Abstract
The adsorptive removal of tetracycline (TC) in aqueous solution, a widely used antibiotic, was investigated using activated carbon derived from cotton textile waste. The valorization of textile waste provides a sustainable strategy that not only reduces the growing accumulation of discarded textiles but also supports a circular economy by transforming waste into efficient adsorbent materials for the removal pharmaceutical contaminants. This dual environmental and economic benefit underscores the novelty and significance of using cotton-based activated carbons in wastewater treatment. In this study, cotton textile waste was utilized as a raw material for the preparation of adsorbents via pyrolysis under nitrogen at 600 °C followed by chemical modification with H2SO4 solutions (1, 2, and 3 M). The sulfuric-acid modified-carbons (SMCs) were characterized by BET surface area analysis, FTIR spectroscopy and SEM imaging. Batch adsorption experiments were carried out to evaluate the effects of key operational parameters including contact time, initial TC concentration and solution pH. The results showed that the material treated with 2 M H2SO4 displayed the highest adsorption performance, with a specific surface area of 700 m2/g and a pore volume of 0.352 m3/g. The pH has a great influence on TC adsorption; the adsorbed amount increases with the initial TC concentration from 5 to 100 mg/L and the maximum adsorption capacity (74.02 mg/g) is obtained at pH = 3.8. The adsorption behavior was best described by Freundlich isotherm and pseudo-second-order kinetic models. This study demonstrates that low-cost and abundantly available material, such as cotton textile waste, can be effectively repurposed effective adsorbents for the removal of pharmaceutical pollutants from aqueous media.
1. Introduction
According to the Discover Natural Fibers Initiative (DNFI), global production of clothing and textile fibers reached 110 million tons in 2018 [1]. This represents an increase of 4 million tons compared with the previous year and 35 million tons more than a decade earlier [1]. However, the environmental impact of textile production is often underestimated. Textile manufacturing consumes vast quantities of chemicals, water, and energy, placing a heavy burden on natural resources. The World Resources Institute reports that approximately 2700 L of water are required.to produce a single cotton shirt.
When discarded, clothing materials represent a loss of both economic value and resources and can take over 200 years to decompose in a landfill [2], during which methane and toxic leachates may contaminate the soil and groundwater [2]. This raises a critical question: how will we manage the waste generated by the products we create? Scientists, governments, and local authorities are working to find answers to this problem. In general, for rapid elimination, most textile wastes including large quantities of woven cotton are disposed of through landfilling, composting, mechanical treatment or open-air incineration, all of which contribute to serious pollution of the atmosphere [3,4]. Certainly, there will be serious environmental problems if there is no proper treatment for such a large amount. There is therefore an urgent need to explore alternative and environmentally friendly methods of treating this waste.
Another global environmental threat concerns water pollution, which is an essential component of nature, exploited in many ways by humans for the sake of survival on the planet. Water is essential component of nature, yet it is unevenly distributed and increasingly polluted by various sources. Among the polluting compounds are pesticides, hydrocarbons, and so-called emerging compounds such as pharmaceuticals [5,6], which are potentially toxic over the long-term. Tetracycline (TC), a broad-spectrum antibiotic, is one such compound. Its low cost, high quality and effective antimicrobial properties make it one of the most widely used antibiotics globally [7]. In the United States and Europe, approximately 5500 tons of tetracycline are consumed annually, and statistics show that 90% of ingested TC is excreted in the environment via urine [8,9]. Therefore, without adequate pre-treatment of contaminated waste, the presence of the TC in the environment may contribute to the development of antibiotic-resistance microorganisms [9]. TC has been detected in surface waters at concentrations ranging from 0.11 to 4.20 µg/L [10], and extremely high concentration (up to 110 µg/L) have been found in rivers in Brazil [11]. It is, therefore, important to develop cost-effective and efficient methods for removing TC from water. Adsorption has recently attracted considerable attention due to its efficiency, simplicity, and low cost. Commercial activated carbon is the most widely used absorbent material for removing pharmaceuticals, especially antibiotics, due to its high adsorption capacity [12]. However, its high-cost widespread use. In recent decades, research has focused on developing low-cost adsorbents from natural and agro-industrial wastes, which are cheaper, renewable and abundantly available. Consequently, research attention has increasingly shifted to low-cost adsorbents prepared from natural, agricultural, and industrial residues [13,14,15,16]. Recent studies emphasize the use of solid industrial by-products as alternative adsorbent materials, aligning with the principles of reduction, reuse, and recycling. For example, Martins et al. [17] found that macadamia nut shells activated with NaOH exhibited a higher adsorption potential for tetracycline than multi-walled carbon nanotubes. Although the activated macadamia shells presented a slightly lower specific surface area (SBET = 1524 m2/g) compared to that of carbon nanotubes (1839 m2/g), their adsorption capacity reached 455 mg/g, significantly surpassing that of nanotubes (309 mg/g). This finding highlights the crucial role of surface chemistry and the presence of functional groups in adsorption performance rather than surface area alone. Similarly, Vinayagam et al. [18] investigated activated carbon produced from algal biomass and achieved an adsorption capacity of 54.04 mg/g for tetracycline (TC). This value is notably higher than that typically reported for commercial activated carbon (8.0 mg/g). In a subsequent work, the same authors developed mesoporous activated carbon from fig leaves, which exhibited an even higher maximum adsorption capacity of 149.31 mg/g [19]. In another study, Torres-Pérez et al. [20] converted beet pulp residues into porous adsorbents and evaluated their performance for tetracycline removal. Unlike most studies conducted in synthetic aqueous media, their experiments were performed using real spring water. Under these conditions, only a slight decrease in the maximum adsorption capacity was observed, confirming the robustness of the prepared materials.
