, F.M.; Taslim, N.A.; Wewengkang, D.S.; Sabrina, N.; Arifin, G.R.; Mantik, K.E.K.; Bahar, M.R.; et al. Sea Grapes Extract Improves Blood Glucose, Total Cholesterol, and PGC-1α in Rats Fed on Cholesterol- and Fat-Enriched Diet. F1000Research 2021, 10, 718. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Firani, N.K.; Prijadi, B.; Irnandi, D.F.; Riawan, W.; Yusuf, M.; Amar, N.; Chandra, L.A.; Yusuf, V.M.; Subali, A.D.; et al. Kombucha Drink Enriched with Sea Grapes (Caulerpa racemosa) as a Potential Functional Beverage to Contrast Obesity: An in Vivo and in Vitro Approach. Clin. Nutr. ESPEN 2022, 49, 232–240. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Nurkolis, F.; Hardinsyah, H.; Taslim, N.A.; Sabrina, N.; Ibrahim, F.M.; Visnu, J.; Kumalawati, D.A.; Febriana, S.A.; Sudargo, T.; et al. Metabolomic Assay, Computational Screening, and Pharmacological Evaluation of Caulerpa racemosa as an Anti-Obesity with Anti-Aging by Altering Lipid Profile and Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-α Levels. Front. Nutr. 2022, 9, 939073. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Nurkolis, F.; Augusta, P.S.; Mayulu, N.; Kuswari, M.; Taslim, N.A.; Wewengkang, D.S.; Batubara, S.C.; Ben Gunawan, W. Kombucha Tea from Seagrapes (Caulerpa racemosa) Potential as a Functional Anti-Ageing Food: In Vitro and in Vivo Study. Heliyon 2021, 7, e07944. [Google Scholar] [CrossRef]
- La China, S.; De Vero, L.; Anguluri, K.; Brugnoli, M.; Mamlouk, D.; Gullo, M. Kombucha Tea as a Reservoir of Cellulose-Producing Bacteria: Assessing Diversity among Komagataeibacter Isolates. Appl. Sci. 2021, 11, 1595. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-Level Functional Profiling of Metagenomes and Metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef]
- Jakubczyk, K.J.; Piotrowska, G.; Janda, K. Characteristics and Biochemical Composition of Kombucha—Fermented Tea. Med. Ogólna Nauk. Zdrowiu 2020, 26, 94–96. [Google Scholar] [CrossRef]
- Matchado, M.S.; Rühlemann, M.; Reitmeier, S.; Kacprowski, T.; Frost, F.; Haller, D.; Baumbach, J.; List, M. On the Limits of 16S RRNA Gene-Based Metagenome Prediction and Functional Profiling. Microb. Genom. 2024, 10, 001203. [Google Scholar] [CrossRef]
- Jagadeesan, B.; Gerner-Smidt, P.; Allard, M.W.; Leuillet, S.; Winkler, A.; Xiao, Y.; Chaffron, S.; Van Der Vossen, J.; Tang, S.; Katase, M.; et al. The Use of Next-Generation Sequencing for Improving Food Safety: Translation into Practice. Food Microbiol. 2019, 79, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Augusta, P.S.; Nurkolis, F.; Noor, S.L.; Permatasari, H.K.; Hardinsyah; Taslim, N.A.; Batubara, S.C.; Mayulu, N.; Wewengkang, D.S.; Rotinsulu, H. Probiotic Beverage: The Potential of Anti-Diabetes within Kombucha Tea Made from Sea Grapes (Ceulerpa racemosa) Containing High Antioxidant and Polyphenol Total. Proc. Nutr. Soc. 2021, 80, E149. [Google Scholar] [CrossRef]
- Puspitasari, Y.; Palupi, R.; Nurikasari, M. Analisis Kandungan Vitamin C Teh Kombucha Berdasarkan Lama Fermentasi Sebagai Alternatif Minuman Untuk Antioksidan. Glob. Health Sci. 2017, 2, 245–253. Available online: https://jurnal.csdforum.com/index.php/GHS/article/view/137 (accessed on 30 August 2025). [CrossRef]
- Løvdal, T.; Lunestad, B.T.; Myrmel, M.; Rosnes, J.T.; Skipnes, D. Microbiological Food Safety of Seaweeds. Foods 2021, 10, 2719. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Xu, R.; Tian, J.; Li, T.; Chen, S. Comparative Analysis of the Nutrient Composition of Caulerpa Lentillifera from Various Cultivation Sites. Foods 2025, 14, 474. [Google Scholar] [CrossRef]
