Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites
Abstract
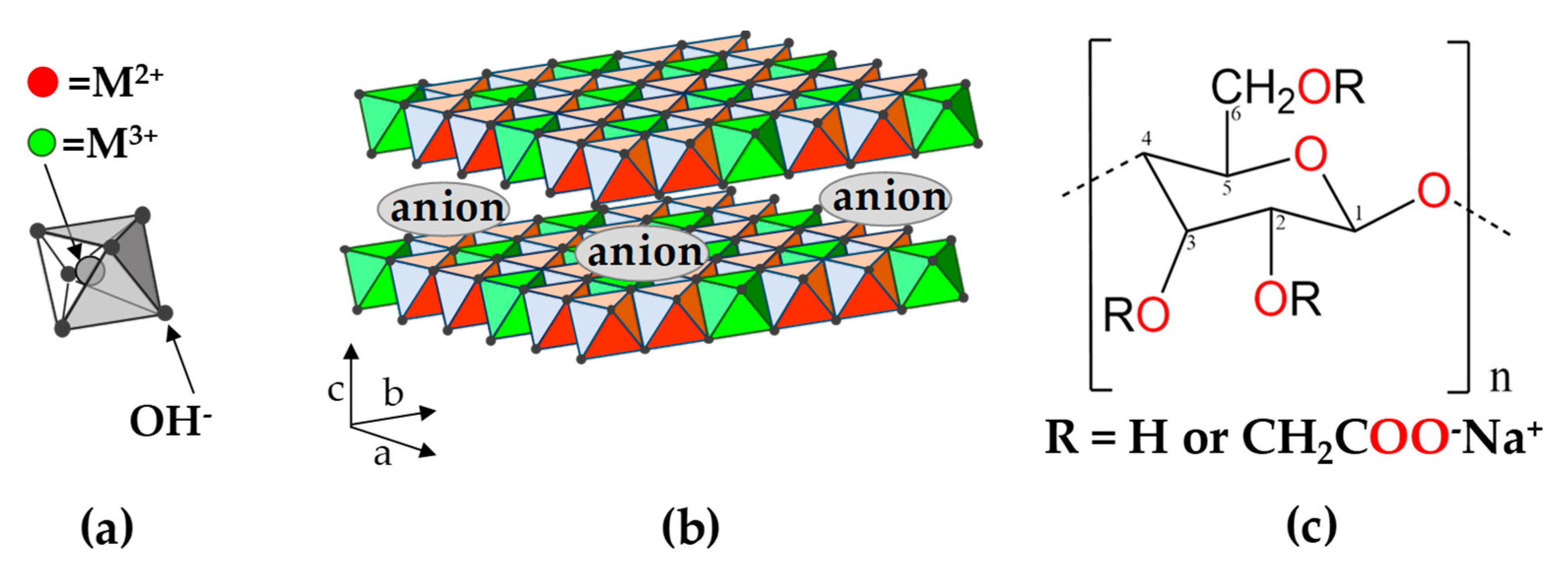
1. Introduction
2. Materials and Methods
2.1. Chemicals
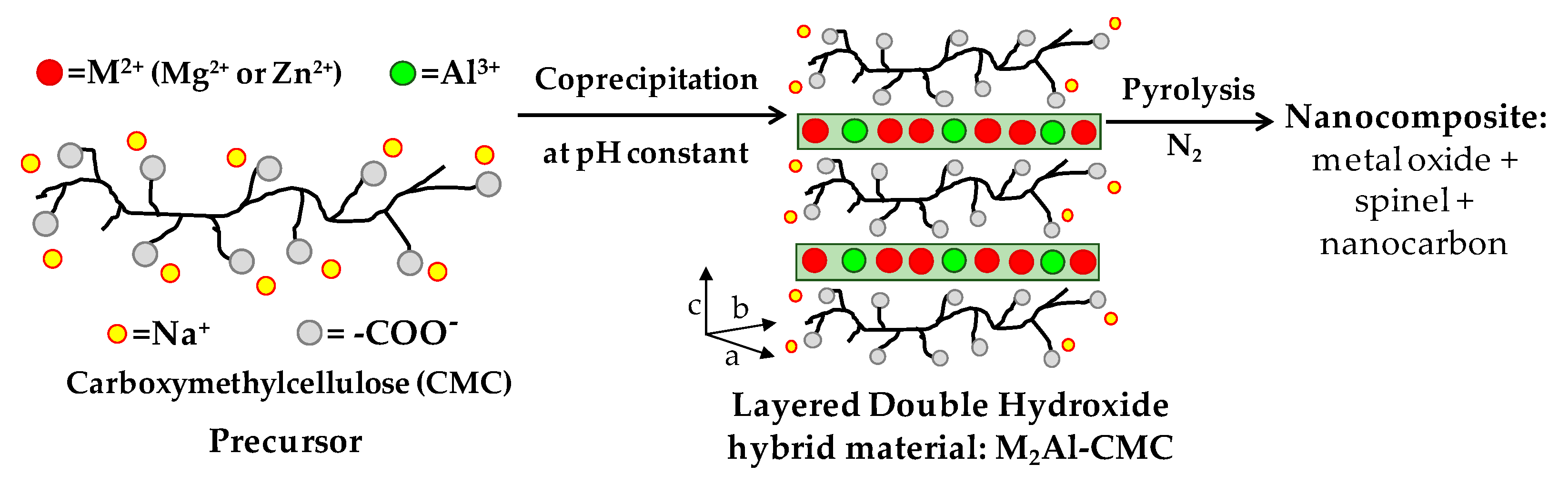
2.2. Preparation of LDH-CMC Hybrid Materials
2.3. Preparation of MMO/C Composites
2.4. Physical Measurements
3. Results and Discussion
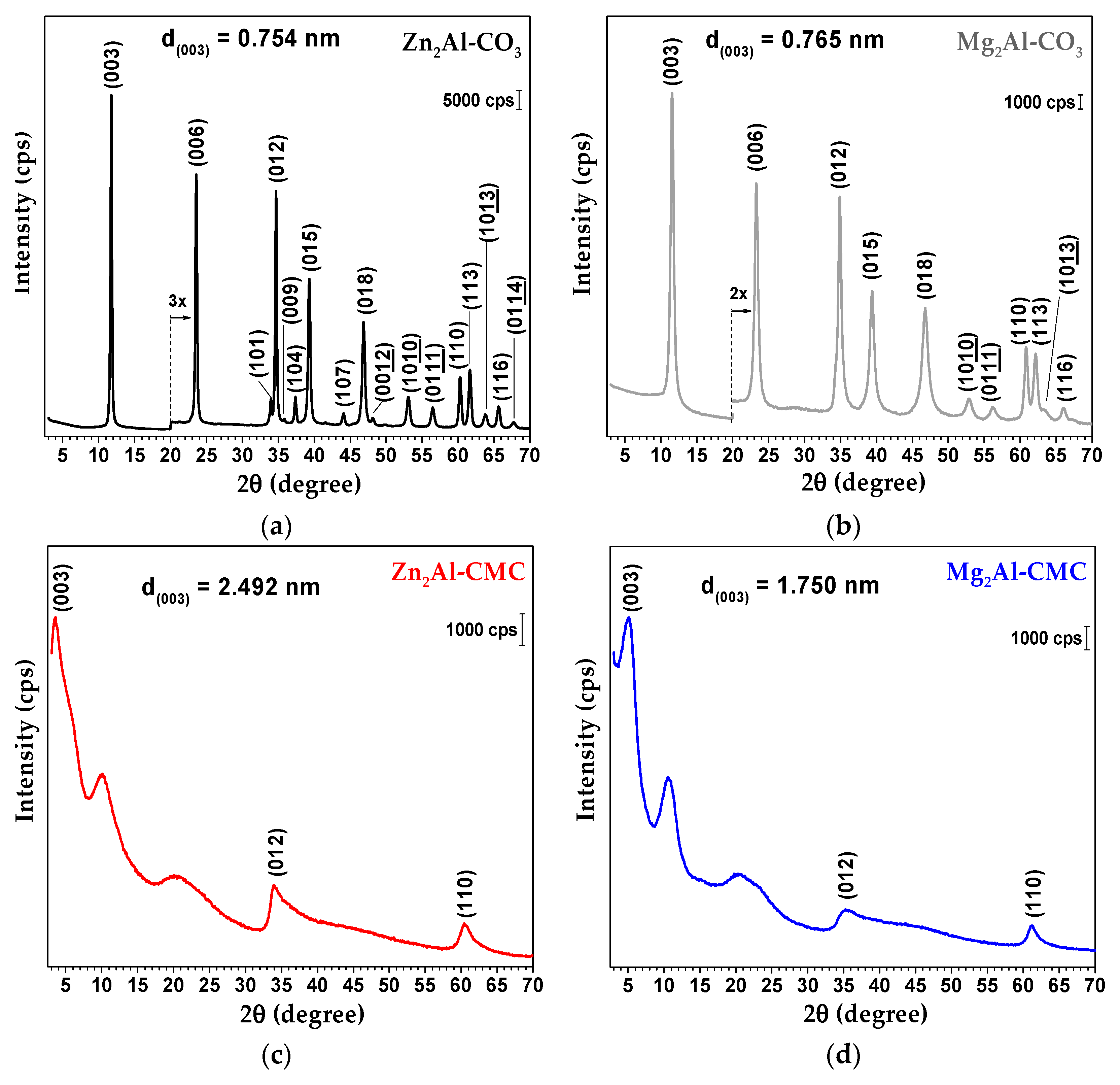
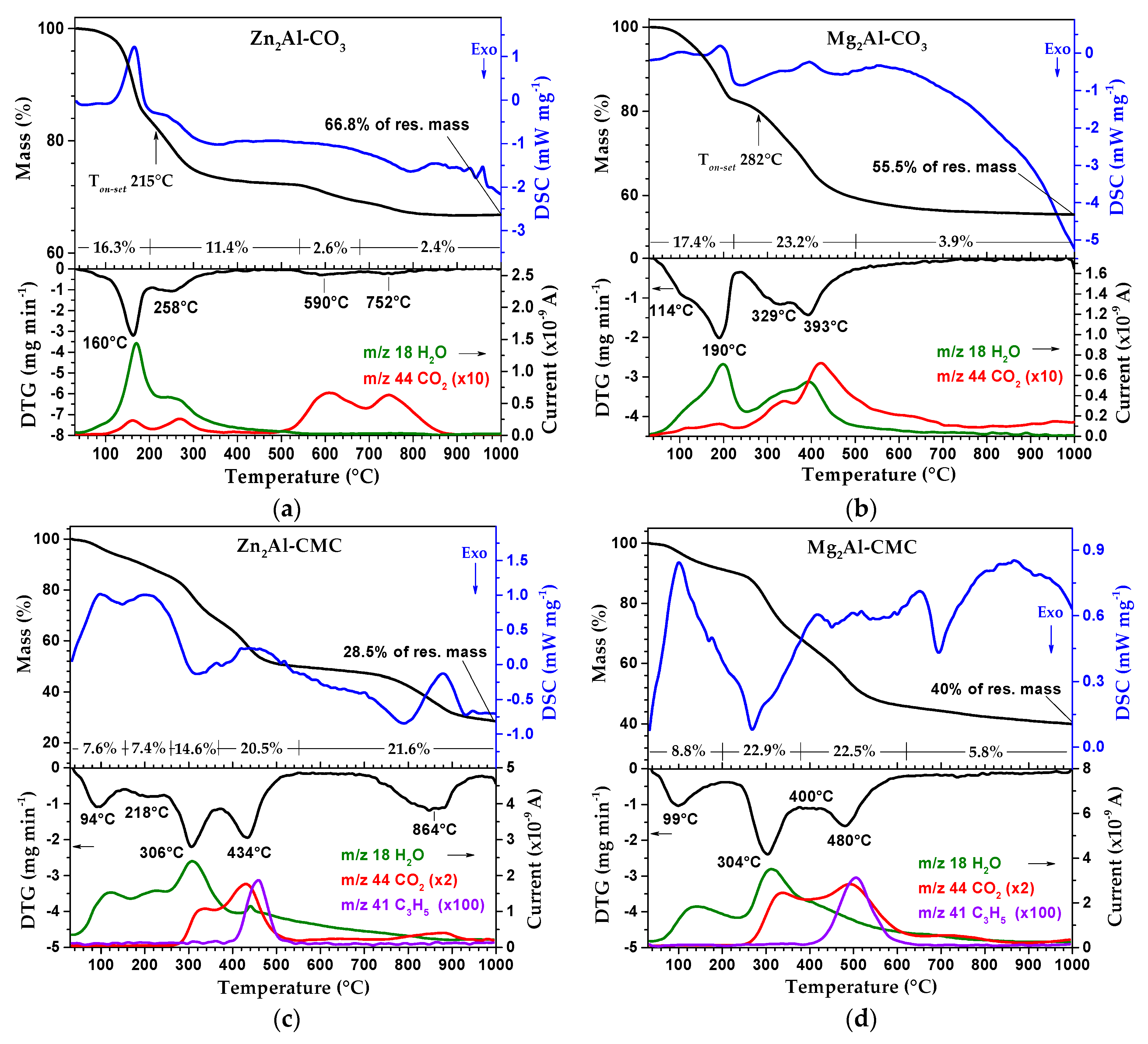
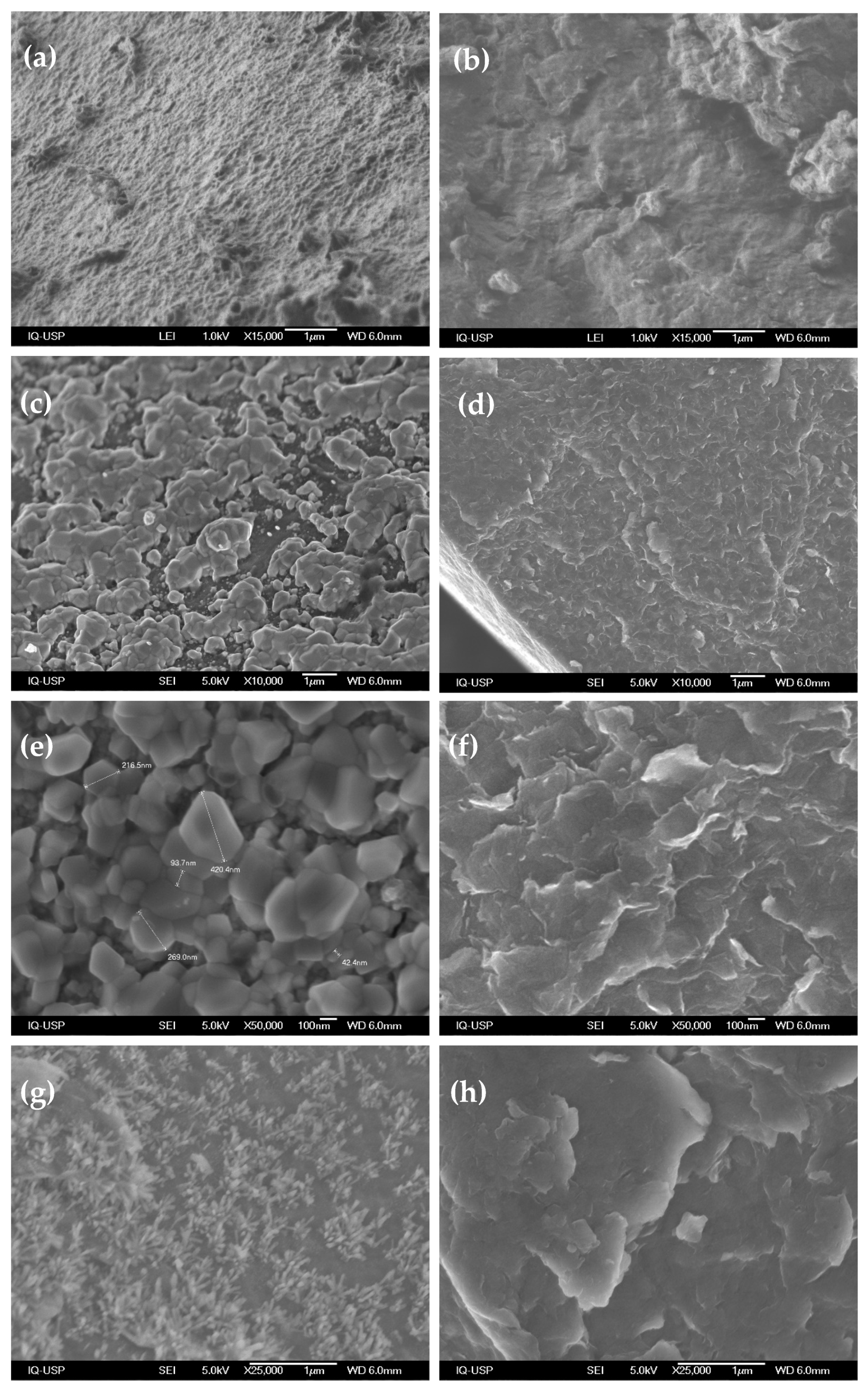
3.1. Characterization of LDH-CMC Hybrid Materials
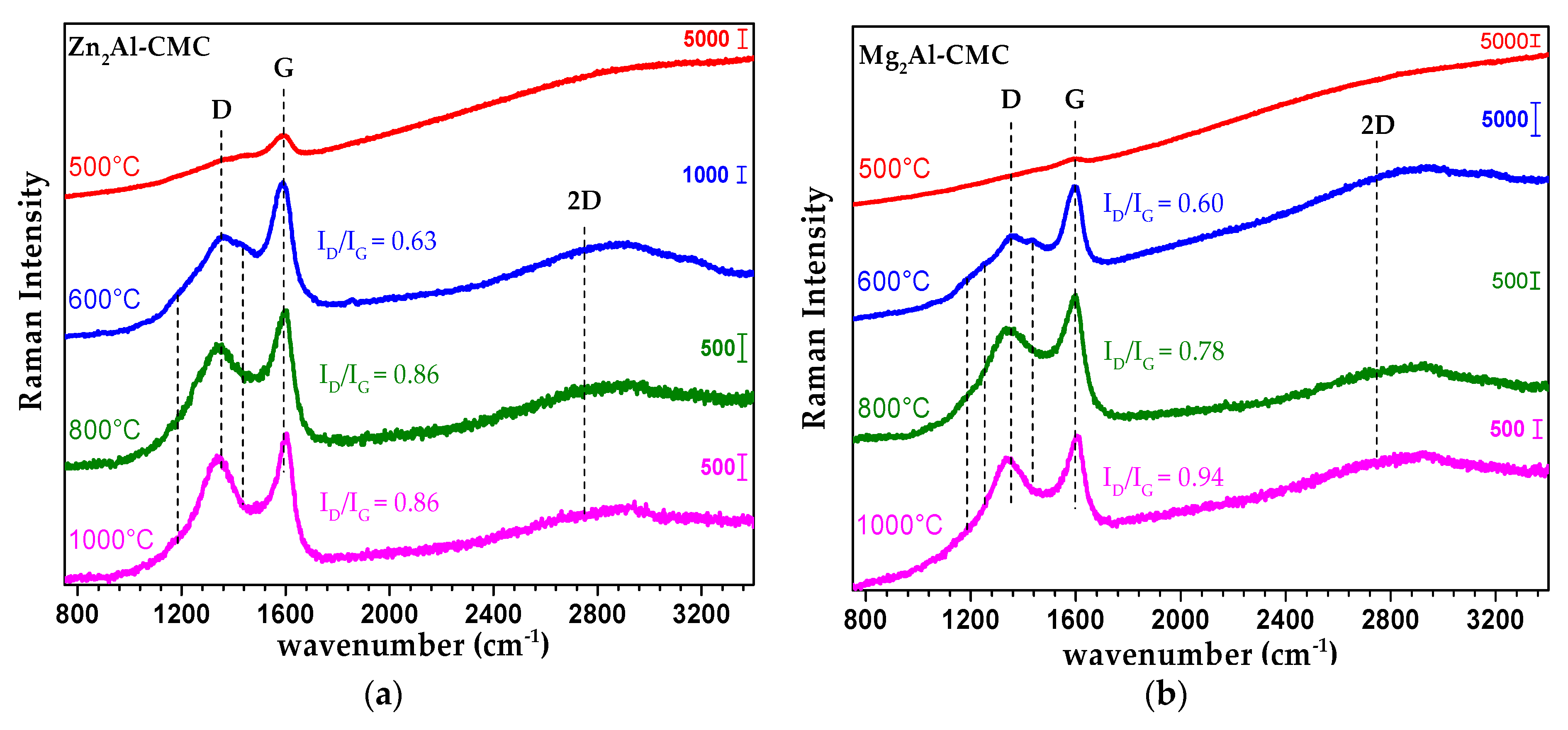
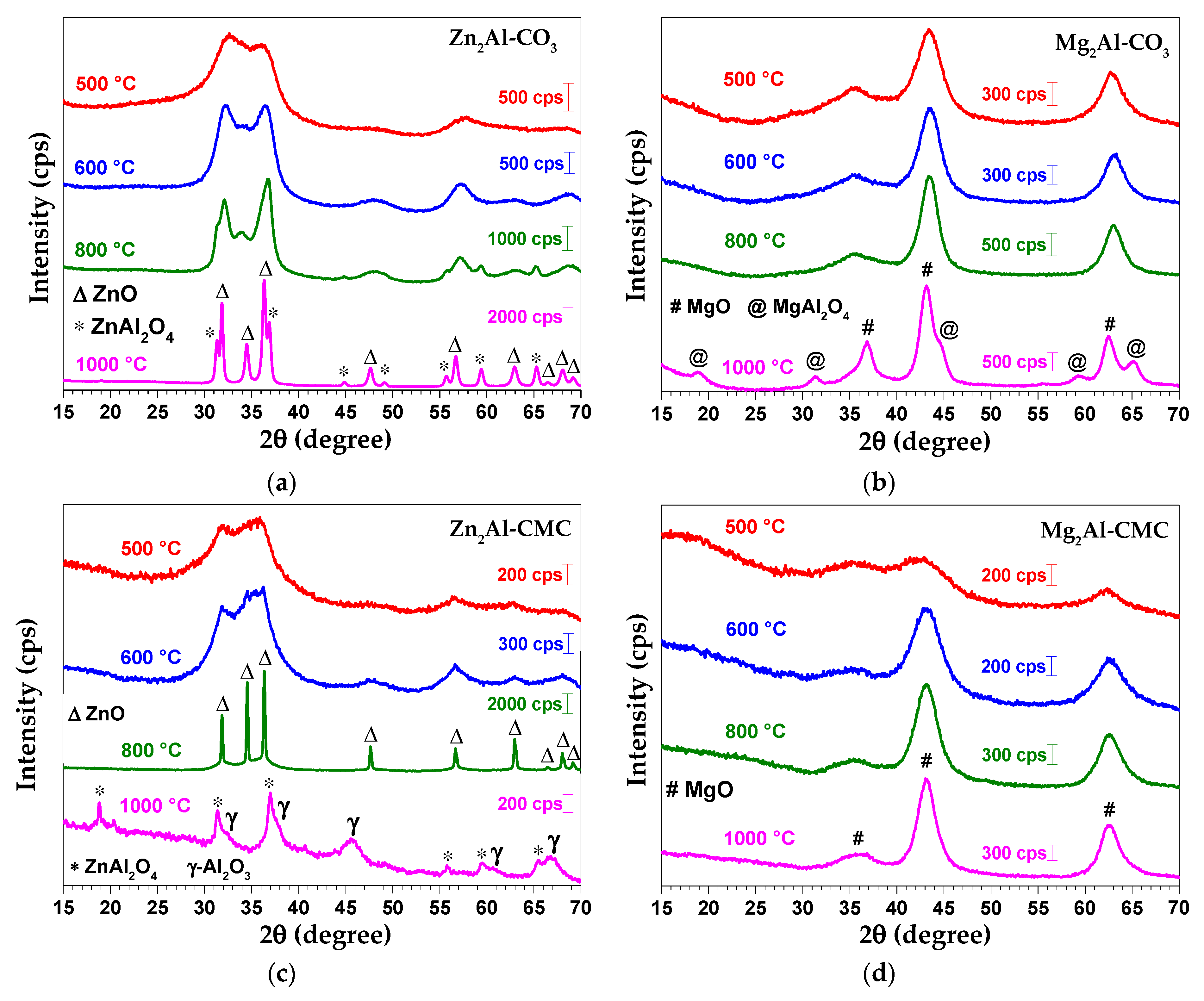
3.2. Characterization of MMO/C Composites
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Miner. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Structure & Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 119, pp. 1–87. ISBN 3540282793. [Google Scholar]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Li, T.; Miras, H.; Song, Y.-F. Polyoxometalate (POM)-Layered Double Hydroxides (LDH) Composite Materials: Design and Catalytic Applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef]
