The Constrained Disorder Principle: A Paradigm Shift for Accurate Interactome Mapping and Information Analysis in Complex Biological Systems
Abstract
1. Introduction
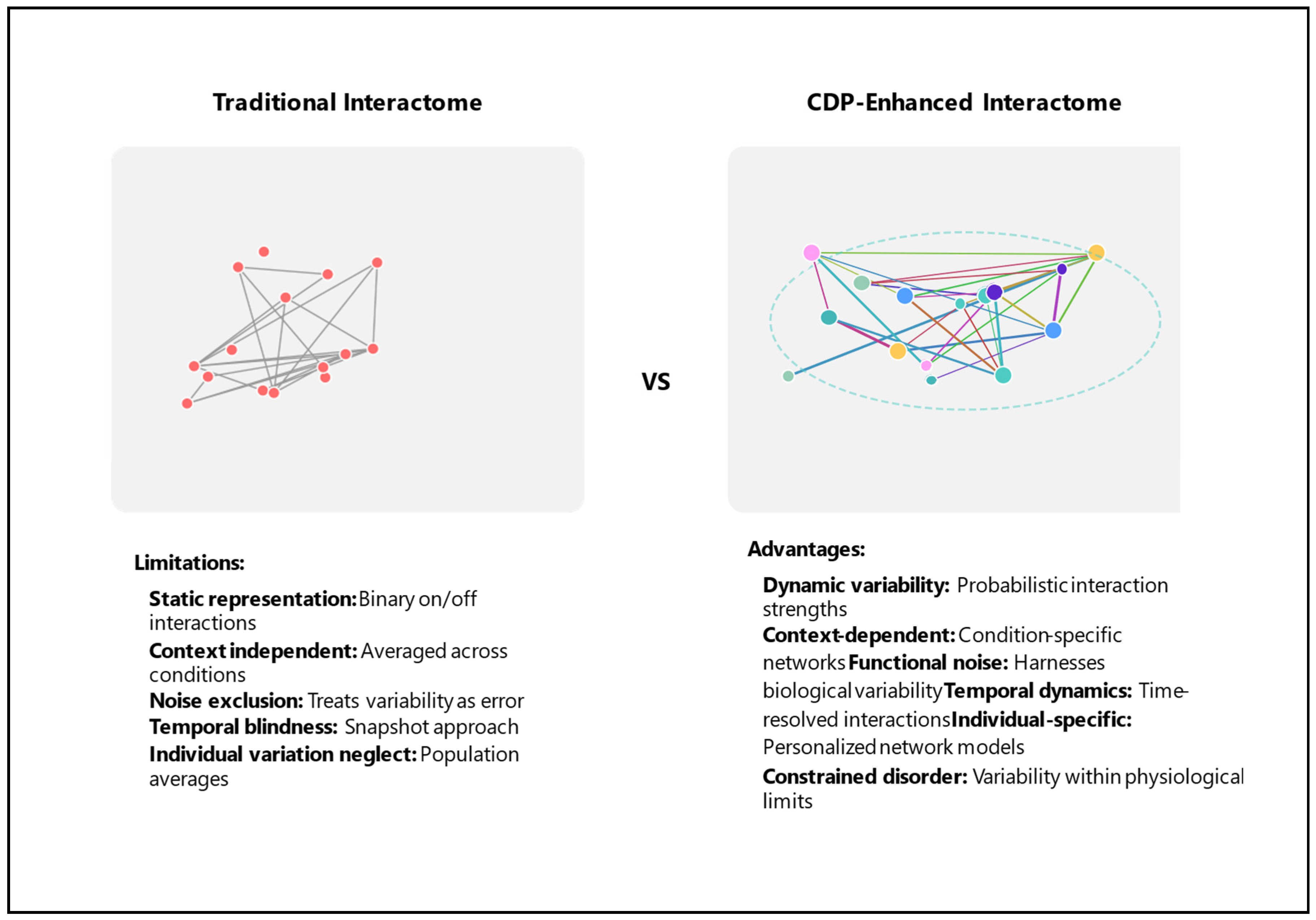
2. The Traditional Interactome: Achievements and Limitations
2.1. Historical Development and Current Methodologies
2.2. Major Achievements
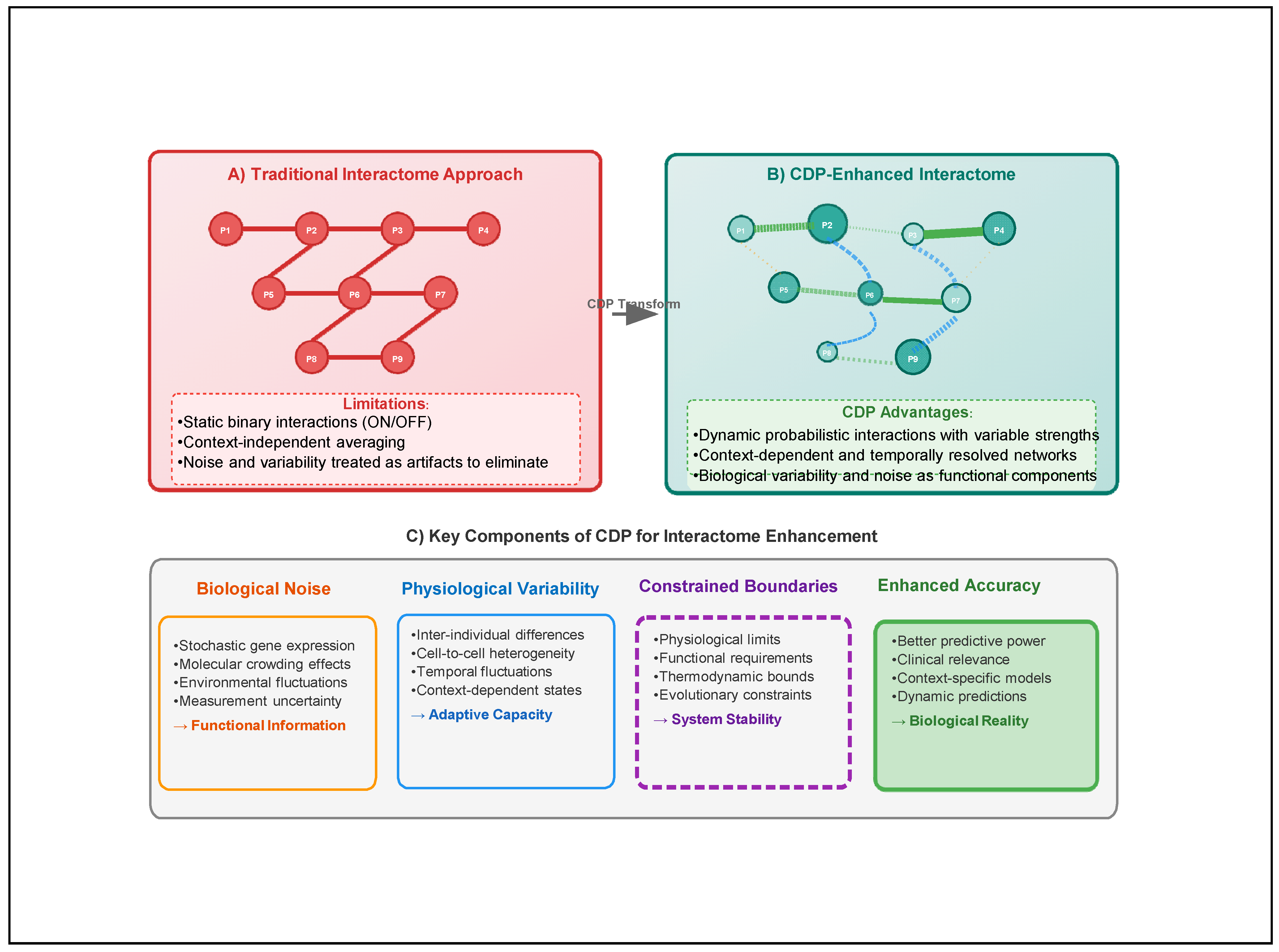
2.3. Fundamental Limitations
2.4. Consequences of These Limitations
3. The Constrained Disorder Principle: Theoretical Foundation
3.1. Conceptual Framework
3.2. Mathematical Formulation
- Energy constraints: Ensuring the system stays within thermodynamically feasible states.
- Stoichiometric constraints: Maintaining proper ratios of molecular components.
- Regulatory constraints: Meeting feedback control requirements.
- Spatial constraints: Respecting cellular compartmentalization.
