Effects of Enzymatic Hydrolysis Combined with Ultrasonic Treatment on the Properties of an Apple Juice Enriched with Apple Bagasse
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Preparation of Apple Bagasse
2.3. Determination of DF Composition in Apple Bagasse
2.4. Enzymatic Hydrolysis and Ultrasonic Treatment of Bagasse-Enriched Juices
2.5. Analysis of Bagasse-Enriched Juices
2.5.1. Particle Size Distribution
2.5.2. pH and Color
2.5.3. Viscosity
2.5.4. Galacturonic Acid Content
2.5.5. Antioxidant Properties
- Total phenolic content
- b.
- DPPH assay
2.5.6. Glucose Content
2.5.7. Glucose Retardation Kinetics In Vitro
2.5.8. Sensory Evaluation
2.5.9. Glycemic Response In Vivo
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fiber Composition of Apple Bagasse
3.2. Analysis of the Properties of Bagasse-Enriched Juices
3.2.1. Particle Size and Color
3.2.2. Viscosity
3.2.3. Galacturonic Acid Content and pH
3.2.4. Antioxidant Properties
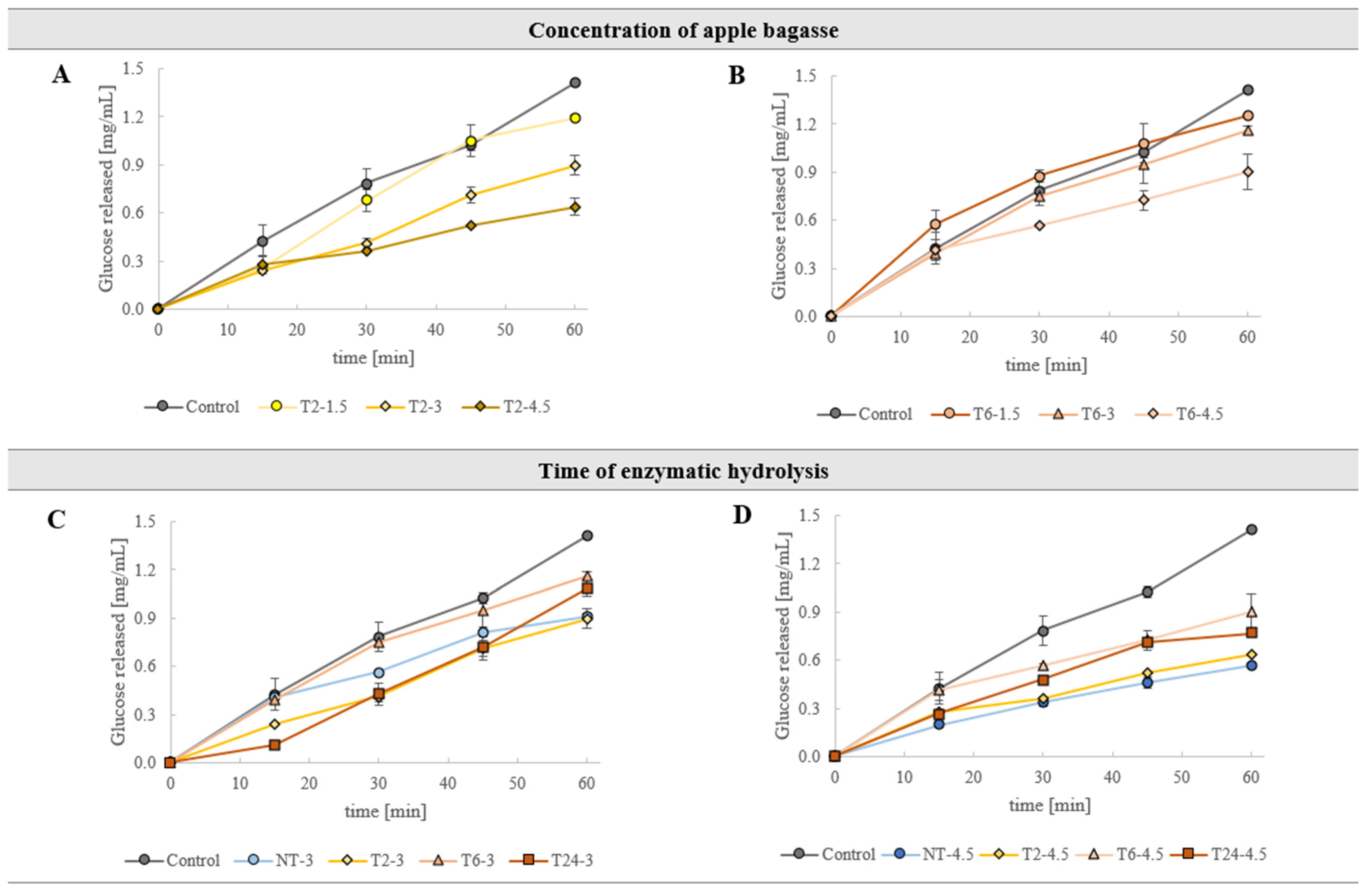
3.2.5. Glucose Retardation Kinetics In Vitro
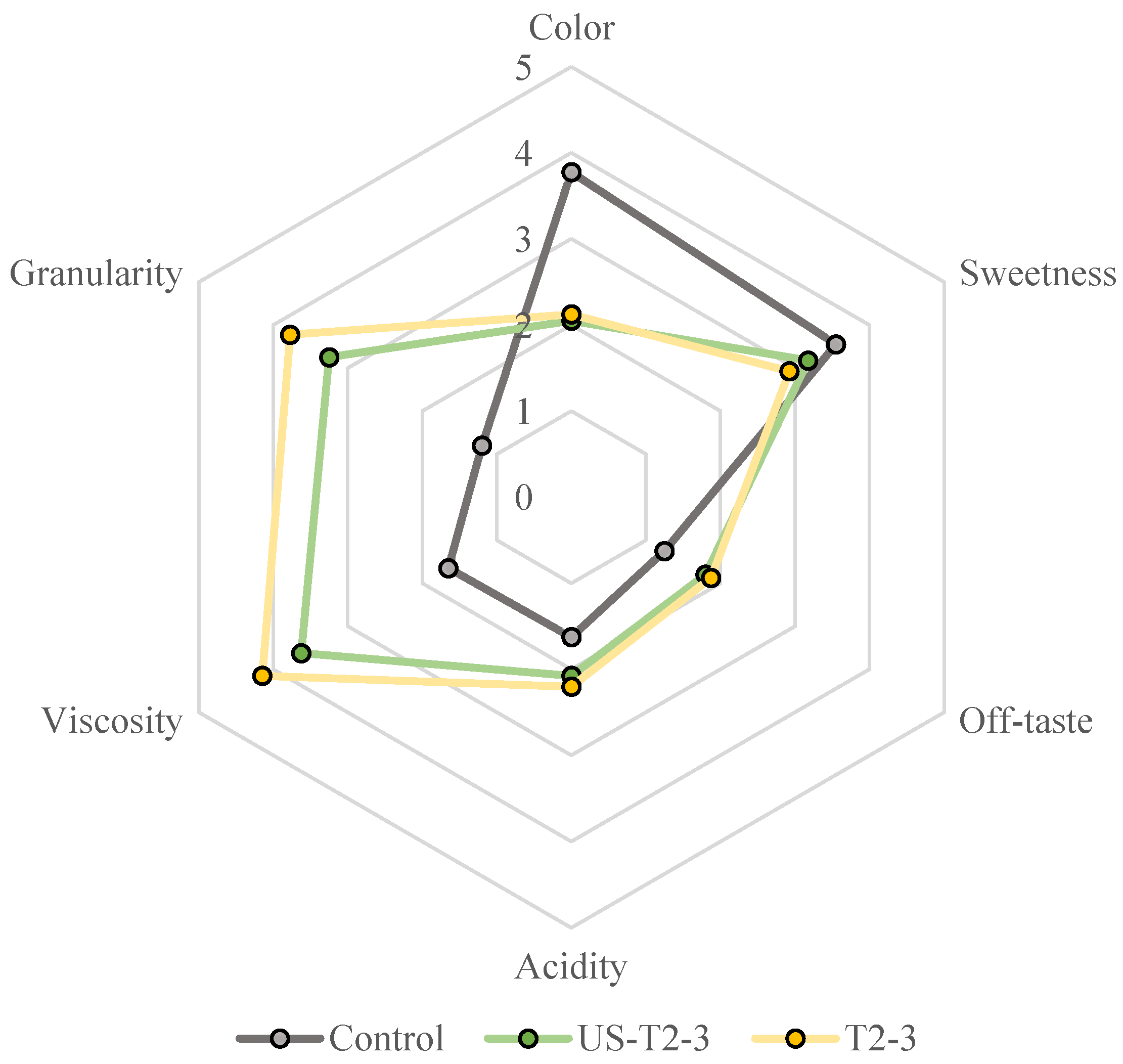
3.2.6. Sensory Evaluation
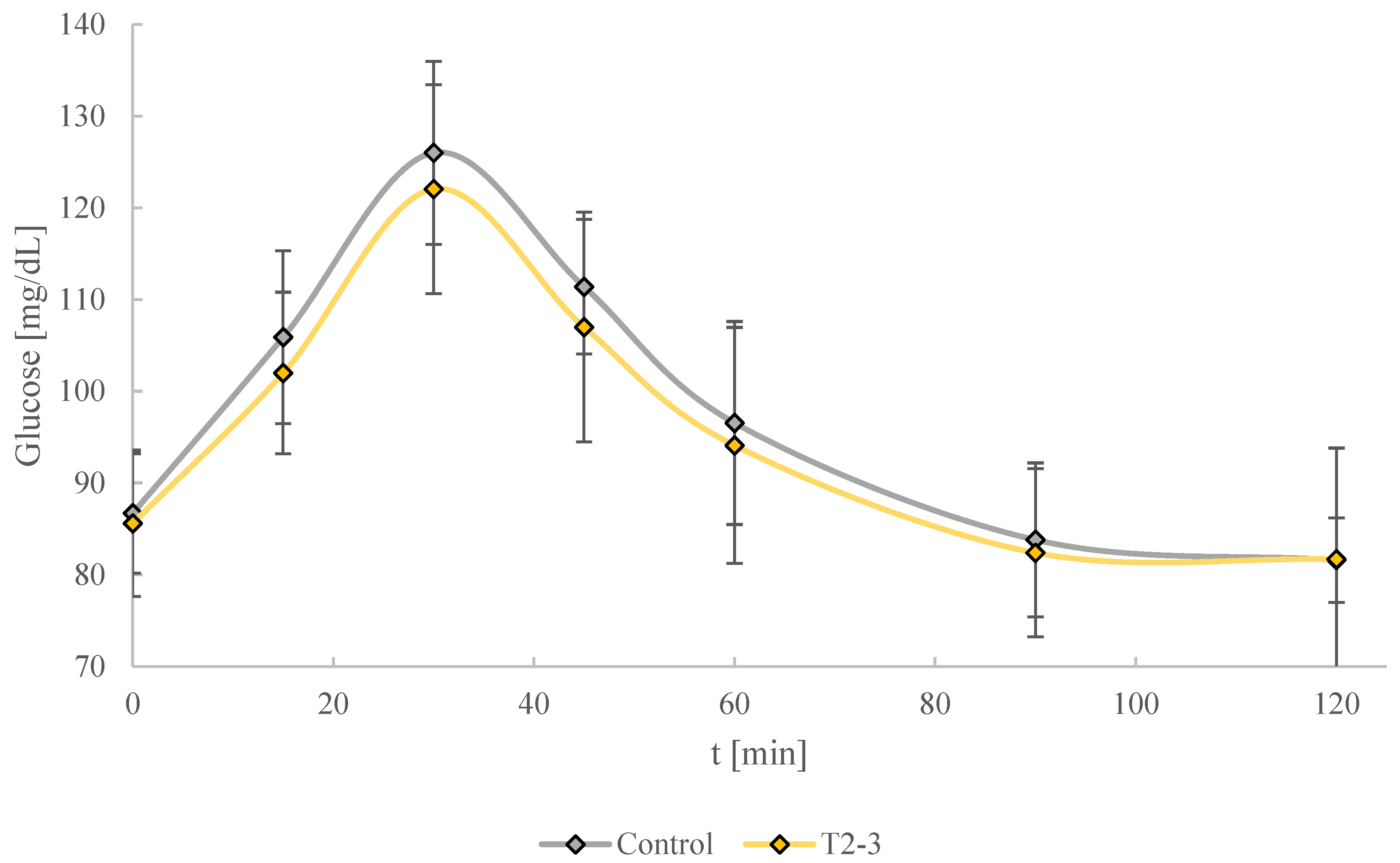
3.2.7. Glycemic Response in Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims on foods. Off. J. Eur. Union 2006, L 404, 9–25. [Google Scholar]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and waste reduction as an integral part of a circular economy. Front. Environ. Sci. 2017, 5, 21. [Google Scholar] [CrossRef]
- Collier, B. Glucose Control and the Inflammatory Response. Nutr. Clin. Pr. 2008, 23, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Mootha, V.K.; Dwyer, A.A.; Hardin, M.; Lee, H.; Eriksson, K.F.; Tripathy, D.; Yialamas, M.; Groop, L.; Elahi, D.; et al. Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men. Diabetes Care July 2005, 28, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Bano, G. Glucose homeostasis, obesity and diabetes. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 715–726. [Google Scholar] [CrossRef]
- Espírito-Santo, A.; Lagazzo, A.; Sousa, A.; Perego, P.; Converti, A.; Oliveira, M.N. Rheology, spontaneous whey separation, microstructure and sensorial characteristics of probiotic yoghurts enriched with passion fruit fiber. Food Res. Int. 2013, 50, 224–231. [Google Scholar] [CrossRef]
- Tomic, N.; Dojnov, B.; Miocinovic, J.; Tomasevic, I.; Smigic, N.; Djekic, I.; Vujcic, Z. Enrichment of yoghurt with insoluble dietary fiber from triticale—A sensory perspective. LWT Food Sci. Technol. 2017, 80, 59–66. [Google Scholar] [CrossRef]
