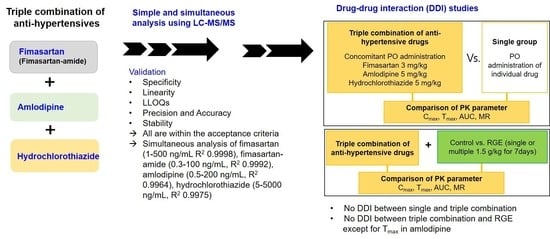
Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LC-MS/MS Analysis and Validation of Fimasartan, Fimasartan-Amide, Amlodipine, and Hydrochlorothiazide
2.3. Analytical Validation
2.4. Pharmacokinetic Study
2.5. Intestinal Permeability of Fimasartan, Amlodipine, and Hydrochlorothiazide
2.6. Data Analysis and Statistics
3. Results
3.1. Simultaneous Analysis of Fimasartan, Fimasartan-Amide, Amlodipine, and Hydrochlorothiazide
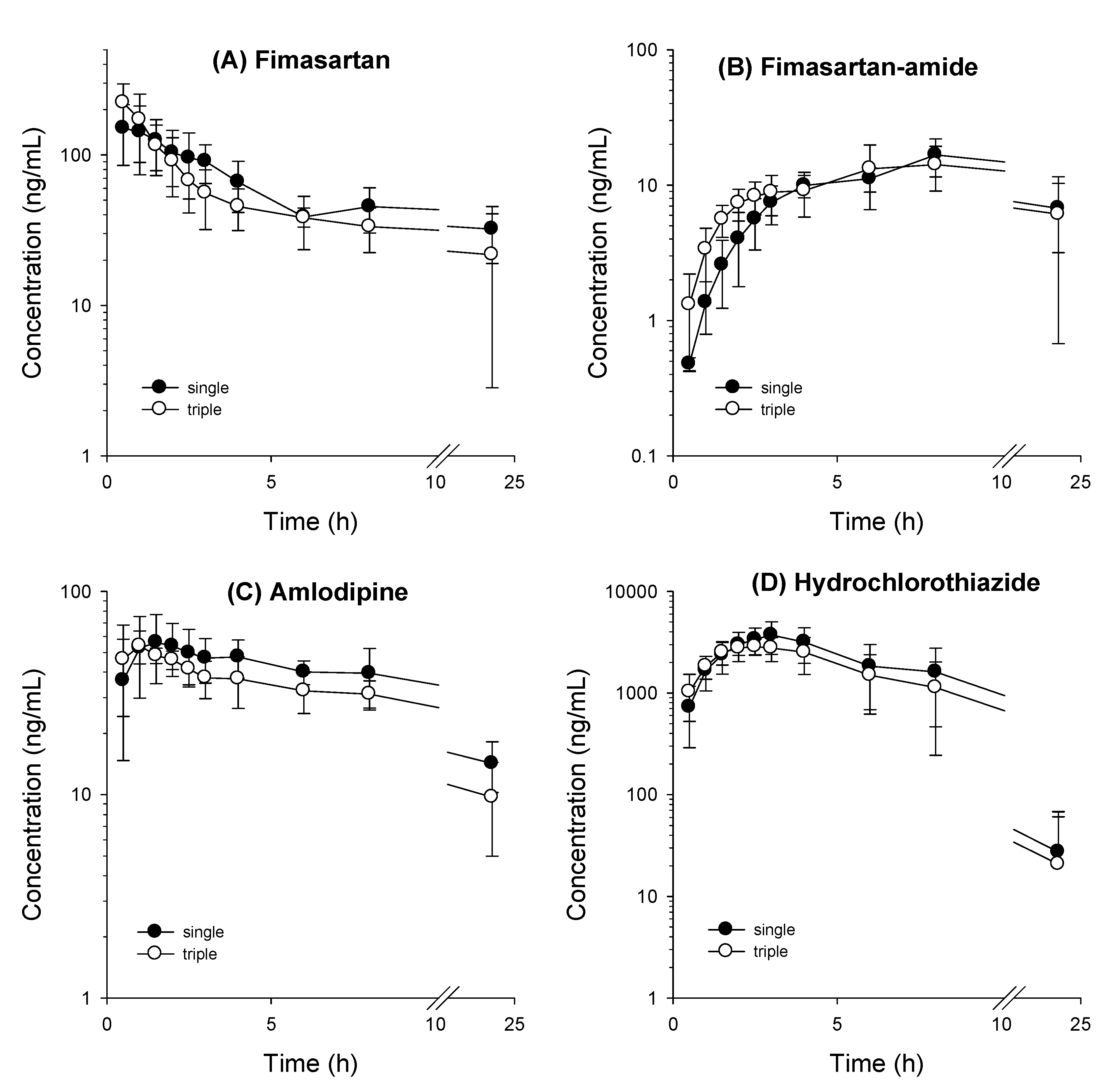
3.2. Comparative Pharmacokinetics of Fimasartan, Amlodipine, and Hydrochlorothiazide Following Oral Administration as Monotherapy or Combination Doses
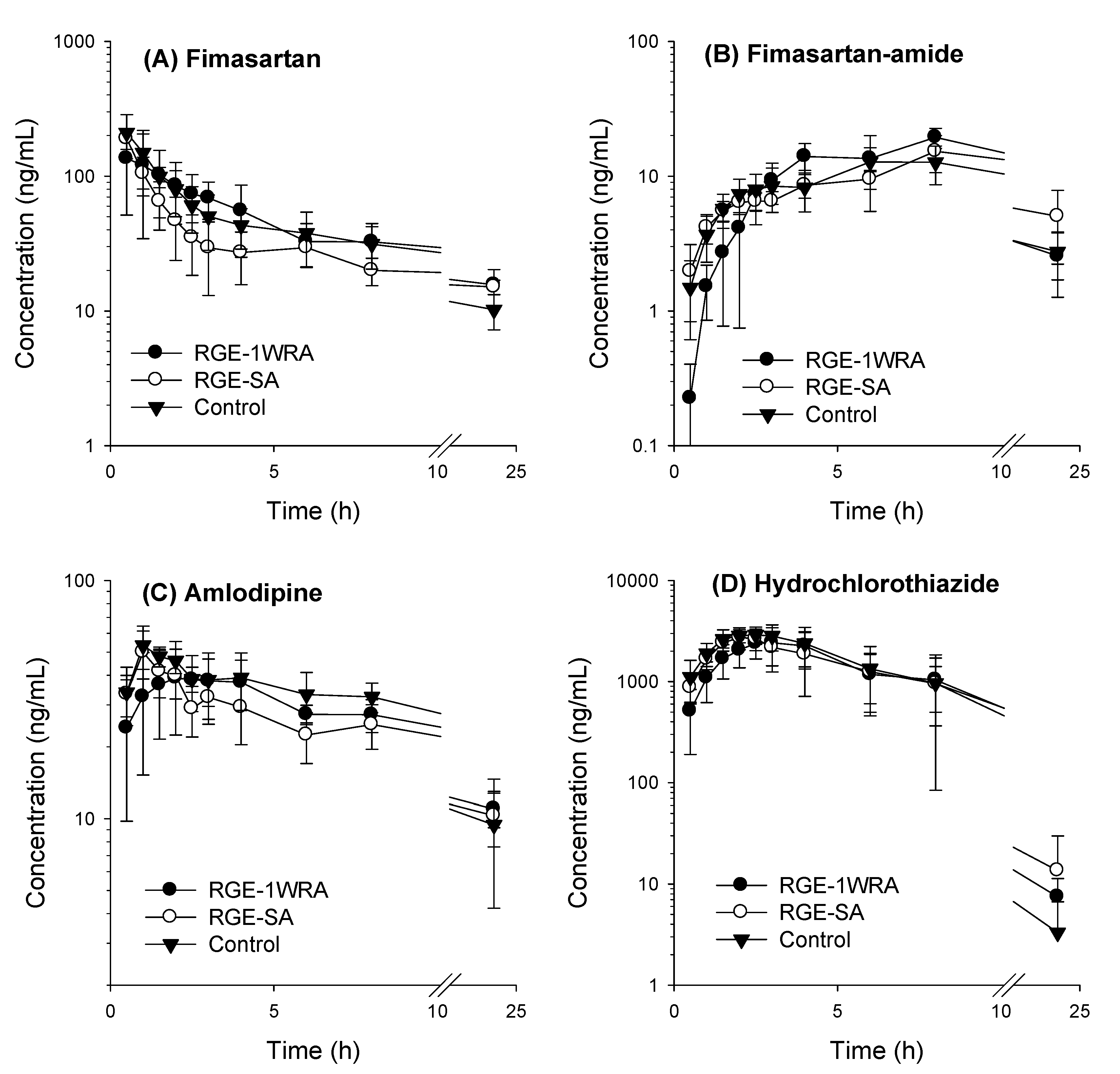
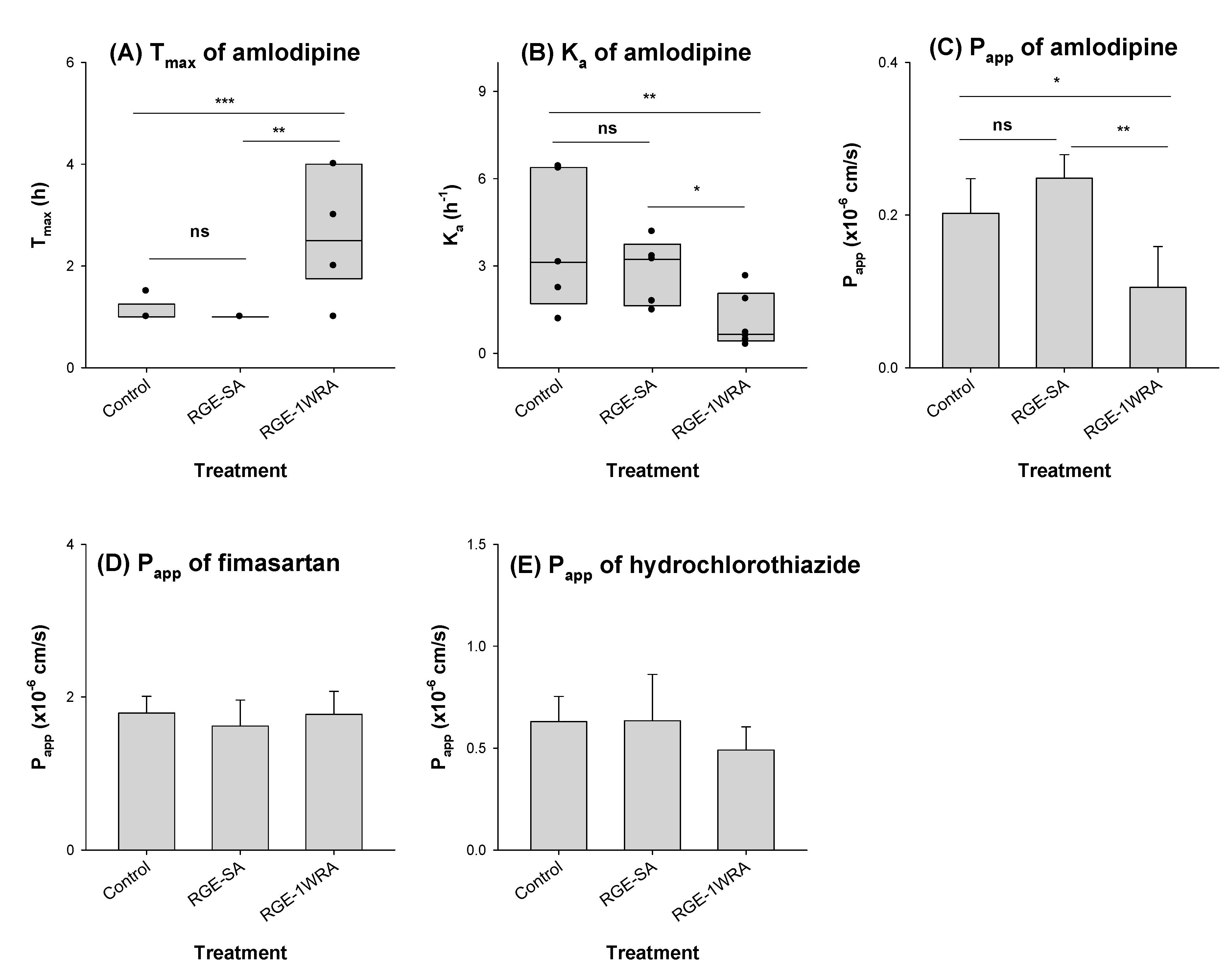
3.3. Effect of RGE on the Pharmacokinetics of Fimasartan, Amlodipine, and Hydrochlorothiazide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhee, S.J.; Lee, H.A.; Lee, S.; Kim, E.; Jeon, I.; Song, I.S.; Yu, K.S. Physiologically based pharmacokinetic modeling of fimasartan, amlodipine, and hydrochlorothiazide for the investigation of drug–drug interaction potentials. Pharm. Res. 2018, 35, 236. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.Y.; Baek, S.H.; Kim, W.; Park, C.G.; Park, S.W.; Oh, B.H.; Kim, S.H.; Kim, J.J.; Shin, J.H.; Yoo, B.S.; et al. Efficacy of fimasartan/hydrochlorothiazide combination in hypertensive patients inadequately controlled by fimasartan monotherapy. Drug Des. Devel. Ther. 2015, 9, 2847–2854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, S.; Kim, T.E.; Yoon, S.H.; Cho, J.Y.; Shin, S.G.; Jang, I.J.; Yu, K.S. Pharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteers. J. Cardiovasc. Pharmacol. 2011, 57, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Oh, B.H. Fimasartan: A new angiotensin receptor blocker. Drugs 2016, 76, 1015–1022. [Google Scholar] [CrossRef]
- Jeon, H.; Lim, K.S.; Shin, K.H.; Kim, J.; Yoon, S.H.; Cho, J.Y.; Shin, S.G.; Jang, I.J.; Yu, K.S. Assessment of the drug-drug interactions between fimasartan and hydrochlorothiazide in healthy volunteers. J. Cardiovasc. Pharmacol. 2012, 59, 84–91. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.; Oh, J.; Lee, S.; Jang, I.-J.; Lee, D.; Yu, K.S. Pharmacokinetic comparison between a fixed-dose combination of fimasartan/amlodipine/hydrochlorothiazide 60/10/25 mg and a corresponding loose combination of fimasartan/amlodipine 60/25 mg and hydrochlorothiazide 25 mg in healthy subjects. Transl. Clin. Pharmacol. 2021, 29, 53. [Google Scholar] [CrossRef]
