Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health
Abstract
1. Periodontal Diseases and Tobacco Smoking
2. Electronic Nicotine Delivery Systems (ENDS) Aerosol Constituents in Comparison to Those Found in Combustible Tobacco Smoke
3. In Vitro Studies on Effects of E-Cigs on Oral Cells and Tissues
4. Studies on Oral and Periodontal Tissues
4.1. Studies on Direct Health Effects of ENDS in the Oral Cavity
4.2. Studies on Other Effects of ENDS in the Oral Cavity
5. Concluding Remarks

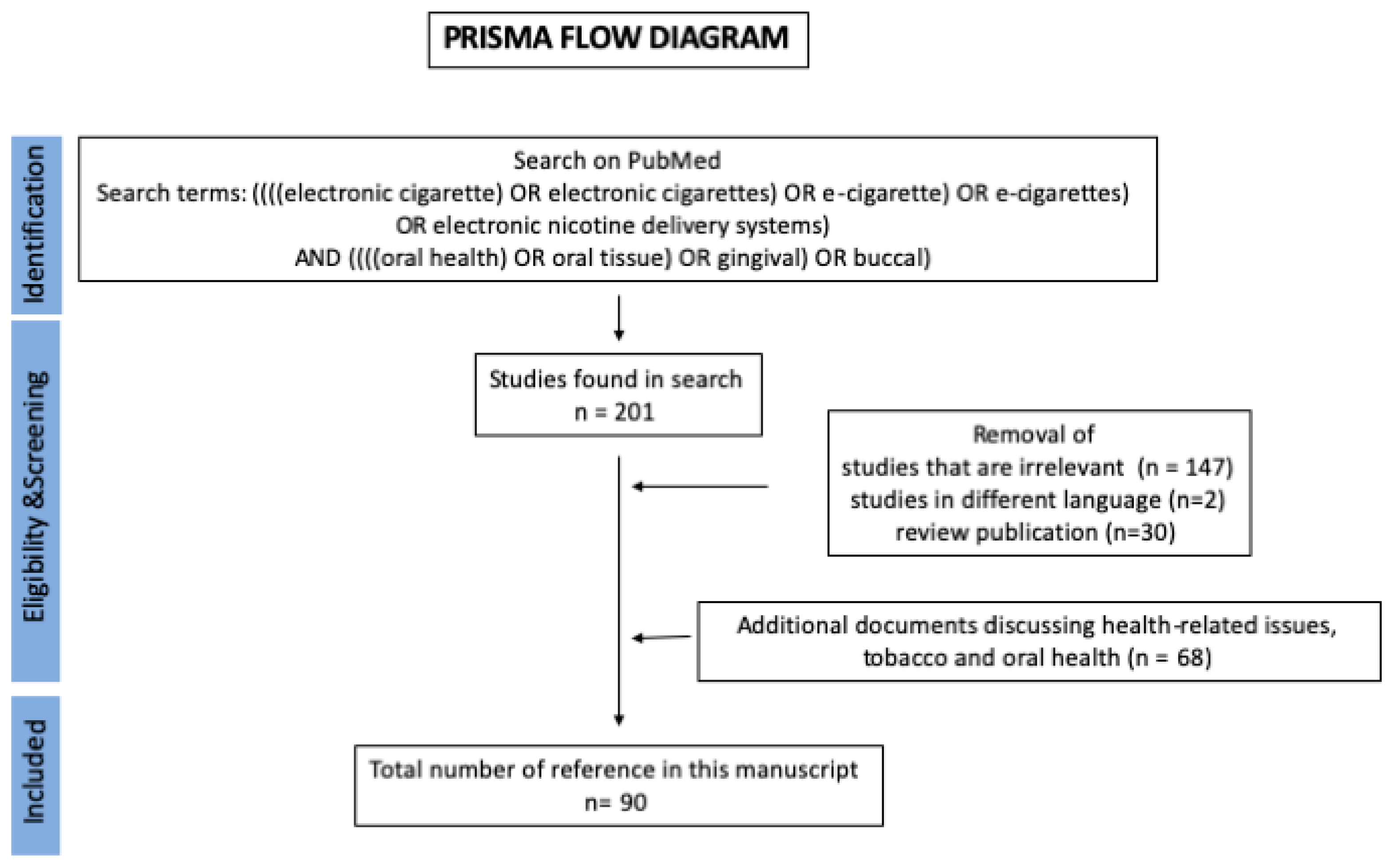
6. Methods
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BER | Base Excision Repair Mechanisms |
| BOP | Bleeding on Probing |
| e-cigs | Electronic Cigarettes |
| e-liquid | E-cig Liquid |
| ENDS | Electronic Nicotine Delivery Systems |
| GCF | Gingival Crevicular Fluid |
| HGF | Human Gingival Fibroblasts |
| HnB | Heat not Burn |
| NNN | N’-nitrosonornicotine |
| OMLs | Oral Mucosal Lesions |
| OSCC | Oral Squamous Cell Carcinoma |
| ROS | Reactive Oxygen Species |
References
- Albandar, J.M.; Brunelle, J.A.; Kingman, A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J. Periodontol. 1999, 70, 351. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodontal Res. 1993, 28, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Preber, H. The influence of cigarette smoking on the development of experimental gingivitis. J. Periodontal Res. 1986, 21, 668–676. [Google Scholar] [CrossRef]
- Bergström, J.; Boström, L. Tobacco smoking and periodontal hemorrhagic responsiveness. J. Clin. Periodontol. 2001, 28, 680–685. [Google Scholar] [CrossRef]
- Morozumi, T.; Kubota, T.; Sato, T.; Okuda, K.; Yoshie, H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J. Clin. Periodontol. 2004, 31, 267–272. [Google Scholar] [CrossRef]
- Nair, P.; Sutherland, G.; Palmer, R.M.; Wilson, R.F.; Scott, D.A. Gingival bleeding on probing increases after quitting smoking. J. Clin. Periodontol. 2003, 30, 435–437. [Google Scholar] [CrossRef]
- Razali, M.; Palmer, R.M.; Coward, P.; Wilson, R.F. A retrospective study of periodontal disease severity in smokers and non-smokers. Br. Dent. J. 2005, 198, 495–498. [Google Scholar] [CrossRef]
- Müller, H.P.; Stadermann, S.; Heinecke, A. Gingival recession in smokers and non-smokers with minimal periodontal disease. J. Clin. Periodontol. 2002, 29, 129–136. [Google Scholar] [CrossRef]
- Giorgetti, A.P.O.; César Neto, J.B.; Casati, M.Z.; Sallum, E.A.; Nociti Júnior, F.H. Cigarette smoke inhalation influences bone healing of post-extraction tooth socket: A histometric study in rats. Braz. Dent. J. 2012, 23, 228–234. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Campos, M.L.G.; Benatti, B.B.; Marques, M.R.; Casati, M.Z.; Nociti, F.H.; Sallum, E.A. The impact of cigarette smoke inhalation on the outcome of enamel matrix derivative treatment in rats: Histometric analysis. J. Periodontol. 2010, 81, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- César-Neto, J.B.; Benatti, B.B.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H. The influence of cigarette smoke inhalation and its cessation on the tooth-supporting alveolar bone: A histometric study in rats. J. Periodontal Res. 2006, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Datta, S.; Maiti, G.P.; Baral, A.; Jha, G.N.; Panda, C.K.; Chowdhury, S.; Ghosh, S.; Roy, B.; Roychoudhury, S. Comprehensive SNP Scan of DNA Repair and DNA Damage Response Genes Reveal Multiple Susceptibility Loci Conferring Risk to Tobacco Associated Leukoplakia and Oral Cancer. PLoS ONE 2013, 8, e56952. [Google Scholar] [CrossRef] [PubMed]
