Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Extraction and Cleanup
2.3. Sample Analysis
2.4. Data Analysis
3. Results
3.1. The Statistical Features of PAH Contents in Soil and Plants
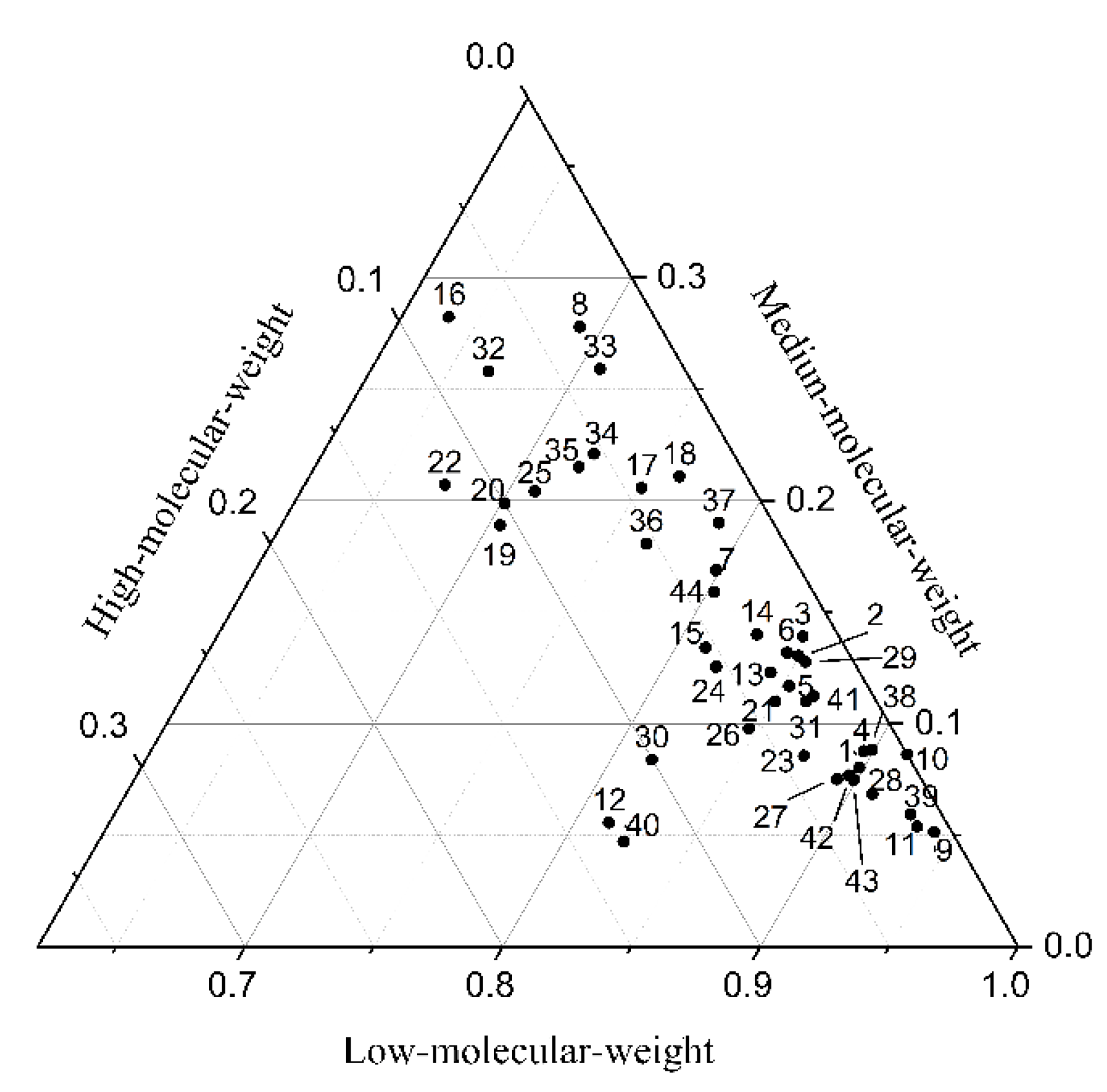
3.2. Distribution and Composition of PAHs in Soil
3.3. Distribution and Composition of PAHs in Plants
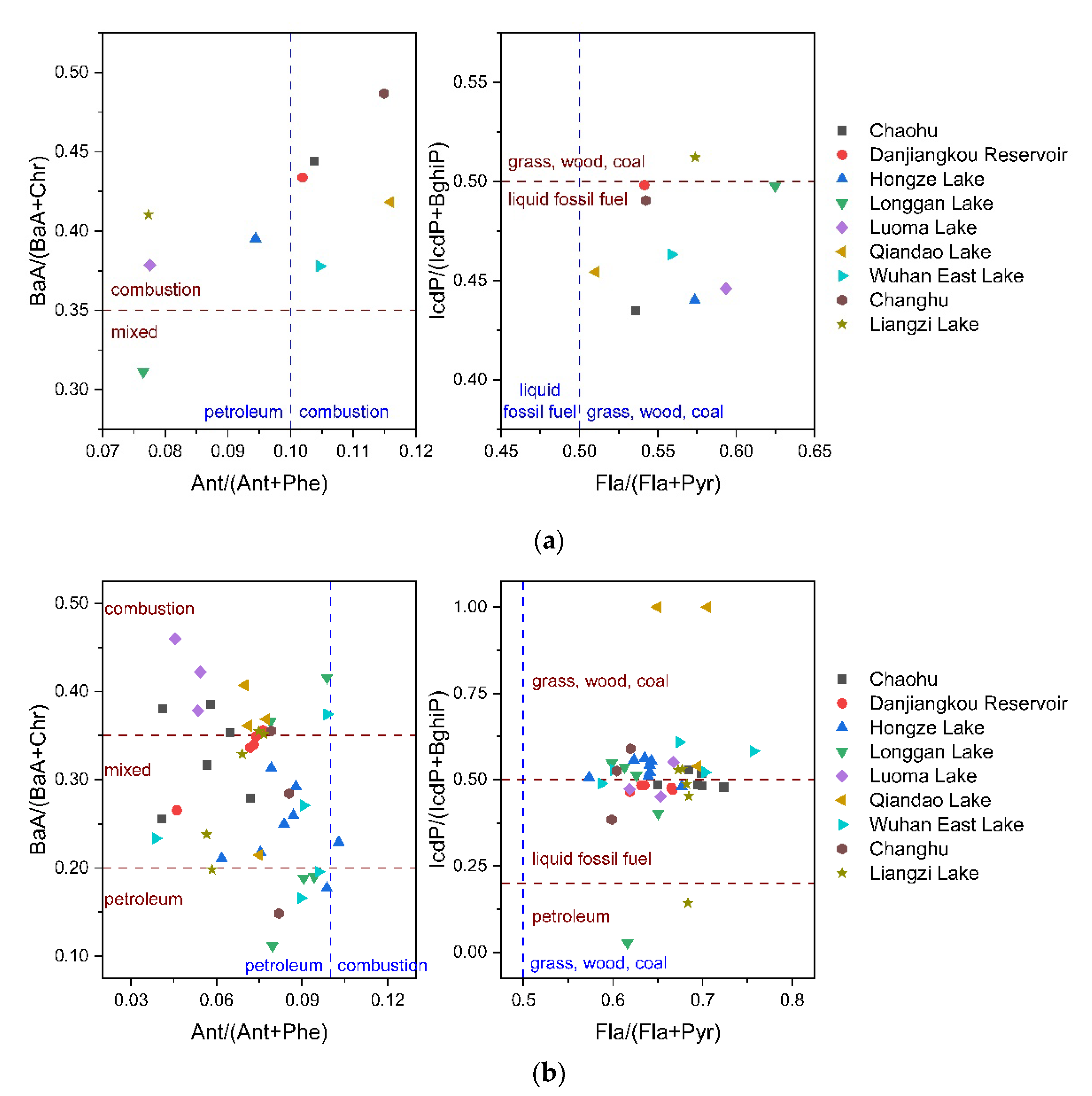
3.4. Possible sources of PAHs in soil and plants
3.5. Relationship between the Distribution and Composition of PAHs in Soil and Plants
3.5.1. Correlation between the distribution of PAHs in soil and plants
3.5.2. Accumulation of PAHs in Plants
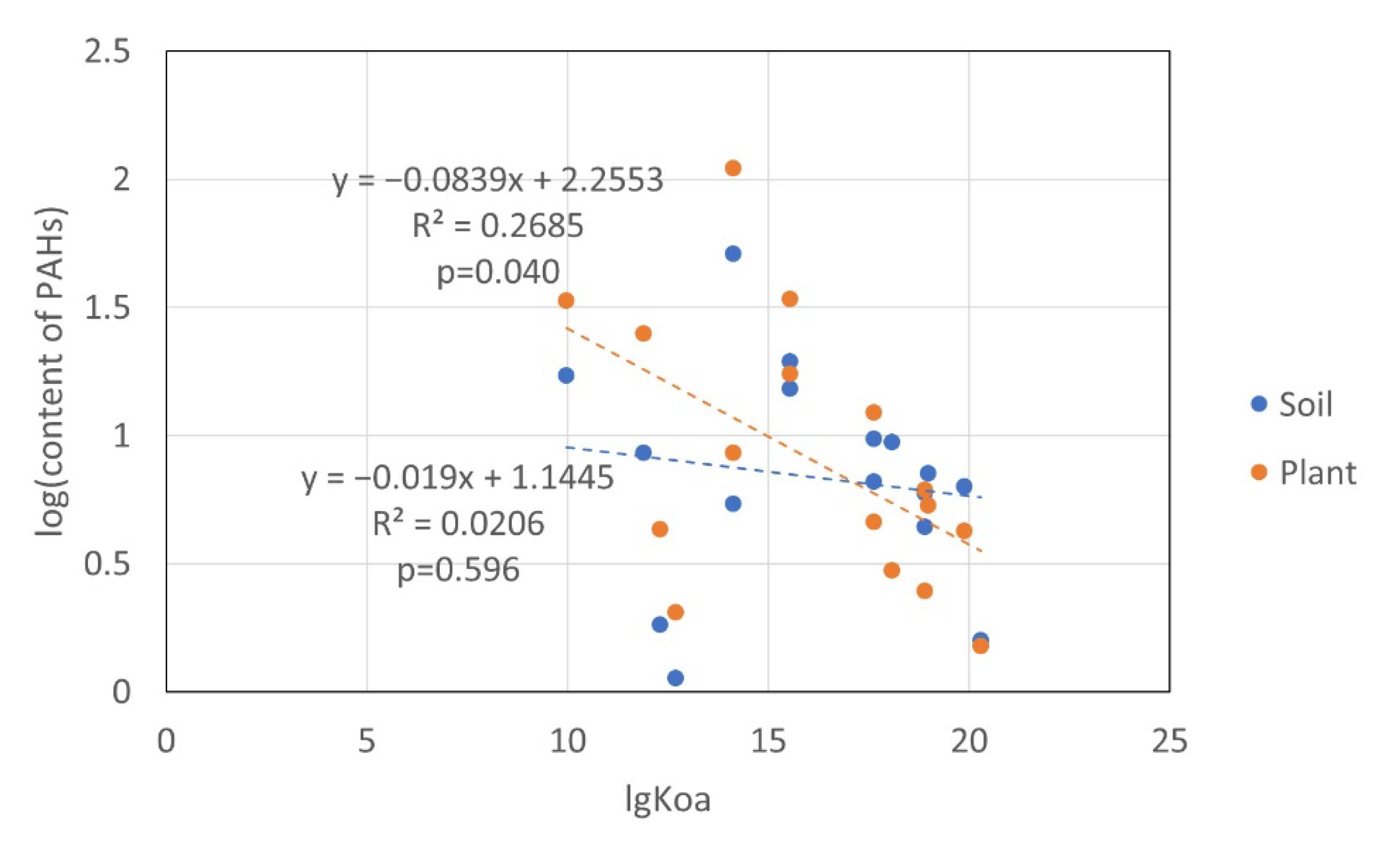
3.5.3. Effects of Physicochemical Properties on the Distribution of PAHs in Soil and Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mastral, A.M.; Callén, M.S. A Review on Polycyclic Aromatic Hydrocarbon (PAH) Emissions from Energy Generation. Environ. Sci. Technol. 2000, 34, 3051–3057. [Google Scholar] [CrossRef]
- Luo, J.; Han, Y.; Zhao, Y.; Huang, Y.; Liu, X.; Tao, S.; Liu, J.; Huang, T.; Wang, L.; Chen, K.; et al. Effect of northern boreal forest fires on PAH fluctuations across the arctic. Environ. Pollut. 2020, 261, 114186. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.M.; Winkler, J.; Oulehla, J.; Vaverková, M.D. Effect of Application of Soil Amendments on the PAHs Level in the Fire-Affected Forest Soil. J. Ecol. Eng. 2022, 23, 26–38. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Mohammed, M.O.A.; Song, W.W.; Ma, Y.L.; Liu, L.Y.; Ma, W.L.; Li, W.L.; Li, Y.F.; Wang, F.Y.; Qi, M.Y.; Lv, N.; et al. Distribution patterns, infiltration and health risk assessment of PM2.5-bound PAHs in indoor and outdoor air in cold zone. Chemosphere 2016, 155, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.K.; Baker, J.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O.; George, S.C. The Potential of Oxygenates to Increase the Risk of Exposure to Polycyclic Aromatic Hydrocarbons through Groundwater Contamination. Water 2022, 14, 739. [Google Scholar] [CrossRef]
- Qian, W.; Dequan, L.; Yiran, X.; Fei, P.; Juan-Ying, L.; Feng, W.; Yanping, C.; Ruihua, S.; Siquan, T. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in the seafood from an important fishing area in the East China Sea and a comparison between seafood from different origins. Environ. Monit. Assess. 2022, 194, 528. [Google Scholar] [CrossRef]
- Wentao, W.; Simonich, S.L.M.; Xue, M.; Zhao, J.; Zhang, N.; Wang, R.; Cao, J.; Tao, S. Concentrations, sources and spatial distribution of polycyclic aromatic hydrocarbons in soils from Beijing, Tianjin and surrounding areas, north China. Environ. Pollut. 2010, 158, 1245–1251. [Google Scholar]