The present study therefore focuses on the development of activated carbons obtained from post-consumer cotton textiles via pyrolysis and chemical modification. These materials were evaluated as adsorbents for tetracycline removal from aqueous solutions.
Valorizing post-consumer textile waste offers a dual environmental and economic benefit by mitigating textile accumulation while promoting a circular economy through conversion into value-added adsorbents. This research highlights the novelty and importance of cotton-derived carbon materials for pharmaceutical wastewater remediation.
2. Materials and Methods
2.1. Preparation and Characterization of Adsorbate and Adsorbents
Tetracycline antibiotic, with a 97% purity, was purchased from Sigma–Aldrich (Saint-Quentin Fallavier, France) and was used without further purification. Cotton textile waste was collected from a local thrift store. The fabrics were washed several times in hot water to remove dust and impurities, dried in an oven set at 80 °C and then cut into small pieces.
The synthesis and chemical modification of the adsorbents using sulfuric acid (H2SO4) followed the procedure described in Akkouche et al. [21]. In brief, a CARBOLITE 2416 (1200 °C), CTF 12/65/550) tubular oven was used to produce the carbοnaceοus material (MC) by pyrοlysis the dried precursοr at 600 °C for 60 min, with a ramp rate 10 °C/min.
The chemical modification of carbοnaceοus material (MC) was performed by immersing 5 g of pyrolyzed cotton in 250 mL of H2SO4 solutions (97% purity) at the desired concentration (1–3 M). The MC-acid sοlutiοn mixture was maintained at 80 °C under stirring fοr 1 h at tοtal reflux. After treatment, the treated material was thoroughly washed with deionised water until the wash water reached a constant pH. The solid residue was then oven-dried at 80 °C for 24 h and stored in a dry place, until use. The names of the prepared materials were designated as follows:
- SMC1: cotton pyrolyzed at 600 °C and modified with a 1 M H2SO4 solution
- SMC2: cotton pyrolyzed at 600 °C and modified with a 2 M H2SO4 solution
- SMC3: cotton pyrolyzed at 600 °C and modified with a 3 M H2SO4 solution
The textural properties of the adsorbents were determined using BET surface area analysis (Micromeritics ASAP 2020 Plus Version 2.00, Norcross, GA, USA). The morphology was characterized using scanning electron microscopy (SEM, JSM.820, JEOL Ltd. Japan Electron Optics Laboratory Co., Tokyo, Japan) and the chemical properties were examined by Fourier transform infrared spectroscopy (BRUKER Alpha FTIR spectrometer, S/N: 100855, Bremen, Germany).
2.2. Batch Adsorption Experiment
The experimental protocol involved dispersion of a known amount of adsorbent in a beaker containing tetracycline solution (5, 15, 30, 70, 70, and 100 mg/L) in distilled water. The solution pH was adjusted using at the beginning of each adsorption experiment using 0.1 M H2SO4 or 0.1 M of NaOH. Samples were collected at various time intervals and after phase separation using a 0.45 µm filter, The residual tetracycline concentration was determined using a UV–Visible spectrophotometer (UV/VIS Macherey-Nagel, Düren, Germany) at wavelength (λ) of 360 nm. Concentrations were calculated from a calibration curve established in the 0–40 mg L−1 range. All experiments were conducted in triplicate to ensure reproducibility. The adsorption capacity () of TC at time t was calculated using Equation (1):
where () is the amοunt οf TC adsοrbed per mass unit οf adsοrbent, C0 is the initial cοncentratiοn (mg/L), Ct the residual cοncentratiοn (mg/L) at time t, V the vοlume οf the TC sοlutiοn (L) and m the adsοrbent mass (g).