- Vellappally, S.; Fiala, Z.; Smejkalova, J.; Jacob, V.; Somanathan, R. Smoking related systemic and oral diseases. Acta Med. Hradec Kral. 2007, 50, 161–166. [Google Scholar] [CrossRef][Green Version]
- Chiu, C.T.; Li, C.F.; Li, J.R.; Wang, J.; Chuang, C.Y.; Chiang, W.F.; Huang, S.C.; Chang, S.W. Candida invasion and influences in smoking patients with multiple oral leucoplakias—A retrospective study. Mycoses 2011, 54, e377–e383. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Cigarette smoking: Cancer risks, carcinogens, and mechanisms. Langenbeck’s Arch. Surg. 2006, 39, 603–613. [Google Scholar] [CrossRef]
- Erdemir, E.O.; Erdemir, A. The Detection of Salivary Minerals in Smokers and Non-Smokers With Chronic Periodontitis by the Inductively Coupled Plasma-Atomic Emission Spectrophotometry Technique. J. Periodontol. 2006, 77, 990–995. [Google Scholar] [CrossRef]
- Opeodu, O.; Arowojolu, M.; Dosumu, E.; Fawole, O. A comparative study of the oral hygiene status of smokers and non-smokers in Ibadan, Oyo state. Niger. Med. J. 2013, 54, 240–243. [Google Scholar] [CrossRef]
- Pejčić, A.; Obradović, R.; Kesić, L.; Kojović, D. Smoking and periodontal disease a review. Med. Biol. 2007, 14, 53–59. [Google Scholar]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef]
- Bekki, K.; Uchiyama, S.; Ohta, K.; Inaba, Y.; Nakagome, H.; Kunugita, N. Carbonyl compounds generated from electronic cigarettes. Int. J. Environ. Res. Public Health 2014, 11, 11192–11200. [Google Scholar] [CrossRef]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Whitsel, L.P.; Ribisl, K.M.; Bullen, C.; Chaloupka, F.; Piano, M.R.; Robertson, R.M.; McAuley, T.; Goff, D.; Benowitz, N. Electronic cigarettes: A policy statement from the American Heart Association. Circulation 2014, 130, 1418–1436. [Google Scholar] [CrossRef] [PubMed]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef] [PubMed]
- D’Ruiz, C.D.; Graff, D.W.; Yan, X.S. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes Health behavior, health promotion and society. BMC Public Health 2015, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef]
- Moore, K.; Young II, H.; Ryan, M.F. FDA Public Health Focus—Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted By FDA. Open J. Emerg. Med. 2015, 3. [Google Scholar] [CrossRef][Green Version]
- Food and Drug Administration. FDA Warns of Health Risks Posed by E-Cigarettes; Food and Drug Administration: Champlain, NY, USA, 2009.
- McCauley, L.; Markin, C.; Hosmer, D. An unexpected consequence of electronic cigarette use. Chest 2012, 141, 1110–1113. [Google Scholar] [CrossRef]
- El Mubarak, M.A.; Danika, C.; Vlachos, N.S.; Farsalinos, K.; Poulas, K.; Sivolapenko, G. Development and validation of analytical methodology for the quantification of aldehydes in e-cigarette aerosols using UHPLC-UV. Food Chem. Toxicol. 2018. [Google Scholar] [CrossRef]