- P(x) is the probability of observing state x
- f(x) represents the intrinsic variability function, capturing the natural tendencies of the system
- g(C(x)) represents the constraint weighting function, which modulates probability based on how well constraints are satisfied
- Z is the normalization constant ensuring that probabilities sum to unity
3.3. Information-Theoretic Perspective
3.4. Biological Implications
4. Applying the Constrained Disorder Principle to Interactome Analysis
4.1. Reconceptualizing Molecular Interactions
4.2. Incorporating Physiological Variability
4.3. The CDP Accounts for Embracing Biological Noise
4.4. Dynamic Network Models
5. Methodological Advances for CDP-Based Interactomics
5.1. Experimental Approaches
5.2. Computational Methods
5.3. Data Integration Strategies
6. The CDP Accounts for the Importance of Variability in Biological Systems
6.1. Molecular Level Evidence
6.2. Cellular Level Evidence
6.3. Systems Level Evidence
7. Case Studies Demonstrating How CDP-Enhanced Interactome Analysis Could Potentially Address System Malfunctions
7.1. Cancer Network Dynamics
7.2. Neurodegenerative Diseases
7.3. Immune System Function
8. Technical Implementation of CDP-Based Interactome Models
8.1. Data Collection Protocols
8.2. Statistical Analysis Methods
8.3. Network Representation
9. Validation and Benchmarking
9.1. Validation Strategies
9.2. Benchmarking Approaches
10. Future Directions and Challenges
10.1. Technical Challenges
10.2. Methodological Developments
10.3. Applications and Impact
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Przytycka, T.M.; Singh, M.; Slonim, D.K. Toward the dynamic interactome: It’s about time. Brief. Bioinform. 2010, 11, 15–29. [Google Scholar] [CrossRef]
- Vidal, M.; Cusick, M.E.; Barabási, A.L. Interactome networks and human disease. Cell 2011, 144, 986–998. [Google Scholar] [CrossRef]
- Vitorino, R. Transforming Clinical Research: The Power of High-Throughput Omics Integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.E.; Adames, N.R.; Rogers, M.F.; Kraikivski, P.; Ibele, A.; Nurzynski-Loth, K.; Kudlow, E.; Murali, T.M.; Tyson, J.J.; Peccoud, J. Genetic interactions derived from high-throughput phenotyping of 6589 yeast cell cycle mutants. Npj Syst. Biol. Appl. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; Dar, R.D. Internetwork connectivity of molecular networks across species of life. Sci. Rep. 2021, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.L.; Oltvai, Z.N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef]
- Ortiz-Vilchis, P.; De-la-Cruz-García, J.-S.; Ramirez-Arellano, A. Identification of Relevant Protein Interactions with Partial Knowledge: A Complex Network and Deep Learning Approach. Biology 2023, 12, 140. [Google Scholar] [CrossRef]
- Broderick, K.; Moutaoufik, M.T.; Saccon, T.; Malty, R.; Amin, S.; Phanse, S.; Joseph, T.P.; Zilocchi, M.; Hosseinnia, A.; Istace, Z.; et al. Human protein interaction networks of ancestral and variant SARS-CoV-2 in organ-specific cells and bodily fluids. Nat. Commun. 2025, 16, 5784. [Google Scholar] [CrossRef]
- Cusick, M.E.; Klitgord, N.; Vidal, M.; Hill, D.E. Interactome: Gateway into systems biology. Hum. Mol. Genet. 2005, 14, R171–R181. [Google Scholar] [CrossRef]
- Yakubu, R.R.; Nieves, E.; Weiss, L.M. The Methods Employed in Mass Spectrometric Analysis of Posttranslational Modifications (PTMs) and Protein-Protein Interactions (PPIs). Adv. Exp. Med. Biol. 2019, 1140, 169–198. [Google Scholar] [CrossRef]
- Nayar, G.; Altman, R.B. Heterogeneous network approaches to protein pathway prediction. Comput. Struct. Biotechnol. J. 2024, 23, 2727–2739. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2022, 51, D638–D646. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Murakami, Y.; Mizuguchi, K. Recent developments of sequence-based prediction of protein-protein interactions. Biophys. Rev. 2022, 14, 1393–1411. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; van Oudenaarden, A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell 2008, 135, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Balazsi, G.; van Oudenaarden, A.; Collins, J.J. Cellular decision making and biological noise: From microbes to mammals. Cell 2011, 144, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The constrained disorder principle defines living organisms and provides a method for correcting disturbed biological systems. Comput. Struct. Biotechnol. J. 2022, 20, 6087–6096. [Google Scholar] [CrossRef]
- Ilan, Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J. Biosci. 2019, 44, 1–12. [Google Scholar] [CrossRef]
- Ilan, Y. Generating randomness: Making the most out of disordering a false order into a real one. J. Transl. Med. 2019, 17, 49. [Google Scholar] [CrossRef]
- Ilan, Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J. Comput. Biol. 2020, 27, 20–29. [Google Scholar] [CrossRef]
- Ilan, Y. Order Through Disorder: The Characteristic Variability of Systems. Front. Cell Dev. Biol. 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The constrained-disorder principle defines the functions of systems in nature. Front. Netw. Physiol. 2024, 4, 1361915. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Giot, L.; Cagney, G.; Mansfield, T.A.; Judson, R.S.; Knight, J.R.; Lockshon, D.; Narayan, V.; Srinivasan, M.; Pochart, P.; et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 2000, 403, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Fields, S.; Song, O. A novel genetic system to detect protein-protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Gavin, A.C.; Bösche, M.; Krause, R.; Grandi, P.; Marzioch, M.; Bauer, A.; Schultz, J.; Rick, J.M.; Michon, A.M.; Cruciat, C.M.; et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 2002, 415, 141–147. [Google Scholar] [CrossRef]
- Brückner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009, 10, 2763–2788. [Google Scholar] [CrossRef]
- Feuer, E.; Zimran, G.; Shpilman, M.; Mosquna, A. A Modified Yeast Two-Hybrid Platform Enables Dynamic Control of Expression Intensities to Unmask Properties of Protein–Protein Interactions. ACS Synth. Biol. 2022, 11, 2589–2598. [Google Scholar] [CrossRef]
- Ferro, E.; Trabalzini, L. The yeast two-hybrid and related methods as powerful tools to study plant cell signalling. Plant Mol. Biol. 2013, 83, 287–301. [Google Scholar] [CrossRef]
- Shoemaker, B.A.; Panchenko, A.R. Deciphering protein-protein interactions. Part II. Computational methods to predict protein and domain interaction partners. PLoS Comput. Biol. 2007, 3, e43. [Google Scholar] [CrossRef]
- Ding, Z.; Kihara, D. Computational Methods for Predicting Protein-Protein Interactions Using Various Protein Features. Curr. Protoc. Protein Sci. 2018, 93, e62. [Google Scholar] [CrossRef]
- Grassmann, G.; Miotto, M.; Desantis, F.; Di Rienzo, L.; Tartaglia, G.G.; Pastore, A.; Ruocco, G.; Monti, M.; Milanetti, E. Computational Approaches to Predict Protein–Protein Interactions in Crowded Cellular Environments. Chem. Rev. 2024, 124, 3932–3977. [Google Scholar] [CrossRef]
- Keshava Prasad, T.S.; Goel, R.; Kandasamy, K.; Keerthikumar, S.; Kumar, S.; Mathivanan, S.; Telikicherla, D.; Raju, R.; Shafreen, B.; Venugopal, A.; et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009, 37, D767–D772. [Google Scholar] [CrossRef]
- Chatr-Aryamontri, A.; Oughtred, R.; Boucher, L.; Rust, J.; Chang, C.; Kolas, N.K.; O’Donnell, L.; Oster, S.; Theesfeld, C.; Sellam, A.; et al. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 2017, 45, D369–D379. [Google Scholar] [CrossRef]
- Peri, S.; Navarro, J.D.; Kristiansen, T.Z.; Amanchy, R.; Surendranath, V.; Muthusamy, B.; Gandhi, T.K.; Chandrika, K.N.; Deshpande, N.; Suresh, S.; et al. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004, 32, D497–D501. [Google Scholar] [CrossRef]
- Xian, L.; Wang, Y. Advances in Computational Methods for Protein–Protein Interaction Prediction. Electronics 2024, 13, 1059. [Google Scholar] [CrossRef]
- Jeong, H.; Mason, S.P.; Barabási, A.L.; Oltvai, Z.N. Lethality and centrality in protein networks. Nature 2001, 411, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Janjić, V.; Pržulj, N. Biological function through network topology: A survey of the human diseasome. Brief. Funct. Genom. 2012, 11, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, S.; Liu, C.M.; Fernie, A.R.; Yan, S. Recent Advances in Mass Spectrometry-Based Protein Interactome Studies. Mol. Cell. Proteom. 2025, 24, 100887. [Google Scholar] [CrossRef]
- Zhu, X.; Gerstein, M.; Snyder, M. Getting connected: Analysis and principles of biological networks. Genes. Dev. 2007, 21, 1010–1024. [Google Scholar] [CrossRef]
- Oti, M.; Brunner, H.G. The modular nature of genetic diseases. Clin. Genet. 2007, 71, 1–11. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based Approaches in Pharmacology. Mol. Inform. 2017, 36, 1700048. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, W.; Liu, G.; Tang, Y. Network-Based Methods for Prediction of Drug-Target Interactions. Front. Pharmacol. 2018, 9, 1134. [Google Scholar] [CrossRef]