- Khan, S.; Aijun, L.; Zhang, S.; Hu, Q.; Zhu, Y.G. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Liu, J.; Cheng, Y.; Zhai, F.; Sun, Z.; Han, L. Enhancing Salix viminalis L.–mediated phytoremediation of polycyclic aromatic hydrocarbon–contaminated soil by inoculation with Crucibulum laeve (white-rot fungus). Environ. Sci. Pollut. Res. Int. 2020, 27, 41326–41341. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.R.; Karlson, U. Diffuse PAH contamination of surface soils: Environmental occurrence, bioavailability, and microbial degradation. Appl. Microbiol. Biotechnol. 2007, 76, 533–543. [Google Scholar] [CrossRef]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Visualizing the Air-To-Leaf Transfer and Within-Leaf Movement and Distribution of Phenanthrene: Further Studies Utilizing Two-Photon Excitation Microscopy. Environ. Sci. Technol. 2006, 40, 907–916. [Google Scholar] [CrossRef]
- Holoubek, I.; Kořínek, P.; Šeda, Z.; Schneiderová, E.; Holoubková, I.; Pacl, A.; Tříska, J.; Cudlın, P.; Čáslavský, J. The use of mosses and pine needles to detect persistent organic pollutants at local and regional scales. Environ. Pollut. 2000, 109, 283–292. [Google Scholar] [CrossRef]
- Pokhrel, B.; Gong, P.; Wang, X.; Chen, M.; Wang, C.; Gao, S. Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere 2018, 200, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Apiratikul, R.; Pongpiachan, S.; Deelaman, W. Spatial distribution, sources and quantitative human health risk assessments of polycyclic aromatic hydrocarbons in urban and suburban soils of Chile. Environ. Geochem. Health 2021, 43, 2851–2870. [Google Scholar] [CrossRef]
- Akporhonor, E.E.; Emoyan, O.O.; Agbaire, P.O. Concentrations, origin, and human health risk of polycyclic aromatic hydrocarbons in anthropogenic impacted soils of the Niger Delta, Nigeria. Environ. Forensics 2022, 23, 127–140. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Li, H.; Xu, S.; Du, J.; Chen, Y.; Ma, C.; Tang, J. Concentration, distribution, source apportionment, and risk assessment of surrounding soil PAHs in industrial and rural areas: A comparative study. Ecol. Indic. 2021, 125, 107513. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Xia, Q.; Rong, G.; Zhang, J. Ecological risk and early warning of soil compound pollutants (HMs, PAHs, PCBs and OCPs) in an industrial city, Changchun, China. Environ. Pollut. 2021, 272, 116038. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Wang, Q.; Wang, J.; Liu, W.; Luo, Y.; Christie, P. Enhanced bioremediation of PAH-contaminated soil by wheat bran and microbial community response. Arch. Für Acker- Und Pflanzenbau Und Bodenkd. 2020, 66, 1089–1102. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.; Chen, J.; Wang, J.; Hou, L.; Zhou, Y. Enhanced phytoremediation of PAHs and cadmium contaminated soils by a Mycobacterium. Sci. Total Environ. 2021, 754, 141198. [Google Scholar] [CrossRef] [PubMed]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Parrish, Z.D.; White, J.C.; Isleyen, M.; Gent, M.P.; Iannucci-Berger, W.; Eitzer, B.D.; Kelsey, J.W.; Mattina, M.I. Accumulation of weathered polycyclic aromatic hydrocarbons (PAHs) by plant and earthworm species. Chemosphere 2006, 64, 609–618. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, R.; Jin, C.; Dai, Y. Efficiency of five ornamental plant species in the phytoremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecol. Eng. 2015, 75, 384–391. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Chen, W. Phytoremediation potential of Bermuda grass (Cynodon dactylon (L.) pers.) in soils co-contaminated with polycyclic aromatic hydrocarbons and cadmium. Ecotoxicol. Environ. Saf. 2022, 234, 113389. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Dashti, N.; Khanafer, M.; Al-Awadhi, H.; Radwan, S. Bioremediation of soils saturated with spilled crude oil. Sci. Rep. 2020, 10, 1116–1119. [Google Scholar] [CrossRef]
- Qi, P.; Qu, C.; Albanese, S.; Lima, A.; Cicchella, D.; Hope, D.; Cerino, P.; Pizzolante, A.; Zheng, H.; Li, J. Investigation of polycyclic aromatic hydrocarbons in soils from Caserta provincial territory, southern Italy: Spatial distribution, source apportionment, and risk assessment. J. Hazard. Mater. 2020, 383, 121158. [Google Scholar] [CrossRef]
- Liao, Q.; Liu, H.; Lu, C.; Liu, J.; Waigi, M.G.; Ling, W. Root exudates enhance the PAH degradation and degrading gene abundance in soils. Sci. Total Environ. 2021, 764, 144436. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, P.; Su, J.; da Silva, E.B.; de Oliveira, L.M. PAHs in urban soils of two Florida cities: Background concentrations, distribution, and sources. Chemosphere 2019, 214, 220–227. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Walker, T.R.; Wang, Y.; Wu, H.; Wang, X.; Luo, Q. Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban soils from 23 cities in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 73401–73413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, X.; Yves, U.J.; Zhan, H.; Wu, Y. Status, source and health risk assessment of polycyclic aromatic hydrocarbons in street dust of an industrial city, NW China. Ecotoxicol. Environ. Saf. 2014, 106, 11–18. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, W.; Yang, C.; Liu, W.; Xu, F. Spatiotemporal toxicity assessment of suspended particulate matter (SPM)–bound polycyclic aromatic hydrocarbons (PAHs) in Lake Chaohu, China: Application of a source-based quantitative method. Sci. Total Environ. 2020, 727, 138690. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, S.; Feng, Y.; Lin, N.; Lu, B.; Zhang, Z.; Cui, F.; Xing, B.; Hammond, S.K. Concentrations of polycyclic aromatic hydrocarbons in resuspendable fraction of settled bus dust and its implications for human exposure. Environ. Pollut. 2015, 198, 1–7. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Liao, H.; Li, Y. Spatiotemporal distribution of atmospheric polycyclic aromatic hydrocarbon emissions during 2013–2017 in mainland China. Sci. Total Environ. 2021, 789, 148003. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Dumanoglu, Y.; Kara, M.; Altiok, H.; Bayram, A.; Elbir, T.; Odabasi, M. Spatial and temporal variation and air–soil exchange of atmospheric PAHs and PCBs in an industrial region. Atmos. Pollut. Res. 2012, 3, 435–449. [Google Scholar] [CrossRef]