- Rives, V.; Angeles Ulibarri, M. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Leroux, F.; Taviot-Guého, C. Fine tuning between organic and inorganic host structure: New trends in layered double hydroxide hybrid assemblies. J. Mater. Chem. 2005, 15, 3628. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Patel, R.; Park, J.T.; Patel, M.; Dash, J.K.; Gowd, E.B.; Karpoormath, R.; Mishra, A.; Kwak, J.; Kim, J.H. Transition-metal-based layered double hydroxides tailored for energy conversion and storage. J. Mater. Chem. A 2018, 6, 12–29. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Erastova, V.; Degiacomi, M.T.G.; Fraser, D.; Greenwell, H.C. Mineral surface chemistry control for origin of prebiotic peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem. A Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, F.; Zhang, C.; Zeng, G.; Tan, X.; Yu, Z.; Zhong, Y.; Wang, H.; Cui, F. Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: A review. RSC Adv. 2016, 6, 79415–79436. [Google Scholar] [CrossRef]
- Takehira, K. Recent development of layered double hydroxide-derived catalysts−Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci. 2017, 136, 112–141. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides—Properties, synthesis, and applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Kukrety, A.; Singh, R.K.; Singh, P.; Ray, S.S. Comprehension on the Synthesis of Carboxymethylcellulose (CMC) Utilizing Various Cellulose Rich Waste Biomass Resources. Waste Biomass Valorization 2018, 9, 1587–1595. [Google Scholar] [CrossRef]
- Kang, H.; Huang, G.; Ma, S.; Bai, Y.; Ma, H.; Li, Y.; Yang, X. Coassembly of Inorganic Macromolecule of Exfoliated LDH Nanosheets with Cellulose. J. Phys. Chem. C 2009, 113, 9157–9163. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H. Synthesis and characterization of carboxymethyl cellulose/layered double hydroxide nanocomposites. J. Nanopar. Res. 2013, 15, 1563. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H.; Aghazadeh, M. Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int. J. Biol. Macromol. 2015, 79, 269–277. [Google Scholar] [CrossRef]
