An Overview of Natural Polymers as Reinforcing Agents for 3D Printing
Abstract
:1. Introduction
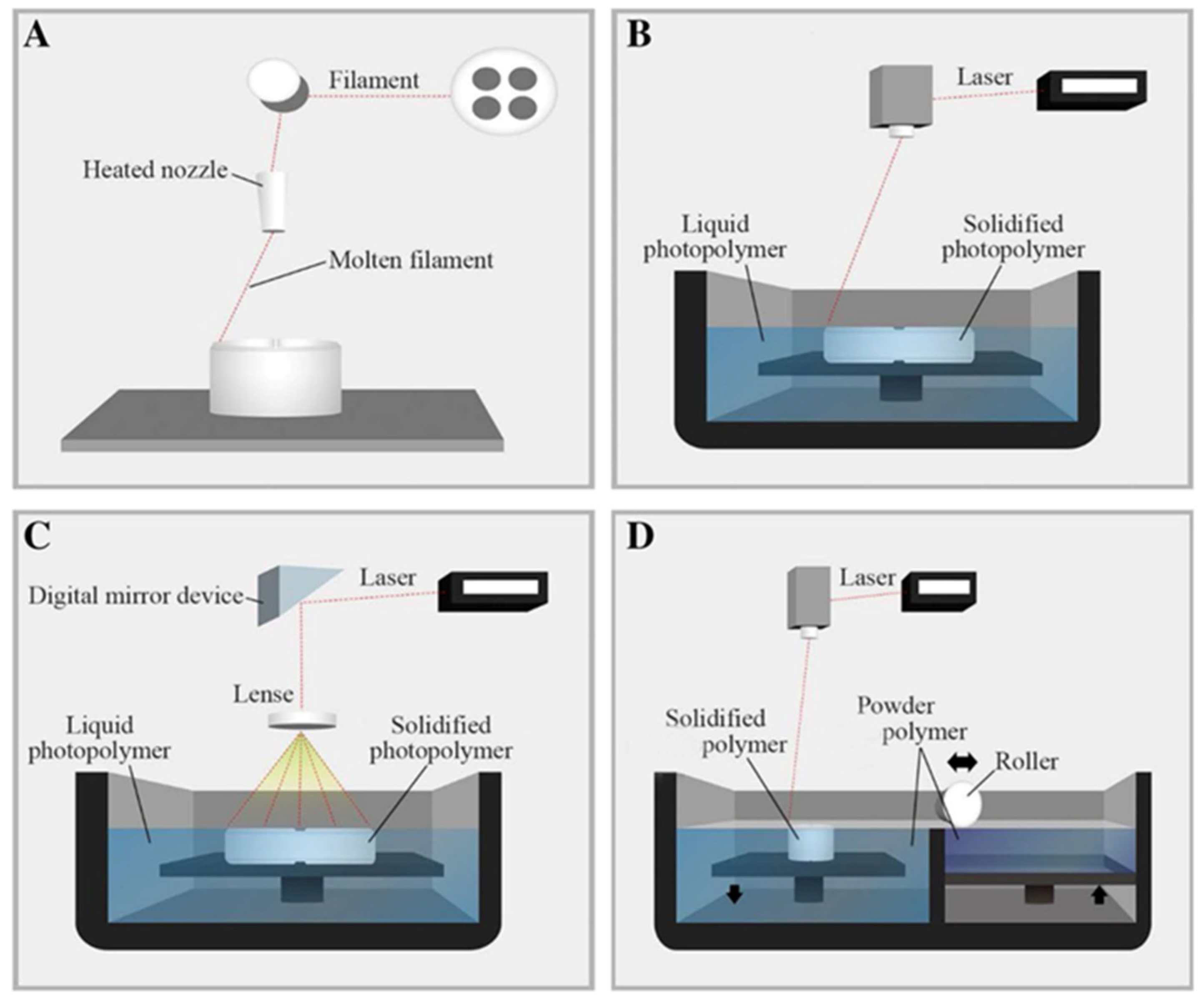
2. Natural Fillers as Reinforcement for 3D Printing
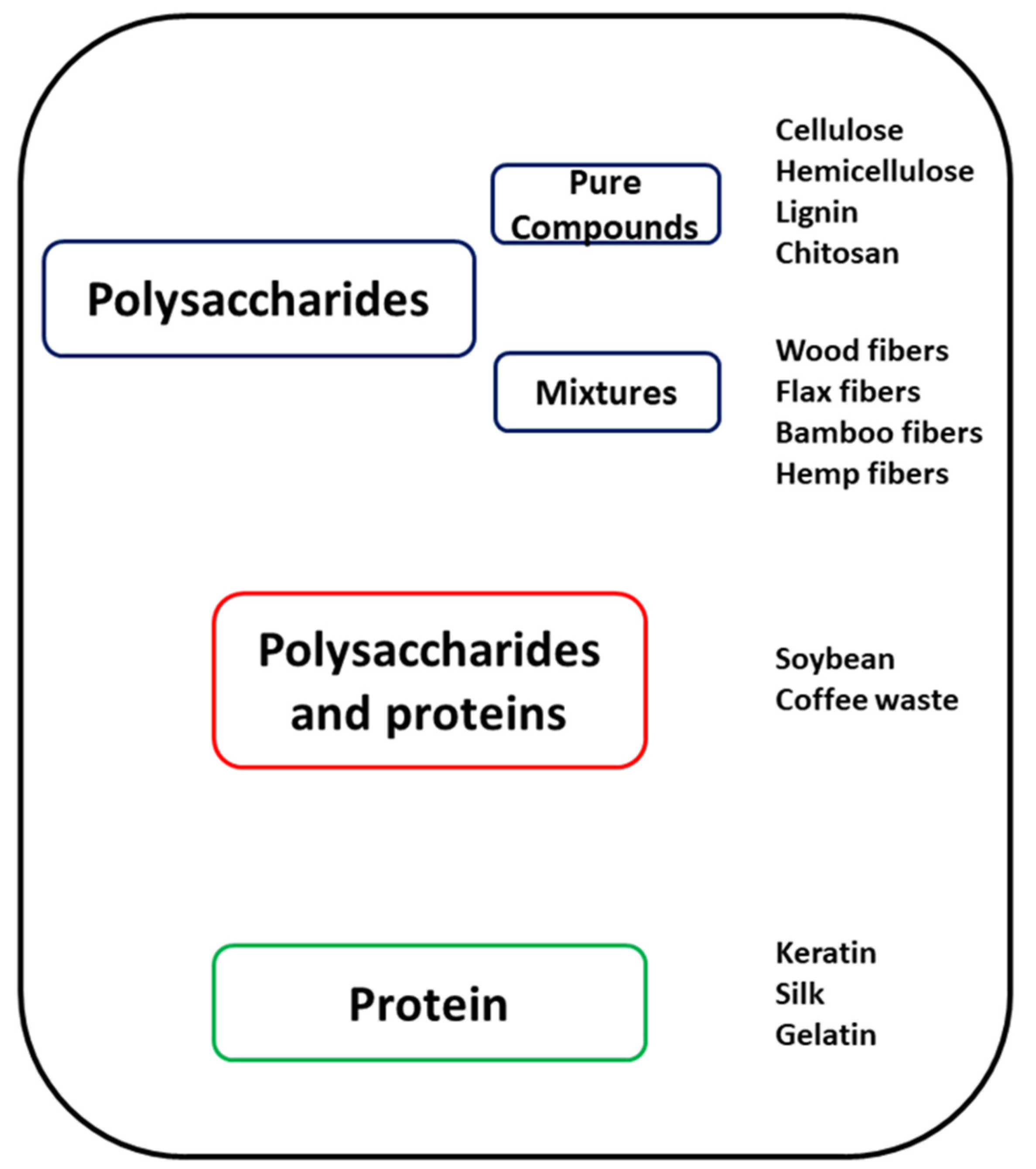
3. Natural Materials Used as Fillers in 3D Printing
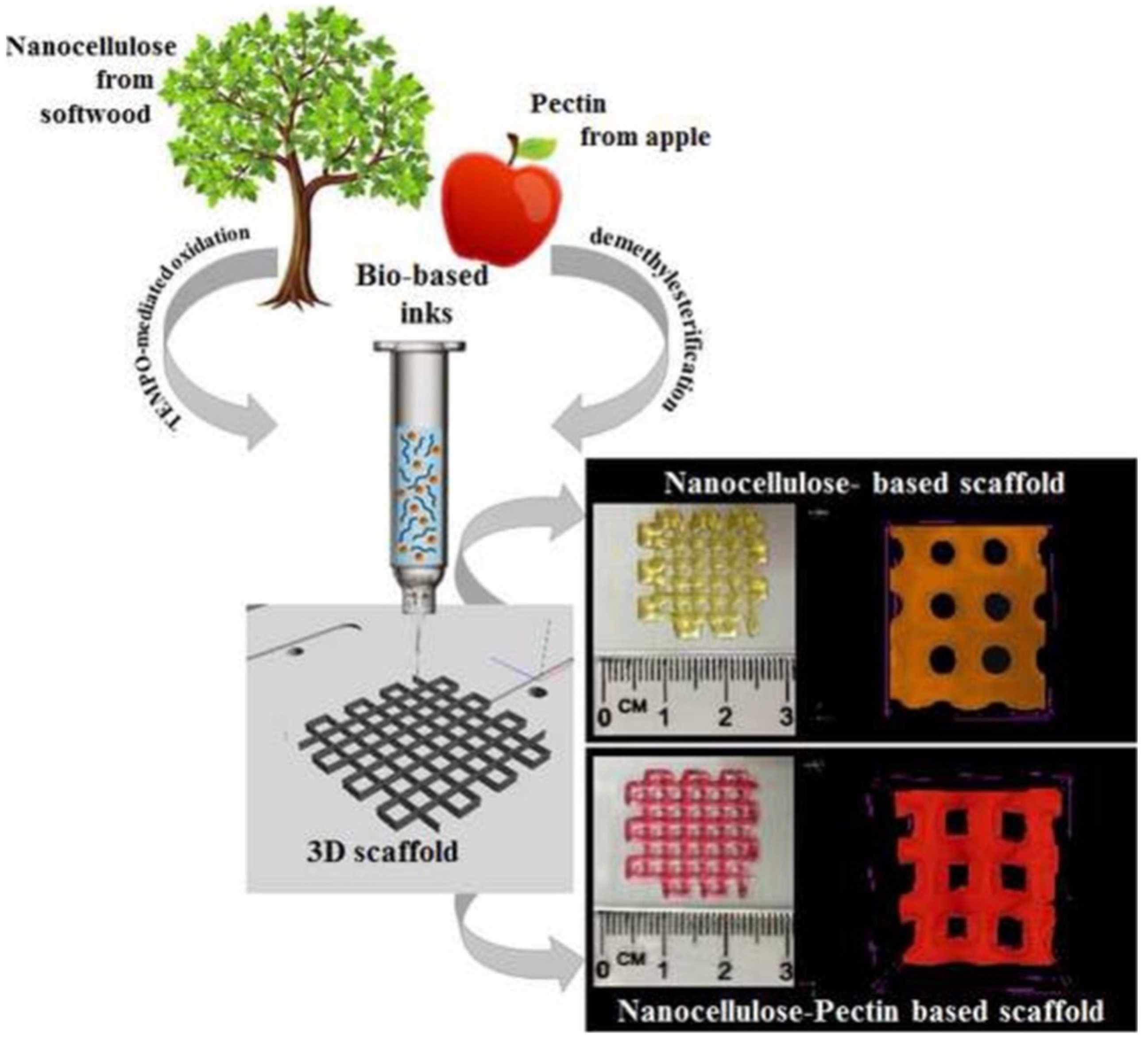
3.1. Cellulose
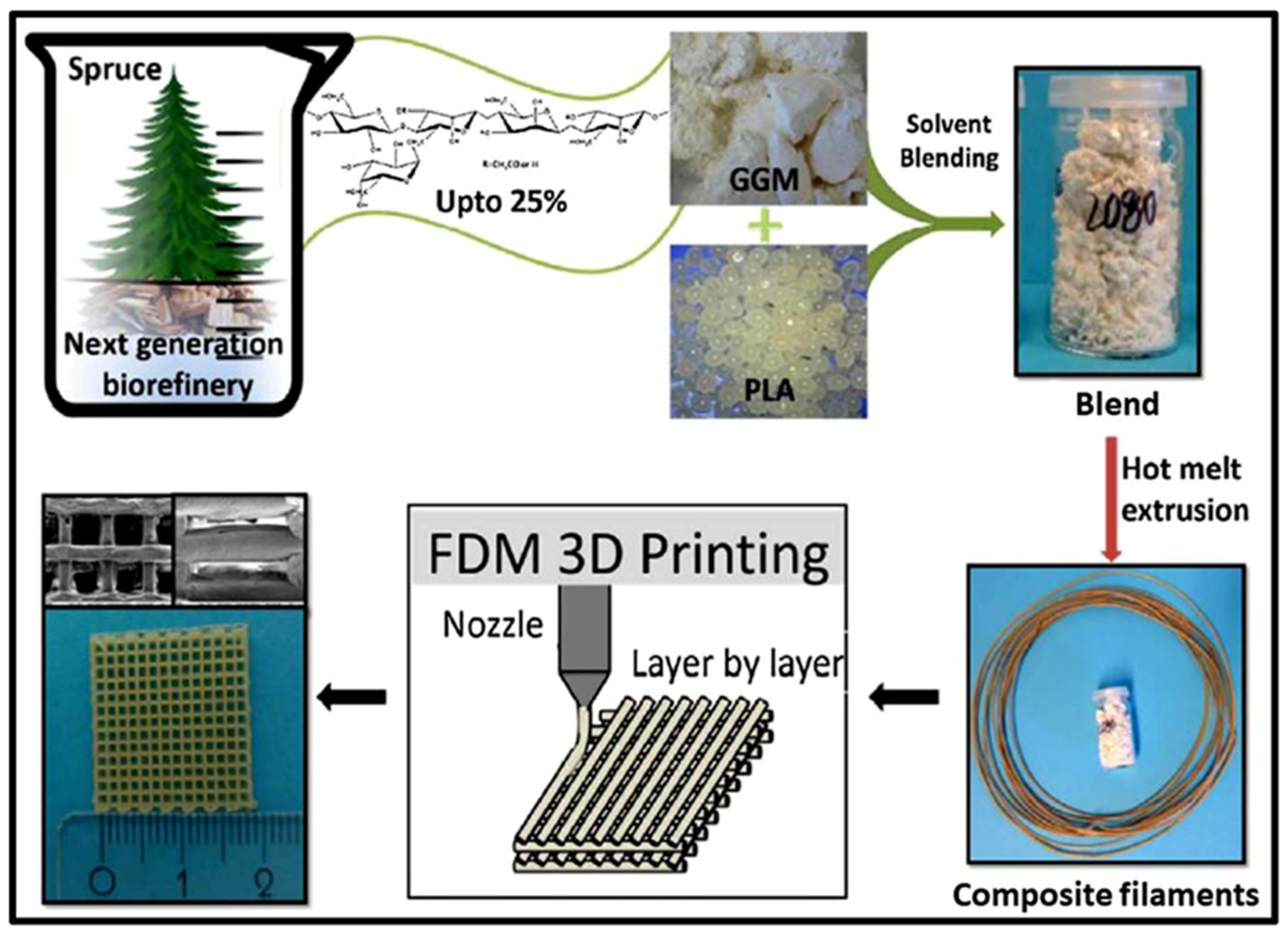
3.2. Hemicellulose
3.3. Lignin
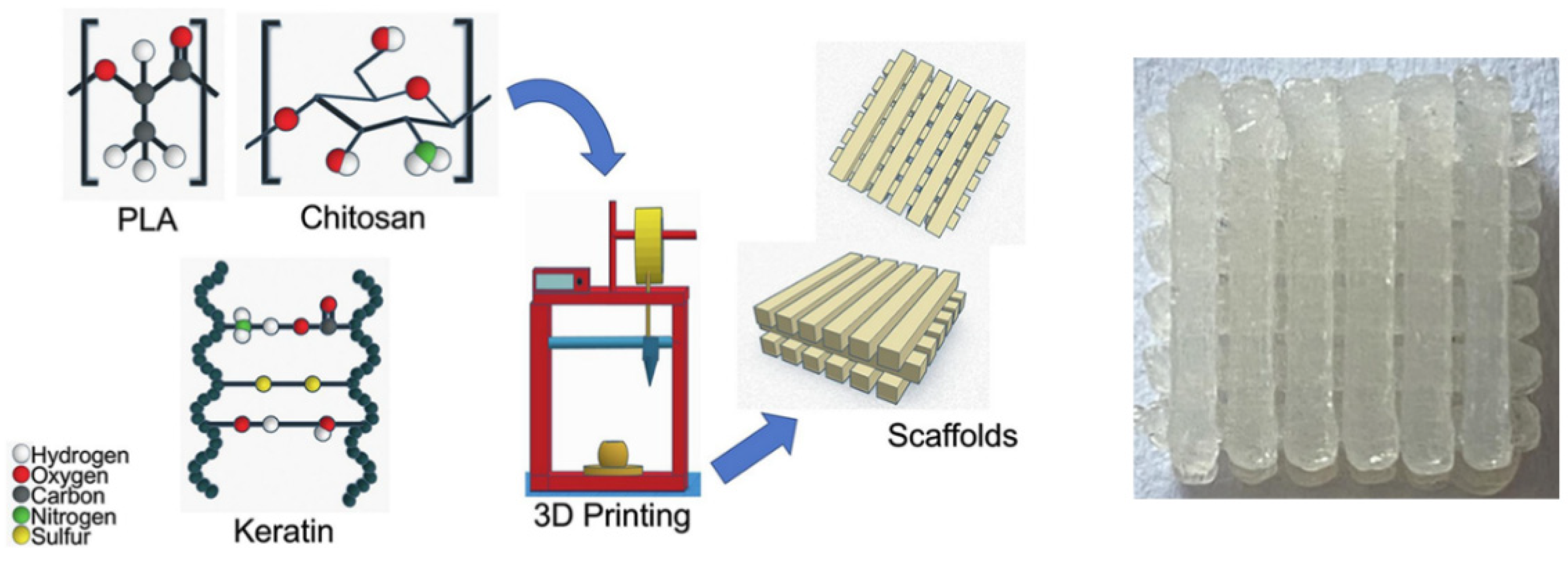
3.4. Chitosan
3.5. Wood Fibers/Flour
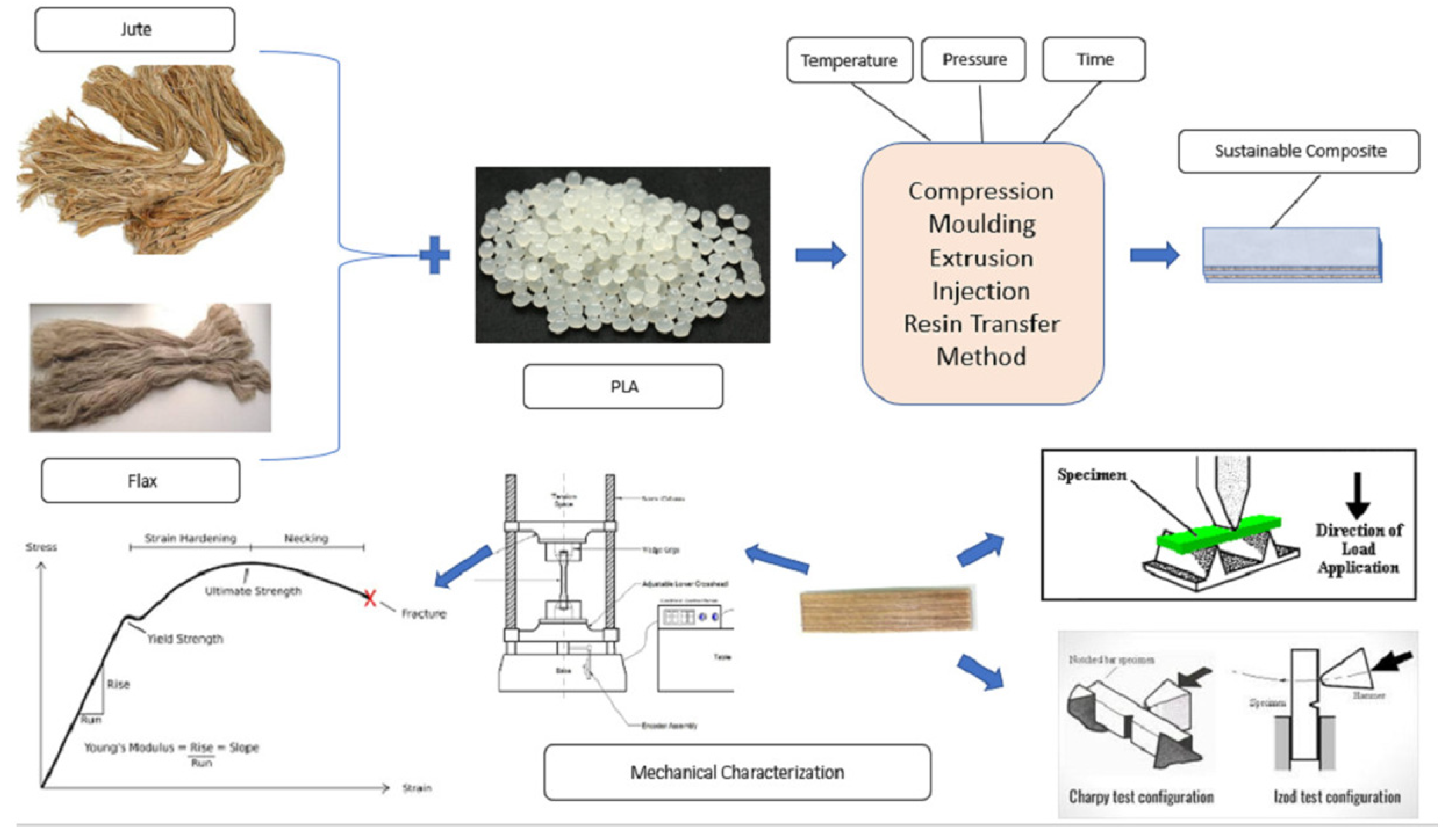
3.6. Flax/Bamboo Fibers
3.7. Hemp Fibers
3.8. Soybean
3.9. Coffee Waste
3.10. Keratin
3.11. Silk
3.12. Gelatin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D Printing with Cellulose Materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical Applications of 3D Printing Technology: Current Understanding and Future Perspectives. J. Pharm. Investig. 2018, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.D.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d Printing Technologies Applied for Food Design: Status and Prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D Printing to Innovate Biopolymer Materials for Demanding Applications: A Review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D Printing by Fused Deposition Modeling of Single- and Multi-Compartment Hollow Systems for Oral Delivery—A Review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D Printing of Polymers Containing Natural Fillers: A Review of Their Mechanical Properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.K.; Goda, K.; Sreekala, M.S. Part One Introduction to Polymer Composites. Polym. Compos. 2012, 1, 16. [Google Scholar]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Sudamrao Getme, A.; Patel, B. A Review: Bio-Fiber’s as Reinforcement in Composites of Polylactic Acid (PLA). Mater. Today Proc. 2020, 26, 2116–2122. [Google Scholar] [CrossRef]
- Deb, D.; Jafferson, J.M. Natural Fibers Reinforced FDM 3D Printing Filaments. Mater. Today Proc. 2021, 46, 1308–1318. [Google Scholar] [CrossRef]
- Nehete, J.Y.; Bhambar, R.S.; Narkhede, M.R.; Gawali, S.R. Natural Proteins: Sources, Isolation, Characterization and Applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Sundararajan, P.R. Cellulose. In The Polysaccharides; Elsevier: Amsterdam, The Netherlands, 1983; pp. 11–95. ISBN 978-0-12-065602-8. [Google Scholar]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Wang, B.; Ding, G.; Chen, K.; Jia, S.; Wei, J.; Wang, Y.; He, R.; Shao, Z. A Physical and Chemical Double Enhancement Strategy for 3D Printing of Cellulose Reinforced Nanocomposite. J. Appl. Polym. Sci. 2020, 137, 49164. [Google Scholar] [CrossRef]
- Shariatnia, S.; Veldanda, A.; Obeidat, S.; Jarrahbashi, D.; Asadi, A. Atomization of Cellulose Nanocrystals Aqueous Suspensions in Fused Deposition Modeling: A Scalable Technique to Improve the Strength of 3D Printed Polymers. Compos. Part B Eng. 2019, 177, 107291. [Google Scholar] [CrossRef]