- Musa Bandowe, B.A.; Wei, C.; Han, Y.; Cao, J.; Zhan, C.; Wilcke, W. Polycyclic aromatic compounds (PAHs, oxygenated PAHs, nitrated PAHs and azaarenes) in soils from China and their relationship with geographic location, land use and soil carbon fractions. Sci. Total Environ. 2019, 690, 1268–1276. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Shao, T.; Wang, R.; Chen, Z.; Wei, P.; Xu, Z.; Li, D.; Fu, L.; Wang, F. A Study on PAHs in the surface soil of the region around Qinghai Lake in the Tibet plateau: Evaluation of distribution characteristics, sources and ecological risks. Environ. Res. Commun. 2021, 3, 041005. [Google Scholar] [CrossRef]
- Ping, L.F.; Luo, Y.M.; Zhang, H.B.; Li, Q.B.; Wu, L.H. Distribution of polycyclic aromatic hydrocarbons in thirty typical soil profiles in the Yangtze River Delta region, east China. Environ. Pollut. 2007, 147, 358–365. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhang, F.; Liu, X.; Zhou, M. Contamination and health risk assessment of PAHs in farmland soils of the Yinma River Basin, China. Ecotoxicol. Environ. Saf. 2018, 156, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Fang, H.; Zhang, T.; Wang, X. Polycyclic aromatic hydrocarbons in the leaves of twelve plant species along an urbanization gradient in Shanghai, China. Environ. Sci. Pollut. Res. 2017, 24, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Wang, S.; Luo, C.; Yin, H.; Zhang, G. Characterisation and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soils and plants around e-waste dismantling sites in southern China. Environ. Sci. Pollut. Res. 2017, 24, 22173–22182. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Yang, J.; Sun, P.; Zhang, X.; Wei, X.Y.; Huang, Y.P.; Du, W.N.; Qadeer, A.; Liu, M. Source apportionment of PAHs in roadside agricultural soils of a megacity using positive matrix factorization receptor model and compound-specific carbon isotope analysis. J. Hazard. Mater. 2021, 403, 123592. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, M.K.; Lee, S.E.; Go, H.J.; Cho, B.C.; Lee, Y.S.; Choi, S.D. Impact of traffic volumes on levels, patterns, and toxicity of polycyclic aromatic hydrocarbons in roadside soils. Environ. Sci. Processes Impacts 2019, 21, 174–182. [Google Scholar] [CrossRef]
- Kumar, V.; Kothiyal, N.C. Distribution behavior and carcinogenic level of some polycyclic aromatic hydrocarbons in roadside soil at major traffic intercepts within a developing city of India. Environ. Monit. Assess. 2012, 184, 6239–6252. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Zhou, Q.; Xu, Y.; Wang, L.; Liang, X.; Sun, Y.; Qin, X. Polycyclic Aromatic Hydrocarbon (PAH) Contamination in the Urban Topsoils of Shenyang, China. Soil Sediment Contam. Int. J. 2012, 21, 901–917. [Google Scholar] [CrossRef]