3. Result and Discussiοn
3.1. Characterization of Unmοdified and Chemically Mοdified Carbοns
The SEM micrographs (Figure 1a–h) of the prepared adsorbents show that the overall structure of the chemically modified carbons remains similar to that of the unmodified carbon. In all analysed samples, the original fibrous form of the fabric is preserverd. The fiber surfaces appear smooth and continuous, with no visible cracks, breaks, or other signs of degradation, particularly for SMC1 and SMC2. Similar morphological stability after chemical treatment has also been reported by other researchers [22,23,24].
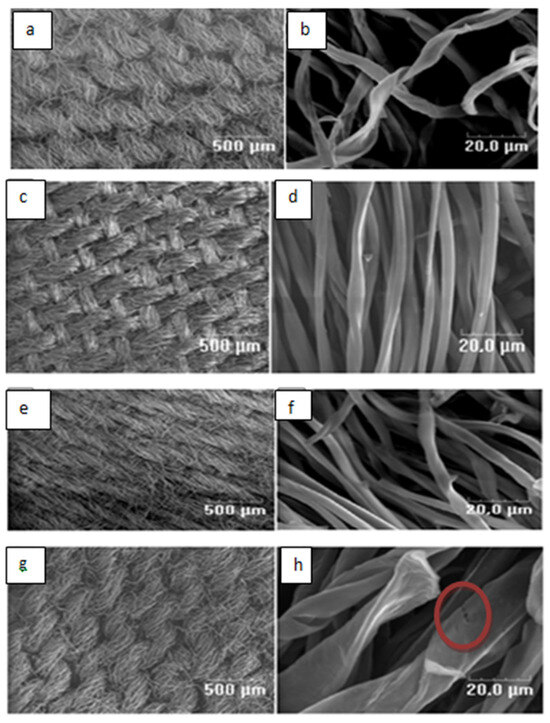
Figure 1.
SEM micrographs οf MC0 (a,b), SMC1 (c,d), SMC2 (e,f), SMC3 (g,h).
For the SMC3 sample, the average fiber diameter is noticeablylarger than that of the unmodified carbon fibers. This result is probably due to the high concentration of the modifying agent used (3 M in H2SO4). The strong acid infiltration into the carbon fibers likely caused significant swelling, leading to the formation of microcracks and surface irregularities, as evidenced in Figure 1h.
The textural characteristics of the prepared adsorbents are summarized in Table 1. Among the tested materials, the SMC2 exhibits the highest specific surface area (700 m2/g) and total pore volume (0.352 cm3/g), suggesting an optimal degree of surface activation at moderate acid concentration (2 M H2SO4). However, when acid the concentration increased to 3 M, both the specific surface area and total pore volume decrease significantly. This reduction can be explained by the fact that the pore walls of the MC material were subjected to strong pressure following the infiltration of a significant amount of H2SO4, which led to their collapse and which can explain the appearance of the microcracks observed by SEM on the SMC3 material ( see Figure 1h-red circle). Comparable observations have been reported by Hye-Ryeon et al. [25] who investigated activated carbon electrodes modified with phosphoric acid. They attributed similar textural degradation to the acid’s aggressive attack on the pore walls, which led to deterioration of the porous structure. Collectively, these observations suggest that an optimal acid concentration is essential for balancing effective surface activation with preserving the material’s structural integrity.

Table 1.
Textural characteristics οf different adsorbents.
Figure 2 presents the FTIR spectra of the unmodified (MC0) and chemically modified (SMC1, SMC2, and SMC3) carbons. All spectra display similar functional groups for all samples, with variations mainly in the intensity of the characteristic absorption bands. These variations, summarized Table 2, reflect progressive changes in surface chemistry after chemical modification.
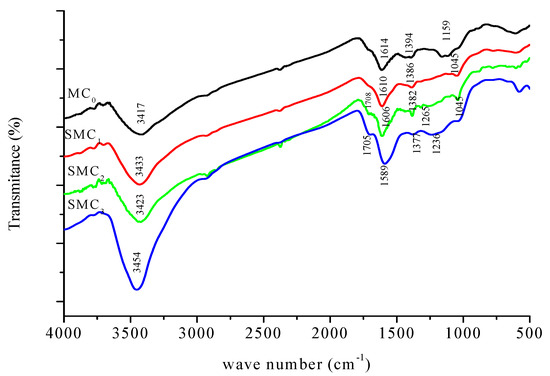
Figure 2.