- Lisko, J.G.; Tran, H.; Stanfill, S.B.; Blount, B.C.; Watson, C.H. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in E-Cigarette cartridges and refill solutions. Nicotine Tob. Res. 2015, 17, 1270–1278. [Google Scholar] [CrossRef]
- Javed, F.; Kellesarian, S.V.; Sundar, I.K.; Romanos, G.E.; Rahman, I. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis. 2017, 23, 1052–1057. [Google Scholar] [CrossRef]
- Varlet, V.; Farsalinos, K.; Augsburger, M.; Thomas, A.; Etter, J.F. Toxicity assessment of refill liquids for electronic cigarettes. Int. J. Environ. Res. Public Health 2015, 12, 4796–4815. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Voudris, V.; Poulas, K. E-cigarettes generate high levels of aldehydes only in “dry puff” conditions. Addiction 2015, 110, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, S.H.; Weng, M.; Wang, H.T.; Huang, W.C.; Lepor, H.; Wu, X.R.; Chen, L.C.; Tang, M. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA 2018, 115, e1560–e1569. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.; Ramchandraprasad, M.; Bajaj, P.; Rao, N.; Agarwal, E. Protein carbonyl: An oxidative stress marker in gingival crevicular fluid in healthy, gingivitis, and chronic periodontitis subjects. Contemp. Clin. Dent. 2013, 4, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Baltacioǧlu, E.; Akalin, F.A.; Alver, A.; Deǧer, O.; Karabulut, E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch. Oral Biol. 2008, 53, 716–722. [Google Scholar] [CrossRef]
- Lerner, C.A.; Rutagarama, P.; Ahmad, T.; Sundar, I.K.; Elder, A.; Rahman, I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016, 477, 620–625. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Chen, S.X.; Law, S.; Van Demark, M.; Poirier, C.; Justice, M.J.; Hubbard, W.C.; Kim, E.S.; Lai, X.; Wang, M.; et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L175–L187. [Google Scholar] [CrossRef]
- Bustamante, G.; Ma, B.; Yakovlev, G.; Yershova, K.; Le, C.; Jensen, J.; Hatsukami, D.K.; Stepanov, I. Presence of the Carcinogen N′-Nitrosonornicotine in Saliva of E-cigarette Users. Chem. Res. Toxicol. 2018, 31, 731–738. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W. Alzheimer’s Disease Neuroimaging Initiative Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2014, 10, s122–s145. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Wang, H.T.; Weng, M.; Chin, C.; Huang, W.; Lepor, H.; Wu, X.R.; Rom, W.N.; Chen, L.C.; Tang, M. Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget 2015, 6, 33226–33236. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Chang, Y. Regulation of nicotine-induced cyclooxygenase-2 protein expression in human gingival fibroblasts. Acta Pharmacol. Sin. 2006, 27, 409–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, Y.; Svoboda, K.K.H. Nicotine inhibits human gingival fibroblast migration via modulation of Rac signalling pathways. J. Clin. Periodontol. 2005, 32, 1200–1207. [Google Scholar] [CrossRef]
- Poggi, P.; Rota, M.T.; Boratto, R. The volatile fraction of cigarette smoke induces alterations in the human gingival fibroblast cytoskeleton. J. Periodontal Res. 2002, 37, 230–235. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, M.; Song, F.; Windsor, L.J. Effects of cigarette smoke condensate and nicotine on human gingival fibroblast-mediated collagen degradation. J. Periodontol. 2011, 82, 1071–1079. [Google Scholar] [CrossRef]