- Hashim, I.B.; Khalil, A.H.; Afifi, H.S. Quality characteristics and consumer acceptance of yogurt fortified with date fiber. J. Dairy Sci. 2009, 92, 5403–5407. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef]
- Song, L.-w.; Qi, J.-r.; Liao, J.-s.; Yang, X.-q. Enzymatic and enzyme-physical modification of citrus fiber by xylanase and planetary ball milling treatment. Food Hydrocoll. 2021, 121, 107015. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Vodeničarová, M.; Dřímalová, G.; Hromádková, Z.; Malovíková, A.; Ebringerová, A. Xyloglucan degradation using different radiation sources: A comparative study. Ultrason. Sonochem. 2006, 13, 157–164. [Google Scholar] [CrossRef]
- Manthei, A.; Elez-Martínez, P.; Soliva-Fortuny, R.; Murciano-Martínez, P. Ultrasonication and enzymatic treatment of apple and orange bagasses: Molecular characterization of released oligosaccharides and modification of techno-functional and health-related properties. LWT 2024, 194, 115816. [Google Scholar] [CrossRef]
- Manthei, A.; Elez-Martínez, P.; Soliva-Fortuny, R.; Murciano-Martínez, P. Prebiotic potential of pectin and cello-oligosaccharides from apple bagasse and orange peel produced by high-pressure homogenization and enzymatic hydrolysis. Food Chem. 2024, 435, 137583. [Google Scholar] [CrossRef]
- Sarpong, F.; Yu, X.; Zhou, C.; Hongpeng, Y.; Uzoejinwa, B.B.; Bai, J.; Wu, B.; Ma, H. Influence of anti-browning agent pretreatment on drying kinetics, enzymes inactivation and other qualities of dried banana (Musa ssp.) under relative humidity-convective air dryer. J. Food Meas. Charact. 2018, 12, 1229–1241. [Google Scholar] [CrossRef]
- Alvarez, S.; Alvarez, R.; Riera, F.A.; Coca, J. Influence of depectinization on apple juice ultrafiltration. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 377–382. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Viacava, G.; Ponce, A.; Moreira, M. Enhancing quality attributes of fiber-enriched strawberry juice by application of vanillin or geraniol. LWT 2016, 72, 90–98. [Google Scholar] [CrossRef]