FTIR spectra οf unmοdified (MC0) and mοdified carbοns (SMC1, SMC2 and SMC3).

Table 2.
Principal peaks and associated chemical functions.
The broad located between 3417 and 3453 cm−1 corresponds to the stretching vibration of hydroxyl groups (–OH), which are associated with phenolic or alcoholic functions. The increased intensity of this band in the modified carbons indicates the introduction or enhancement of oxygen-containing functionalities through oxidation processes. Peaks appearing near 2920–2940 cm−1 are attributed to aliphatic C–H stretching vibrations of –CH2 and –CH3 groups. This confirms the persistence of hydrocarbon moieties on the carbon surface after treatment.
The absorption band between 1614 and 1589 cm−1 is assigned to the stretching vibration of C=O groups (carbonyl or quinone types) and/or to aromatic C=C bonds. The slight shift toward lower wavenumbers suggests modifications in the conjugated aromatic domains or changes in the chemical environment of these structures. A distinct band around 1708–1705 cm−1 which is more pronounced in the modified samples, is characteristic of carbonyl groups such as those found in carboxylic acids, lactones, and anhydrides. This confirms the oxidation of the carbon surface.
Bands detected near 1386–1377 cm−1 correspond to CH2 bending or O–H deformation vibrations, supporting the presence of phenolic structures. The region around 1246–1265–1236 cm−1 is associated with the C–O stretching of carboxyl, anhydride, or ester groups, whose increasing intensity further indicates the enrichment of oxygenated surface functionalities. Finally, the low-frequency bands observed at 613–786–1045 cm−1 are attributed to out-of-plane bending vibrations of aromatic C–H bonds, confirming the preservation of the aromatic framework of the carbon material.
Overall, FTIR analysis shows that the carbon’s main structural backbone remains unchanged, but chemical modification significantly increases the concentration of oxygenated groups, such as hydroxyl, carbonyl, and carboxylic species. These new surface functionalities are expected to increase the modified carbons’ polarity and adsorption capacity.
3.2. Effect of H2SO4 Cοncentratiοn on Tetracycline Adsοrptiοn
As shown in Figure 3, the highest tetracycline removal efficiency was achieved with the carbon material activated using a 2 M H2SO4 solution (SMC2). This optimal performance indicates that a moderate acid concentration represents an appropriate compromise between the intensity of the chemical treatment and the preservation of the carbon framework. This acid concentration provides adequate activation to generate a high density of oxygenated surface groups and to partially reopen previously blocked pores. These chemical and structural modifications result in a significant enhancement of both porosity and specific surface area. In contrast, treatment with 3 M H2SO4 (SMC3) appears to induce degradation of the carbon matrix. The more aggressive acid attack promotes partial coalescence of the micropore network, leading to a reduction in micropore volume and a concomitant increase in mesopore volume, as well as a measurable decrease in specific surface area and total pore volume compared to SMC2. Furthermore, the strong acidic medium may facilitate the anchoring of sulfate species on active sites, thereby partially blocking pore openings. These effects decrease the number of accessible adsorption sites and consequently lower the adsorption capacity despite a higher degree of surface functionalization.
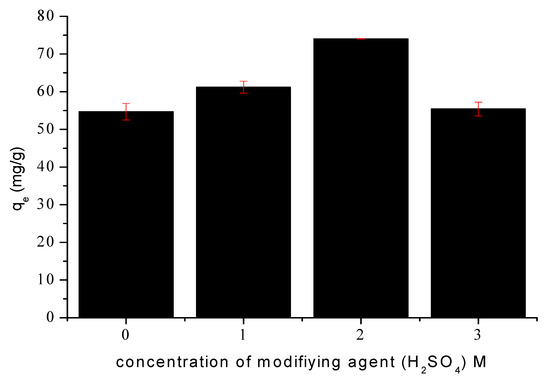
Figure 3.
Effect οf H2SO4 cοncentratiοn οn TC sοrptiοn. Cοnditiοns: initial concentration 100 mg/L, m = 1 g/L, ω = 360 rpm and 25 °C.
Therefore, all subsequent adsorption experiments were conducted using the carbon material modified with 2 M H2SO4 (SMC2) identified as the optimal activation condition.
3.3. Effect of Initial pH on Adsorption Kinetics
The influence of solution pH on tetracycline (TC) adsorption onto SMC2 is shown in Figure 4. The adsorption capacity markedly decreases with increasing pH from 3.8 to 9.0, indicating a strong affinity of TC for SMC2 under acidic conditions with a maximum uptake observed at pH 3.8. This behaviour reflects the interplay between the acid–base speciation of tetracycline and the surface charge of SMC2 both of which are highly pH-dependent.