- Bondeson, D.; Oksman, K. Polylactic Acid/Cellulose Whisker Nanocomposites Modified by Polyvinyl Alcohol. Compos. Part Appl. Sci. Manuf. 2007, 38, 2486–2492. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Bras, J.; Fortunati, E.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(Lactic Acid)/Natural Rubber/Cellulose Nanocrystal Bionanocomposites Part I. Processing and Morphology. Carbohydr. Polym. 2013, 96, 611–620. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and Thermal Properties of Poly(Lactic Acid)/Cellulose Whiskers Nanocomposite Materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Goussé, C.; Chanzy, H.; Excoffier, G.; Soubeyrand, L.; Fleury, E. Stable Suspensions of Partially Silylated Cellulose Whiskers Dispersed in Organic Solvents. Polymer 2002, 43, 2645–2651. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface Acetylation of Bacterial Cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and Chiral-Nematic Self-Ordering of Cellulose Microcrystals Suspensions in Nonpolar Solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(Ethylene Glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Murphy, C.A.; Collins, M.N. Microcrystalline Cellulose Reinforced Polylactic Acid Biocomposite Filaments for 3D Printing. Polym. Compos. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yu, T.; Wang, N.; Wang, C.; Wang, H. Preparation of Polylactic Acid/TEMPO-Oxidized Bacterial Cellulose Nanocomposites for 3D Printing via Pickering Emulsion Approach. Compos. Commun. 2019, 16, 162–167. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Lungu, A.; Stancu, I.-C.; Serafim, A.; Heggset, E.; Syverud, K.; Iovu, H. Bioinspired 3D Printable Pectin-Nanocellulose Ink Formulations. Carbohydr. Polym. 2019, 220, 12–21. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical Properties of Cellulose Nanofiber (CNF) Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Dong, J.; Mei, C.; Han, J.; Lee, S.; Wu, Q. 3D Printed Poly(Lactic Acid) Composites with Grafted Cellulose Nanofibers: Effect of Nanofiber and Post-Fabrication Annealing Treatment on Composite Flexural Properties. Addit. Manuf. 2019, 28, 621–628. [Google Scholar] [CrossRef]
- Dong, J.; Li, M.; Zhou, L.; Lee, S.; Mei, C.; Xu, X.; Wu, Q. The Influence of Grafted Cellulose Nanofibers and Postextrusion Annealing Treatment on Selected Properties of Poly(Lactic Acid) Filaments for 3D Printing. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 847–855. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Meng, X.; Lu, Y.; Kunc, V.; Love, L.J.; Peter, W.H.; Ozcan, S. High Modulus Biocomposites via Additive Manufacturing: Cellulose Nanofibril Networks as “Microsponges”. Compos. Part B Eng. 2019, 173, 106817. [Google Scholar] [CrossRef]