- Perkins, J.R.; Diboun, I.; Dessailly, B.H.; Lees, J.G.; Orengo, C. Transient Protein-Protein Interactions: Structural, Functional, and Network Properties. Structure 2010, 18, 1233–1243. [Google Scholar] [CrossRef]
- Snider, J.; Kotlyar, M.; Saraon, P.; Yao, Z.; Jurisica, I.; Stagljar, I. Fundamentals of protein interaction network mapping. Mol. Syst. Biol. 2015, 11, 848. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Zhang, Z. Competition-cooperation relationship networks characterize the competition and cooperation between proteins. Sci. Rep. 2015, 5, 11619. [Google Scholar] [CrossRef]
- Bossi, A.; Lehner, B. Tissue specificity and the human protein interaction network. Mol. Syst. Biol. 2009, 5, 260. [Google Scholar] [CrossRef]
- Wright, S.N.; Colton, S.; Schaffer, L.V.; Pillich, R.T.; Churas, C.; Pratt, D.; Ideker, T. State of the interactomes: An evaluation of molecular networks for generating biological insights. Mol. Syst. Biol. 2025, 21, 1–29. [Google Scholar] [CrossRef]
- Alexopoulos, L.G.; Saez-Rodriguez, J.; Cosgrove, B.D.; Lauffenburger, D.A.; Sorger, P.K. Networks Inferred from Biochemical Data Reveal Profound Differences in Toll-like Receptor and Inflammatory Signaling between Normal and Transformed Hepatocytes. Mol. Cell. Proteom. 2010, 9, 1849–1865. [Google Scholar] [CrossRef]
- Armingol, E.; Officer, A.; Harismendy, O.; Lewis, N.E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 2021, 22, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Simkus, A.; Coolen-Maturi, T.; Coolen, F.P.A.; Bendtsen, C. Statistical Perspectives on Reproducibility: Definitions and Challenges. J. Stat. Theory Pract. 2025, 19, 40. [Google Scholar] [CrossRef]
- Gencel, M.; Cofino, G.M.; Hui, C.; Sahaf, Z.; Gauthier, L.; Matta, C.; Gagné-Leroux, D.; Tsang, D.K.L.; Philpott, D.P.; Ramathan, S.; et al. Quantifying the intra- and inter-species community interactions in microbiomes by dynamic covariance mapping. Nat. Commun. 2025, 16, 6314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ng, S.K.; Sung, J.J.Y.; Goh, W.W.B.; Wong, S.H. Data pre-processing for analyzing microbiome data—A mini review. Comput. Struct. Biotechnol. J. 2023, 21, 4804–4815. [Google Scholar] [CrossRef] [PubMed]
- de Lichtenberg, U.; Jensen, L.J.; Brunak, S.; Bork, P. Dynamic complex formation during the yeast cell cycle. Science 2005, 307, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Morris, A.; Townsend-Teague, A.; Tichit, L.; Habermann, B.; Barrat, A. Inferring cell cycle phases from a partially temporal network of protein interactions. Cell Rep. Methods 2023, 3, 100397. [Google Scholar] [CrossRef]
- Ghiassian, S.D.; Menche, J.; Barabási, A.L. A DIseAse MOdule Detection (DIAMOnD) algorithm derived from a systematic analysis of connectivity patterns of disease proteins in the human interactome. PLoS Comput. Biol. 2015, 11, e1004120. [Google Scholar] [CrossRef]
- Ke, X.; Cortina-Borja, M.; Silva, B.; Lowe, R.; Rakyan, V.; Balding, D. Integrated analysis of genome-wide genetic and epigenetic association data for identification of disease mechanisms. Epigenetics 2013, 8, 1236–1244. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sharma, G.; Karmakar, S.; Banerjee, S. Multi-OMICS approaches in cancer biology: New era in cancer therapy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167120. [Google Scholar] [CrossRef]
- Hart, G.T.; Ramani, A.K.; Marcotte, E.M. How complete are current yeast and human protein-interaction networks? Genome Biol. 2006, 7, 120. [Google Scholar] [CrossRef]
- Kosoglu, K.; Aydin, Z.; Tuncbag, N.; Gursoy, A.; Keskin, O. Structural coverage of the human interactome. Brief. Bioinform. 2024, 25, bbad496. [Google Scholar] [CrossRef]
- Nada, H.; Choi, Y.; Kim, S.; Jeong, K.S.; Meanwell, N.A.; Lee, K. New insights into protein–protein interaction modulators in drug discovery and therapeutic advance. Signal Transduct. Target. Ther. 2024, 9, 341. [Google Scholar] [CrossRef]
- Braun, P.; Tasan, M.; Dreze, M.; Barrios-Rodiles, M.; Lemmens, I.; Yu, H.; Sahalie, J.M.; Murray, R.R.; Roncari, L.; de Smet, A.-S.; et al. An experimentally derived confidence score for binary protein-protein interactions. Nat. Methods 2009, 6, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Caufield, J.H.; Uetz, P. Making the Right Choice: Critical Parameters of the Y2H Systems. Methods Mol. Biol. 2018, 1794, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Stibius, K.; Sneppen, K. Modeling the Two-Hybrid Detector: Experimental Bias on Protein Interaction Networks. Biophys. J. 2007, 93, 2562–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Wang, B.; Pan, B.; Zhang, Z.; Shen, M.; Zhao, W.; Zhang, T.; Li, S.; Liu, L. Leveraging Network Target Theory for Efficient Prediction of Drug-Disease Interactions: A Transfer Learning Approach. Adv. Sci. 2025, 12, e2409130. [Google Scholar] [CrossRef]
- Maron, B.A.; Wang, R.S.; Shevtsov, S.; Drakos, S.G.; Arons, E.; Wever-Pinzon, O.; Huggins, G.S.; Samokhin, A.O.; Oldham, W.M.; Aguib, Y.; et al. Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat. Commun. 2021, 12, 873. [Google Scholar] [CrossRef]
- Gerassy-Vainberg, S.; Starosvetsky, E.; Gaujoux, R.; Blatt, A.; Maimon, N.; Gorelik, Y.; Pressman, S.; Alpert, A.; Bar-Yoseph, H.; Dubovik, T.; et al. A personalized network framework reveals predictive axis of anti-TNF response across diseases. Cell Rep. Med. 2024, 5, 101300. [Google Scholar] [CrossRef]
- Granzotto, A.; Vissel, B.; Sensi, S.L. Lost in translation: Inconvenient truths on the utility of mouse models in Alzheimer’s disease research. elife 2024, 13, e90633. [Google Scholar] [CrossRef]
- Sah, P.; Otterstatter, M.; Leu, S.T.; Leviyang, S.; Bansal, S. Revealing mechanisms of infectious disease spread through empirical contact networks. PLoS Comput. Biol. 2021, 17, e1009604. [Google Scholar] [CrossRef]
- Galindez, G.; Sadegh, S.; Baumbach, J.; Kacprowski, T.; List, M. Network-based approaches for modeling disease regulation and progression. Comput. Struct. Biotechnol. J. 2023, 21, 780–795. [Google Scholar] [CrossRef]
- Jia, Z.C.; Yang, X.; Wu, Y.K.; Li, M.; Das, D.; Chen, M.X.; Wu, J. The Art of Finding the Right Drug Target: Emerging Methods and Strategies. Pharmacol. Rev. 2024, 76, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, S.K.; Kim, S. AI-driven drug discovery using a context-aware hybrid model to optimize drug-target interactions. Sci. Rep. 2025, 15, 35719. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinical Outcomes. Front. Digit. Health 2020, 2, 569178. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Improving Global Healthcare and Reducing Costs Using Second-Generation Artificial Intelligence-Based Digital Pills: A Market Disruptor. Int. J. Environ. Res. Public Health 2021, 18, 811. [Google Scholar] [CrossRef]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef]
- Hurvitz, N.; Ilan, Y. The Constrained-Disorder Principle Assists in Overcoming Significant Challenges in Digital Health: Moving from “Nice to Have” to Mandatory Systems. Clin. Pract. 2023, 13, 994–1014. [Google Scholar] [CrossRef]
- Sigawi, T.; Ilan, Y. Using Constrained-Disorder Principle-Based Systems to Improve the Performance of Digital Twins in Biological Systems. Biomimetics 2023, 8, 359. [Google Scholar] [CrossRef]
- Sigawi, T.; Lehmann, H.; Hurvitz, N.; Ilan, Y. Constrained Disorder Principle-Based Second-Generation Algorithms Implement Quantified Variability Signatures to Improve the Function of Complex Systems. J. Bioinform. Syst. Biol. 2023, 6, 82–89. [Google Scholar] [CrossRef]
- Ivanov, P.C.; Amaral, L.A.N.; Goldberger, A.L.; Stanley, H.E. Stochastic feedback and the regulation of biological rhythms. Europhys. Lett. 1998, 43, 363. [Google Scholar] [CrossRef]
- El-Haj, M.; Kanovitch, D.; Ilan, Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: A novel platform for designing personalized immunotherapies. Immunol. Res. 2019, 67, 337–347. [Google Scholar] [CrossRef]
- Ilan, Y. Randomness in microtubule dynamics: An error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019, 43, 739–748. [Google Scholar] [CrossRef]
- Ilan, Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J. Cell Physiol. 2019, 234, 7923–7937. [Google Scholar] [CrossRef] [PubMed]
- Ilan-Ber, T.; Ilan, Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, E.; Kenig, A.; Ilan, Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol. Res. Perspect. 2020, 8, e00616. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front. Immunol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Ilan, Y. Microtubules as a potential platform for energy transfer in biological systems: A target for implementing individualized, dynamic variability patterns to improve organ function. Mol. Cell Biochem. 2023, 478, 375–392. [Google Scholar] [CrossRef]
- Ilan, Y. Enhancing the plasticity, proper function and efficient use of energy of the Sun, genes and microtubules using variability. Clin. Transl. Discov. 2022, 2, e103. [Google Scholar] [CrossRef]
- Shabat, Y.; Lichtenstein, Y.; Ilan, Y. Short-Term Cohousing of Sick with Healthy or Treated Mice Alleviates the Inflammatory Response and Liver Damage. Inflammation 2021, 44, 518–525. [Google Scholar] [CrossRef]