- Deelaman, W.; Pongpiachan, S.; Tipmanee, D.; Choochuay, C.; Iadtem, N.; Suttinun, O.; Wang, Q.; Xing, L.; Li, G.; Han, Y.; et al. Source identification of polycyclic aromatic hydrocarbons in terrestrial soils in Chile. J. South Am. Earth Sci. 2020, 99, 102514. [Google Scholar] [CrossRef]
- Zhang, X.L.; Tao, S.; Liu, W.X.; Yang, Y.; Zuo, Q.; Liu, S.Z. Source Diagnostics of Polycyclic Aromatic Hydrocarbons Based on Species Ratios: A Multimedia Approach. Environ. Sci. Technol. 2005, 39, 9109–9114. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Breivik, K. Model-based evaluation of the use of polycyclic aromatic hydrocarbons molecular diagnostic ratios as a source identification tool. Environ. Pollut. 2014, 184, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Biache, C.; Mansuy-Huault, L.; Faure, P. Impact of oxidation and biodegradation on the most commonly used polycyclic aromatic hydrocarbon (PAH) diagnostic ratios: Implications for the source identifications. J. Hazard. Mater. 2014, 267, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ratola, N.; Amigo, J.M.; Lacorte, S.; Barceló, D.; Psillakis, E.; Alves, A. Comparison of PAH Levels and Sources in Pine Needles from Portugal, Spain, and Greece. Anal. Lett. 2012, 45, 508–525. [Google Scholar] [CrossRef]
- Fasani, D.; Fermo, P.; Barroso, P.J.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Analytical Method for Biomonitoring of PAH Using Leaves of Bitter Orange Trees (Citrus aurantium): A Case Study in South Spain. Water Air Soil Pollut. 2016, 227, 1. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Terzi, E.; Cai, Q.-Y. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere 2007, 69, 1337–1339. [Google Scholar] [CrossRef]
- Lang, C.; Tao, S.; Wang, X.; Zhang, G.; Fu, J. Modeling polycyclic aromatic hydrocarbon composition profiles of sources and receptors in the Pear River Delta, China. Environ. Toxicol. Chem. Int. J. 2008, 27, 4–9. [Google Scholar] [CrossRef]
- Buczyńska, A.J.; Geypens, B.; Van Grieken, R.; De Wael, K. Stable carbon isotopic ratio measurement of polycyclic aromatic hydrocarbons as a tool for source identification and apportionment—A review of analytical methodologies. Talanta 2013, 105, 435–450. [Google Scholar] [CrossRef]
- Gao, P.; Li, H.; Wilson, C.P.; Townsend, T.G.; Xiang, P.; Liu, Y.; Ma, L.Q. Source identification of PAHs in soils based on stable carbon isotopic signatures. Crit. Rev. Environ. Sci. Technol. 2018, 48, 923–948. [Google Scholar] [CrossRef]
- Ya, M.; Wu, Y.; Wang, X.; Li, Y.; Su, G. The importance of compound-specific radiocarbon analysis in source identification of polycyclic aromatic hydrocarbons: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 937–978. [Google Scholar] [CrossRef]