- Choi, M.K.; Song, I.S. Interactions of ginseng with therapeutic drugs. Arch. Pharm. Res. 2019, 42, 862–878. [Google Scholar] [CrossRef]
- Jeurissen, S.M.F.; Buurma-Rethans, E.J.M.; Beukers, M.H.; Jansen-van der Vliet, M.; van Rossum, C.T.M.; Sprong, R.C. Consumption of plant food supplements in the Netherlands. Food Funct. 2018, 9, 179–190. [Google Scholar] [CrossRef]
- Park, J.B.; Sung, K.-C.; Kang, S.-M.; Cho, E.J. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb Study). Am. J. Cardiovas. Drugs 2013, 13, 47–56. [Google Scholar] [CrossRef][Green Version]
- Park, D.H.; Yun, G.Y.; Eun, H.S.; Joo, J.S.; Kim, J.S.; Kang, S.H.; Moon, H.S.; Lee, E.S.; Lee, B.S.; Kim, K.H. Fimasartan-induced liver injury in a patient with no adverse reactions on other types of angiotensin II receptor blockers: A case report. Medicine 2017, 96, e8905. [Google Scholar] [CrossRef]
- Real, M.; Barnhill, M.S.; Higley, C.; Rosenberg, J.; Lewis, J.H. Drug-induced liver injury: Highlights of the recent literature. Drug Saf. 2019, 42, 365–387. [Google Scholar] [CrossRef]
- Cho, E.J.; Sung, K.C.; Kang, S.M.; Shin, M.S.; Joo, S.J.; Park, J.B. Fimasartan reduces clinic and home pulse pressure in elderly hypertensive patients: A K-MetS study. PLoS ONE 2019, 14, e0214293. [Google Scholar] [CrossRef]
- Kim, C.; Kim, M.Y.; Kang, D.R.; Kim, J.Y.; Park, J.B. The efficacy of fimasartan for cardiovascular events and metabolic syndrome (K-MetS Study): Rationale, design and participant characteristics. Pulse 2014, 1, 177–185. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, J.W.; Park, S.; Yoo, T.H.; Kim, Y.J.; Ryu, D.R.; Chin, H.J. FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC): Study protocol for randomized controlled trial. Trials. 2017, 18, 632. [Google Scholar] [CrossRef]
- Shin, B.S.; Kim, T.H.; Paik, S.H.; Chi, Y.H.; Lee, J.H.; Tan, H.K.; Choi, Y.; Kim, M.; Yoo, S.D. Simultaneous determination of fimasartan, a novel antihypertensive agent, and its active metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 1208–1214. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, J.; Park, J.H.; Lee, C.H.; Choi, M.K.; Song, I.S. Effect of lactic acid bacteria on the pharmacokinetics and metabolism of ginsenosides in mice. Pharmaceutics 2021, 13, 1496. [Google Scholar] [CrossRef]