- Rocha, M.A.; Petersen, P.A.D.; Teixeira-Neto, E.; Petrilli, H.M.; Leroux, F.; Taviot-Gueho, C.; Constantino, V.R.L. Layered double hydroxide and sulindac coiled and scrolled nanoassemblies for storage and drug release. RSC Adv. 2016, 6, 16419–16436. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. From layered double hydroxides to ZnO-based mixed metal oxides by thermal decomposition: Transformation mechanism and UV-blocking properties of the product. Chem. Mater. 2010, 22, 3933–3942. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. NPJ 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.Z.; Elhalil, A.; Tounsadi, H.; Abdennouri, M.; Sadiq, M.; Qourzal, S.; Zouhri, A.; Barka, N. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal. J. Mater. Res. Technol. 2017, 6, 271–283. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical Behavior of a Natural Polyelectrolyte Hydrogel: Chitosan/Carboxymethylcellulose Hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef]
- Lee, C.M.; Mohamed, N.M.A.; Watts, H.D.; Kubicki, J.D.; Kim, S.H. Sum-frequency-generation vibration spectroscopy and density functional theory calculations with dispersion corrections (DFT-D2) for cellulose Iα and Iβ. J. Phys. Chem. B 2013, 117, 6681–6692. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in I(β) cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. Beattie in Situ Particle Film ATR FTIR Spectroscopy of Carboxymethyl Cellulose Adsorption on Talc: Binding Mechanism, pH Effects, and Adsorption Kinetics. Langmuir 2008, 24, 8036–8044. [Google Scholar] [CrossRef]
- Schenzel, K.; Fischer, S. NIR FT Raman spectroscopy—A rapid analytical tool for detecting the transformation of cellulose polymorphs. Cellulose 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Barsberg, S. Prediction of vibrational spectra of polysaccharides-simulated IR spectrum of cellulose based on density functional theory (DFT). J. Phys. Chem. B 2010, 114, 11703–11708. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.; Vasko, P.D.; Koenig, J.L. Infrared and raman spectra of the cellulose from the cell wall of valonia ventricosa. J. Appl. Phys. 1970, 41, 4375–4379. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H. Characterization of polymer complexes by thermal and ir spectral analyses. Polym. Plast. Technol. Eng. 2000, 39, 887–904. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1−xAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Belloto, M.; Bernadette, R.; Olivier, C.; John, L.; Dominique, B.; Elkaim, E. A Reexamination of Hydrotalcite Chemistry. J. Phys. Chem. 1996, 100, 8527–8534. [Google Scholar] [CrossRef]
- Warringham, R.; Mitchell, S.; Murty, R.; Schäublin, R.; Crivelli, P.; Kenvin, J.; Pérez-Ramírez, J. Mapping the Birth and Evolution of Pores upon Thermal Activation of Layered Hydroxides. Chem. Mater. 2017, 29, 4052–4062. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbé, N. Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Graf, G.G. Zinc. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 717–739. [Google Scholar]
- Mardones, J.P. Processing of Spinel-Bearing Compounds for Zinc Extraction. Master’s Thesis, Delft University of Technology, Faculty of Civil Engineering and Geosciences, Delft, The Netherlands, 2012. [Google Scholar]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Vittal, R.; Ho, K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
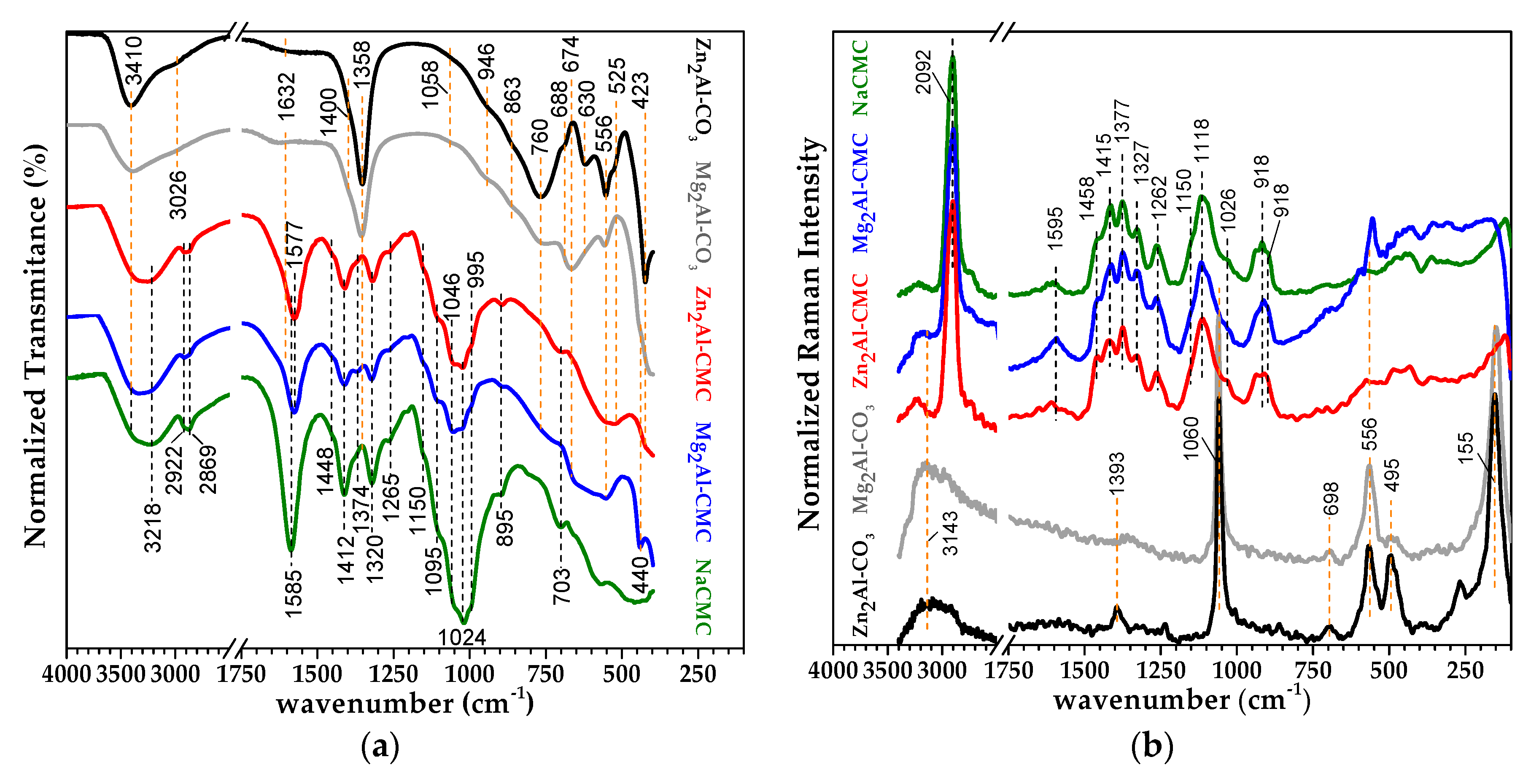
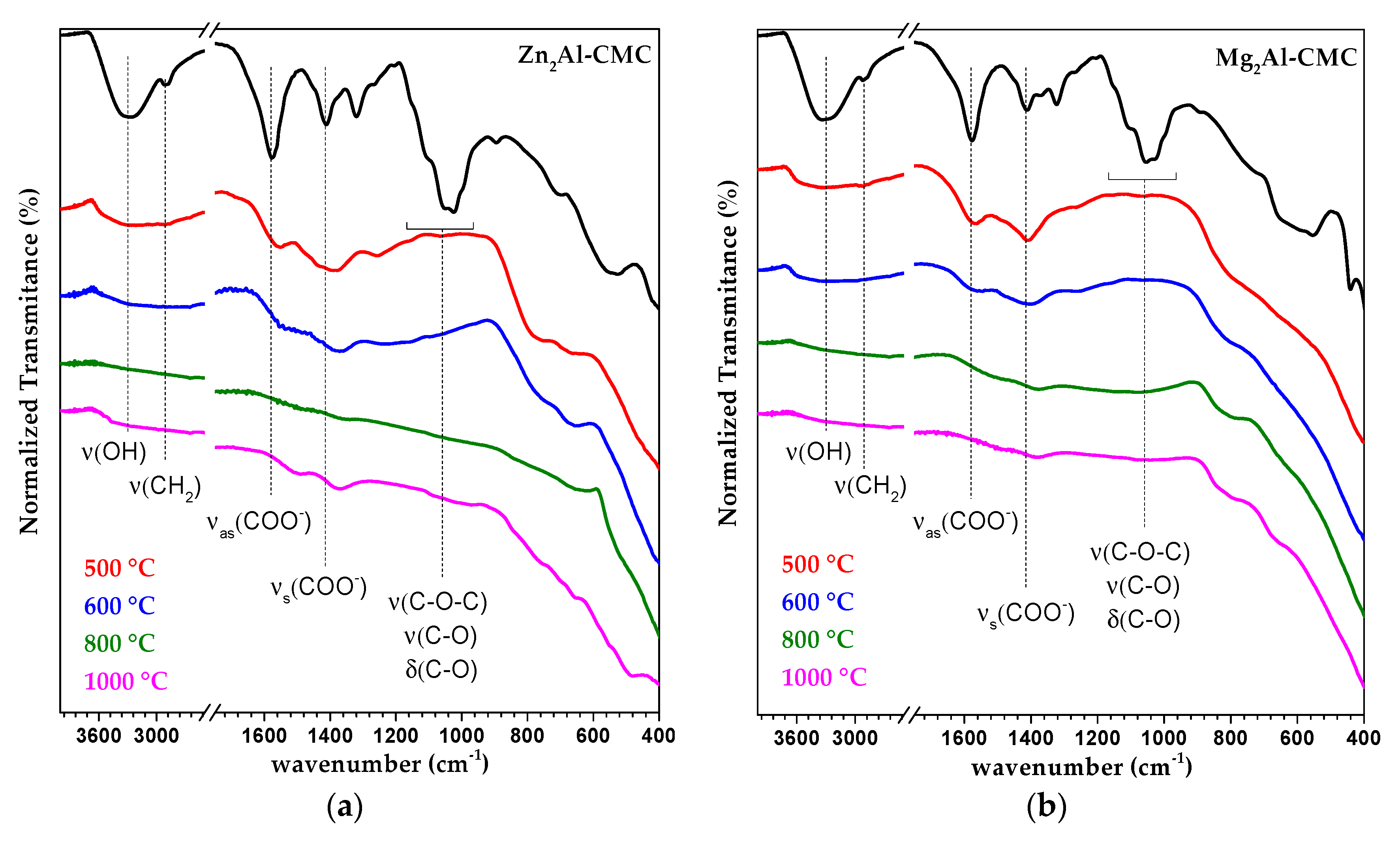
| Raman | IR | Tentative of Attribution | Reference |
|---|---|---|---|
| 3400–3200 | 3600–3300 (bb) | νOH free or hydrogen bonded | [28,29,30] |
| 2902 | -- | νCH | [28,30] |
| -- | 2922 | νasC(6)H2 | [28] |
| -- | 2869 | νsC(6)H2 | [28] |
| 1595 | 1585 | νasCOO- | [31] |
| 1458(sh) | -- | δscC(6)H2 | [30,32] |
| -- | 1448(sh) | δscCH2 and δipCOH | [29,32] |
| 1415 | -- | δCH2 and δCCH, δHCO, δCOH | [30,32] |
| -- | 1412 | νsCOO- | [31] |
| 1377 | 1374(sh) | δCH2 and δCCH, δHCO, δCOH | [30,32] |
| 1327 | 1320 | δwC(6)H2 and δCCH, δHCO, δCOH | [30,32] |
| 1262 | 1264 | δtwC(6)H2 and δCCH, δHCO, δCOH | [30,32] |
| 1150(sh) | 1150(sh) | νasC-C and νasC-O (ring breathing) | [29,32] |
| 1118 | -- | νsC(1)-O-C(4) and s ring breathing | [32] |
| -- | 1095(sh) | νC(2)-O | [29,33] |
| -- | 1046(sh) | δC(6)-O and δC(5)-O | [33] |
| 1026(sh) | 1019 | δC(3)-O | [33] |
| -- | 995 (sh) | νC(1)-O | [33] |
| 918 | -- | δC(5)C(6)-H and δHC(6)O | [30] |
| 896 | 895 | δC(1)-H | [32] |
| 703 | δrkCH2 and δoopCOH (C6 and C3) | [29,34] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, V.R.; Duarte, A.; Perotti, G.F.; Constantino, V.R.L. Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites. ChemEngineering 2019, 3, 55. https://doi.org/10.3390/chemengineering3020055
Magri VR, Duarte A, Perotti GF, Constantino VRL. Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites. ChemEngineering. 2019; 3(2):55. https://doi.org/10.3390/chemengineering3020055
Chicago/Turabian StyleMagri, Vagner R., Alfredo Duarte, Gustavo F. Perotti, and Vera R.L. Constantino. 2019. "Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites" ChemEngineering 3, no. 2: 55. https://doi.org/10.3390/chemengineering3020055
APA StyleMagri, V. R., Duarte, A., Perotti, G. F., & Constantino, V. R. L. (2019). Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites. ChemEngineering, 3(2), 55. https://doi.org/10.3390/chemengineering3020055