- Alagic, S.C.; Jovanović, V.P.S.; Mitić, V.D.; Cvetković, J.S.; Petrović, G.M.; Stojanović, G.S. Bioaccumulation of HMW PAHs in the roots of wild blackberry from the Bor region (Serbia): Phytoremediation and biomonitoring aspects. Sci. Total Environ. 2016, 562, 561–570. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ma, Q.; Sun, C.; Ha, S.; Zhang, F. Human health risk assessment and source diagnosis of polycyclic aromatic hydrocarbons (PAHs) in the corn and agricultural soils along main roadside in Changchun, China. Hum. Ecol. Risk Assess. 2016, 22, 706–720. [Google Scholar] [CrossRef]
- Paraíba, L.C.; Queiroz, S.C.N.; Maia, A.D.H.N.; Ferracini, V.L. Bioconcentration factor estimates of polycyclic aromatic hydrocarbons in grains of corn plants cultivated in soils treated with sewage sludge. Sci. Total Environ. 2010, 408, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Sojinu, O.S.; Sonibare, O.O.; Gayawan, E. Investigating Polycyclic Aromatic Hydrocarbons Profiles in Higher Plants Using Statistical Models. Int. J. Phytoremediation 2013, 15, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, K.; Wang, J.; Bao, H.; Luo, W.; Zhang, H.; Hong, H. Accumulation and translocation of phenanthrene, anthracene and pyrene in winter wheat affected by soil water content. Ecotoxicol. Environ. Saf. 2019, 183, 109567. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, X.; Chu, S.; Wang, H.; Zhang, Z.; Xi, N.; Gan, J. Cation-π Interactions with Coexisting Heavy Metals Enhanced the Uptake and Accumulation of Polycyclic Aromatic Hydrocarbons in Spinach. Environ. Sci. Technol. 2020, 54, 7261–7270. [Google Scholar] [CrossRef]
- Lin, H.; Tao, S.; Zuo, Q.; Coveney, R.M. Uptake of polycyclic aromatic hydrocarbons by maize plants. Environ. Pollut. 2007, 148, 614–619. [Google Scholar] [CrossRef]
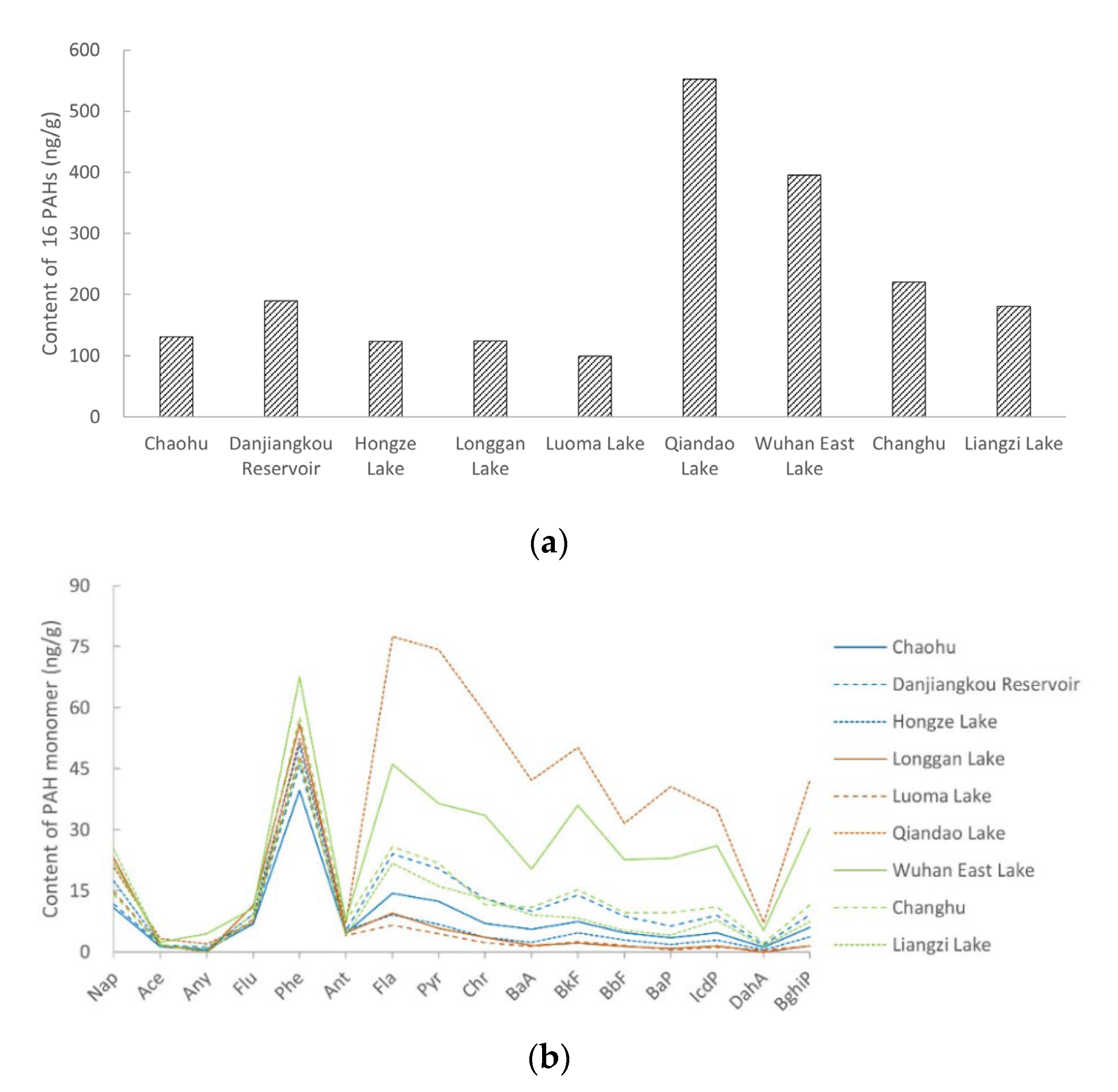
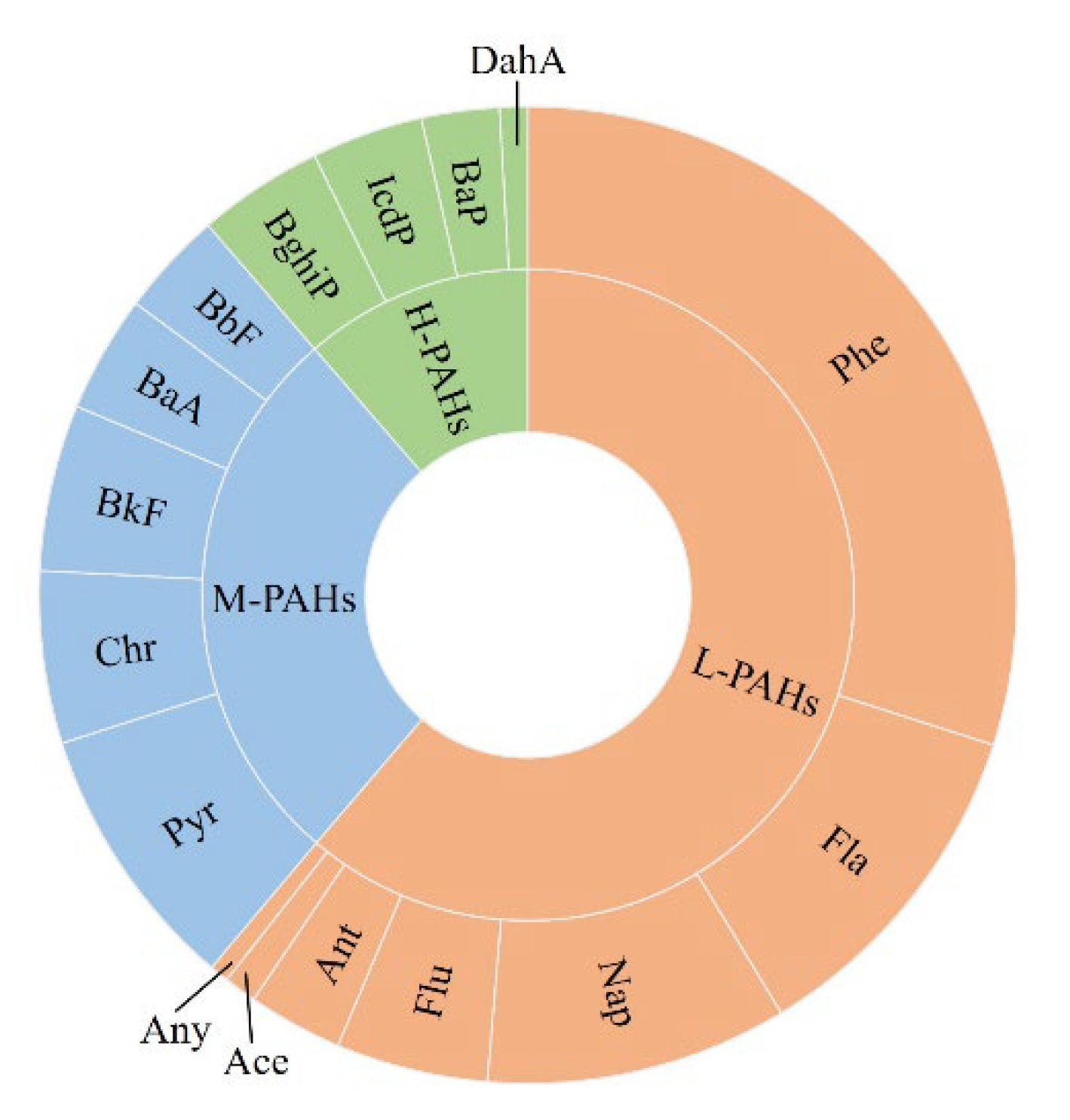
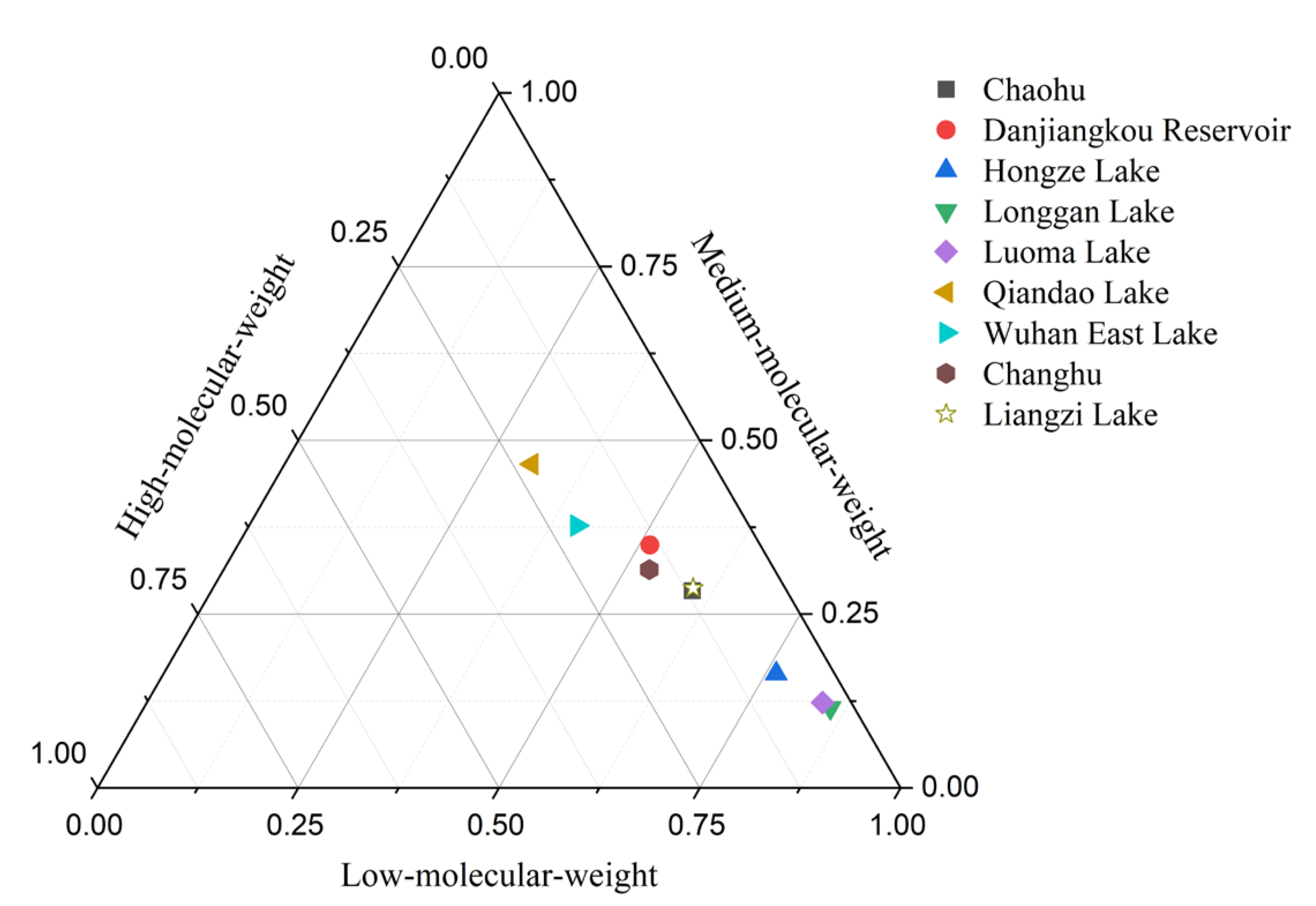

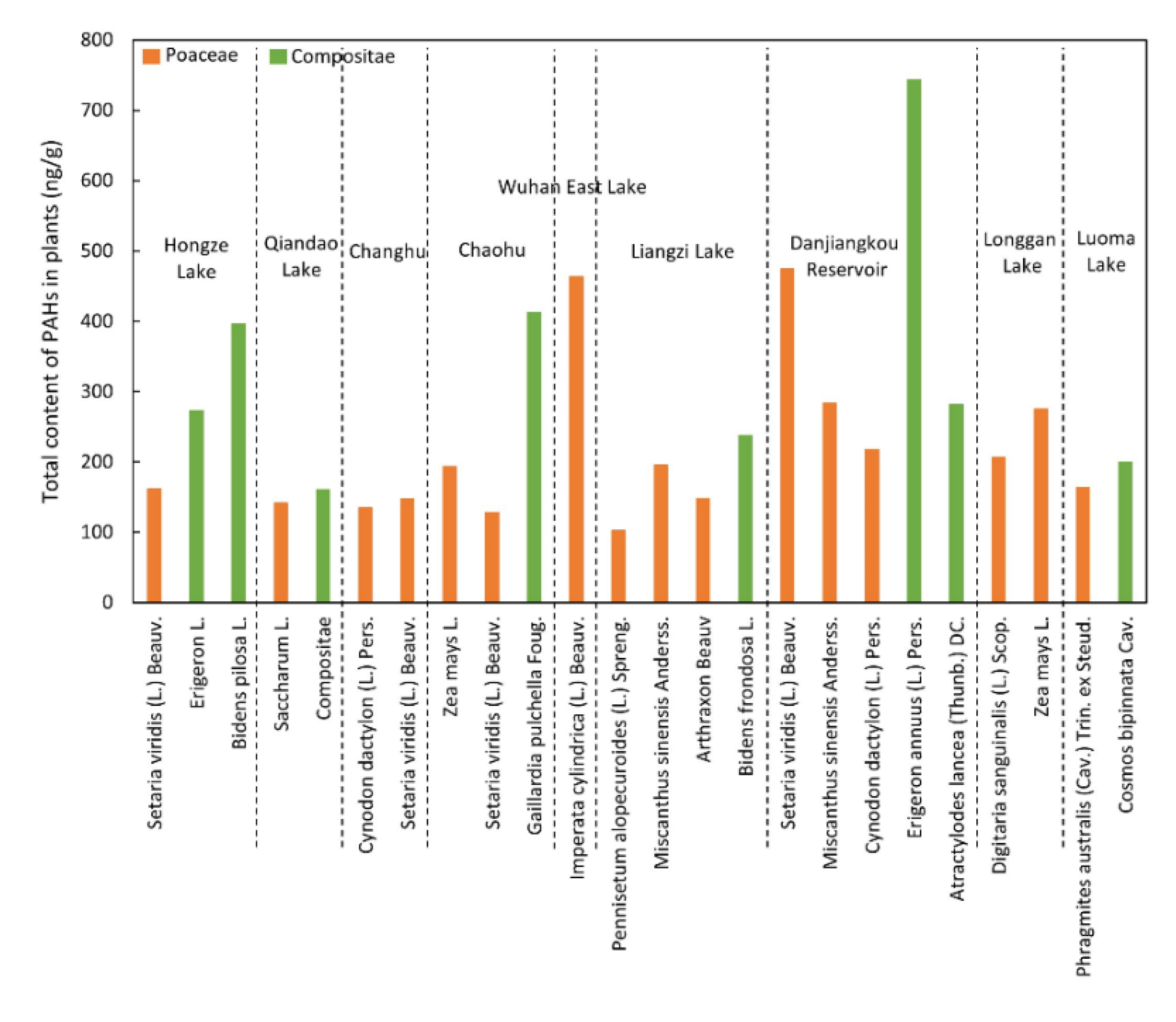


| PAHs 2 | Mean | Median | Maximum | Minimum | Detection Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | Plant | Soil | Plant | Soil | Plant | Soil | Plant | Soil | Plant | |
| Nap | 17.1 | 33.5 | 17.5 | 31.2 | 25.3 | 61.4 | 10.9 | 17.2 | 100% | 100% |
| Ace | 1.83 | 4.29 | 1.71 | 4.13 | 3.25 | 6.59 | 1.30 | 2.65 | 100% | 100% |
| Any | 1.13 | 2.04 | 0.50 | 1.27 | 4.46 | 14.7 | ND 1 | ND 1 | 66.7% | 88.6% |
| Flu | 8.57 | 24.9 | 8.11 | 22.6 | 11.5 | 47.8 | 6.86 | 14.3 | 100% | 100% |
| Phe | 51.2 | 110 | 50.9 | 105 | 67.6 | 226 | 39.7 | 57.1 | 100% | 100% |
| Ant | 5.39 | 8.57 | 5.22 | 8.12 | 7.90 | 20.8 | 3.96 | 4.06 | 100% | 100% |
| Fla | 19.5 | 34.0 | 21.8 | 19.4 | 77.5 | 208 | 6.56 | 7.39 | 100% | 100% |
| Pyr | 15.2 | 17.4 | 16.2 | 10.5 | 74.3 | 104 | 4.49 | 3.55 | 100% | 100% |
| Chr | 9.72 | 12.3 | 11.6 | 8.79 | 58.7 | 49.2 | 2.27 | 1.52 | 100% | 100% |