- Johnson, G.K.; Organ, C.C. Prostaglandin E2and interleukin-1 concentrations in nicotine-exposed oral keratinocyte cultures. J. Periodontal Res. 1997, 32, 447–454. [Google Scholar] [CrossRef]
- Wendell, K.J.; Stein, S.H. Regulation of cytokine production in human gingival fibroblasts following treatment with nicotine and lipopolysaccharide. J. Periodontol. 2001, 72, 1038–1044. [Google Scholar] [CrossRef]
- Tanur, E.; McQuade, M.J.; McPherson, J.C.; Al-Hashimi, I.H.; Rivera-Hidalgo, F. Effects of Nicotine on the Strength of Attachment of Gingival Fibroblasts to Glass and Non-Diseased Human Root Surfaces. J. Periodontol. 2000, 71, 717–722. [Google Scholar] [CrossRef]
- Tipton, D.A.; Dabbous, M.K. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J. Periodontol. 1995, 66, 1056–1064. [Google Scholar] [CrossRef]
- Austin, G.W.; Cuenin, M.F.; Hokett, S.D.; Peacock, M.E.; Sutherland, D.E.; Erbland, J.F.; Billman, M.A. Effect of nicotine on fibroblast beta 1 integrin expression and distribution in vitro. J. Periodontol. 2001, 72, 438–444. [Google Scholar] [CrossRef]
- Wisniewski, D.J.; Ma, T.; Schneider, A. Nicotine induces oral dysplastic keratinocyte migration via Fatty Acid Synthase-dependent Epidermal Growth Factor Receptor activation. Exp. Cell Res. 2018, 370, 343–352. [Google Scholar] [CrossRef]
- Bollu, L.R.; Katreddy, R.R.; Blessing, A.M.; Pham, N.; Zheng, B.; Wu, X.; Weihua, Z. Intracellular activation of EGFR by fatty acid synthase dependent palmitoylation. Oncotarget 2015, 6, 34992–35003. [Google Scholar] [CrossRef]
- Wisniewski, D.J.; Ma, T.; Schneider, A. Advances in the Chemopreventive Targeting of Oral Carcinogenesis. Curr. Oral Health Rep. 2015, 2, 63–72. [Google Scholar] [CrossRef]
- Costa, V.; Kowalski, L.P.; Coutinho-Camillo, C.M.; Begnami, M.D.; Calsavara, V.F.; Neves, J.I.; Kaminagakura, E. EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int. J. Oral Maxillofac. Surg. 2018, 47, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; di Giacomo, V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. [Google Scholar] [CrossRef]
- Tinti, F.; Soory, M. Mechanisms for redox actions of nicotine and glutathione in cell culture, relevant to periodontitis. Sci. Rep. 2012. [Google Scholar] [CrossRef]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Zanetti, F.; Sewer, A.; Scotti, E.; Titz, B.; Schlage, W.K.; Leroy, P.; Kondylis, A.; Vuillaume, G.; Iskandar, A.R.; Guedj, E.; et al. Assessment of the impact of aerosol from a potential modified risk tobacco product compared with cigarette smoke on human organotypic oral epithelial cultures under different exposure regimens. Food Chem. Toxicol. 2018, 115, 148–169. [Google Scholar] [CrossRef]
- Sánchez-Pérez, A.; Moya-Villaescusa, M.J.; Caffesse, R.G. Tobacco as a risk factor for survival of dental implants. J. Periodontol. 2007, 78, 351–359. [Google Scholar] [CrossRef]
- Rouabhia, M.; Alanazi, H.; Park, H.J.; Gonçalves, R.B. Cigarette Smoke and E-Cigarette Vapor Dysregulate Osteoblast Interaction With Titanium Dental Implant Surface. J. Oral Implantol. 2018, 45, 2–11. [Google Scholar] [CrossRef]