- Shavandi, A.; Hosseini, S.; Okoro, O.V.; Nie, L.; Eghbali Babadi, F.; Melchels, F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv. Healthc. Mater. 2020, 9, 2001472. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Cellulose, Hemicellulose, Lignin, and Their Derivatives as Multi-Components of Bio-Based Feedstocks for 3D Printing. Carbohydr. Polym. 2020, 250, 116881. [Google Scholar] [CrossRef]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct Cryo Writing of Aerogels Via 3D Printing of Aligned Cellulose Nanocrystals Inspired by the Plant Cell Wall. Colloids Interfaces 2019, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.; Xu, C. Surface Engineered Biomimetic Inks Based on UV Cross-Linkable Wood Biopolymers for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef] [Green Version]
- Bahçegül, E.G.; Bahçegül, E.; Özkan, N. 3D Printing of Hemicellulosic Biopolymers Extracted from Lignocellulosic Agricultural Wastes. ACS Appl. Polym. Mater. 2020, 2, 2622–2632. [Google Scholar] [CrossRef]
- Xu, W.; Pranovich, A.; Uppstu, P.; Wang, X.; Kronlund, D.; Hemming, J.; Öblom, H.; Moritz, N.; Preis, M.; Sandler, N.; et al. Novel Biorenewable Composite of Wood Polysaccharide and Polylactic Acid for Three Dimensional Printing. Carbohydr. Polym. 2018, 187, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ebers, L.-S.; Arya, A.; Bowland, C.C.; Glasser, W.G.; Chmely, S.C.; Naskar, A.K.; Laborie, M.-P. 3D Printing of Lignin: Challenges, Opportunities and Roads Onward. Biopolymers 2021, 112, e23431. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Nuruddin; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and Characterization of Lignin from Different Biomass Resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/Lignin Blends: Interactions, Properties, Applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A General Method to Improve 3D-Printability and Inter-Layer Adhesion in Lignin-Based Composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Mimini, V.; Sykacek, E.; Syed Hashim, S.N.A.; Holzweber, J.; Hettegger, H.; Fackler, K.; Potthast, A.; Mundigler, N.; Rosenau, T. Compatibility of Kraft Lignin, Organosolv Lignin and Lignosulfonate With PLA in 3D Printing. J. Wood Chem. Technol. 2019, 39, 14–30. [Google Scholar] [CrossRef]
- Wasti, S.; Triggs, E.; Farag, R.; Auad, M.; Adhikari, S.; Bajwa, D.; Li, M.; Ragauskas, A.J. Influence of Plasticizers on Thermal and Mechanical Properties of Biocomposite Filaments Made from Lignin and Polylactic Acid for 3D Printing. Compos. Part B Eng. 2021, 205, 108483. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.; Fong, M.; Stewart, S.; Irwin, N.; Rial-Hermida, M.; Donnelly, R.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yang, Z.; Chmely, S.; Wang, Q.; Wang, S.; Xie, Y. Lignin-Coated Cellulose Nanocrystal Filled Methacrylate Composites Prepared via 3D Stereolithography Printing: Mechanical Reinforcement and Thermal Stabilization. Carbohydr. Polym. 