- Schneider, V.S.; Bark, J.M.; Winnischofer, S.M.B.; Dos Santos, E.F.; Iacomini, M.; Cordeiro, L.M.C. Dietary fibres from guavira pomace, a co-product from fruit pulp industry: Characterization and cellular antioxidant activity. Food Res. Int. 2020, 132, 109065. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Leite, A.K.F.; da Silva, A.R.A.; Fernandes, F.A.N.; Rodrigues, S. Sonication Effect on Bioactive Compounds of Cashew Apple Bagasse. Food Bioprocess Technol. 2017, 10, 1854–1864. [Google Scholar] [CrossRef]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, J.; Xu, Y. Co-preparation of pectin and cellulose from apple pomace by a sequential process. J. Food Sci. Technol. 2019, 56, 4091–4100. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Mudgil, D. The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. [Google Scholar]
- Luo, X.; Wang, Q.; Fang, D.; Zhuang, W.; Chen, C.; Jiang, W.; Zheng, Y. Modification of insoluble dietary fibers from bamboo shoot shell: Structural characterization and functional properties. Int. J. Biol. Macromol. 2018, 120, 1461–1467. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review and assessment of hydrodynamic cavitation as a technology for the future. Ultrason. Sonochem. 2005, 12, 21–27. [Google Scholar] [CrossRef]
- Chaplin, M.F. Fibre and water binding. Proc. Nutr. Soc. 2003, 62, 223–227. [Google Scholar] [CrossRef]
- Dikeman, C.L.; Fahey, G.C. Viscosity as related to dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary fibre for glycaemia control: Towards a mechanistic understanding. Bioact. Carbohydrates Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martínez, M.C.; Ortuño, J. Relationship between Physical and Hydration Properties of Soluble and Insoluble Fiber of Artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Du, H.; Yang, H.; Wang, X.; Zhu, F.; Tang, D.; Cheng, J.; Liu, X. Effects of mulberry pomace on physicochemical and textural properties of stirred-type flavored yogurt. J. Dairy Sci. 2021, 104, 12403–12414. [Google Scholar] [CrossRef]
- Paquet, É.; Hussain, R.; Bazinet, L.; Makhlouf, J.; Lemieux, S.; Turgeon, S.L. Effect of processing treatments and storage conditions on stability of fruit juice based beverages enriched with dietary fibers alone and in mixture with xanthan gum. LWT 2014, 55, 131–138. [Google Scholar] [CrossRef]
- Vaikousi, H.; Biliaderis, C.G. Processing and formulation effects on rheological behavior of barley β-glucan aqueous dispersions. Food Chem. 2005, 91, 505–516. [Google Scholar] [CrossRef]
- Martău, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Rashid, R.; Wani, S.M.; Manzoor, S.; Masoodi, F.; Dar, M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2022, 398, 133871. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Cordoba, M.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K.; Meletharayil, G.H. Application of hydrodynamic cavitation to improve antioxidant activity in sorghum flour and apple pomace. Food Bioprod. Process. 2016, 100, 335–343. [Google Scholar] [CrossRef]