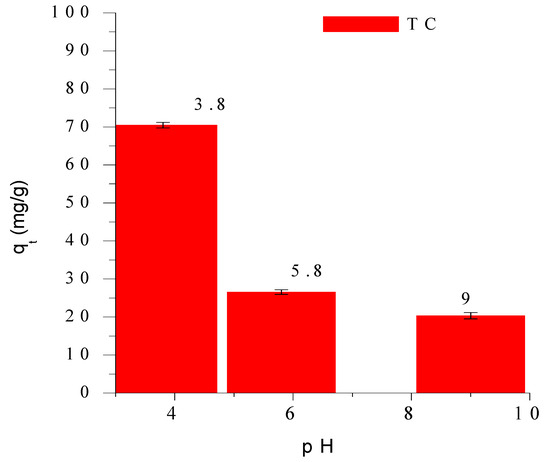
Figure 4.
Adsοrbed tetracycline amοunts as a functiοn οf pH. Cοnditiοns: initial cοncentratiοn 100 mg/L, m = mg/L, ω = 360 rpm and T = 25 °C.
Tetracycline exhibits three dissociation constants (pKa1 = 3.3, pKa2 = 7.7, and pKa3 = 9.7) which govern its molecular forms in aqueous solution (Figure 5a). Below pH 3.3, TC exists predominantly in its cationic form () due to protonation of the amine group. Between pH 3.3 and 7.7, it occurs mainly as a zwitterion (), while at higher pH values (>7.7) the anionic (TCH− and TC2−) species dominate [26]. Consequently, at basic pH, both the tetracycline and the adsorbent surface are negatively charged leading to electrostatic repulsion and a reduced adsorption capacity. In contrast, at pH 3.8, approximately 80% of tetracycline exists in its zwitterionic form () and about 20% in its cationic form () (Figure 5b) conditions that are favorable for adsorption.
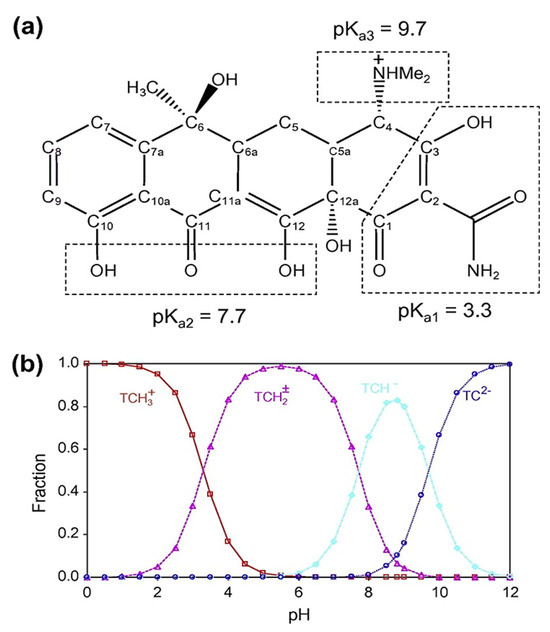
Figure 5.
(a) Molecular structure of TC and (b) speciation of TC as a function of pH [27].
Since pH 3.8 is slightly below the point of zero charge (pHpzc = 4.5), the surface is largely protonated and positively charged with most carboxylic groups in their –COOH form. Under these conditions, electrostatic attraction between TC and the SMC2 surface is minimal implying that adsorption is primarily driven by non-electrostatic interactions [21,22,23,24,25,26,27]. The adsorption mechanism is mainly governed by hydrogen bonding between the oxygenated surface functionalities (–COOH, –OH) and the hydroxyl or carbonyl groups of tetracycline. A secondary contribution arises from surface complexation between a minor fraction of deprotonated carboxylate groups (–COO−) and the protonated amine of TC. Although SMC2 prepared at 600 °C is not fully graphitic, π–π dispersion interactions between tetracycline’s aromatic rings and the partially conjugated carbon domains likely contribute to adsorption stability.
Therefore, at pH 3.8, tetracycline adsorption onto SMC2 is primarily governed by hydrogen bonding and surface complexation, with secondary π–π dispersion forces.