- Ilan, Y. Making use of noise in biological systems. Prog. Biophys. Mol. Biol. 2023, 178, 83–90. [Google Scholar] [CrossRef]
- Rotnemer-Golinkin, D.; Ilan, Y. Personalized-Inherent Variability in a Time-Dependent Immune Response: A Look into the Fifth Dimension in Biology. Pharmacology 2022, 107, 417–422. [Google Scholar] [CrossRef]
- Ilan, Y. Using the Constrained Disorder Principle to Navigate Uncertainties in Biology and Medicine: Refining Fuzzy Algorithms. Biology 2024, 13, 830. [Google Scholar] [CrossRef]
- Ilan, Y. The Constrained Disorder Principle Overcomes the Challenges of Methods for Assessing Uncertainty in Biological Systems. J. Pers. Med. 2025, 15, 10. [Google Scholar] [CrossRef]
- Ilan, Y. The constrained disorder principle and the law of increasing functional information: The elephant versus the Moeritherium. Comput. Struct. Biotechnol. Rep. 2025, 2, 100040. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Abbott, D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 2009, 5, e1000348. [Google Scholar] [CrossRef] [PubMed]
- Adar, O.; Shakargy, J.D.; Ilan, Y. The Constrained Disorder Principle: Beyond Biological Allostasis. Biology 2025, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The Relationship Between Biological Noise and Its Application: Understanding System Failures and Suggesting a Method to Enhance Functionality Based on the Constrained Disorder Principle. Biology 2025, 14, 349. [Google Scholar] [CrossRef]
- Azmanov, H.; Ross, E.L.; Ilan, Y. Establishment of an Individualized Chronotherapy, Autonomic Nervous System, and Variability-Based Dynamic Platform for Overcoming the Loss of Response to Analgesics. Pain. Physician 2021, 24, 243–252. [Google Scholar]
- Ilan, Y. Constrained disorder principle-based variability is fundamental for biological processes: Beyond biological relativity and physiological regulatory networks. Progress Biophys. Mol. Biol. 2023, 180, 37–48. [Google Scholar] [CrossRef]
- Kessler, A.; Weksler-Zangen, S.; Ilan, Y. Role of the Immune System and the Circadian Rhythm in the Pathogenesis of Chronic Pancreatitis: Establishing a Personalized Signature for Improving the Effect of Immunotherapies for Chronic Pancreatitis. Pancreas 2020, 49, 1024–1032. [Google Scholar] [CrossRef]
- Ishay, Y.; Kolben, Y.; Kessler, A.; Ilan, Y. Role of circadian rhythm and autonomic nervous system in liver function: A hypothetical basis for improving the management of hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G400–G412. [Google Scholar] [CrossRef]
- Kolben, Y.; Weksler-Zangen, S.; Ilan, Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes. Rev. 2021, 22, e13108. [Google Scholar] [CrossRef] [PubMed]
- Kenig, A.; Kolben, Y.; Asleh, R.; Amir, O.; Ilan, Y. Improving Diuretic Response in Heart Failure by Implementing a Patient-Tailored Variability and Chronotherapy-Guided Algorithm. Front. Cardiovasc. Med. 2021, 8, 695547. [Google Scholar] [CrossRef] [PubMed]
- Potruch, A.; Khoury, S.T.; Ilan, Y. The role of chronobiology in drug-resistance epilepsy: The potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure 2020, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Isahy, Y.; Ilan, Y. Improving the long-term response to antidepressants by establishing an individualized platform based on variability and chronotherapy. Int. J. Clin. Pharmacol. Ther. 2021, 59, 768–774. [Google Scholar] [CrossRef]
- Khoury, T.; Ilan, Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: A Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front. Immunol. 2019, 10, 2726. [Google Scholar] [CrossRef]
- Khoury, T.; Ilan, Y. Platform introducing individually tailored variability in nerve stimulations and dietary regimen to prevent weight regain following weight loss in patients with obesity. Obes. Res. Clin. Pract. 2021, 15, 114–123. [Google Scholar] [CrossRef]
- Kenig, A.; Ilan, Y. A Personalized Signature and Chronotherapy-Based Platform for Improving the Efficacy of Sepsis Treatment. Front. Physiol. 2019, 10, 1542. [Google Scholar] [CrossRef]
- Ilan, Y. Why targeting the microbiome is not so successful: Can randomness overcome the adaptation that occurs following gut manipulation? Clin. Exp. Gastroenterol. 2019, 12, 209–217. [Google Scholar] [CrossRef]
- Gelman, R.; Bayatra, A.; Kessler, A.; Schwartz, A.; Ilan, Y. Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: An algorithm-based method for overcoming resistance to antiviral agents. Emerg. Microbes Infect. 2020, 9, 1397–1406. [Google Scholar] [CrossRef]
- Ishay, Y.; Potruch, A.; Schwartz, A.; Berg, M.; Jamil, K.; Agus, S.; Ilan, Y. A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: An adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage. Biomed. Pharmacother. 2021, 143, 112228. [Google Scholar] [CrossRef]
- Ilan, Y.; Spigelman, Z. Establishing patient-tailored variability-based paradigms for anti-cancer therapy: Using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat. Res. Commun. 2020, 25, 100240. [Google Scholar] [CrossRef]
- Hurvitz, N.; Azmanov, H.; Kesler, A.; Ilan, Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021, 29, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Digital Medical Cannabis as Market Differentiator: Second-Generation Artificial Intelligence Systems to Improve Response. Front. Med. 2021, 8, 788777. [Google Scholar] [CrossRef] [PubMed]
- Gelman, R.; Berg, M.; Ilan, Y. A Subject-Tailored Variability-Based Platform for Overcoming the Plateau Effect in Sports Training: A Narrative Review. Int. J. Environ. Res. Public. Health 2022, 19, 1722. [Google Scholar] [CrossRef] [PubMed]
- Azmanov, H.; Bayatra, A.; Ilan, Y. Digital Analgesic Comprising a Second-Generation Digital Health System: Increasing Effectiveness by Optimizing the Dosing and Minimizing Side Effects. J. Pain. Res. 2022, 15, 1051–1060. [Google Scholar] [CrossRef]
- Hurvitz, N.; Elkhateeb, N.; Sigawi, T.; Rinsky-Halivni, L.; Ilan, Y. Improving the effectiveness of anti-aging modalities by using the constrained disorder principle-based management algorithms. Front. Aging 2022, 3, 1044038. [Google Scholar] [CrossRef]
- Kolben, Y.; Azmanov, H.; Gelman, R.; Dror, D.; Ilan, Y. Using chronobiology-based second-generation artificial intelligence digital system for overcoming antimicrobial drug resistance in chronic infections. Ann. Med. 2023, 55, 311–318. [Google Scholar] [CrossRef]
- Lehmann, H.; Arkadir, D.; Ilan, Y. Methods for Improving Brain-Computer Interface: Using A Brain-Directed Adjuvant and A Second-Generation Artificial Intelligence System to Enhance Information Streaming and Effectiveness of Stimuli. Int. J. Appl. Biol. Pharm. Technol. 2023, 14, 42–52. [Google Scholar] [CrossRef]
- Adar, O.; Hollander, A.; Ilan, Y. The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation. Adv. Respir. Med. 2023, 91, 350–367. [Google Scholar] [CrossRef]
- Ilan, Y. The Constrained Disorder Principle Accounts for The Structure and Function of Water as An Ultimate Biosensor and Bioreactor in Biological Systems. Int. J. Appl. Biol. Pharm. Technol. 2023, 14, 31–41. [Google Scholar] [CrossRef]
- Sigawi, T.; Hamtzany, O.; Shakargy, J.D.; Ilan, Y. The Constrained Disorder Principle May Account for Consciousness. Brain Sci. 2024, 14, 209. [Google Scholar] [CrossRef]
- Ilan, Y. Special Issue “Computer-Aided Drug Discovery and Treatment”. Int. J. Mol. Sci. 2024, 25, 2683. [Google Scholar] [CrossRef]
- Hurvitz, N.; Dinur, T.; Revel-Vilk, S.; Agus, S.; Berg, M.; Zimran, A.; Ilan, Y. A Feasibility Open-Labeled Clinical Trial Using a Second-Generation Artificial-Intelligence-Based Therapeutic Regimen in Patients with Gaucher Disease Treated with Enzyme Replacement Therapy. J. Clin. Med. 2024, 13, 3325. [Google Scholar] [CrossRef]
- Ilan, Y. Free Will as Defined by the Constrained Disorder Principle: A Restricted, Mandatory, Personalized, Regulated Process for Decision-Making. Integr. Psychol. Behav. Sci. 2024, 58, 1843–1875. [Google Scholar] [CrossRef]
- Ilan, Y. The Constrained Disorder Principle Defines Mitochondrial Variability and Provides A Platform for A Novel Mechanism for Improved Functionality of Complex Systems. Fortune J. Health Sci. 2024, 7, 338–347. [Google Scholar] [CrossRef]
- Sigawi, T.; Israeli, A.; Ilan, Y. Harnessing Variability Signatures and Biological Noise May Enhance Immunotherapies’ Efficacy and Act as Novel Biomarkers for Diagnosing and Monitoring Immune-Associated Disorders. Immunotargets Ther. 2024, 13, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol. Ther. Methods Clin. Dev. 2020, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Bayatra, A.; Nasserat, R.; Ilan, Y. Overcoming Low Adherence to Chronic Medications by Improving their Effectiveness Using a Personalized Second-generation Digital System. Curr. Pharm. Biotechnol. 2024, 25, 2078–2088. [Google Scholar] [CrossRef]
- Gelman, R.; Hurvitz, N.; Nesserat, R.; Kolben, Y.; Nachman, D.; Jamil, K.; Agus, S.; Asleh, R.; Amir, O.; Berg, M.; et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. Biomed. Pharmacother. 2023, 161, 114334. [Google Scholar] [CrossRef]