- Dhakal, S.; Pandey, D.; van der Heide, M.E.; Nørgaard, J.V.; Vrhovsek, U.; Khanal, P. Effect of Different Drying Methods on the Nutritional Composition and Phenolic Compounds of the Brown Macroalga, Fucus Vesiculosus (Fucales, Phaeophyceae). J. Appl. Phycol. 2024, 36, 3649–3663. [Google Scholar] [CrossRef]
- Khaerah, A.; Halijah, H.; Nawir, N. Perbandingan Total Mikroba Kombucha Dengan Variasi Jenis Teh Dan Lama Fermentasi. Bionature 2020, 21, 26–34. [Google Scholar] [CrossRef]
- Azizah, N.; Al-Barrii, A.N.; Mulyani, S. Pengaruh Lama Fermentasi Terhadap Kadar Alkohol, pH, Dan Produksi Gas Pada Proses Fermentasi Bioetanol Dari Whey Dengan Substitusi Kulit Nanas. J. Apl. Teknol. Pangan 2012, 1, 72–77. [Google Scholar]
- Guerra, V.; Beule, L.; Lehtsaar, E.; Liao, H.-L.; Karlovsky, P. Improved Protocol for DNA Extraction from Subsoils Using Phosphate Lysis Buffer. Microorganisms 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Chen, A.; Li, S.; Tao, R.; Chen, K.; Huang, P.; Li, L.; Huang, J.; Li, C.; et al. Comparison of the Full-Length Sequence and Sub-Regions of 16S RRNA Gene for Skin Microbiome Profiling. mSystems 2024, 9, e0039924. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Edgar, R.C.; Flyvbjerg, H. Error Filtering, Pair Assembly and Error Correction for Next-Generation Sequencing Reads. Bioinformatics 2015, 31, 3476–3482. [Google Scholar] [CrossRef]
- Czech, L.; Barbera, P.; Stamatakis, A. Genesis and Gappa: Processing, Analyzing and Visualizing Phylogenetic (Placement) Data. Bioinformatics 2020, 36, 3263–3265. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Siagian, K.D.; Lantang, D.; Dirgantara, S.; Simaremare, E.S. Uji Aktivitas Antifungi Anggur Laut (Caulerpa sp.) Asal Pulau Ambai Serui Terhadap Fungi Candida Krusei Dan Candida Albicans. Pharmacy 2018, 15, 16. [Google Scholar] [CrossRef]
- Yulianto, S.; Jamilatun, M.; Jamilatun, M.; Pangesti, F.A.; Pangesti, F.A. Analisis Kadar Vitamin C Wedang Jeruk Nipis (Citrus aurantifolia) Berdasarkan Variasi Suhu Menggunakan Metode Spektrofotometri Uv—Vis. J. Formil (Forum Ilm.) Kesmas Respati 2022, 7, 208. [Google Scholar] [CrossRef]
- Mulyani, E.; Herlina, H.; Suci, K. Penetapan Kadar Tanin Ekstrak Daun Pagoda (Clerodendrum Paniculantum) Dengan Metode Spektrofotometri Visible Dan Titrasi Permanganometri. Lumbung Farm. J. Ilmu Kefarmasian 2022, 3, 7. [Google Scholar] [CrossRef]
- Ramadhani, N.; Samudra, A.G.; Pratiwi, L.W.I. Analisis Penetapan Kadar Flavonoid Sari Jeruk Kalamansi (Citrofortunella Microcarpa) Dengan Metode Spektrofotometri UV-VIS. J. Mandala Pharmacon Indones. 2020, 6, 53–58. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (Dpph) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Suffys, S.; Richard, G.; Burgeon, C.; Werrie, P.-Y.; Haubruge, E.; Fauconnier, M.-L.; Goffin, D. Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles. Foods 2023, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and Technological Parameters Impacting the Chemical Composition and Sensory Quality of Kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tesvichian, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Buakeaw, A.; Puthong, S.; Thitiprasert, S.; Mekboonsonglarp, W.; Liangsakul, J.; Sopon, A.; et al. Sulfated Polysaccharides from Caulerpa Lentillifera: Optimizing the Process of Extraction, Structural Characteristics, Antioxidant Capabilities, and Anti-Glycation Properties. Heliyon 2024, 10, e24444. [Google Scholar] [CrossRef]