- Irfan, M.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Youn, S.H.; Kwak, Y.S.; Han, C.K.; Haidere, M.F.; Kim, J.K.; Min, H.; Jung, Y.J.; Hosseinzadeh, H.; Hyun, S.H.; et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J. Ginseng Res. 2021, 45, 32–40. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Gui, Q.F.; Xu, Z.R.; Xu, K.Y.; Yang, Y.M. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Medicine 2016, 95, e2584. [Google Scholar] [CrossRef]
- Lee, H.W.; Lim, H.J.; Jun, J.H.; Choi, J.; Lee, M.S. Ginseng for Treating Hypertension: A systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Curr. Vasc. Pharmacol. 2017, 15, 549–556. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, S.; Chung, M.-Y.; Choi, H.-K.; Hwang, J.-T.; Park, J.H. Effects of panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng Res. 2022, 46, 188–205. [Google Scholar] [CrossRef]
- Hur, M.-H.; Lee, M.-S.; Yang, H.-J.; Kim, C.; Bae, I.-L.; Ernst, E. Ginseng for reducing the blood pressure in patients with hypertension: A systematic review and meta-analysis. J. Ginseng Res. 2010, 34, 342–347. [Google Scholar] [CrossRef]
- Stavro, P.M.; Woo, M.; Heim, T.F.; Leiter, L.A.; Vuksan, V. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension 2005, 46, 406–411. [Google Scholar] [CrossRef]
- Lee, K.H.; Bae, I.Y.; Park, S.I.; Park, J.-D.; Lee, H.G. Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine–fructose. J. Ginseng Res. 2016, 40, 237–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagar, H.; Kang, S.K.; Choi, S.W.; Song, H.-J.; Choi, S.-J.; Piao, S.; Kim, S.; Lee, I.; Kim, C.-S. Antihypertensive effects of Rg3-enriched Korean vitamin ginseng in spontaneously hypertensive rats. Nat. Prod. Commun. 2020, 15, 173–179. [Google Scholar] [CrossRef]
- Jovanovski, E.; Smircic-Duvnjak, L.; Komishon, A.; Au-Yeung, F.R.; Sievenpiper, J.L.; Zurbau, A.; Jenkins, A.L.; Sung, M.-K.; Josse, R.; Li, D. Effect of coadministration of enriched Korean Red Ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L) on cardiometabolic outcomes in type-2 diabetes: A randomized controlled trial. J. Ginseng Res. 2021, 45, 546–554. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, S.; Lee, W.; Jin, S.; Kwon, M.; Shin, C.H.; Choi, M.K.; Song, I.S. Herb-drug interaction of red ginseng extract and ginsenoside Rc with valsartan in rats. Molecules 2020, 25, 622. [Google Scholar] [CrossRef]