- Sigawi, T.; Gelman, R.; Maimon, O.; Yossef, A.; Hemed, N.; Agus, S.; Berg, M.; Ilan, Y.; Popovtzer, A. Improving the response to lenvatinib in partial responders using a Constrained-Disorder-Principle-based second-generation artificial intelligence-therapeutic regimen: A proof-of-concept open-labeled clinical trial. Front. Oncol. 2024, 14, 1426426. [Google Scholar] [CrossRef]
- Hurvitz, N.; Lehman, H.; Hershkovitz, Y.; Kolben, Y.; Jamil, K.; Agus, S.; Berg, M.; Aamar, S.; Ilan, Y. A constrained disorder principle-based second-generation artificial intelligence digital medical cannabis system: A real-world data analysis. J. Public Health Res. 2025, 14, 22799036251337640. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Chaos Me! Amazon Digital Services LLC-Kdp Print US: Seattle, WA, USA, 2019. [Google Scholar]
- Shabat, Y.; Ilan, Y. Correlations between components of the immune system. F1000Research 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Shabat, Y.; Rotnemer-Golinkin, D.; Zolotarov, L.; Ilan, Y. Inter-organ correlations in inflammation regulation: A novel biological paradigm in a murine model. J. Med. Life 2025, 18, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Gupta, S.P.; Masand, N.; Balasubramanian, K. Experimental and computational models to understand protein-ligand, metal-ligand and metal-DNA interactions pertinent to targeted cancer and other therapies. Eur. J. Med. Chem. Rep. 2024, 10, 100133. [Google Scholar] [CrossRef]
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering protein function: From classification to complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef]
- Vauquelin, G.; Charlton, S.J. Exploring avidity: Understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 2013, 168, 1771–1785. [Google Scholar] [CrossRef]
- White, Z.T.; Smith, A.M.; Vernerey, F.J. Mechanical cooperation between time-dependent and covalent bonds in molecular damage of polymer networks. Commun. Phys. 2025, 8, 265. [Google Scholar] [CrossRef]
- Samih, A.; de Moura Ferreira, M.A.; Nikoloski, Z. Gene expression and protein abundance: Just how associated are these molecular traits? Biotechnol. Adv. 2026, 86, 108720. [Google Scholar] [CrossRef]
- Munro, V.; Kelly, V.; Messner, C.B.; Kustatscher, G. Cellular control of protein levels: A systems biology perspective. Proteomics 2024, 24, 2200220. [Google Scholar] [CrossRef]
- Clerc, I.; Sagar, A.; Barducci, A.; Sibille, N.; Bernadó, P.; Cortés, J. The diversity of molecular interactions involving intrinsically disordered proteins: A molecular modeling perspective. Comput. Struct. Biotechnol. J. 2021, 19, 3817–3828. [Google Scholar] [CrossRef]
- Monti, A.; Vitagliano, L.; Caporale, A.; Ruvo, M.; Doti, N. Targeting Protein–Protein Interfaces with Peptides: The Contribution of Chemical Combinatorial Peptide Library Approaches. Int. J. Mol. Sci. 2023, 24, 7842. [Google Scholar] [CrossRef]
- Filipov, V.; Arleo, A.; Miksch, S. Are We There Yet? A Roadmap of Network Visualization from Surveys to Task Taxonomies. Comput. Graph. Forum 2023, 42, e14794. [Google Scholar] [CrossRef]
- Virolainen, S.J.; VonHandorf, A.; Viel, K.; Weirauch, M.T.; Kottyan, L.C. Gene-environment interactions and their impact on human health. Genes Immun. 2023, 24, 1–11. [Google Scholar] [CrossRef]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic gene expression in a single cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, V.; Nita-Lazar, A. Recent Advancements in Subcellular Proteomics: Growing Impact of Organellar Protein Niches on the Understanding of Cell Biology. J. Proteome Res. 2024, 23, 2700–2722. [Google Scholar] [CrossRef] [PubMed]
- Raser, J.M.; O’Shea, E.K. Noise in gene expression: Origins, consequences, and control. Science 2005, 309, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Razooky, B.S.; Dar, R.D.; Weinberger, L.S. Dynamics of protein noise can distinguish between alternate sources of gene-expression variability. Mol. Syst. Biol. 2012, 8, 607. [Google Scholar] [CrossRef]
- Bertaux, F.; Marguerat, S.; Shahrezaei, V. Division rate, cell size and proteome allocation: Impact on gene expression noise and implications for the dynamics of genetic circuits. R. Soc. Open Sci. 2018, 5, 172234. [Google Scholar] [CrossRef]
- Raynold, A.A.M.; Li, D.; Chang, L.; Gautrot, J.E. Competitive binding and molecular crowding regulate the cytoplasmic interactome of non-viral polymeric gene delivery vectors. Nat. Commun. 2021, 12, 6445. [Google Scholar] [CrossRef]
- Nguyen, J.; Lara-Gutiérrez, J.; Stocker, R. Environmental fluctuations and their effects on microbial communities, populations and individuals. FEMS Microbiol. Rev. 2021, 45, fuaa068. [Google Scholar] [CrossRef]
- Lai, K.S.; Goh, Z.Z.; Ghazali, S.M. The effects of temperature and pH change on the snapping sound characteristic of Alpheus edwardsii. J. Environ. Biol. 2021, 42, 832–839. [Google Scholar] [CrossRef]
- Harton, M.D.; Batchelor, E. Determining the Limitations and Benefits of Noise in Gene Regulation and Signal Transduction through Single Cell, Microscopy-Based Analysis. J. Mol. Biol. 2017, 429, 1143–1154. [Google Scholar] [CrossRef]
- Pilpel, Y. Noise in Biological Systems: Pros, Cons, and Mechanisms of Control. In Yeast Systems Biology. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 759, pp. 407–425. [Google Scholar] [CrossRef]
- Eling, N.; Morgan, M.D.; Marioni, J.C. Challenges in measuring and understanding biological noise. Nat. Rev. Genet. 2019, 20, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.G.; Pefani, D.E.; Pateras, I.S.; Trougakos, I.P. Integrating the DNA damage and protein stress responses during cancer development and treatment. J. Pathol. 2018, 246, 12–40. [Google Scholar] [CrossRef]
- Bhandary, S.; Kaur, T.; Banerjee, T.; Dutta, P.S. Network resilience of FitzHugh-Nagumo neurons in the presence of nonequilibrium dynamics. Phys. Rev. E 2021, 103, 022314. [Google Scholar] [CrossRef]
- Duan, G.; Walther, D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol. 2015, 11, e1004049. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Vistain, L.; Van Phan, H.; Keisham, B.; Jordi, C.; Chen, M.; Reddy, S.T.; Tay, S. Quantification of extracellular proteins, protein complexes and mRNAs in single cells by proximity sequencing. Nat. Methods 2022, 19, 1578–1589. [Google Scholar] [CrossRef]
- Ma, R.; Huang, J.; Jiang, T.; Ma, W. A mini-review of single-cell Hi-C embedding methods. Comput. Struct. Biotechnol. J. 2024, 23, 4027–4035. [Google Scholar] [CrossRef]
- Rahmati, S.; Emili, A. Proximity Labeling: Precise Proteomics Technology for Mapping Receptor Protein Neighborhoods at the Cancer Cell Surface. Cancers 2025, 17, 179. [Google Scholar] [CrossRef]
- Qian, Y.; Celiker, O.T.; Wang, Z.; Guner-Ataman, B.; Boyden, E.S. Temporally multiplexed imaging of dynamic signaling networks in living cells. Cell 2023, 186, 5656–5672.e5621. [Google Scholar] [CrossRef] [PubMed]
- Reicher, A.; Reiniš, J.; Ciobanu, M.; Růžička, P.; Malik, M.; Siklos, M.; Kartysh, V.; Tomek, T.; Koren, A.; Rendeiro, A.F.; et al. Pooled multicolour tagging for visualizing subcellular protein dynamics. Nat. Cell Biol. 2024, 26, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, D.; Smith, G.R.; Bouhaddou, M.; Stern, A.D.; Erskine, J.; Birtwistle, M.R. Network inference from perturbation time course data. NPJ Syst. Biol. Appl. 2022, 8, 42. [Google Scholar] [CrossRef]
- Yu, H.; Qian, W.; Song, Y.; Welch, J.D. PerturbNet predicts single-cell responses to unseen chemical and genetic perturbations. Mol. Syst. Biol. 2025, 21, 960–982. [Google Scholar] [CrossRef]
- Krieger, J.M.; Sorzano, C.O.S.; Carazo, J.M.; Bahar, I. Protein dynamics developments for the large scale and cryoEM: Case study of ProDy 2.0. Acta Crystallogr. D Struct. Biol. 2022, 78, 399–409. [Google Scholar] [CrossRef]
- Sanches, P.H.G.; de Melo, N.C.; Porcari, A.M.; de Carvalho, L.M. Integrating Molecular Perspectives: Strategies for Comprehensive Multi-Omics Integrative Data Analysis and Machine Learning Applications in Transcriptomics, Proteomics, and Metabolomics. Biology 2024, 13, 848. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Qi, L.; Zalloua, P. From omics to AI—Mapping the pathogenic pathways in type 2 diabetes. FEBS Lett. 2025. early view. [Google Scholar] [CrossRef]
- Bielza, C.; Larrañaga, P. Bayesian networks in neuroscience: A survey. Front. Comput. Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef]
- Wang, Q.-A.; Lu, A.-W.; Ni, Y.-Q.; Wang, J.-F.; Ma, Z.-G. Bayesian Network in Structural Health Monitoring: Theoretical Background and Applications Review. Sensors 2025, 25, 3577. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liang, L.; Liu, L.; Tang, M.J. Graph Neural Networks and Their Current Applications in Bioinformatics. Front. Genet. 2021, 12, 690049. [Google Scholar] [CrossRef]
- Sartori, F.; Codicè, F.; Caranzano, I.; Rollo, C.; Birolo, G.; Fariselli, P.; Pancotti, C. A Comprehensive Review of Deep Learning Applications with Multi-Omics Data in Cancer Research. Genes 2025, 16, 648. [Google Scholar] [CrossRef]