2017, 169, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Michailidou, G.; Terzopoulou, Z.; Kehagia, A.; Michopoulou, A.; Bikiaris, D.N. Preliminary Evaluation of 3D Printed Chitosan/Pectin Constructs for Biomedical Applications. Mar. Drugs 2021, 19, 36. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Prakash, C.; Ramakrishna, S.; Lamberti, L.; Pruncu, C.I. 3D Printed Biodegradable Composites: An Insight into Mechanical Properties of PLA/Chitosan Scaffold. Polym. Test. 2020, 89, 106722. [Google Scholar] [CrossRef]
- Zhang, S.; Bhagia, S.; Li, M.; Meng, X.; Ragauskas, A.J. Wood-Reinforced Composites by Stereolithography with the Stress Whitening Behavior. Mater. Des. 2021, 206, 109773. [Google Scholar] [CrossRef]
- Kariz, M.; Sernek, M.; Obućina, M.; Kuzman, M.K. Effect of Wood Content in FDM Filament on Properties of 3D Printed Parts. Mater. Today Commun. 2018, 14, 135–140. [Google Scholar] [CrossRef]
- Bi, H.; Ren, Z.; Guo, R.; Xu, M.; Song, Y. Fabrication of Flexible Wood Flour/Thermoplastic Polyurethane Elastomer Composites Using Fused Deposition Molding. Ind. Crop. Prod. 2018, 122, 76–84. [Google Scholar] [CrossRef]
- Guo, R.; Ren, Z.; Bi, H.; Song, Y.; Xu, M. Effect of Toughening Agents on the Properties of Poplar Wood Flour/Poly (Lactic Acid) Composites Fabricated with Fused Deposition Modeling. Eur. Polym. J. 2018, 107, 34–45. [Google Scholar] [CrossRef]
- Le Duigou, A.; Castro, M.; Bevan, R.; Martin, N. 3D Printing of Wood Fibre Biocomposites: From Mechanical to Actuation Functionality. Mater. Des. 2016, 96, 106–114. [Google Scholar] [CrossRef]
- Petchwattana, N.; Channuan, W.; Naknaen, P.; Narupai, B. 3D Printing Filaments Prepared from Modified Poly(Lactic Acid)/Teak Wood Flour Composites: An Investigation on the Particle Size Effects and Silane Coupling Agent Compatibilisation. J. Phys. Sci. 2019, 30, 169–188. [Google Scholar] [CrossRef]
- Faludi, G.; Dora, G.; Renner, K.; Móczó, J.; Pukánszky, B. Improving Interfacial Adhesion in Pla/Wood Biocomposites. Compos. Sci. Technol. 2013, 89, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, Q.; Mi, Y. Bamboo Fiber-reinforced Polypropylene Composites: A Study of the Mechanical Properties. J. Appl. Polym. Sci. 1998, 69, 1891–1899. [Google Scholar] [CrossRef]
- Pozo Morales, A.; Güemes, A.; Fernandez-Lopez, A.; Carcelen Valero, V.; De La Rosa Llano, S. Bamboo–Polylactic Acid (PLA) Composite Material for Structural Applications. Materials 2017, 10, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Duigou, A.; Barbé, A.; Guillou, E.; Castro, M. 3D Printing of Continuous Flax Fibre Reinforced Biocomposites for Structural Applications. Mater. Des. 2019, 180, 107884. [Google Scholar] [CrossRef]