- Jin, S.; Lee, S.; Jeon, J.H.; Kim, H.; Choi, M.K.; Song, I.S. Enhanced intestinal permeability and plasma concentration of metformin in rats by the repeated administration of red ginseng extract. Pharmaceutics 2019, 11, 189. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, M.; Choi, M.K.; Song, I.S. Effects of red ginseng extract on the pharmacokinetics and elimination of methotrexate via Mrp2 regulation. Molecules 2018, 23, 2948. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Han, Y.J.; Lee, W.; Kang, B.; Choi, M.K.; Han, Y.H.; Song, I.S. Effect of red ginseng extract on the pharmacokinetics and efficacy of metformin in streptozotocin-induced diabetic rats. Pharmaceutics 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, S.; Bashir, M.; Chi, Y.H.; Paik, S.H.; Lee, J.H.; Choi, H.J.; Choi, J.H.; Yoo, S.D.; Bulitta, J.B. Pharmacokinetics and metabolite profiling of fimasartan, a novel antihypertensive agent, in rats. Xenobiotica 2014, 44, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.Y.; Ryu, C.S.; Chi, Y.H.; Paik, S.H.; Kim, S.K. Role of cytochrome P450 enzymes in fimasartan metabolism in vitro. Food Chem. Toxicol. 2018, 115, 375–384. [Google Scholar] [CrossRef]
- Ryu, S.H.; Kim, J.W.; Kim, Y.S.; Lee, S.-H.; Cho, Y.-B.; Lee, H.K.; Kim, Y.G.; Jeong, W.-S.; Kim, K.-B. Negligible pharmacokinetic interaction of red ginseng and antihypertensive agent amlodipine in Sprague-Dawley rats. J. Toxicol. Environ. Health Part A 2014, 77, 1372–1383. [Google Scholar] [CrossRef]
- Shah, J.V.; Parekh, J.M.; Shah, P.A.; Shah, P.V.; Sanyal, M.; Shrivastav, P.S. Application of an LC–MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study. J. Pharm. Anal. 2017, 7, 309–316. [Google Scholar] [CrossRef]
- Kaza, M.; Karaźniewicz-Łada, M.; Kosicka, K.; Siemiątkowska, A.; Rudzki, P.J. Bioanalytical method validation: New FDA guidance vs. EMA guideline. Better or worse? J. Pharm. Biomed. Anal. 2019, 165, 381–385. [Google Scholar] [CrossRef]
- Pradhan, A.; Gupta, V.; Sethi, R. Fimasartan: A new armament to fight hypertension. J. Family Med. Prim. Care 2019, 8, 2184. [Google Scholar] [CrossRef]