- Farrokhi, Z.; Pirgazi, J.; Sorkhi, A.G. An effective deep learning and graph neural network approach for accurate prediction of LncRNA-disease associations. Biomed. Signal Process. Control 2026, 112, 108431. [Google Scholar] [CrossRef]
- Khazaal, A.; Vafaee, F. From Static to Dynamic: Exploring Temporal Networks in Systems Biology. arXiv 2025, arXiv:2505.10741. [Google Scholar] [CrossRef]
- Sarıyüce, A.E. A powerful lens for temporal network analysis: Temporal motifs. Discov. Data 2025, 3, 14. [Google Scholar] [CrossRef]
- Hasanaj, E.; Póczos, B.; Bar-Joseph, Z. Recovering time-varying networks from single-cell data. Bioinformatics 2025, 41, i628–i636. [Google Scholar] [CrossRef] [PubMed]
- Alber, M.; Buganza Tepole, A.; Cannon, W.R.; De, S.; Dura-Bernal, S.; Garikipati, K.; Karniadakis, G.; Lytton, W.W.; Perdikaris, P.; Petzold, L.; et al. Integrating machine learning and multiscale modeling—Perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. Npj Digit. Med. 2019, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Garfinkel, A.; Weiss, J.N.; Nivala, M. Multi-scale Modeling in Biology: How to Bridge the Gaps between Scales? Prog. Biophys. Mol. Biol. 2011, 107, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Fogo, G.M.; Torres Torres, F.J.; Speas, R.L.; Anzell, A.R.; Sanderson, T.H. Agent-based modeling of neuronal mitochondrial dynamics using intrinsic variables of individual mitochondria. iScience 2025, 28, 112390. [Google Scholar] [CrossRef]
- Siddique, S.; Haque, M.A.; George, R.; Gupta, K.D.; Gupta, D.; Faruk, M.J.H. Survey on Machine Learning Biases and Mitigation Techniques. Digital 2024, 4, 1–68. [Google Scholar] [CrossRef]
- Kiar, G.; Mumford, J.A.; Xu, T.; Vogelstein, J.T.; Glatard, T.; Milham, M.P. Why experimental variation in neuroimaging should be embraced. Nat. Commun. 2024, 15, 9411. [Google Scholar] [CrossRef]
- Bertolini, R.; Finch, S.J.; Nehm, R.H. Quantifying variability in predictions of student performance: Examining the impact of bootstrap resampling in data pipelines. Comput. Educ. Artif. Intell. 2022, 3, 100067. [Google Scholar] [CrossRef]
- Gel, Y.R.; Lyubchich, V.; Ramirez Ramirez, L.L. Bootstrap quantification of estimation uncertainties in network degree distributions. Sci. Rep. 2017, 7, 5807. [Google Scholar] [CrossRef]
- Athieniti, E.; Spyrou, G.M. A guide to multi-omics data collection and integration for translational medicine. Comput. Struct. Biotechnol. J. 2023, 21, 134–149. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, W.; Tan, X.; Bao, Y.-J. Network-based multi-omics integrative analysis methods in drug discovery: A systematic review. BioData Min. 2025, 18, 27. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Motsinger-Reif, A.A.; Reif, D.M.; Akhtari, F.S.; House, J.S.; Campbell, C.R.; Messier, K.P.; Fargo, D.C.; Bowen, T.A.; Nadadur, S.S.; Schmitt, C.P.; et al. Gene-environment interactions within a precision environmental health framework. Cell Genom. 2024, 4, 100591. [Google Scholar] [CrossRef]
- Hayat, U.; Ke, C.; Wang, L.; Zhu, G.; Fang, W.; Wang, X.; Chen, C.; Li, Y.; Wu, J. Using Quantitative Trait Locus Mapping and Genomic Resources to Improve Breeding Precision in Peaches: Current Insights and Future Prospects. Plants 2025, 14, 175. [Google Scholar] [CrossRef]
- Wu, S.; Chen, D.; Snyder, M.P. Network biology bridges the gaps between quantitative genetics and multi-omics to map complex diseases. Curr. Opin. Chem. Biol. 2022, 66, 102101. [Google Scholar] [CrossRef]
- Chapal, M.; Mintzer, S.; Brodsky, S.; Carmi, M.; Barkai, N. Resolving noise–control conflict by gene duplication. PLOS Biol. 2019, 17, e3000289. [Google Scholar] [CrossRef]
- Niazi, S.K. Protein Catalysis Through Structural Dynamics: A Comprehensive Analysis of Energy Conversion in Enzymatic Systems and Its Computational Limitations. Pharmaceuticals 2025, 18, 951. [Google Scholar] [CrossRef]
- Cui, X.; Ge, L.; Chen, X.; Lv, Z.; Wang, S.; Zhou, X.; Zhang, G. Beyond static structures: Protein dynamic conformations modeling in the post-AlphaFold era. Brief. Bioinform. 2025, 26, bbaf340. [Google Scholar] [CrossRef]
- Campitelli, P.; Kazan, I.C.; Hamilton, S.; Ozkan, S.B. Dynamic Allostery: Evolution’s Double-Edged Sword in Protein Function and Disease. J. Mol. Biol. 2025, 437, 169175. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, J.; Wu, S.; Tang, W.; Zhang, J.R.; Zhu, W.; Zhu, J.J.; Chen, Z. Single molecule-driven nanomotors reveal the dynamic-disordered chemomechanical transduction of active enzymes. Sci. Adv. 2025, 11, eads0446. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Valueva, A.A.; Ershova, M.O.; Pleshakova, T.O. AFM for Studying the Functional Activity of Enzymes. Biomolecules 2025, 15, 574. [Google Scholar] [CrossRef]
- Whitney, P.H.; Lionnet, T. The method in the madness: Transcriptional control from stochastic action at the single-molecule scale. Curr. Opin. Struct. Biol. 2024, 87, 102873. [Google Scholar] [CrossRef]
- Lenstra, T.L.; Rodriguez, J.; Chen, H.; Larson, D.R. Transcription Dynamics in Living Cells. Annu. Rev. Biophys. 2016, 45, 25–47. [Google Scholar] [CrossRef]
- Tuğrul, M.; Paixão, T.; Barton, N.H.; Tkačik, G. Dynamics of Transcription Factor Binding Site Evolution. PLoS Genet. 2015, 11, e1005639. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Y.; Zhu, J.; Cheng, X.; Liu, L.; Hammerschmidt, K.; Zhou, J.; Cai, Z. Bet hedging in a unicellular microalga. Nat. Commun. 2024, 15, 2063. [Google Scholar] [CrossRef]
- Spratt, M.R.; Lane, K. Navigating Environmental Transitions: The Role of Phenotypic Variation in Bacterial Responses. mBio 2022, 13, e0221222. [Google Scholar] [CrossRef]
- Villa Martín, P.; Muñoz, M.A.; Pigolotti, S. Bet-hedging strategies in expanding populations. PLoS Comput. Biol. 2019, 15, e1006529. [Google Scholar] [CrossRef]
- Burggren, W.W.; Mendez-Sanchez, J.F. “Bet hedging” against climate change in developing and adult animals: Roles for stochastic gene expression, phenotypic plasticity, epigenetic inheritance and adaptation. Front. Physiol. 2023, 14, 1245875. [Google Scholar] [CrossRef]
- Suzuki, Y.; Asakawa, N. Stochastic Resonance in Organic Electronic Devices. Polymers 2022, 14, 747. [Google Scholar] [CrossRef]
- White, O.; Babič, J.; Trenado, C.; Johannsen, L.; Goswami, N. The Promise of Stochastic Resonance in Falls Prevention. Front. Physiol. 2019, 9, 1865. [Google Scholar] [CrossRef]
- Harrison, D.L.; Fang, Y.; Huang, J. T-Cell Mechanobiology: Force Sensation, Potentiation, and Translation. Front. Phys. 2019, 7, 45. [Google Scholar] [CrossRef]
- Gatlin, V.; Gupta, S.; Romero, S.; Chapkin, R.S.; Cai, J.J. Exploring cell-to-cell variability and functional insights through differentially variable gene analysis. Npj Syst. Biol. Appl. 2025, 11, 29. [Google Scholar] [CrossRef]
- Sherman, M.S.; Lorenz, K.; Lanier, M.H.; Cohen, B.A. Cell-to-cell variability in the propensity to transcribe explains correlated fluctuations in gene expression. Cell Syst. 2015, 1, 315–325. [Google Scholar] [CrossRef]
- Herrera, J.; Bensussen, A.; García-Gómez, M.L.; Garay-Arroyo, A.; Álvarez-Buylla, E.R. A system-level model reveals that transcriptional stochasticity is required for hematopoietic stem cell differentiation. NPJ Syst. Biol. Appl. 2024, 10, 145. [Google Scholar] [CrossRef]
- Malik, S.; Stokes Iii, J.; Manne, U.; Singh, R.; Mishra, M.K. Understanding the significance of biological clock and its impact on cancer incidence. Cancer Lett. 2022, 527, 80–94. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Vakali, E.; Rigopoulos, D.; Dinas, P.C.; Drosatos, I.A.; Theodosiadi, A.G.; Vazeou, A.; Stergiou, G.; Kollias, A. Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review. J. Clin. Med. 2023, 12, 2362. [Google Scholar] [CrossRef]
- Ahsan, M.; Abualait, T.; Al-Subaiei, M.; Al Muslem, W.; Aldokhayyil, M.; Nuhmani, S.; Alzahrani, A. Determining the characteristics of gait variability with a preferred walking speed in hypertensive and normotensive participants. Clin. Epidemiol. Glob. Health 2023, 23, 101344. [Google Scholar] [CrossRef]
- Iliopoulou, C.; Makridis, M.A.; Kouvelas, A. Improving transit network resilience against disruptions through path redundancy. Socio-Econ. Plan. Sci. 2025, 100, 102228. [Google Scholar] [CrossRef]
- Qi, X.; Mei, G. Network Resilience: Definitions, approaches, and applications. J. King Saud. Univ.-Comput. Inf. Sci. 2024, 36, 101882. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, H.; Chen, Y.; Wei, L.; Liu, J.; Nielsen, J.; Chen, Y.; Xu, N. Relieving metabolic burden to improve robustness and bioproduction by industrial microorganisms. Biotechnol. Adv. 2024, 74, 108401. [Google Scholar] [CrossRef]
- Alcalde Cuesta, F.; González Sequeiros, P.; Lozano Rojo, Á. Exploring the topological sources of robustness against invasion in biological and technological networks. Sci. Rep. 2016, 6, 20666. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.; Zhang, B. FW-PSO Algorithm to Enhance the Invulnerability of Industrial Wireless Sensor Networks Topology. Sensors 2020, 20, 1114. [Google Scholar] [CrossRef] [PubMed]
- Eigenfeld, M.; Schwaminger, S.P. Cellular variability as a driver for bioprocess innovation and optimization. Biotechnol. Adv. 2025, 79, 108528. [Google Scholar] [CrossRef]