- Zhao, W.; Subbiah, V.; Xie, C.; Yang, Z.; Shi, L.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and Bioavailability of Phenolic Compounds in Seaweed. Food Rev. Int. 2022, 39, 5729–5760. [Google Scholar] [CrossRef]
- Tarhan Kuzu, K.; Aykut, G.; Tek, S.; Yatmaz, E.; Germec, M.; Yavuz, I.; Turhan, I. Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes 2023, 11, 2100. [Google Scholar] [CrossRef]
- Ningsih, D.R.; Bintoro, V.P.; Nurwantoro, N. Analisis Total Padatan Terlarut, Kadar Alkohol, Nilai PH Dan Total Asam Pada Kefir Optima Dengan Penambahan High Fructose Syrup (HFS). J. Teknol. Pang. 2018, 2, 84–89. [Google Scholar] [CrossRef]
- Nur, M.; Hasriadi, H.; Ita Juwita, A.; Mursida, M. Korelasi Waktu Fermentasi Terhadap Arus Listrik Albedo Dan Flavedo Jeruk Pamelo (Citrus Maxima). Agrokompleks 2021, 21, 33–39. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Ledford, R.A.; Steinkraus, K.H. Determination and Characterization of the Antimicrobial Activity of the Fermented TeaKombucha. LWT Food Sci. Technol. 1998, 31, 291–296. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of Tea Polyphenols Solution with Different PH at Different Temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Dewi, R.S. Analisis Metagenomik Bakteri Pada Kombucha Anggur Laut (Caulerpa racemosa) Berdasarkan Gen 16S RRNA; UIN Sunan Kalijaga: Yogyakarta, Indonesia, 2025. [Google Scholar]
- Phung, L.T.; Kitwetcharoen, H.; Chamnipa, N.; Boonchot, N.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Changes in the Chemical Compositions and Biological Properties of Kombucha Beverages Made from Black Teas and Pineapple Peels and Cores. Sci. Rep. 2023, 13, 7859. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- Wu, L.-H.; Lu, Z.-M.; Zhang, X.-J.; Wang, Z.-M.; Yu, Y.-J.; Shi, J.-S.; Xu, Z.-H. Metagenomics Reveals Flavour Metabolic Network of Cereal Vinegar Microbiota. Food Microbiol. 2017, 62, 23–31. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Santosa, G.W.; Djunaedi, A.; Susanto, A.B.; Pringgenies, D.; Ariyanto, D.; Pranoton, A.K. The Potential Two Types of Green Macroalgae (Caulerpa racemose and Caulerpa lentillifera) as a Natural Food Preservative from Jepara Beach, Indonesia. Trends Sci. 2024, 21, 7394. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Z.; Jiang, Q.; Hu, X.; Zhang, X.; Yu, P.; Yang, F.; Liu, S.; Xia, W. Metabolomics Ravels Flavor Compound Formation and Metabolite Transformation in Rapid Fermentation of Salt-Free Fish Sauce from Catfish Frames Induced by Mixed Microbial Cultures. Food Chem. 2025, 463, 141246. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yang, Y.; Guo, Z.; Dai, S.; Jiang, M.; Zhu, Y.; Wang, Y.; Yu, Z.; Wang, K.; Rong, C.; et al. A Review on the Interaction of Acetic Acid Bacteria and Microbes in Food Fermentation: A Microbial Ecology Perspective. Foods 2024, 13, 2534. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zuo, L.; Li, P.; Lou, H.; Zhao, R. Revealing the Characteristics and Correlations among Microbial Communities, Functional Genes, and Vital Metabolites through Metagenomics in Henan Mung Bean Sour. Microorganisms 2025, 13, 845. [Google Scholar] [CrossRef]