| BaA | 6.63 | 4.61 | 9.10 | 2.69 | 42.2 | 18.2 | 1.38 | 0.98 | 100% | 100% |
| BkF | 9.42 | 2.98 | 8.33 | 2.14 | 50.2 | 10.2 | 2.11 | ND 1 | 100% | 93.2% |
| BbF | 5.94 | 6.14 | 5.25 | 3.69 | 31.6 | 20.9 | 1.33 | 0.95 | 100% | 100% |
| BaP | 4.39 | 2.48 | 4.11 | 1.36 | 40.6 | 11.7 | 0.52 | ND 1 | 100% | 81.8% |
| IcdP | 6.33 | 4.25 | 7.75 | 2.45 | 35.0 | 20.0 | 1.19 | ND 1 | 100% | 97.7% |
| DahA | 1.59 | 1.50 | 1.42 | ND 1 | 7.15 | 34.6 | ND 1 | ND 1 | 88.9% | 31.8% |
| BghiP | 7.12 | 5.32 | 7.38 | 2.46 | 42.0 | 41.2 | 1.46 | ND 1 | 100% | 93.2% |
| 3 | 190 | 275 | 181 | 234 | 552 | 743 | 99.2 | 123 | ||
| Source | Ant/(Ant+Phe) | BaA/(BaA+Chr) | Fla/(Fla+Pyr) | IcdP/(IcdP+BghiP) |
|---|---|---|---|---|
| Petroleum | <0.1 | <0.2 | <0.4 | <0.2 |
| Combustion | >0.1 | >0.35 | ||
| Liquid fossil fuel combustion | 0.4–0.5 | 0.2–0.5 | ||
| Grass, wood, coal combustion | >0.5 | >0.5 |
| Principal Component | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Nap | 0.46 | 0.43 | −0.30 | −0.36 |
| Ace | 0.61 | 0.61 | −0.053 | −0.27 |
| Any | 0.46 | 0.17 | 0.63 | 0.39 |
| Flu | 0.52 | 0.77 | 0.18 | 0.15 |
| Phe | 0.84 | 0.24 | 0.30 | −0.16 |
| Ant | 0.65 | 0.46 | −0.40 | −0.13 |
| Fla | 0.77 | −0.25 | 0.43 | −0.31 |
| Pyr | 0.84 | −0.22 | 0.29 | −0.35 |
| Chr | 0.84 | −0.34 | 0.076 | −0.20 |
| BaA | 0.92 | −0.29 | 0.008 | −0.044 |
| BkF | 0.89 | −0.24 | −0.19 | 0.22 |
| BbF | 0.92 | −0.31 | −0.12 | 0.11 |
| BaP | 0.86 | −0.21 | −0.21 | 0.35 |
| IcdP | 0.84 | −0.20 | −0.27 | 0.37 |
| DahA | 0.15 | 0.53 | 0.35 | 0.37 |
| BghiP | 0.50 | 0.29 | −0.44 | 0.12 |
| In Soil | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nap | Ace | Any | Flu | Phe | Ant | Fla | Pyr | Chr | BaA | BkF | BbF | BaP | IcdP | DahA | BghiP | ||
| In palnts | Nap | −0.042 | 0.057 | 0.180 | 0.223 | 0.275 | 0.279 | 0.028 | −0.037 | −0.035 | −0.037 | −0.117 | −0.117 | −0.037 | −0.037 | −0.034 | −0.034 |
| Ace | 0.201 | 0.277 | 0.109 | 0.255 | 0.158 | 0.135 | 0.013 | −0.028 | 0.057 | −0.028 | −0.054 | −0.054 | −0.028 | −0.028 | −0.048 | −0.048 | |
| Any | −0.151 | −0.008 | 0.383 * | −0.137 | −0.042 | 0.106 | 0.297 | 0.285 | 0.317 * | 0.285 | 0.109 | 0.109 | 0.285 | 0.285 | 0.303 * | 0.303 * | |
| Flu | 0.149 | 0.129 | 0.009 | 0.240 | 0.063 | 0.027 | −0.101 | −0.135 | −0.065 | −0.135 | −0.052 | −0.052 | −0.135 | −0.135 | −0.170 | −0.170 | |
| Phe | −0.013 | 0.025 | 0.097 | 0.122 | −0.076 | 0.015 | −0.063 | −0.072 | 0.007 | −0.072 | −0.060 | −0.060 | −0.072 | −0.072 | −0.095 | −0.095 | |
| Ant | 0.216 | 0.257 | 0.124 | 0.553 ** | 0.327 * | 0.370 * | −0.068 | −0.077 | −0.035 | −0.077 | −0.458 ** | −0.458 ** | −0.077 | −0.077 | −0.140 | −0.140 | |
| Fla | −0.313 * | −0.110 | 0.343 * | −0.052 | −0.235 | 0.101 | 0.035 | 0.086 | 0.170 | 0.086 | −0.185 | −0.185 | 0.086 | 0.086 | 0.092 | 0.092 | |
| Pyr | −0.280 | −0.128 | 0.293 | 0.082 | −0.134 | 0.148 | −0.025 | 0.015 | 0.080 | 0.015 | −0.299 * | −0.299 * | 0.015 | 0.015 | 0.017 | 0.017 | |