- Yang, E.; Lee, S.; Lee, H.; Hwang, I.; Jang, I.J.; Yu, K.S.; Lee, S. Pharmacokinetic comparison between fixed-dose combination of fimasartan/amlodipine 60/10 mg and the corresponding loose combination through partial replicated crossover study in healthy subjects. Transl. Clin. Pharmacol. 2019, 27, 134–140. [Google Scholar] [CrossRef]
- Kumar, A.; Dwivedi, S.P.; Prasad, T. Method validation for simultaneous quantification of olmesartan and hydrochlorothiazide in human plasma using LC-MS/MS and its application through bioequivalence study in healthy volunteers. Front. Pharmacol. 2019, 10, 810. [Google Scholar] [CrossRef]
- Madureira, T.V.; Barreiro, J.C.; Rocha, M.J.; Cass, Q.B.; Tiritan, M.E. Pharmaceutical trace analysis in aqueous environmental matrices by liquid chromatography–ion trap tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7033–7042. [Google Scholar] [CrossRef]
- Gadepalli, S.G.; Deme, P.; Kuncha, M.; Sistla, R. Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats. J. Pharm. Anal. 2014, 4, 399–406. [Google Scholar] [CrossRef]
- Hao, K.; Chen, Y.; Zhao, X.; Liu, X. Pharmacokinetic-pharmacodynamic model of the antihypertensive interaction between telmisartan and hydrochlorothiazide in spontaneously hypertensive rats. J. Pharm. Pharmacol. 2014, 66, 1112–1121. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. The potential for interaction of hydrochlorothiazide with garlic in rats. Chem. Biol. Interact. 2009, 181, 472–479. [Google Scholar] [CrossRef]
- Fujimura, A.; Shiga, T.; Ohashi, K.-i.; Ebihara, A. Chronopharmacology of amlodipine in rats. Life Sci. 1993, 53, 595–602. [Google Scholar] [CrossRef]
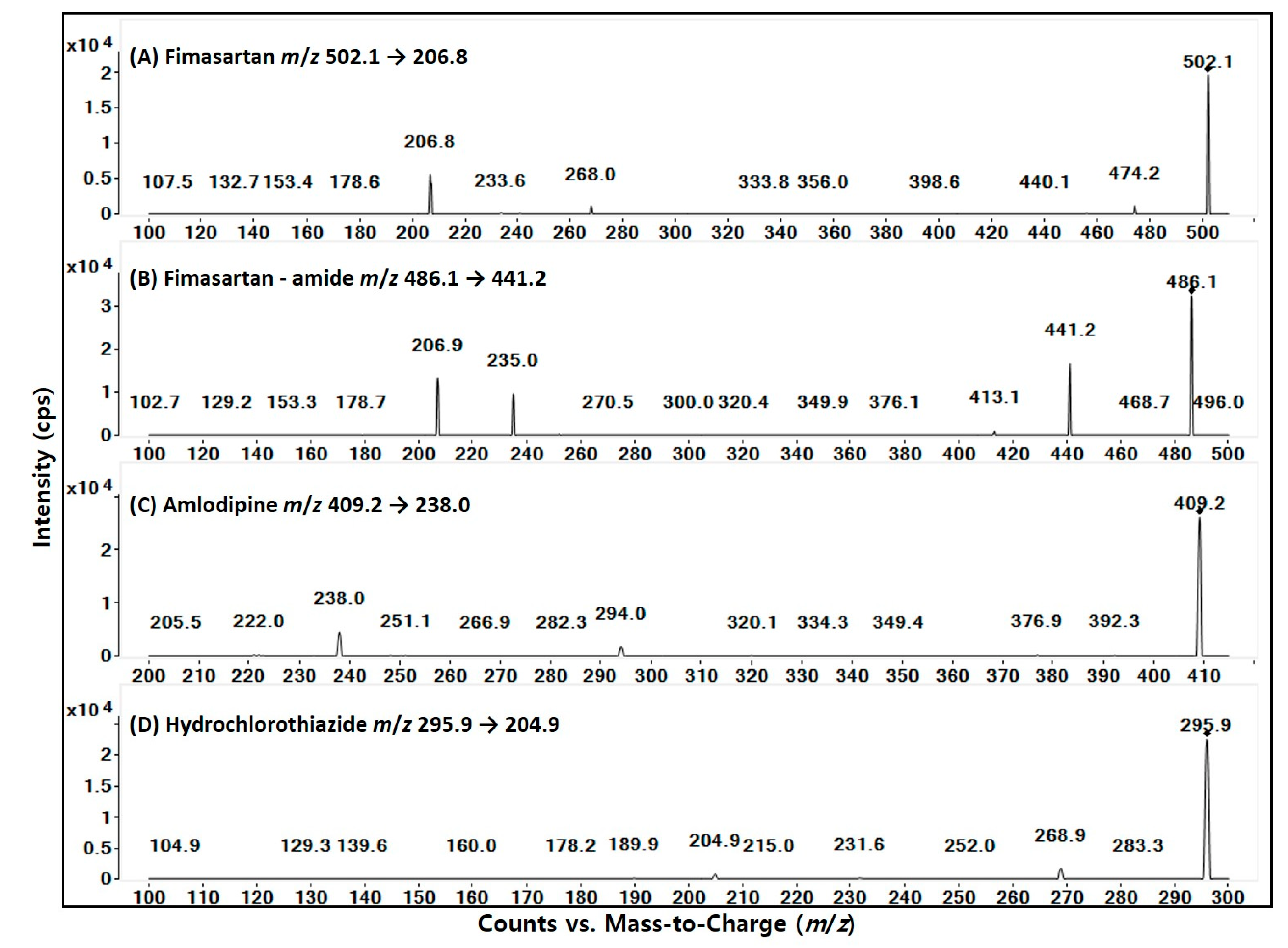

| Compounds | Fimasartan | Fimasartan-Amide | Amlodipine | Hydrochlorothiazide |
|---|---|---|---|---|
| MRM Transitions (m/z) | 502.1 → 206.8 | 486.1 → 441.2 | 409.2 → 238.0 | 295.9 → 204.9 |
| Ionization Mode | positive | positive | positive | negative |
| Fragmentwr (V) | 125 | 135 | 105 | 125 |
| Collision Energy (eV) | 25 | 10 | 8 | 20 |
| Linear Range (ng/mL) | 1–500 | 0.3–100 | 0.5–200 | 5–5000 |
| LLOQ (ng/mL) | 1 | 0.3 | 0.5 | 5 |
| Representative Equation | Y = 0.03874x + 0.002912 | Y = 0.00978 + 0.000351 | Y = 0.01158x + 0.002238 | Y = 0.00083x + 0.000086 |
| R2 | 0.9998 | 0.9992 | 0.9964 | 0.9975 |
| Analytes | Nominal Concentration (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 6) | ||||