- Al Amri, M.D.; Kellesarian, S.V.; Abduljabbar, T.S.; Al Rifaiy, M.Q.; Al Baker, A.M.; Al-Kheraif, A.A. Comparison of Peri-Implant Soft Tissue Parameters and Crestal Bone Loss Around Immediately Loaded and Delayed Loaded Implants in Smokers and Non-Smokers: 5-Year Follow-Up Results. J. Periodontol. 2017, 88, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Raes, S.; Rocci, A.; Raes, F.; Cooper, L.; De Bruyn, H.; Cosyn, J. A prospective cohort study on the impact of smoking on soft tissue alterations around single implants. Clin. Oral Implant. Res. 2015, 26, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Rothem, D.E.; Rothem, L.; Soudry, M.; Dahan, A.; Eliakim, R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J. Bone Miner. Metab. 2009, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Ribeiro, É.D.P.; Sallum, A.W.; Tatakis, D.N. Free Gingival Grafts: Graft Shrinkage and Donor-Site Healing in Smokers and Non-Smokers. J. Periodontol. 2010, 81, 692–701. [Google Scholar] [CrossRef]
- Berley, J.; Yamano, S.; Sukotjo, C. The Effect of Systemic Nicotine on Osseointegration of Titanium Implants in the Rat Femur. J. Oral Implantol. 2010, 36, 185–193. [Google Scholar] [CrossRef]
- Yamano, S.; Berley, J.A.; Kuo, W.P.; Gallucci, G.O.; Weber, H.P.; Sukotjo, C. Effects of nicotine on gene expression and osseointegration in rats. Clin. Oral Implant. Res. 2010, 21, 1353–1359. [Google Scholar] [CrossRef]
- Seo, A.D.; Kim, D.C.; Yu, H.J.; Kang, M.J. Accidental ingestion of E-cigarette liquid nicotine in a 15-month-old child: An infant mortality case of nicotine intoxication. Korean J. Pediatr. 2016, 59, 490–493. [Google Scholar] [CrossRef]
- Schipper, E.M.; De Graaff, L.C.G.; Koch, B.C.P.; Brkic, Z.; Wilms, E.B.; Alsma, J.; Schuit, S.C.E. A new challenge: Suicide attempt using nicotine fillings for electronic cigarettes. Br. J. Clin. Pharmacol. 2014, 78, 1469–1471. [Google Scholar] [CrossRef]
- Willershausen, I.; Wolf, T.; Weyer, V.; Sader, R.; Ghanaati, S.; Willershausen, B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head Face Med. 2014, 15, 10–39. [Google Scholar] [CrossRef] [PubMed]
- Huilgol, P.; Bhatt, S.P.; Biligowda, N.; Wright, N.C.; Wells, J.M. Association of e-cigarette use with oral health: A populationbased cross-sectional questionnaire study. J. Public Health (UK) 2019, 41, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The association between electronic-cigarette use and self-reported oral symptoms including cracked or broken teeth and tongue and/or inside-cheek pain among adolescents: A cross-sectional study. PLoS ONE 2017, 12, e0180506. [Google Scholar] [CrossRef] [PubMed]
- Vora, M.V.; Chaffee, B.W. Tobacco-use patterns and self-reported oral health outcomes. J. Am. Dent. Assoc. 2019, 150, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Akinkugbe, A.A. Cigarettes, E-cigarettes, and Adolescents’ Oral Health: Findings from the Population Assessment of Tobacco and Health (PATH) Study. JDR Clin. Transl. Res. 2019, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, S.A.; Alasqah, M.N.; Michelogiannakis, D.; Al-Kheraif, A.A.; Romanos, G.E.; Javed, F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette- and waterpipe-smokers and E-cig users. Environ. Toxicol. Pharmacol. 2018, 61, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Rahman, I.; Romanos, G.E. Comparison of Periodontal Parameters and Self-Perceived Oral Symptoms among Cigarette-Smokers, Individuals Vaping Electronic-Cigarettes and Never-Smokers: A Pilot Study. J. Periodontol. 2017, 88, 1059–1065. [Google Scholar] [CrossRef]