| Chr | −0.205 | −0.066 | 0.399 ** | 0.140 | 0.014 | 0.284 | 0.152 | 0.185 | 0.212 | 0.185 | −0.394 ** | −0.394 ** | 0.185 | 0.185 | 0.171 | 0.171 | |
| BaA | −0.388 ** | −0.207 | 0.417 ** | −0.031 | −0.148 | 0.199 | 0.114 | 0.174 | 0.201 | 0.174 | −0.299 * | −0.299 * | 0.174 | 0.174 | 0.187 | 0.187 | |
| BkF | −0.327 * | −0.179 | 0.427 ** | 0.119 | 0.003 | 0.289 | 0.091 | 0.129 | 0.139 | 0.129 | −0.391 ** | −0.391 ** | 0.129 | 0.129 | 0.132 | 0.132 | |
| BbF | −0.386 ** | −0.244 | 0.423 ** | 0.050 | −0.091 | 0.234 | 0.089 | 0.128 | 0.146 | 0.128 | −0.345 * | −0.345 * | 0.128 | 0.128 | 0.129 | 0.129 | |
| BaP | −0.491 ** | −0.301 * | 0.485 ** | −0.152 | −0.194 | 0.161 | 0.168 | 0.195 | 0.219 | 0.195 | −0.145 | −0.145 | 0.195 | 0.195 | 0.216 | 0.216 | |
| IcdP | −0.416 ** | −0.277 | 0.441 ** | −0.137 | −0.215 | 0.106 | 0.190 | 0.235 | 0.259 | 0.235 | −0.182 | −0.182 | 0.235 | 0.235 | 0.255 | 0.255 | |
| DahA | −0.383 * | −0.098 | 0.510 * | −0.392 * | −0.255 | 0.127 | 0.345 * | 0.358 * | 0.403 ** | 0.358 * | 0.186 | 0.186 | 0.358 * | 0.358 * | 0.358 * | 0.358 * | |
| BghiP | −0.261 | −0.278 | 0.239 | −0.064 | −0.217 | −0.067 | 0.114 | 0.100 | 0.144 | 0.100 | −0.059 | −0.059 | 0.100 | 0.100 | 0.109 | 0.109 | |
| Plant Concentration Factor (PCF) | ||||
|---|---|---|---|---|
| Bidens pilosa L. | Gaillardia pulchella Foug. | Corn near Longgan Lake | Corn near Chaohu | |
| Nap | 1.97 | 2.35 | 1.99 | 2.30 |
| Ace | 3.38 | 2.85 | 3.32 | 2.89 |
| Any | 3.23 | 29.2 | − 1 | 6.11 |
| Flu | 2.75 | 6.96 | 3.93 | 2.98 |
| Phe | 2.79 | 4.66 | 2.55 | 2.67 |
| Ant | 1.77 | 1.73 | 3.37 | 1.38 |
| Fla | 6.01 | 3.73 | 1.39 | 1.69 |
| Pyr | 6.02 | 1.85 | 1.45 | 0.855 |
| Chr | 11.7 | 1.49 | 0.586 | 0.741 |
| BaA | 4.77 | 1.15 | 0.923 | 0.430 |
| BkF | 1.08 | 0.570 | 0.511 | 0.273 |
| BbF | 3.94 | 1.67 | 1.87 | 0.902 |
| BaP | 1.73 | 1.57 | 0.00 | 0.528 |
| IcdP | 1.60 | 1.46 | 0.805 | 0.719 |
| DahA | 0.00 | 2.50 | 0.00 | 0.00 |
| BghiP | 1.22 | 1.20 | 28.3 | 0.588 |
| lg(PAHs in Soil) | lg(PAHs in Plants) | ||
|---|---|---|---|
| lgKow | r | −0.071 | −0.461 |
| p | 0.794 | 0.073 | |
| lgKoa | r | −0.143 | −0.518 * |
| p | 0.596 | 0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; He, W.; Wu, R.; Xu, F. Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China. Toxics 2022, 10, 577. https://doi.org/10.3390/toxics10100577
Zhao Z, He W, Wu R, Xu F. Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China. Toxics. 2022; 10(10):577. https://doi.org/10.3390/toxics10100577
Chicago/Turabian StyleZhao, Zhiwei, Wei He, Ruilin Wu, and Fuliu Xu. 2022. "Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China" Toxics 10, no. 10: 577. https://doi.org/10.3390/toxics10100577
APA StyleZhao, Z., He, W., Wu, R., & Xu, F. (2022). Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China. Toxics, 10(10), 577. https://doi.org/10.3390/toxics10100577