- Liao, T.; Li, X.-R.; Fan, L.; Zhang, B.; Zheng, W.-M.; Hua, J.-J.; Li, L.; Mahror, N.; Cheng, L.-H. Nature of Back Slopping Kombucha Fermentation Process: Insights from the Microbial Succession, Metabolites Composition Changes and Their Correlations. Front. Microbiol. 2024, 15, 1433127. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.-P.; Taillandier, P.; Beaufort, S. Metabolome-Microbiome Signatures in the Fermented Beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Zhang, H.; Dou, Z.; Bi, W.; Yang, C.; Wu, X.; Wang, L. Multi-Omics Study of Sulfur Metabolism Affecting Functional Microbial Community Succession during Aerobic Solid-State Fermentation. Bioresour. Technol. 2023, 387, 129664. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant Activities of Kombucha Prepared from Three Different Substrates and Changes in Content of Probiotics during Storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A Review on Health Benefits of Kombucha Nutritional Compounds and Metabolites. CyTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Content of Organic Acids and Tea Polyphenols during Kombucha Tea Fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Fukaya, M.; Takemura, H.; Tayama, K.; Okumura, H.; Kawamura, Y.; Horinouchi, S.; Beppu, T. The AarC Gene Responsible for Acetic Acid Assimilation Confers Acetic Acid Resistance on Acetobacter Aceti. J. Ferment. Bioeng. 1993, 76, 270–275. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. Microbial Biosynthesis and Secretion of L-Malic Acid and Its Applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Tabata, K.; Oba, T.; Kusumoto, K.; Mitsuiki, S.; Kadokura, T.; Nakazato, A. Characteristics of the High Malic Acid Production Mechanism in Saccharomyces Cerevisiae Sake Yeast Strain No. 28. J. Biosci. Bioeng. 2012, 114, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, M.; Zhang, X.; Li, X.; Zhang, R.; Yan, Y.; Lang, F.; Zheng, Y.; Wang, M. Unraveling the Metabolic Network of Organic Acids in Solid-State Fermentation of Chinese Cereal Vinegar. Food Sci. Nutr. 2021, 9, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial, and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of Substrates, Regulations, Composition, and Biological Properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha Tea Beverage: Microbiological Characteristic, Antioxidant Activity, and Phytochemical Composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Marwati; Syahrumsyah, H.; Handria, R. Pengaruh Konsentrasi Gula Dan Strater Terhadap Mutu Teh Kombucha. J. Teknol. Pertan. 2013, 8, 49–53. [Google Scholar]
- Anggraini, A.C.; Retnaningrum, E. Efektivitas Dan Kualitas Produk Fermentasi Kombucha Dengan Kombinasi Substrat Teh Daun Sukun (Artocarpus Altilis (Parkinson) Fosberg) Dan Lemon (Citrus limon (L.) Burm. f.). J. Pengolah. Pangan 2023, 8, 97–106. [Google Scholar] [CrossRef]
- Kurniawan, M.B.; Ginting, S.; Nurminah, M. Pengaruh Penambahan Gula Dan Starter Terhadap Karakteristik Minuman Teh Kombucha Daun Gambir (Uncaria Gambir Roxb). J. Rekayasa Pangan Dan Pertan. 2017, 5, 251–257. [Google Scholar]
- Jamilah, V. Pengaruh Variasi Konsentrasi Starter Terhadap Kualitas Teh Kombucha (Studi Eksperimen Sebagai Sumber Belajar Peserta Didik Pada Materi Bioteknologi Untuk Sekolah Menenegah Atas Kelas XII Semester Genap). Ph.D. Thesis, UIN Raden Intan Lampung, Bandar Lampung, Indonesia, 2019. [Google Scholar]
- Zubaidah, E.; Yurista, S.; Rahmadani, N.R. Characteristic of Physical, Chemical, and Microbiological Kombucha from Various Varieties of Apples. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012040. [Google Scholar] [CrossRef]
- Guo, X.; Chen, D.; Huang, P.; Gao, L.; Zhou, W.; Zhang, J.; Zhang, Q. Effects of Tannin-Tolerant Lactic Acid Bacteria in Combination with Tannic Acid on the Fermentation Quality, Protease Activity and Bacterial Community of Stylo Silage. J. Sci. Food Agric. 2025, 105, 2540–2551. [Google Scholar] [CrossRef]