|---|---|---|---|---|---|---|---|
| Measured Concentration (ng/mL) | Precision (CV, %) | Accuracy (%) | Measured Concentration (ng/mL) | Precision (CV, %) | Accuracy (%) | ||
| Fimasartan | 3 | 3.265 | 2.38 | 108.8 | 3.207 | 3.10 | 106.9 |
| 30 | 32.44 | 4.56 | 108.1 | 30.50 | 5.47 | 101.7 | |
| 300 | 316.1 | 3.81 | 105.4 | 318.8 | 5.38 | 106.3 | |
| Fimasartan-amide | 0.5 | 0.528 | 6.77 | 105.6 | 0.528 | 5.28 | 105.5 |
| 3 | 3.263 | 2.73 | 108.8 | 3.172 | 3.79 | 105.7 | |
| 75 | 70.54 | 4.24 | 94.06 | 74.04 | 3.43 | 98.72 | |
| Amlodipine | 1.5 | 1.644 | 4.17 | 109.6 | 1.573 | 7.75 | 104.9 |
| 15 | 15.99 | 5.43 | 106.6 | 15.36 | 2.46 | 102.4 | |
| 150 | 159.3 | 3.23 | 106.2 | 149.6 | 1.96 | 99.73 | |
| Hydrochlorothiazide | 15 | 13.99 | 5.06 | 93.24 | 15.14 | 4.09 | 100.9 |
| 150 | 131.9 | 3.00 | 87.94 | 147.2 | 5.58 | 98.15 | |
| 3000 | 2937 | 4.51 | 97.91 | 3215 | 4.68 | 107.2 | |
| Analyte | Concentration (ng/mL) | Extraction Recovery (%) | CV (%) | Matrix Effects (%) | CV (%) |
|---|---|---|---|---|---|
| Fimasartan | 3 | 90.30 | 10.6 | 35.06 | 13.6 |
| 30 | 75.92 | 8.59 | 37.08 | 13.3 | |
| 300 | 77.62 | 13.4 | 34.11 | 9.44 | |
| Fimasartan-amide | 0.5 | 91.91 | 4.98 | 6.628 | 9.53 |
| 3 | 89.43 | 4.14 | 6.839 | 8.90 | |
| 75 | 96.73 | 6.53 | 8.030 | 10.9 | |
| Amlodipine | 1.5 | 85.55 | 6.77 | 49.35 | 9.30 |
| 15 | 80.00 | 4.83 | 47.18 | 9.11 | |
| 150 | 89.02 | 8.82 | 39.87 | 13.2 | |
| Hydrochlorothiazide | 15 | 93.44 | 5.85 | 12.29 | 6.73 |
| 150 | 93.88 | 9.25 | 9.192 | 11.1 | |
| 3000 | 95.19 | 11.7 | 11.17 | 6.64 | |
| IS | 1 | 97.25 | 7.21 | 89.26 | 6.84 |
| Storage Conditions | Analytes | Concentration (ng/mL) | Precision (CV%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Spiked | Measured | ||||
| Bench-top stability (6 h at 25 °C) | Fimasartan | 3 | 2.86 | 1.09 | 95.42 |
| 300 | 298.4 | 4.12 | 99.48 | ||
| Fimasartan-amide | 0.5 | 0.55 | 3.63 | 110.6 | |
| 75 | 72.24 | 4.87 | 96.32 | ||
| Amlodipine | 1.5 | 1.47 | 2.67 | 98.31 | |
| 150 | 157.6 | 3.49 | 105.1 | ||
| Hydrochlorothiazide | 15 | 13.40 | 4.53 | 89.34 | |
| 3000 | 2864 | 1.16 | 95.47 | ||
| Post-preparative stability (24 h at 6 °C) | Fimasartan | 3 | 3.21 | 4.96 | 106.9 |
| 300 | 293.1 | 6.11 | 97.70 | ||
| Fimasartan-amide | 0.5 | 0.56 | 6.28 | 111.2 | |
| 75 | 71.14 | 3.74 | 94.86 | ||