- Jutkus, R.A.L.; Li, N.; Taylor, L.S.; Mauer, L.J. Effect of temperature and initial moisture content on the chemical stability and color change of various forms of Vitamin C. Int. J. Food Prop. 2015, 18, 862–879. [Google Scholar] [CrossRef]
- Buchweitz, M.; Speth, M.; Kammerer, D.; Carle, R. Stabilisation of strawberry (Fragaria x ananassa Duch.) anthocyanins by different pectins. Food Chem. 2013, 141, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.; Dorta, E.; Lobo, M.G.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of Banana (Musa acuminata Colla AAA) Peel Extract as an Antioxidant Source in Orange Juices. Plant Foods Hum. Nutr. 2017, 72, 60–66. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Composition and antioxidant capacity of a novel beverage produced with green tea and minimally-processed byproducts of broccoli. Innov. Food Sci. Emerg. Technol. 2011, 12, 361–368. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Tyrakowska, B. Quality of commercial apple juices evaluated on the basis of the polyphenol content and the TEAC antioxidant activity. J. Food Sci. 2003, 68, 1844–1849. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, W.; Cao, J.; He, K.; Liu, Y.; Bai, X. Microstructure characteristics of tea seed dietary fibre and its effect on cholesterol, glucose and nitrite ion adsorption capacities in vitro: A comparison study among different modifications. Int. J. Food Sci. Technol. 2020, 55, 1781–1791. [Google Scholar] [CrossRef]
- Dhital, S.; Dolan, G.; Stokes, J.R.; Gidley, M.J. Enzymatic hydrolysis of starch in the presence of cereal soluble fibre polysaccharides. Food Funct. 2014, 5, 579–586. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.-C.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Axten, L.; Wohlers, M.; Wegrzyn, T. Using phytochemicals to enhance health benefits of milk: Impact of polyphenols on flavor profile. J. Food Sci. 2008, 73, H122–H126. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crop. Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Effect of addition of phenolic compounds recovered from apple pomace on cider quality. LWT 2019, 100, 348–354. [Google Scholar] [CrossRef]