- Charlet, K.; Jernot, J.P.; Gomina, M.; Bréard, J.; Morvan, C.; Baley, C. Influence of an Agatha Flax Fibre Location in a Stem on Its Mechanical, Chemical and Morphological Properties. Compos. Sci. Technol. 2009, 69, 1399–1403. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, K. PLA Toughened by Bamboo Cellulose Nanowhiskers: Role of Silane Compatibilization on the PLA Bionanocomposite Properties. Compos. Sci. Technol. 2017, 148, 59–69. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R. Physical and mechanical properties of natural fibers. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–83. ISBN 978-0-08-100411-1. [Google Scholar]
- Zhang, H.; Liu, D.; Huang, T.; Hu, Q.; Lammer, H. Three-Dimensional Printing of Continuous Flax Fiber-Reinforced Thermoplastic Composites by Five-Axis Machine. Materials 2020, 13, 1678. [Google Scholar] [CrossRef] [Green Version]
- Badouard, C.; Traon, F.; Denoual, C.; Mayer-Laigle, C.; Paës, G.; Bourmaud, A. Exploring Mechanical Properties of Fully Compostable Flax Reinforced Composite Filaments for 3D Printing Applications. Ind. Crop. Prod. 2019, 135, 246–250. [Google Scholar] [CrossRef]
- El-Sabbagh, A.; Steuernagel, L.; Ziegmann, G.; Meiners, D.; Toepfer, O. Processing Parameters and Characterisation of Flax Fibre Reinforced Engineering Plastic Composites with Flame Retardant Fillers. Compos. Part B Eng. 2014, 62, 12–18. [Google Scholar] [CrossRef]
- Depuydt, D.; Balthazar, M.; Hendrickx, K.; Six, W.; Ferraris, E.; Desplentere, F.; Ivens, J.; Van Vuure, A.W. Production and Characterization of Bamboo and Flax Fiber Reinforced Polylactic Acid Filaments for Fused Deposition Modeling (FDM). Polym. Compos. 2019, 40, 1951–1963. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef] [PubMed]
- Karche, T.; Singh, M.R. The Application of Hemp (Cannabis sativa L.) for a Green Economy: A Review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to Emerging Industrial Applications of Cannabis (Cannabis sativa L.). Rend. Lincei Sci. Fis. E Nat. 2021, 32, 233–243. [Google Scholar] [CrossRef]
- Coppola, B.; Garofalo, E.; Di Maio, L.; Scarfato, P.; Incarnato, L. Investigation on the Use of PLA/Hemp Composites for the Fused Deposition Modelling (FDM) 3D Printing. AIP Conf. Proc. 2018, 1981, 020086. [Google Scholar] [CrossRef]
- Mazzanti, V.; Pariante, R.; Bonanno, A.; Ruiz de Ballesteros, O.; Mollica, F.; Filippone, G. Reinforcing Mechanisms of Natural Fibers in Green Composites: Role of Fibers Morphology in a PLA/Hemp Model System. Compos. Sci. Technol. 2019, 180, 51–59. [Google Scholar] [CrossRef]
- Xiao, X.; Chevali, V.S.; Song, P.; He, D.; Wang, H. Polylactide/Hemp Hurd Biocomposites as Sustainable 3D Printing Feedstock. Compos. Sci. Technol. 2019, 184, 107887. [Google Scholar] [CrossRef]
- Koushki, P.; Kwok, T.-H.; Hof, L.; Wuthrich, R. Reinforcing Silicone with Hemp Fiber for Additive Manufacturing. Compos. Sci. Technol. 2020, 194, 108139. [Google Scholar] [CrossRef]