| Amlodipine | 1.5 | 1.41 | 7.27 | 94.04 | |
| 150 | 150.3 | 6.65 | 100.2 | ||
| Hydrochlorothiazide | 15 | 14.36 | 8.42 | 95.73 | |
| 3000 | 2973 | 9.40 | 99.11 | ||
| Three freeze–thaw cycle stability | Fimasartan | 3 | 3.22 | 2.81 | 107.4 |
| 300 | 322.6 | 4.17 | 107.5 | ||
| Fimasartan-amide | 0.5 | 0.56 | 0.35 | 111.0 | |
| 75 | 78.53 | 5.84 | 104.7 | ||
| Amlodipine | 1.5 | 1.53 | 1.89 | 101.7 | |
| 150 | 166.8 | 2.43 | 111.2 | ||
| Hydrochlorothiazide | 15 | 13.21 | 3.60 | 88.08 | |
| 3000 | 3118 | 6.74 | 103.9 | ||
| Drugs | PK Parameters | Single | Triple | p-Value |
|---|---|---|---|---|
| Fimasartan | Cmax (ng/mL) | 170.1 ± 51.7 | 222.3 ± 73.9 | 0.240 |
| Tmax (h) | 1.7 ± 1.4 | 0.5 ± 0.0 | 0.065 | |
| AUClast (ng∙h/mL) | 1218 ± 253 | 994.8 ± 367 | 0.589 | |
| AUCinf (ng∙h/mL) | 2219 ± 591 | 1344 ± 868 | 0.126 | |
| T1/2 (h) | 22.1 ± 12.3 | 23.0 ± 25.2 | 0.631 | |
| MRT (h) | 8.9 ± 1.5 | 7.5 ± 1.9 | 0.093 | |
| Fimasartan-amide | Cmax (ng/mL) | 16.9 ± 5.1 | 17.0 ± 4.8 | 0.699 |
| Tmax (h) | 7.7 ± 0.8 | 10.0 ± 4.8 | 0.923 | |
| AUClast (ng∙h/mL) | 254.3 ± 68.6 | 236.1 ± 82.3 | 0.522 | |
| AUCinf (ng∙h/mL) | NC | NC | ||
| T1/2 (h) | NC | NC | ||
| MRT (h) | 10.6 ± 1.5 | 10.4 ± 1.5 | 0.394 | |
| MR | 0.2 | 0.2 | ||
| Amlodipine | Cmax (ng/mL) | 64.9 ± 15.4 | 61.6 ± 11.5 | 0.310 |
| Tmax (h) | 1.4 ± 0.9 | 1.1 ± 0.7 | 0.394 | |
| AUClast (ng∙h/mL) | 699.7 ± 249 | 625.2 ± 103 | 0.485 | |
| AUCinf (ng∙h/mL) | 1018 ± 269 | 798.8 ± 197 | 0.065 | |
| T1/2 (h) | 11.3 ± 1.9 | 10.7 ± 4.8 | 0.818 | |
| MRT (h) | 7.8 ± 1.8 | 7.9 ± 0.9 | 0.589 | |
| Hydrochlorothiazide | Cmax (ng/mL) | 3785 ± 1263 | 3289 ± 391 | 0.423 |
| Tmax (h) | 3.3 ± 0.6 | 2.8 ± 1.0 | 0.406 | |
| AUClast (μg∙h/mL) | 31.5 ± 15.8 | 24.7 ± 11.1 | 0.423 | |
| AUCinf (μg∙h/mL) | 31.6 ± 15.9 | 24.8 ± 11.1 | 0.423 | |
| T1/2 (h) | 2.7 ± 0.7 | 2.6 ± 1.2 | 0.262 | |
| MRT (h) | 5.5 ± 0.7 | 5.1 ± 1.0 | 0.522 |
| Drugs | PK Parameters | RGE Treatment (1.5 g/kg) | p-Value | ||
|---|---|---|---|---|---|
| Control | RGE-SA | RGE-1WRA | |||
| Fimasartan | Cmax (ng/mL) | 210.1 ± 756 | 190.1 ± 32.4 | 147.2 ± 72.3 | 0.181 |
| Tmax (h) | 0.50 ± 0.00 | 0.50 ± 0.00 | 1.25 ± 1.41 | 0.169 | |
| AUClast (ng∙h/mL) | 840.9 ± 258.9 | 643.3 ± 122.0 | 903.5 ± 299.9 | 0.075 | |
| AUCinf (ng∙h/mL) | 999.9 ± 268.9 | 1245.3 ± 334.5 | 1284.6 ± 314.4 | 0.336 | |