- Liu, C.-M.; Liang, R.-H.; Dai, T.-T.; Ye, J.-P.; Zeng, Z.-C.; Luo, S.-J.; Chen, J. Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocoll. 2016, 57, 55–61. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Manthei, A.; Drusch, S. Enzymatic pre-treatment defines the water-binding and rheological properties of dynamic ultra-high-pressure homogenised pea hull suspensions. Food Hydrocoll. 2024, 157, 110454. [Google Scholar] [CrossRef]
- Vrolix, R.; Mensink, R.P. Variability of the glycemic response to single food products in healthy subjects. Contemp. Clin. Trials 2010, 31, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Whelan, W.J.; Hollar, D.; Agatston, A.; Dodson, H.J.; Tahal, D.S. The glycemic response is a personal attribute. IUBMB Life 2010, 62, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Borgström, B.; Dahlqvist, A.; Lundh, G.; Sjövall, J. Studies of intestinal digestion and absorption in the human. J. Clin. Investig. 1957, 36, 1521–1536. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of invitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Thondre, P.S.; Shafat, A.; Clegg, M.E. Molecular weight of barley β-glucan influences energy expenditure, gastric emptying and glycaemic response in human subjects. Br. J. Nutr. 2013, 110, 2173–2179. [Google Scholar] [CrossRef]
- Wood, P.J.; Braaten, J.T.; Scott, F.W.; Riedel, K.D.; Wolynetz, M.S.; Collins, M.W. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 1994, 72, 731–743. [Google Scholar] [CrossRef]
- Törrönen, R.; McDougall, G.J.; Dobson, G.; Stewart, D.; Hellström, J.; Mattila, P.; Pihlava, J.-M.; Koskela, A.; Karjalainen, R. Fortification of blackcurrant juice with crowberry: Impact on polyphenol composition, urinary phenolic metabolites, and postprandial glycemic response in healthy subjects. J. Funct. Foods 2012, 4, 746–756. [Google Scholar] [CrossRef]
- Zhao, C.; Wan, X.; Zhou, S.; Cao, H. Natural Polyphenols: A Potential Therapeutic Approach to Hypoglycemia. eFood 2020, 1, 107–118. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]

 ) and DPPH scavenging rate (
) and DPPH scavenging rate ( ) of apple juices without apple bagasse (control) and enriched with 3 % pectin or different concentrations of bagasse (1.5 %, 3 %, 4.5 %) applying different treatment conditions. NT—not-treated; T2/T6/T24—enzymatically treated for 2/6/24 h; US-T2—juice; ultrasonically treated and subsequently hydrolyzed with enzymes for 2 h; the following number, i.e., 1.5, 3, 4.5, indicate the concentration of apple bagasse/pectin in the juice.