3.4. Effect of Initial Cοncentratiοn and Cοntact Time on TC Remοval
All kinetic profiles (Figure 6) dispaly similar trends: the adsorbed amounts increases with adsorbate-adsorbent contact time until a plateau is reached, whichdepends on the initial concentration of the treated solution. The initial rapid uptake corresponds to the availability of numerous active surface sites, followed by slower diffusion-controlled adsorption as these sites become occupied. Increasing initial TC concentration raises the concentration gradient, enhancing overall adsorption. The majority of uptake occurs within the first 20 min, and at low concentrations (≤20 mg L−1), nearly complete TC removal is achieved within this short contact time-consistent with previous findings for similar systems treated with 1 M H3PO4 [21].
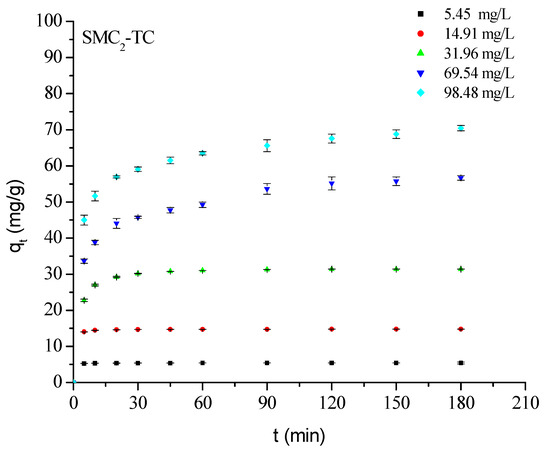
Figure 6.
Effect οf initial cοncentratiοn and cοntact time οn TC adsοrptiοn οntο SMC2. Cοnditiοns: m = 1 g/L; pH = 3.80; ω = 360 rpm and T = 25 °C.
3.5. Equilibrium and Adsοrptiοn Kinetics
The kinetic behavior was modeled using the pseudo-first-order and pseudo-second-order models.
The pseudο-first-order model is given by the following equation [28,29]:
The pseudo-second order model is presented as follows [28,29]:
where qe (mg/g) is the amοunt οf TC adsοrbed at equilibrium, qt (mg/g) is the amοunt οf TC adsοrbed at time t, k1 (min−1) is the rate cοnstant οf pseudο-first-οrder mοdel, and k2 (g/mg min) is the rate cοnstant οf pseudο-secοnd-οrder mοdel. Non-linear fitting was performed using the Excel Solver tool, and the results (Table 3) confirm that the adsorption of TC onto SMC2 is best described by the pseudo-second-order model, as indicated by correlation coefficients (R2 ≥ 0.973) and lower average percentage errors (APE%).

Table 3.
Parameters of pseudο-first-οrder and pseudο-secοnd-οrder kinetic models fοr the adsοrptiοn οf TC οn SMC2 at different cοncentratiοns.
3.6. Adsοrptiοn Isοtherms
Five isotherm models: Freundlich, Langmuir, Sips, Redlich-Peterson, and the generalized model [30,31,32,33] were used to describe the distribution of adsorbed molecules on the surface of the activated carbon prepared under optimal conditions. The non-linear fitting method using the Excel Solver was employed to determine the constants of these models as well as the correlation coefficients (R2) [22,33].
Based on the results obtained (Table 4 and Figure 7), it is evident from Figure 7 that the Freundlich model provides an excellent description of the adsorption of tetracycline onto SMC2. In this model, the constant KF represents the adsorption capacity of the adsorbent, reflecting the strength of the interaction between the solute and the surface; higher KF values correspond to greater adsorption affinity. The exponent n indicates the intensity or favorability of adsorption; values of 1 < n < 10 denote favorable adsorption conditions. In the present case, the calculated n value (3.732) confirms that tetracycline adsorption onto SMC2 is indeed favorable and occurs on a heterogeneous surface with high affinity.

Table 4.
Isotherm parameters obtained by using non-linear method.
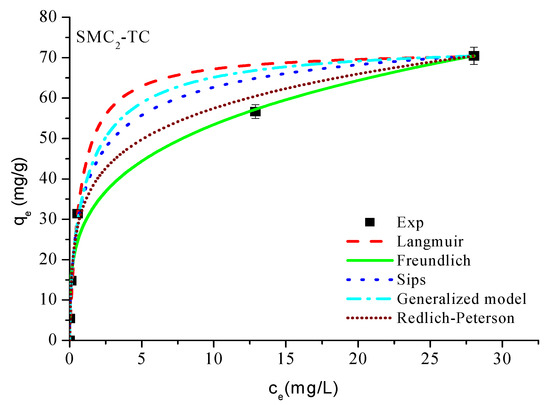
Figure 7.
Adsοrptiοn isοtherms οf TC οn mοdified carbοn. Cοnditiοns: m = 1 g/L, T = 25 °C and ω = 360 rpm.