| T1/2 (h) | 10.68 ± 3.33 | 21.63 ± 4.77 | 22.06 ± 16.7 | 0.104 | |
| MRT (h) | 7.47 ± 0.60 | 8.05 ± 0.42 | 7.93 ± 0.58 | 0.105 | |
| Fimasartan-amide | Cmax (ng/mL) | 15.80 ± 4.82 | 15.32 ± 4.71 | 19.93 ± 2.81 | 0.181 |
| Tmax (h) | 7.20 ± 1.10 | 8.00 ± 0.00 | 7.33 ± 1.63 | 0.343 | |
| AUClast (ng∙h/mL) | 193.3 ± 52.7 | 227.2 ± 50.9 | 257.8 ± 30.2 | 0.135 | |
| AUCinf (ng∙h/mL) | 235.6 ± 93.9 | NC | NC | NC | |
| T1/2 (h) | 6.18 ± 1.32 | NC | NC | NC | |
| MRT (h) | 8.69 ± 0.71 | 9.98 ± 1.60 | 8.49 ± 0.82 | 0.294 | |
| MR | 0.24 ± 0.10 | 0.37 ± 0.12 | 0.31 ± 0.11 | 0.120 | |
| Amlodipine | Cmax (ng/mL) | 54.38 ± 8.96 | 49.97 ± 11.4 | 48.50 ± 10.5 | 0.560 |
| Tmax (h) | 1.10 ± 0.22 | 1.00 ± 0.00 | 2.67 ± 1.21 | 0.008 | |
| AUClast (ng∙h/mL) | 630.5 ± 108.2 | 515.4 ± 95.8 | 557.3 ± 74.5 | 0.279 | |
| AUCinf (ng∙h/mL) | 795.6 ± 218.4 | 788.5 ± 247.2 | 774.8 ± 82.1 | 0.940 | |
| T1/2 (h) | 10.22 ± 5.09 | 12.44 ± 1.96 | 13.46 ± 2.91 | 0.138 | |
| MRT (h) | 7.90 ± 0.93 | 8.62 ± 0.88 | 8.75 ± 0.94 | 0.319 | |
| Hydrochlorothiazide | Cmax (ng/mL) | 3309.8 ± 433.4 | 3071.6 ± 583.1 | 2668.9 ± 861.2 | 0.426 |
| Tmax (h) | 2.60 ± 0.96 | 2.30 ± 0.97 | 3.42 ± 0.66 | 0.101 | |
| AUClast (μg∙h/mL) | 22.90 ± 11.4 | 20.90 ± 7.96 | 20.91 ± 9.92 | 0.940 | |
| AUCinf (μg∙h/mL) | 23.05 ± 11.4 | 21.01 ± 8.06 | 21.01 ± 10.0 | 0.916 | |
| T1/2 (h) | 2.65 ± 1.29 | 2.77 ± 0.64 | 2.71 ± 0.65 | 0.560 | |
| MRT (h) | 4.89 ± 1.03 | 5.29 ± 0.60 | 5.47 ± 0.66 | 0.426 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-Y.; Jeon, J.-H.; Park, J.-H.; Lee, J.; Pang, M.; Choi, M.-K.; Song, I.-S. Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract. Toxics 2022, 10, 576. https://doi.org/10.3390/toxics10100576
Jeon S-Y, Jeon J-H, Park J-H, Lee J, Pang M, Choi M-K, Song I-S. Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract. Toxics. 2022; 10(10):576. https://doi.org/10.3390/toxics10100576
Chicago/Turabian StyleJeon, So-Yeon, Ji-Hyeon Jeon, Jin-Hyang Park, Jihoon Lee, Minyeong Pang, Min-Koo Choi, and Im-Sook Song. 2022. "Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract" Toxics 10, no. 10: 576. https://doi.org/10.3390/toxics10100576
APA StyleJeon, S.-Y., Jeon, J.-H., Park, J.-H., Lee, J., Pang, M., Choi, M.-K., & Song, I.-S. (2022). Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract. Toxics, 10(10), 576. https://doi.org/10.3390/toxics10100576