) of apple juices without apple bagasse (control) and enriched with 3 % pectin or different concentrations of bagasse (1.5 %, 3 %, 4.5 %) applying different treatment conditions. NT—not-treated; T2/T6/T24—enzymatically treated for 2/6/24 h; US-T2—juice; ultrasonically treated and subsequently hydrolyzed with enzymes for 2 h; the following number, i.e., 1.5, 3, 4.5, indicate the concentration of apple bagasse/pectin in the juice.
 ) and DPPH scavenging rate (
) and DPPH scavenging rate ( ) of apple juices without apple bagasse (control) and enriched with 3 % pectin or different concentrations of bagasse (1.5 %, 3 %, 4.5 %) applying different treatment conditions. NT—not-treated; T2/T6/T24—enzymatically treated for 2/6/24 h; US-T2—juice; ultrasonically treated and subsequently hydrolyzed with enzymes for 2 h; the following number, i.e., 1.5, 3, 4.5, indicate the concentration of apple bagasse/pectin in the juice.
) of apple juices without apple bagasse (control) and enriched with 3 % pectin or different concentrations of bagasse (1.5 %, 3 %, 4.5 %) applying different treatment conditions. NT—not-treated; T2/T6/T24—enzymatically treated for 2/6/24 h; US-T2—juice; ultrasonically treated and subsequently hydrolyzed with enzymes for 2 h; the following number, i.e., 1.5, 3, 4.5, indicate the concentration of apple bagasse/pectin in the juice.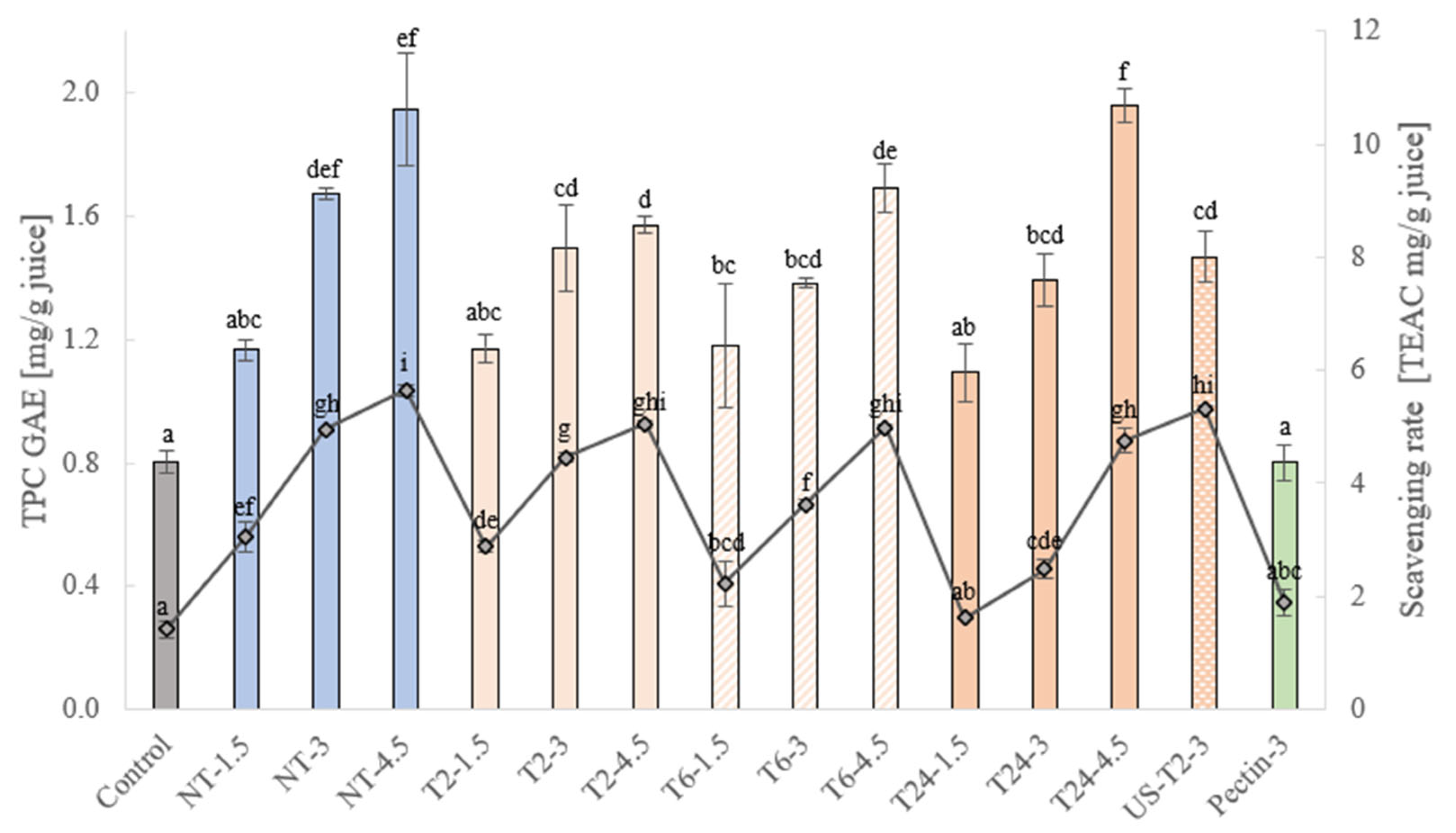
| IDF [%] | SDF [%] | Cellulose [%] | Hemicellulose [%] | Lignin [%] | |
|---|---|---|---|---|---|
| Apple bagasse | 37.40 ± 1.27 | 7.45 ± 0.49 | 19.92 ± 0.98 | 9.11 ± 1.12 | 4.26 ± 0.45 |
| D50 | D90 | pH | Viscosity [mPa·s] | L*-Value (Whiteness) | a*-Value (Red-Green) | b*-Value (Yellow-Blue) | Color Difference ΔE | |
|---|---|---|---|---|---|---|---|---|
| Control | 9.94 ± 0.06 a | 23.43 ± 0.15 a | 3.87 | 1.83 ± 0.01 a | 33.70 ± 0.00 a | −1.83 ± 0.00 ab | 4.78 ± 0.01 a | |
| NT-1.5 | 356.57 ± 4.04 b | 771.67 ± 1.15 b | 3.76 | 5.98 ± 0.1 a | 39.38 ± 0.02 b | −2.18 ± 0.01 cd | 10.16 ± 0.01 b | 7.84 ± 0.02 a |
| NT-3 | 340.33 ± 4.04 bc | 760.00 ± 4.36 b | 3.65 | 257.50 ± 12.02 b | 41.35 ± 0.00 c | −1.44 ± 0.00 f | 12.12 ± 0.02 c | 10.62 ± 0.02 b |