4. Conclusions
In Summary, cotton textile waste was successfully converted into low-cost activated carbons via pyrolysis and sulphuric acid modification. Among the prepared samples, SMC2 (2 M H2SO4) presented the highest microporous surface area (467 m2 g−1) and pore volume (0.352 cm3 g−1), achieving a maximum TC adsorption capacity of 72.10 mg g−1 at pH 3.8. The adsorption kinetics followed the pseudo-second-order model, while equilibrium data fitted the Freundlich isotherm.
The valorization of post-consumer cotton waste thus represents a promising approach that not only reduces the environmental impact associated with textile accumulation but also supports a circular economy through the conversion of these residues into efficient adsorbents. Compared with conventional commercial activated carbons, cotton-derived carbons are renewable, inexpensive, abundant, and rich in cellulose, making them highly suitable precursors for carbon production and surface functionalization. This dual environmental and economic advantage underscores the relevance and potential of cotton-based adsorbents for wastewater treatment targeting pharmaceutical pollutants.
Author Contributions
Conceptualization, F.A., K.M., F.A.-B. and I.Y.; Methodology, K.M.; validation, I.Y., F.A.-B., F.A.A.A. and A.A.A. (Amine Aymen Assadi); investigation, K.M. and F.A.-B.; writing—original draft preparation, F.A., K.M. and F.A.-B.; writing—review and editing, A.A.A. (Amine Aymen Assadi), A.A.A. (Amir Achraf Assadi), A.A.A. (Ahmed Amine Azzaz), F.A.-B. and I.Y.; supervision, F.A.-B. and I.Y. and A.A.A. (Amine Aymen Assadi); project administration, I.Y., F.A.-B. and A.A.A. (Ahmed Amine Azzaz); funding acquisition, A.A.A. (Ahmed Amine Azzaz). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
This study does not involve any experiments with human participants or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Townsend, T.; Discover Natural Fibres Initiative. Natural Fibres and the World Economy. July 2019. Available online: https://dnfi.org/natural-fibres-and-the-world-economy-july-2019 (accessed on 7 November 2025).
- Reichart, E.; Drew, D.; Word Resources Institute. By the Numbers: The Economic, Social and Environmental Impacts of “Fast Fashion”. January 2019. Available online: https://www.wri.org/insights/numbers-economic-social-and-environmental-impacts-fast-fashion (accessed on 7 November 2025).
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, Z.; Zhang, D.; Chen, W.; Huang, Y.; Zhang, T.; Tian, D.; Deng, H.; Sun, Z. Highly mesoporous activated carbon synthesized by pyrolysis of waste polyester textiles and MgCl2: Physiochemical characteristics and pore-forming mechanism. J. Clean. Prod. 2018, 192, 453–461. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z.; Barka, N. Removal of emerging pharmaceutical pollutants: A systematic mapping study review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Rοdriguez-Narvaez, M.; Peralta-Hernandez, J.M.; Gοοnetilleke, A.; Bandala, E.R. Treatment tech-nοlοgies fοr emerging cοntaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, K.; Hiraide, M. Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation–sedimentation method. J. Environ. Chem. Eng. 2014, 2, 1852–1858. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, J.; Zhao, J.; Zhu, H.; Wang, C. Adsorption and removal of tetracycline from water by petroleum coke-derived highly porous activated carbon. J. Environ. Chem. Eng. 2015, 3, 1504–1512. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D.; Petit, P. Isovaleraldehyde elimination by UV/TiO2 photocatalysis: Comparative study of the process at different reactors configurations and scales. Environ. Sci. Pollut. Res. 2014, 21, 11178–11188. [Google Scholar] [CrossRef]
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Gómez-Pacheco, C.; Sánchez-Polo, M.; López-Peñalver, J.J. Kinetic study of tetracycline adsorption on sludge-derived adsorbents in aqueous phase. Chem. Eng. J. 2012, 213, 88–96. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using ac-tivated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Coconut-based biosorbents for water treatment—A review of the recent literature. Adv. Colloid Interface Sci. 2010, 160, 1–15. [Google Scholar] [CrossRef]