- Etaati, A.; Pather, S.; Fang, Z.; Wang, H. The Study of Fibre/Matrix Bond Strength in Short Hemp Polypropylene Composites from Dynamic Mechanical Analysis. Compos. Part B Eng. 2014, 62, 19–28. [Google Scholar] [CrossRef]
- Walter, R. Fehr Soybean. In Hybridization of Crop Plants; Fehr, W.R., Hadley, H.H., Eds.; American Society of Agronomy and Crop Science Society of America Publishers: Madison, WI, USA, 1980; pp. 589–599. [Google Scholar]
- Jegadeesan, S.; Yu, K. Food Grade Soybean Breeding, Current Status and Future Directions. In Legume Crops: Prospects, Production and Uses; IntechOpen Limited: London, UK, 2020. [Google Scholar]
- Zhao, L.; Cheng, S.; Liu, S.; Gao, X. Improvement of the Addition Amount and Dispersion of Hydroxyapatite in the Poly(Lactic Acid) Matrix by the Compatibilizer-Epoxidized Soybean Oil. J. Mater. Res. 2020, 35, 1523–1530. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Cheng, J.; Shang, Q.; Hu, Y.; Liu, C.; Hu, L.; Zhou, Y. High-Performance 3D Printing UV-Curable Resins Derived from Soybean Oil and Gallic Acid. Green Chem. 2021, 23, 5911–5923. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Konuray, O.; Ramis, X.; Serra, À.; De la Flor, S. Enhancement of 3D-Printable Materials by Dual-Curing Procedures. Materials 2021, 14, 107. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, J.; Lei, D.; Su, J. 3D Printing of a Dual-Curing Resin with Cationic Curable Vegetable Oil. Ind. Eng. Chem. Res. 2020, 59, 11381–11388. [Google Scholar] [CrossRef]
- Guit, J.; Tavares, M.B.L.; Hul, J.; Ye, C.; Loos, K.; Jager, J.; Folkersma, R.; Voet, V.S.D. Photopolymer Resins with Biobased Methacrylates Based on Soybean Oil for Stereolithography. ACS Appl. Polym. Mater. 2020, 2, 949–957. [Google Scholar] [CrossRef]
- Balla, V.K.; Tadimeti, J.G.D.; Kate, K.H.; Satyavolu, J. 3D Printing of Modified Soybean Hull Fiber/Polymer Composites. Mater. Chem. Phys. 2020, 254, 123452. [Google Scholar] [CrossRef]
- Balla, V.K.; Tadimeti, J.G.D.; Sudan, K.; Satyavolu, J.; Kate, K.H. First Report on Fabrication and Characterization of Soybean Hull Fiber: Polymer Composite Filaments for Fused Filament Fabrication. Prog. Addit. Manuf. 2021, 6, 39–52. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of Spent Coffee Grounds: A Review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on Utilization and Composition of Coffee Silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Chaudhry, S.A. (Eds.) Composites for Environmental Engineering, 1st ed.; Scrivener Publishing LLC: Hoboken, NJ, USA, 2020; Chapter 10; pp. 319–345. ISBN 978-1-119-55535-3. [Google Scholar]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of Coffee Wastes as Reinforcement in Polyhydroxybutyrate (PHB) Based Composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of Spent Coffee Grounds Filler on the Physical and Mechanical Properties of Poly(Lactic Acid) Bio-Composite Films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Ortiz-Barajas, D.L.; Arévalo-Prada, J.A.; Fenollar, O.; Rueda-Ordóñez, Y.J.; Torres-Giner, S. Torrefaction of Coffee Husk Flour for the Development of Injection-Molded Green Composite Pieces of Polylactide with High Sustainability. Appl. Sci. 2020, 10, 6468. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee Silverskin as a Multifunctional Waste Filler for High-Density Polyethylene Green Composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, Y.; Ning, J.; Hao, C.; Rock, M.; Amer, M.; Feng, S.; Falahati, M.; Wang, L.-J.; Chen, R.K.; et al. No Such Thing as Trash: A 3D-Printable Polymer Composite Composed of Oil-Extracted Spent Coffee Grounds and Polylactic Acid with Enhanced Impact Toughness. ACS Sustain. Chem. Eng. 2019, 7, 15304–15310. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Sun, S.; Chan, H.; Lu, H.; Nilghaz, A.; Tian, J.; Cao, R. From Brown to Colored: Polylactic Acid Composite with Micro/Nano-Structured White Spent Coffee Grounds for Three-Dimensional Printing. Int. J. Biol. Macromol. 2021, 174, 300–308. [Google Scholar] [CrossRef]
- Patrucco, A.; Visai, L.; Fassina, L.; Magenes, G.; Tonin, C. Keratin-based matrices from wool fibers and human hair. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–403. ISBN 978-0-12-816872-1. [Google Scholar]
- Flores-Hernandez, C.G.; Velasco-Santos, C.; Rivera-Armenta, J.L.; Gomez-Guzman, O.; Yañez-Limon, J.M.; Olivas-Armendariz, I.; Lopez-Barroso, J.; Martinez-Hernandez, A.L. Additive Manufacturing of Green Composites: Poly (Lactic Acid) Reinforced with Keratin Materials Obtained from Angora Rabbit Hair. J. Appl. Polym. Sci. 2021, 138, 50321. [Google Scholar] [CrossRef]
- Flores-Hernandez, C.G.; Velasco-Santos, C.; Hernandez-Zea, A.L.; Gomez-Guzman, O.; Yañez-Limon, J.M.; Rivera-Armenta, J.L.; Martinez-Hernandez, A.L. Low Concentrations for Significant Improvements in Thermal and Thermomechanical Properties of Poly(Lactic Acid)–Keratin Biocomposites Obtained by Extrusion and 3D Printing. J. Nat. Fibers 2020. [Google Scholar] [CrossRef]
- Rojas-Martínez, L.E.; Flores-Hernandez, C.G.; López-Marín, L.M.; Martinez-Hernandez, A.L.; Thorat, S.B.; Reyes Vasquez, C.D.; Del Rio-Castillo, A.E.; Velasco-Santos, C. 3D Printing of PLA Composites Scaffolds Reinforced with Keratin and Chitosan: Effect of Geometry and Structure. Eur. Polym. J. 2020, 141, 110088. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Scott, S.M.; Plowman-Holmes, M.I.; Middlewood, P.G.; Recabar, K. Combination and Processing Keratin with Lignin as Biocomposite Materials for Additive Manufacturing Technology. Acta Biomater. 2020, 104, 95–103. [Google Scholar] [CrossRef]
- Singamneni, S.; Velu, R.; Behera, M.P.; Scott, S.; Brorens, P.; Harland, D.; Gerrard, J. Selective Laser Sintering Responses of Keratin-Based Bio-Polymer Composites. Mater. Des. 2019, 183, 108087. [Google Scholar] [CrossRef]