| NT-4.5 | 320.67 ± 2.08 c | 749.67 ± 3.79 b | 3.59 | n.a. | 44.82 ± 0.04 d | −1.19 ± 0.16 g | 15.81 ± 0.10 d | 15.69 ± 0.05 c |
| T2-1.5 | 255.67 ± 16.01 de | 571.00 ± 40.87 c | 3.64 | 2.58 ± 0.14 a | 37.95 ± 0.01 e | −2.49 ± 0.04 e | 8.67 ± 0.00 e | 5.82 ± 0.01 d |
| T2-3 | 264.00 ± 18.68 d | 581.00 ± 40.80 c | 3.46 | 49.55 ± 9.69 ab | 40.04 ± 0.00 f | −2.26 ± 0.01 d | 11.48 ± 0.01 f | 9.25 ± 0.00 e |
| T2-4.5 | 264.00 ± 18.52 d | 578.67 ± 40.05 c | 3.34 | 179.75 ± 44.90 d | 42.68 ± 0.03 g | −1.30 ± 0.01 fg | 15.42 ± 0.05 g | 13.95 ± 0.05 f |
| T6-1.5 | 241.33 ± 10.02 def | 497.67 ± 29.02 c | 3.54 | 2.59 ± 0.29 a | 34.40 ± 0.00 h | −2.00 ± 0.01 bc | 4.24 ± 0.01 h | 0.89 ± 0.00 g |
| T6-3 | 236.67 ± 12.50 def | 501.00 ± 39.51 c | 3.40 | 14.80 ± 2.12 a | 39.08 ± 0.03 i | −2.31 ± 0.02 de | 10.39 ± 0.02 b | 7.80 ± 0.03 b |
| T6-4.5 | 251.00 ± 15.10 def | 550.33 ± 50.80 c | 3.28 | 138.00 ± 28.28 cd | 41.38 ± 0.06 c | −1.66 ± 0.01 a | 13.72 ± 0.05 i | 11.80 ± 0.07 h |
| T24-1.5 | 217.67 ± 8.62 fg | 456.33 ± 25.42 c | 3.49 | 2.64 ± 0.18 a | 44.82 ± 0.20 j | −1.88 ± 0.03 b | 8.27 ± 0.04 j | 4.07 ± 0.14 i |
| T24-3 | 225.00 ± 13.75 efg | 469.33 ± 34.95 c | 3.32 | 14.05 ± 1.06 a | 38.94 ± 0.06 i | −2.15 ± 0.02 cd | 11.07 ± 0.04 k | 8.21 ± 0.06 j |
| T24-4.5 | 220.67 ± 9.02 fg | 468.00 ± 27.84 c | 3.22 | 90.30 ± 7.78 bc | 40.50 ± 0.13 k | −1.68 ± 0.01 a | 12.81 ± 0.18 l | 10.54 ± 0.21 b |
| US-T2-3 | 197.00 ± 8.19 g | 458.00 ± 46.51 c | 3.47 | 29.90 ± 5.52 a | 42.02 ± 0.04 l | −1.87 ± 0.00 b | 12.76 ± 0.06 l | 11.54 ± 0.07 h |
| Pectin-3 | 9.32 ± 0.12 a | 98.07 ± 5.14 a | 3.17 | 51.15 ± 4.60 ab | 36.78 ± 0.09 m | −2.26 ± 0.02 d | 7.01 ± 0.05 m | 3.83 ± 0.10 i |
| Released Glucose in Dialysate [mg/mL] | ||||
|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | |
| Control | 0.425 ± 0.10 ab | 0.784 ± 0.10 ab | 1.027 ± 0.03 abc | 1.415 ± 0.00 a |
| NT-1.5 | 0.212 ± 0.02 bc | 0.655 ± 0.10 abc | 0.951 ± 0.17 abc | 1.273 ± 0.17 ab |
| NT-3 | 0.404 ± 0.00 ab | 0.565 ± 0.00 bcde | 0.814 ± 0.03 abcd | 0.912 ± 0.02 cde |
| NT-4.5 | 0.197 ± 0.02 bc | 0.340 ± 0.03 ef | 0.459 ± 0.03 e | 0.566 ± 0.01 f |
| T2-1.5 | 0.258 ± 0.02 bc | 0.676 ± 0.07 abc | 1.052 ± 0.10 ab | 1.194 ± 0.01 abc |
| T2-3 | 0.240 ± 0.00 c | 0.412 ± 0.03 f | 0.714 ± 0.05 de | 0.897 ± 0.06 ef |