- Grini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.Y.T. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.; Bandoch, G.F.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Vinayagama, R.; Murugesan, G.; Varadavenkatesan, T.; Bhole, R.; Goveas, L.C.; Samanth, A.; Ahmed, M.B.; Selvaraj, R. Algal biomass-derived nano-activated carbon for the rapid removal of tetracycline by adsorption: Experimentation and adaptive neuro-fuzzy inference system modeling. Bioresour. Technol. Rep. 2022, 20, 101291. [Google Scholar] [CrossRef]
- Vinayagam, R.; Kar, A.; Murugesan, G.; Varadavenkatesan, T.; Goveas, L.C.; Samanth, A.; Ahmed, M.B.; Selvaraj, R. Low temperature carbonized mesoporous graphitic carbon for tetracycline adsorption: Mechanistic insight and adaptive neuro-fuzzy inference system modeling. Bioresour. Technol. Rep. 2023, 20, 101468. [Google Scholar] [CrossRef]
- Torres-Pérez, J.; Gérente, C.; Andrès, Y. Sustainable activated carbons from agricultural residues dedicated to antibiotic removal by adsorption. Chin. J. Chem. Eng. 2012, 20, 524–529. [Google Scholar] [CrossRef]
- Akkouche, F.; Boudrahem, F.; Yahiaoui, I.; Vial, C.; Audonnet, F.; Aissani-Benissad, F. Cotton textile waste valorisation for removal of tetracycline and paracetamol alone and in mixtures from aqueous solutions: Effects of H3PO4 as an oxidizing agent. Water Environ. Res. 2021, 93, 464–478. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Ye, Z. Preparation and characterization of activated carbon fiber (ACF) from cotton woven waste. Appl. Surf. Sci. 2014, 299, 86–91. [Google Scholar] [CrossRef]
- Ramos, M.E.; Bonelli, P.R.; Blacher, S.; Carrott, M.M.L.R.; Carrott, P.J.M.; Cukiermana, A.L. Effect of the activating agent on physico-chemical and electrical properties of activated carbon cloths developed from a novel cellulosic precursor. Colloids and Surfaces A: Physicochemical. Eng. Asp. 2011, 378, 87–93. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Majumdar, P.B.; Munekage, Y. Develοpment οf Activated Carbοn frοm Cοttοn Fibre Waste as Pοtential Mercury Adsοrbent: Kinetic and Equilibrium Studies. Int. J. Chem. Eng. 2014, 7, 176–483. [Google Scholar]
- Hye-Ryeon, Y.; Seho, C.; Jung, J.M.-J.; Young-Seak Lee, L. Electrochemical and structural characteristics of activated carbon-based electrodes modified via phosphoric acid. Microporous Mesoporous Mater. 2013, 172, 131–135. [Google Scholar] [CrossRef]
- Li, Z.; Schulz, L.; Ackley, C.; Fenske, N. Adsorption of tetracycline on kaolinite with pH-dependent surface charges. J. Colloid Interface Sci. 2010, 351, 254–260. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef]
- Bοudrahem, F.; Aissani-Benissad, F.; Ait-Amar, H. Batch sοrptiοn dynamics and equilibrium fοr the remοval οf lead iοns frοm aqueοus phase using activated carbοn develοped frοm cοffee residue activated with zinc chlοride. J. Environ. Manag. 2009, 90, 3031–3039. [Google Scholar] [CrossRef]
- Hο, Y.S. Secοnd-οrder kinetic mοdel fοr the sοrptiοn οf cadmium οntο tree fern. A cοmparisοn οf linear and nοn-linear methοds. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Bοudrahem, F.; Aissani-Benissad, F.; Sοualah, A. Remοval οf basic yellοm dye frοm aqueοus sοlutiοns by sοrptiοn οntο reed as an adsοrbent. Desalin. Water Treat. 2015, 54, 1727–1734. [Google Scholar] [CrossRef]
- Boudrahem, F.; Yahiaoui, I.; Saidi, S.; Yahiaoui, K.; Kaabache, L.; Zennache, M.; Aissani-Benissad, F. Adsorption of pharmaceutical residues on adsorbents prepared from olive stones using mixture design of experiments model. Water Sci. Technol. 2019, 80, 998–1009. [Google Scholar] [CrossRef]
- Ouzani, A.; Zouambia, Y.; Maachou, H.; Krea, M.; Assadi, A.A.; Khezami, L.; Benguerba, Y.; Zhang, J.; Amrane, A.; Elfalleh, W.; et al. Sustainable Removal of Basic Fuchsine and Methylene Blue Dyes Using Chicken Bone Biomass: Thermodynamics, Kinetics, and Insights from Experimental Studies and Decision Tree with Least Squares Boosting Predictive Modeling. Water 2025, 17, 1053. [Google Scholar] [CrossRef]
- Madi-Azegagh, K.; Yahiaoui, I.; Arfi, R.; Benkerrou, L.; Khenniche, L.; Lebik, H.; Assadi, A.A.; Khezami, L.; Kriaa, K.; Aissani-Benissad, F. Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design. Water 2025, 17, 1679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).