- Erdoğan, A.N.; Dasmeh, P.; Socha, R.D.; Chen, J.Z.; Life, B.E.; Jun, R.; Kiritchkov, L.; Kehila, D.; Serohijos, A.W.R.; Tokuriki, N. Neutral drift upon threshold-like selection promotes variation in antibiotic resistance phenotype. Nat. Commun. 2024, 15, 10813. [Google Scholar] [CrossRef]
- Nussinov, R.; Yavuz, B.R.; Jang, H. Drug resistance and tumor heterogeneity: Cells and ensembles. Biophys. Rev. 2025, 17, 759–779. [Google Scholar] [CrossRef]
- Hong, S.; Baek, S.H.; Lai, M.K.P.; Arumugam, T.V.; Jo, D.G. Aging-associated sensory decline and Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 93. [Google Scholar] [CrossRef]
- Poon, M.M.L.; Farber, D.L. The Whole Body as the System in Systems Immunology. iScience 2020, 23, 101509. [Google Scholar] [CrossRef]
- Del Pozo-Yauner, L.; Herrera, G.A.; Perez Carreon, J.I.; Turbat-Herrera, E.A.; Rodriguez-Alvarez, F.J.; Ruiz Zamora, R.A. Role of the mechanisms for antibody repertoire diversification in monoclonal light chain deposition disorders: When a friend becomes foe. Front. Immunol. 2023, 14, 1203425. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W. Advances in tumor subclone formation and mechanisms of growth and invasion. J. Transl. Med. 2025, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Tieng, F.Y.F.; Lee, L.H.; Ab Mutalib, N.S. Single-cell RNA-sequencing of circulating tumour cells: A practical guide to workflow and translational applications. Cancer Metastasis Rev. 2025, 44, 75. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.; Garnica, J.; Carmona, S.J. Identification of malignant cells in single-cell transcriptomics data. Commun. Biol. 2025, 8, 1264. [Google Scholar] [CrossRef]
- Cosci, I.; Salizzato, V.; Del Fiore, P.; Pigozzo, J.; Guarneri, V.; Mocellin, S.; Ferlin, A.; Mathlouthi, S.; Piccin, L.; Garofalo, M. Molecular Basis of BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Pharmaceuticals 2025, 18, 1235. [Google Scholar] [CrossRef]
- Singh, K.; Jacobs, B.A. A Network Based Model for Predicting Spatial Progression of Metastasis. Bull. Math. Biol. 2025, 87, 65. [Google Scholar] [CrossRef]
- Brooks, A.; Zhang, Y.; Chen, J.; Zhao, C.-X. Cancer Metastasis-on-a-Chip for Modeling Metastatic Cascade and Drug Screening. Adv. Healthc. Mater. 2024, 13, 2302436. [Google Scholar] [CrossRef]
- Liu, R.-N.; Kang, Y.-M. Stochastic master equation for early protein aggregation in the transthyretin amyloid disease. Sci. Rep. 2020, 10, 12437. [Google Scholar] [CrossRef]
- Panegyres, P.K. Stochasticity, Entropy and Neurodegeneration. Brain Sci. 2022, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Sigawi, T.; Hurvitz, N.; Elkhateeb, N.; Rinsky-Halivni, L.; Ilan, Y. Variability in Exercise is Linked to Improved Age-related Dysfunctions, Suggesting a Potential Role for the Constrained-Disorder Principle-based Second-Generation Artificial Intelligence System. Curr. Aging Sci. 2025, in press. [Google Scholar] [CrossRef]
- Vockert, N.; Machts, J.; Kleineidam, L.; Nemali, A.; Incesoy, E.I.; Bernal, J.; Schütze, H.; Yakupov, R.; Peters, O.; Gref, D.; et al. Cognitive reserve against Alzheimer’s pathology is linked to brain activity during memory formation. Nat. Commun. 2024, 15, 9815. [Google Scholar] [CrossRef] [PubMed]
- Briney, B.S.; Crowe, J.E. Secondary mechanisms of diversification in the human antibody repertoire. Front. Immunol. 2013, 4, 42. [Google Scholar] [CrossRef]
- Jeon, S.; Jeon, Y.; Lim, J.-Y.; Kim, Y.; Cha, B.; Kim, W. Emerging regulatory mechanisms and functions of biomolecular condensates: Implications for therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 4. [Google Scholar] [CrossRef]
- Grossman, Z.; Meyerhans, A.; Bocharov, G. An integrative systems biology view of host-pathogen interactions: The regulation of immunity and homeostasis is concomitant, flexible, and smart. Front. Immunol. 2023, 13, 1061290. [Google Scholar] [CrossRef]
- Lauvau, G.; Soudja, S.M. Mechanisms of Memory T Cell Activation and Effective Immunity. Adv. Exp. Med. Biol. 2015, 850, 73–80. [Google Scholar] [CrossRef]
- Baird, R.G.; Majumder, A.; Menon, R. Dynamic spectral fluorescence microscopy via event-based & CMOS image-sensor fusion. Opt. Express 2025, 33, 2169–2178. [Google Scholar] [CrossRef]
- Fuhr, L.; Abreu, M.; Pett, P.; Relógio, A. Circadian systems biology: When time matters. Comput. Struct. Biotechnol. J. 2015, 13, 417–426. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Nahum-Shani, I.; Dziak, J.J.; Venera, H.; Pfammatter, A.F.; Spring, B.; Dempsey, W. Design of experiments with sequential randomizations on multiple timescales: The hybrid experimental design. Behav. Res. Methods 2024, 56, 1770–1792. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, J.; Qin, L.; Liu, J.; Yang, H.; Dou, G.; Ma, L.; Dong, Y.; Wang, Y. Decoding ecosystem heterogeneity and transcriptional regulation characteristics of multi-subtype renal cell carcinoma. Heliyon 2024, 10, e33196. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Martin, M. Role of intercellular interactions on single cell and population level responses: Considerations for multicellular bioreporter design. Front. Mol. Biosci. 2025, 12, 1595363. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, F.; Yang, X.; Han, T.; Long, Z.; Wen, J.; Huang, J.; Shen, J.; Guo, Q. Single-cell sequencing technology applied to epigenetics for the study of tumor heterogeneity. Clin. Epigenet. 2023, 15, 161. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Wang, W.; Liu, B. Single-cell sequencing: Accurate disease detection. Clin. Transl. Oncol. 2025, 1–20. [Google Scholar] [CrossRef]
- Xia, J.Q.; Sedransk, N.; Feng, X. Variance component analysis of a multi-site study for the reproducibility of multiple reaction monitoring measurements of peptides in human plasma. PLoS ONE 2011, 6, e14590. [Google Scholar] [CrossRef]
- Veríssimo, J. When Fixed and Random Effects Mismatch: Another Case of Inflation of Evidence in Non-Maximal Models. Comput. Brain Behav. 2023, 6, 84–101. [Google Scholar] [CrossRef]
- Arnol, D.; Schapiro, D.; Bodenmiller, B.; Saez-Rodriguez, J.; Stegle, O. Modeling Cell-Cell Interactions from Spatial Molecular Data with Spatial Variance Component Analysis. Cell Rep. 2019, 29, 202–211.e206. [Google Scholar] [CrossRef]
- Wanichthanarak, K.; Jeamsripong, S.; Pornputtapong, N.; Khoomrung, S. Accounting for biological variation with linear mixed-effects modelling improves the quality of clinical metabolomics data. Comput. Struct. Biotechnol. J. 2019, 17, 611–618. [Google Scholar] [CrossRef]
- Hodson, D.; Mistry, H.; Yates, J.; Guzzetti, S.; Davies, M.; Aarons, L.; Ogungbenro, K. Hierarchical cluster analysis and nonlinear mixed-effects modelling for candidate biomarker detection in preclinical models of cancer. Eur. J. Pharm. Sci. 2024, 197, 106774. [Google Scholar] [CrossRef]
- Meier-Schellersheim, M.; Fraser, I.D.C.; Klauschen, F. Multiscale modeling for biologists. Wiley Interdiscip. reviews. Syst. Biol. Med. 2009, 1, 4–14. [Google Scholar] [CrossRef]
- Britten, G.L.; Mohajerani, Y.; Primeau, L.; Aydin, M.; Garcia, C.; Wang, W.-L.; Pasquier, B.; Cael, B.B.; Primeau, F.W. Evaluating the Benefits of Bayesian Hierarchical Methods for Analyzing Heterogeneous Environmental Datasets: A Case Study of Marine Organic Carbon Fluxes. Front. Environ. Sci. 2021, 9, 491636. [Google Scholar] [CrossRef]
- Yu, J.S.; Bagheri, N. Multi-class and multi-scale models of complex biological phenomena. Curr. Opin. Biotechnol. 2016, 39, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Musilek, P.; Reformat, M.Z. Parametric and Nonparametric Machine Learning Techniques for Increasing Power System Reliability: A Review. Information 2024, 15, 37. [Google Scholar] [CrossRef]
- Vakitbilir, N.; Sainbhi, A.S.; Islam, A.; Gomez, A.; Stein, K.Y.; Froese, L.; Bergmann, T.; McClarty, D.; Raj, R.; Zeiler, F.A. Multivariate linear time-series modeling and prediction of cerebral physiologic signals: Review of statistical models and implications for human signal analytics. Front. Netw. Physiol. 2025, 5, 1551043. [Google Scholar] [CrossRef]
- Mienye, I.D.; Swart, T.G.; Obaido, G. Recurrent Neural Networks: A Comprehensive Review of Architectures, Variants, and Applications. Information 2024, 15, 517. [Google Scholar] [CrossRef]
- Wani, A.A. Comprehensive analysis of clustering algorithms: Exploring limitations and innovative solutions. PeerJ Comput. Sci. 2024, 10, e2286. [Google Scholar] [CrossRef]
- Gao, C.X.; Dwyer, D.; Zhu, Y.; Smith, C.L.; Du, L.; Filia, K.M.; Bayer, J.; Menssink, J.M.; Wang, T.; Bergmeir, C.; et al. An overview of clustering methods with guidelines for application in mental health research. Psychiatry Res. 2023, 327, 115265. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Wang, H.; Du, Y. A Clustering Algorithm Based on the Detection of Density Peaks and the Interaction Degree Between Clusters. Appl. Sci. 2025, 15, 3612. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, C.; Sun, Q.; Zhang, Z. A robust multi-scale clustering framework for single-cell RNA-seq data analysis. Sci. Rep. 2025, 15, 18543. [Google Scholar] [CrossRef]