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine (USA) 2016, 95, 5589. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Conti, G.; Majorana, A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol. Scand. 2018, 76, 226–228. [Google Scholar] [CrossRef]
- Franco, T.; Trapasso, S.; Puzzo, L.; Allegra, E. Electronic Cigarette: Role in the Primary Prevention of Oral Cavity Cancer. Clin. Med. Insights Ear Nose Throat 2016, 9, 7–12. [Google Scholar] [CrossRef]
- Wadia, R.; Booth, V.; Yap, H.F.; Moyes, D.L. A pilot study of the gingival response when smokers switch from smoking to vaping. Br. Dent. J. 2016, 221, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.G.; Shephard, B.C.; Hirsch, R.S. The effects of intra-arterial epinephrine and nicotine on gingival circulation. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 577–582. [Google Scholar] [CrossRef]
- Stewart, C.J.; Auchtung, T.A.; Ajami, N.J.; Velasquez, K.; Smith, D.P.; De La Garza, R.; Salas, R.; Petrosino, J.F. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: A pilot study. PeerJ 2018, 6, e4693. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, S.A.; Abduljabbar, T.; Al-Kheraif, A.A.; Alasqah, M.N.; Michelogiannakis, D.; Samaranayake, L.P.; Javed, F. Oral Candida carriage among cigarette- and waterpipe-smokers, and electronic cigarette users. Oral Dis. 2019, 25, 319–326. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-Cigarettes Increase Candida albicans Growth and Modulate its Interaction with Gingival Epithelial Cells. Int. J. Environ. Res. Public Health 2019, 16, 294. [Google Scholar] [CrossRef]
- Tommasi, S.; Caliri, A.W.; Caceres, A.; Moreno, D.E.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef]
- Reuther, W.J.; Hale, B.; Matharu, J.; Blythe, J.N.; Brennan, P.A. Do you mind if i vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue—A pilot study. Br. J. Oral Maxillofac. Surg. 2016, 54, 338–341. [Google Scholar] [CrossRef]
- Harrison, R.; Hicklin, D. Electronic cigarette explosions involving the oral cavity. J. Am. Dent. Assoc. 2016, 147, 891–896. [Google Scholar] [CrossRef]
- Corey, C.G.; Chang, J.T.; Rostron, B.L. Electronic nicotine delivery system (ENDS) battery-related burns presenting to US emergency departments, 2016. Inj. Epidemiol. 2018, 5, 4. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isik Andrikopoulos, G.; Farsalinos, K.; Poulas, K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics 2019, 7, 61. https://doi.org/10.3390/toxics7040061
Isik Andrikopoulos G, Farsalinos K, Poulas K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics. 2019; 7(4):61. https://doi.org/10.3390/toxics7040061
Chicago/Turabian StyleIsik Andrikopoulos, Gozde, Konstantinos Farsalinos, and Konstantinos Poulas. 2019. "Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health" Toxics 7, no. 4: 61. https://doi.org/10.3390/toxics7040061
APA StyleIsik Andrikopoulos, G., Farsalinos, K., & Poulas, K. (2019). Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics, 7(4), 61. https://doi.org/10.3390/toxics7040061