| T2-4.5 | 0.278 ± 0.06 bc | 0.365 ± 0.00 cdef | 0.523 ± 0.01 de | 0.639 ± 0.05 ef |
| T6-1.5 | 0.574 ± 0.09 a | 0.876 ± 0.04 a | 1.081 ± 0.12 a | 1.249 ± 0.00 ab |
| T6-3 | 0.391 ± 0.02 ab | 0.752 ± 0.03 ab | 0.946 ± 0.12 abc | 1.162 ± 0.02 abc |
| T6-4.5 | 0.416 ± 0.07 ab | 0.569 ± 0.01 bcde | 0.726 ± 0.06 bcde | 0.903 ± 0.11 cde |
| T24-1.5 | 0.212 ± 0.16 bc | 0.501 ± 0.03 cdef | 0.701 ± 0.04 cde | 1.018 ± 0.24 bcde |
| T24-3 | 0.111 ± 0.02 c | 0.427 ± 0.07 def | 0.721 ± 0.08 cde | 1.086 ± 0.05 bc |
| T24-4.5 | 0.267 ± 0.11 bc | 0.480 ± 0.08 cdef | 0.709 ± 0.10 cde | 0.768 ± 0.07 def |
| US-T2-3 | 0.423 ± 0.02 ab | 0.583 ± 0.09 bcd | 0.728 ± 0.02 bcde | 0.893 ± 0.06 cde |
| Pectin-3 | 0.197 ± 0.04 bc | 0.536 ± 0.00 bcdef | 0.801 ± 0.10 abcd | 1.043 ± 0.06 bcd |
| Glucose [mg/mL] | |
|---|---|
| Apple bagasse | 1.08 ± 0.02 a* |
| Control | 31.95 ± 0.21 b |
| NT-3 | 35.18 ± 0.18 c |
| T2-3 | 35.60 ± 0.06 c |
| T6-3 | 35.23 ± 0.24 c |
| T24-3 | 35.39 ± 0.24 c |
| US-T2-3 | 36.56 ± 0.41 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthei, A.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of Enzymatic Hydrolysis Combined with Ultrasonic Treatment on the Properties of an Apple Juice Enriched with Apple Bagasse. Beverages 2025, 11, 133. https://doi.org/10.3390/beverages11050133
Manthei A, Elez-Martínez P, Martín-Belloso O, Soliva-Fortuny R. Effects of Enzymatic Hydrolysis Combined with Ultrasonic Treatment on the Properties of an Apple Juice Enriched with Apple Bagasse. Beverages. 2025; 11(5):133. https://doi.org/10.3390/beverages11050133
Chicago/Turabian StyleManthei, Alina, Pedro Elez-Martínez, Olga Martín-Belloso, and Robert Soliva-Fortuny. 2025. "Effects of Enzymatic Hydrolysis Combined with Ultrasonic Treatment on the Properties of an Apple Juice Enriched with Apple Bagasse" Beverages 11, no. 5: 133. https://doi.org/10.3390/beverages11050133
APA StyleManthei, A., Elez-Martínez, P., Martín-Belloso, O., & Soliva-Fortuny, R. (2025). Effects of Enzymatic Hydrolysis Combined with Ultrasonic Treatment on the Properties of an Apple Juice Enriched with Apple Bagasse. Beverages, 11(5), 133. https://doi.org/10.3390/beverages11050133