- Gupta, S.; Alrabaiah, H.; Christophe, M.; Rahimi-Gorji, M.; Nadeem, S.; Bit, A. Evaluation of silk-based bioink during pre and post 3D bioprinting: A review. J. Biomed. Mater. Res. 2021, 109, 279. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Gil, E.S.; Kluge, J.; Numata, K.; Wang, L.; Wang, X.; Kaplan, D.L. Reinforcing silk scaffolds with silk particles. Macromol. Biosci. 2010, 10, 599. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Adhikary, M.J.C.; Kumar, M.; Bhardwaj, N.; Mandal, B.B. Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces. 2016, 8, 30797. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D Printing of Silk Microparticle Reinforced Polycaprolactone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Kalita, S.; Dilley, R.J.; Wang, X.; Liu, X. Silk Particles, Microfibres and Nanofibres: A Comparative Study of Their Functions in 3D Printing Hydrogel Scaffolds. Mater. Sci. Eng. C 2019, 103, 109784. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.; Shin, S.; Lee, H.; Hyun, J. Formation of a Keratin Layer with Silk Fibroin-Polyethylene Glycol Composite Hydrogel Fabricated by Digital Light Processing 3D Printing. J. Ind. Eng. Chem. 2019, 72, 232–240. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable Alginate/Gelatin Hydrogel Reinforced with Carbon Nanofibers as Electrically Conductive Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Choi, C.; Cho, Y.; Kim, G. Gelatin/PVA Scaffolds Fabricated Using a 3D-Printing Process Employed with a Low-Temperature Plate for Hard Tissue Regeneration: Fabrication and Characterizations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.-G.; Kwon, O.H. Reinforced Gelatin-Methacrylate Hydrogels Containing Poly(Lactic-Co-Glycolic Acid) Nanofiber Fragments for 3D Bioprinting. J. Ind. Eng. Chem. 2020, 89, 147–155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, B.; Cambriani, A.; Cespi, M.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. An Overview of Natural Polymers as Reinforcing Agents for 3D Printing. ChemEngineering 2021, 5, 78. https://doi.org/10.3390/chemengineering5040078
Sabbatini B, Cambriani A, Cespi M, Palmieri GF, Perinelli DR, Bonacucina G. An Overview of Natural Polymers as Reinforcing Agents for 3D Printing. ChemEngineering. 2021; 5(4):78. https://doi.org/10.3390/chemengineering5040078
Chicago/Turabian StyleSabbatini, Beatrice, Alessandra Cambriani, Marco Cespi, Giovanni Filippo Palmieri, Diego Romano Perinelli, and Giulia Bonacucina. 2021. "An Overview of Natural Polymers as Reinforcing Agents for 3D Printing" ChemEngineering 5, no. 4: 78. https://doi.org/10.3390/chemengineering5040078
APA StyleSabbatini, B., Cambriani, A., Cespi, M., Palmieri, G. F., Perinelli, D. R., & Bonacucina, G. (2021). An Overview of Natural Polymers as Reinforcing Agents for 3D Printing. ChemEngineering, 5(4), 78. https://doi.org/10.3390/chemengineering5040078