- Dinh, T.; Wong, H.; Lisik, D.; Koren, M.; Tran, D.; Yu, P.S.; Torres-Sospedra, J. Data clustering: A fundamental method in data science and management. Data Sci. Manag. 2025, in press. [Google Scholar] [CrossRef]
- Deritei, D.; Kunšič, N.; Csermely, P. Probabilistic edge weights fine-tune Boolean network dynamics. PLoS Comput. Biol. 2022, 18, e1010536. [Google Scholar] [CrossRef]
- Tanner, J.; Faskowitz, J.; Teixeira, A.S.; Seguin, C.; Coletta, L.; Gozzi, A.; Mišić, B.; Betzel, R.F. A multi-modal, asymmetric, weighted, and signed description of anatomical connectivity. Nat. Commun. 2024, 15, 5865. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, H.; Deng, B.; Li, Z.; Chang, L. Dual-channel hierarchical interactive learning for the prediction of Protein-Ligand binding affinity. Neural Netw. 2026, 193, 107982. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Li, X.; Cai, F.; Ma, X.; Tang, Y.; Xu, C.; Wang, L.; Ren, P.; Liu, L.; et al. Recent advances in molecular representation methods and their applications in scaffold hopping. Npj Drug Discov. 2025, 2, 14. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Sun, M.; Yang, B.; Wang, Y. Learning continuous dynamic network representation with transformer-based temporal graph neural network. Inf. Sci. 2023, 649, 119596. [Google Scholar] [CrossRef]
- Ravandi, B.; Mili, F.; Springer, J. Identifying and using driver nodes in temporal networks. J. Complex Netw. 2019, 7, 720–748. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, X.; Ke, Y.; Lu, Z.; Li, X.; Liu, C. Temporal Community Detection and Analysis with Network Embeddings. Mathematics 2025, 13, 698. [Google Scholar] [CrossRef]
- Conroy, M.; Gillmann, C.; Harvey, F.; Mchedlidze, T.; Fabrikant, S.I.; Windhager, F.; Scheuermann, G.; Tangherlini, T.R.; Warren, C.N.; Weingart, S.B.; et al. Uncertainty in humanities network visualization. Front. Commun. 2024, 8, 1305137. [Google Scholar] [CrossRef]
- Weiskopf, D. Uncertainty Visualization: Concepts, Methods, and Applications in Biological Data Visualization. Front. Bioinform. 2022, 2, 793819. [Google Scholar] [CrossRef]
- Seoni, S.; Jahmunah, V.; Salvi, M.; Barua, P.D.; Molinari, F.; Acharya, U.R. Application of uncertainty quantification to artificial intelligence in healthcare: A review of last decade (2013–2023). Comput. Biol. Med. 2023, 165, 107441. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, X.; Ma, Y.; Piao, H.; Yang, Y.; Hao, X.; Fu, Y.; Wang, L.; Peng, J. Combining sequence and network information to enhance protein–protein interaction prediction. BMC Bioinform. 2020, 21, 537. [Google Scholar] [CrossRef] [PubMed]
- Panditrao, G.; Bhowmick, R.; Meena, C.; Sarkar, R.R. Emerging landscape of molecular interaction networks:Opportunities, challenges and prospects. J. Biosci. 2022, 47, 24. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Huang, S.; Zhang, T.; Gao, B. Application of Multilayer Network Models in Bioinformatics. Front. Genet. 2021, 12, 664860. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Allgaier, J.; Pryss, R. Cross-Validation Visualized: A Narrative Guide to Advanced Methods. Mach. Learn. Knowl. Extr. 2024, 6, 1378–1388. [Google Scholar] [CrossRef]
- Adin, A.; Krainski, E.T.; Lenzi, A.; Liu, Z.; Martínez-Minaya, J.; Rue, H. Automatic cross-validation in structured models: Is it time to leave out leave-one-out? Spat. Stat. 2024, 62, 100843. [Google Scholar] [CrossRef]
- Su, H.; Hao, N. Computational systems biology approaches to cellular aging—Integrating network maps and dynamical models. Quant. Biol. 2025, 13, e70007. [Google Scholar] [CrossRef]
- Argent, R.; Bevilacqua, A.; Keogh, A.; Daly, A.; Caulfield, B. The Importance of Real-World Validation of Machine Learning Systems in Wearable Exercise Biofeedback Platforms: A Case Study. Sensors 2021, 21, 2346. [Google Scholar] [CrossRef]
- Dawson, D.V.; Pihlstrom, B.L.; Blanchette, D.R. Understanding and evaluating meta-analysis. J. Am. Dent. Assoc. 2016, 147, 264–270. [Google Scholar] [CrossRef]
- Choobdar, S.; Ahsen, M.E.; Crawford, J.; Tomasoni, M.; Fang, T.; Lamparter, D.; Lin, J.; Hescott, B.; Hu, X.; Mercer, J.; et al. Assessment of network module identification across complex diseases. Nat. Methods 2019, 16, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.R.; Li, L.; Li, J.J.; Huang, H. Network Modeling in Biology: Statistical Methods for Gene and Brain Networks. Stat. Sci. 2021, 36, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Karollus, A.; Hingerl, J.; Gankin, D.; Grosshauser, M.; Klemon, K.; Gagneur, J. Species-aware DNA language models capture regulatory elements and their evolution. Genome Biol. 2024, 25, 83. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Vidiella, B.; Martínez-Redondo, G.I.; Duran-Nebreda, S.; Fernández, R.; Bombarely, A.; Rojas, A.M.; Bentley, R.A. Structural Changes in Gene Ontology Reveal Modular and Complex Representations of Biological Function. Mol. Biol. Evol. 2025, 42, msaf148. [Google Scholar] [CrossRef]
- Marcot, B.G. Metrics for evaluating performance and uncertainty of Bayesian network models. Ecol. Model. 2012, 230, 50–62. [Google Scholar] [CrossRef]
- Fatima, K.; Shareef, H. Dynamic Bayesian Network Model for Overhead Power Lines Affected by Hurricanes. Forecasting 2025, 7, 11. [Google Scholar] [CrossRef]
- Xin, D. Multi-source heterogeneous data fusion and intelligent prediction modeling for chemical engineering construction projects based on improved transformer architecture. Sci. Rep. 2025, 15, 38806. [Google Scholar] [CrossRef]
- Lu, D.; Yu, S.; Huang, Y.; Gong, X. Multimeric protein interaction and complex prediction: Structure, dynamics and function. Comput. Struct. Biotechnol. J. 2025, 27, 1975–1997. [Google Scholar] [CrossRef]
- Tripathi, N.; Hérisson, J.; Faulon, J.-L. Machine learning in predictive biocatalysis: A comparative review of methods and applications. Biotechnol. Adv. 2025, 84, 108698. [Google Scholar] [CrossRef]
- Krzywanski, J.; Sosnowski, M.; Grabowska, K.; Zylka, A.; Lasek, L.; Kijo-Kleczkowska, A. Advanced Computational Methods for Modeling, Prediction and Optimization—A Review. Materials 2024, 17, 3521. [Google Scholar] [CrossRef]
- Yang, S.A.; Salazar, J.L.; Li-Kroeger, D.; Yamamoto, S. Functional Studies of Genetic Variants Associated with Human Diseases in Notch Signaling-Related Genes Using Drosophila. In Notch Signaling Research. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2022; Volume 2472, pp. 235–276. [Google Scholar] [CrossRef]
- Campos, T.L.; Korhonen, P.K.; Hofmann, A.; Gasser, R.B.; Young, N.D. Harnessing model organism genomics to underpin the machine learning-based prediction of essential genes in eukaryotes—Biotechnological implications. Biotechnol. Adv. 2022, 54, 107822. [Google Scholar] [CrossRef]
- Tzec-Interián, J.A.; González-Padilla, D.; Góngora-Castillo, E.B. Bioinformatics perspectives on transcriptomics: A comprehensive review of bulk and single-cell RNA sequencing analyses. Quant. Biol. 2025, 13, e78. [Google Scholar] [CrossRef]
- Martinović, M.; Dokic, K.; Pudić, D. Comparative Analysis of Machine Learning Models for Predicting Innovation Outcomes: An Applied AI Approach. Appl. Sci. 2025, 15, 3636. [Google Scholar] [CrossRef]
- Rangarajan, R.; Murugan, T.K.; Govindaraj, L.; Venkataraman, V.; Shankar, K. AI driven automation for enhancing sustainability efforts in CDP report analysis. Sci. Rep. 2025, 15, 24266. [Google Scholar] [CrossRef] [PubMed]
- McMichael, R.D.; Blakley, S.M. Simplified Algorithms for Adaptive Experiment Design in Parameter Estimation. Phys. Rev. Appl. 2022, 18, 054001. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.B.; Shelby, S.A.; Veatch, S.L. Super-Resolution Microscopy: Shedding Light on the Cellular Plasma Membrane. Chem. Rev. 2017, 117, 7457–7477. [Google Scholar] [CrossRef]
- Chin, J.L.; Chan, L.C.; Yeaman, M.R.; Meyer, A.S. Tensor-based insights into systems immunity and infectious disease. Trends Immunol. 2023, 44, 329–332. [Google Scholar] [CrossRef]
- Molla, G.; Bitew, M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024, 12, 2750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilan, Y. The Constrained Disorder Principle: A Paradigm Shift for Accurate Interactome Mapping and Information Analysis in Complex Biological Systems. Bioengineering 2025, 12, 1255. https://doi.org/10.3390/bioengineering12111255
Ilan Y. The Constrained Disorder Principle: A Paradigm Shift for Accurate Interactome Mapping and Information Analysis in Complex Biological Systems. Bioengineering. 2025; 12(11):1255. https://doi.org/10.3390/bioengineering12111255
Chicago/Turabian StyleIlan, Yaron. 2025. "The Constrained Disorder Principle: A Paradigm Shift for Accurate Interactome Mapping and Information Analysis in Complex Biological Systems" Bioengineering 12, no. 11: 1255. https://doi.org/10.3390/bioengineering12111255
APA StyleIlan, Y. (2025). The Constrained Disorder Principle: A Paradigm Shift for Accurate Interactome Mapping and Information Analysis in Complex Biological Systems. Bioengineering, 12(11), 1255. https://doi.org/10.3390/bioengineering12111255