- Prajapati, K.; Prajapati, J.; Patel, D.; Patel, R.; Varshnei, A.; Saraf, M.; Goswami, D. Multidisciplinary Advances in Kombucha Fermentation, Health Efficacy, and Market Evolution. Arch. Microbiol. 2024, 206, 366. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Cvetković, D.; Ranitović, A.; Savić, D.; Joković, N.; Vidaković, A.; Pezo, L.; Markov, S. Survival of Wild Strains of Lactobacilli during Kombucha Fermentation and Their Contribution to Functional Characteristics of Beverage. Pol. J. Food Nutr. Sci. 2019, 69, 407–415. [Google Scholar] [CrossRef]
- Rahmi, N.; Khairiah, N.; Rufida, R.; Hidayati, S.; Muis, A. Pengaruh Fermentasi Terhadap Total Fenolik, Aktivitas Penghambatan Radikal Dan Antibakteri Ekstrak Tepung Biji Teratai (Effect of Fermentation on Total Phenolic, Radical Scavenging and Antibacterial Activity of Waterlily (Nymphaea Pubescens Willd.). Biopropal Ind. 2020, 11, 9. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black, and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Rosita, H.; Amaro, D. Pengaruh Konsentrasi Starter SCOBY (Symbiotic Culture of Bacteria and Yeast) Terhadap Mutu Kimia, Mikrobiologi Dan Organoleptik Kombucha Sari Apel. J. Ilmu Dan Teknol. Pangan 2021, 7, 12–22. [Google Scholar]
- Morales, D. Biological Activities of Kombucha Beverages: The Need for Clinical Evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Leal, J.M.M.; Valera, M.J.D.; Hernández, C.R.; González, R.J. Fermented Kombucha Beverages: Microbial and Chemical Analysis. Food Chem. 2018, 265, 1–7. [Google Scholar]
- Sharma, S.; Sahai, S.; Singh, P. GC-MS-Based Chemical Profiling and Antimicrobial Activity of Fatty Acid Esters from Fermented Food Bacteria. J. Appl. Microbiol. 2020, 128, 1442–1451. [Google Scholar]
- Yadav, R.; Tripathi, M.K.; Mishra, A. Role of Sugar Alcohols in Food Industry: Current Applications and Future Aspects. Food Res. Int. 2021, 140. [Google Scholar] [CrossRef]
- Bicas, J.L.; Dionisio, A.P.; Pastore, G.M. Bio-Oxidation of Terpenes: An Approach for Flavour Industry. Chem. Soc. Rev. 2011, 40, 4455–4475. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Biotransformation of Terpenes. Biotechnol. Adv. 2006, 24, 134–142. [Google Scholar] [CrossRef]
- Baskar, A.A.; Numair, K.S.; Alsaif, M.A.; Ignacimuthu, S. β-Sitosterol Prevents Lipid Peroxidation and Improves Antioxidant Status and Histopathological Changes in Rats with Hepatic Injury. Food Chem. Toxicol. 2012, 50, 2409–2415. [Google Scholar]
- Gupta, M.B.; Nath, R.; Srivastava, N.; Shanker, K.; Kishor, K.; Bhargava, K.P. Anti-Inflammatory and Antipyretic Activities of Beta-Sitosterol. Planta Med. 1980, 39, 157–163. [Google Scholar] [CrossRef]

| Trim | Filter | ||
|---|---|---|---|
| truncQ | 2 | maxLen | NA |
| trimLeft | 0 | minLen | 20 |
| trimRight | 0 | maxN | 0 |
| truncLen | 0 | minQ | 0 |
| maxEE | 2 |
| Samples | pH | Soluble Solid Content | Vitamin C Content (mg AAE/mL) | Total Tannin Content | Total Flavonoid Content |
|---|---|---|---|---|---|
| (°Brix) | (mg GAE/mL) | (mg QE/mL) | |||
| K0 | 4.133 ± 0.018 | 17.17 ± 0.14 | 0.4872 ± 0.0011 | 0.1135 ± 0.0007 | 0.0620 ± 0.0009 |
| K1 (10%) | 2.566 ± 0.021 | 11.08 ± 0.80 | 0.2123 ± 0.0184 | 0.2721 ± 0.0223 | 0.0589 ± 0.0008 |
| K2 (15%) | 2.618 ± 0.013 | 11.17 ± 0.80 | 0.2446 ± 0.0156 | 0.3070 ± 0.0079 | 0.0550 ± 0.0013 |
| K3 (20%) | 2.723 ± 0.017 | 10.04 ± 0.07 | 0.2118 ± 0.0077 | 0.3126 ± 0.0075 | 0.0525 ± 0.0030 |
| Samples | IC50 (µg/mL) |
|---|---|
| K0 | 101.470 ± 5.88 |
| K1 (10%) | 69.637 ± 2.45 |
| K2 (15%) | 56.947 ± 2.45 |
| K3 (20%) | 49.572 ± 2.45 |
| No | Compounds Name | Percent Area (%) | Molecular Formula | Functional Group | Compound Group | PubChem CID |
|---|---|---|---|---|---|---|
| C1 | Quinic acid | 2.30 | C7H12O6 | -COOH, -OH | Organic acid | 6508 |
| C2 | Palmitic acid | 1.09 | C16H32O2 | -COOH | Saturated fatty acid | 985 |
| C3 | 2-Propenoic acid | 0.96 | C3H4O2 | -COOH, C=C | Unsaturated fatty acid | 6581 |
| C4 | Glucitol | 1.66 | C6H14O6 | -OH (polyhydroxy) | Sugar alcohol/polyol | 82170 |
| C5 | Hexadecanoic acid | 4.58 | C16H32O2 | -COOH | Saturated fatty acid | 985 |
| C6 | Octadec-9-enoic acid | 3.97 | C18H34O2 | -COOH, C=C | Unsaturated fatty acid | 965 |
| No | Compounds Name | Percent Area (%) | Molecular Formula | Functional Group | Compound Group | PubChem CID |
|---|---|---|---|---|---|---|
| C1 | Phytol | 11.02 | C20H40O | Alcohol (-OH) | Diterpenoid alcohol | 5280435 |
| C2 | Phytol, acetate | 7.05 | C22H42O | Ester | Terpenoid ester | 637195 |
| C3 | Phytyl decanoate | 7.90 | C30H58O2 | Ester | Terpenoid ester | 140471960 |
| C4 | Pregn-4-ene-3,20-dione, 21-(acetyloxy)-9-fluoro-11,17-dihydroxy-2-methyl-, (2beta,11beta)- | 1.07 | C24H33FO6 | Keton, Ester, -OH | Steroid complexes | 31719 |
| C5 | Rhodopin | 0.65 | C40H58O | Alcohol, Polyenes | Carotenoid | 5365880 |
| C6 | ß-Sitosterol | 11.89 | C29H50O | Alcohol (Sterol) | Phytosterol | 222284 |
| C7 | Hexadecanoic acid | 7.25 | C16H32O2 | Carboxylic acid (-COOH) | Fatty acid | 985 |
| C8 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | 4.05 | C35H68O5 | Ester | Esterified fatty acids | 99931 |
| C9 | i-Propyl 11,12-methylene-octadecanoate | 1.04 | C22H42O2 | Ester | Esterified fatty acids | 91692516 |
| C10 | Neophytadiene | 3.20 | C20H38 | Alkenes | Diterpene hydrocarbons | 10446 |
| No | Compounds Name | Persen Area (%) | Molecular Formula | Gugus Fungsi | Kelompok Senyawa | PubChem CID |
|---|---|---|---|---|---|---|
| C1 | Sorbitol | 32.30 | C6H14O6 | Polyhydroxy Alcohol (-OH) | Sugar alcohol/polyol | 5780 |
| C2 | ß-Sitosterol | 1.80 | C29H50O | Steroidal alcohol (-OH) | Phytosterol | 222284 |
| C3 | γ-Sitosterol | 6.45 | C29H50O | Steroidal alcohol (-OH) | Phytosterol | 457801 |
| C4 | Palmitic acid methyl ester | 0.54 | C17H34O2 | Ester (-COOCH3) | Ester fatty acid | 8181 |
| C5 | Carveol | 6.70 | C10H16O | Alcohol, alkenes (C=C) | Monoterpene alcohol | 7438 |
| C6 | α-Terpineol | 10.22 | C10H18O | Alcohol | Monoterpene alcohol | 17100 |
| C7 | Limetol | 1.90 | C10H18O | Alcohol | Monoterpene alcohol | 522514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumalawati, D.A.; Dewi, R.S.; Fitriani, N.R.; Muchtar, S.Z.; Leonardo, J.; Astuti Taslim, N.; Romano, R.; Santini, A.; Nurkolis, F. Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time. Beverages 2025, 11, 134. https://doi.org/10.3390/beverages11050134
Kumalawati DA, Dewi RS, Fitriani NR, Muchtar SZ, Leonardo J, Astuti Taslim N, Romano R, Santini A, Nurkolis F. Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time. Beverages. 2025; 11(5):134. https://doi.org/10.3390/beverages11050134
Chicago/Turabian StyleKumalawati, Dian Aruni, Reza Sukma Dewi, Noor Rezky Fitriani, Scheirana Zahira Muchtar, Juan Leonardo, Nurpudji Astuti Taslim, Raffaele Romano, Antonello Santini, and Fahrul Nurkolis. 2025. "Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time" Beverages 11, no. 5: 134. https://doi.org/10.3390/beverages11050134
APA StyleKumalawati, D. A., Dewi, R. S., Fitriani, N. R., Muchtar, S. Z., Leonardo, J., Astuti Taslim, N., Romano, R., Santini, A., & Nurkolis, F. (2025). Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time. Beverages, 11(5), 134. https://doi.org/10.3390/beverages11050134










