Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment
Abstract
:1. Introduction
2. Speciation of Radiocesium and Its Transformation in Soil–Water Environment
2.1. Release of Hot Particles following Chernobyl and Fukushima Accidents
2.1.1. Chernobyl
2.1.2. Fukushima
2.2. Transformation of Basic Radiocesium Chemical Forms in Soil–Water Environment
2.2.1. Solid–Liquid Distribution of Radiocesium in Soil–Water Environment in Fukushima and Chernobyl
2.2.2. Radiocesium Leaching from Chernobyl Fuel Particles and Fukushima Glassy Hot Particle CsMPs
2.2.3. Radiocesium Fixation by Soils and Sediments and Remobilization
3. Radiocesium Downward Migration in Soil
4. Time Changes of Radiocesium Concentrations in Freshwaters
4.1. Long-Term Dynamics of Radiocesium in Rivers and Lakes and Its Prediction
4.2. Radiocesium Wash-Off from Contaminated Watersheds and Its Dynamics after the Accident
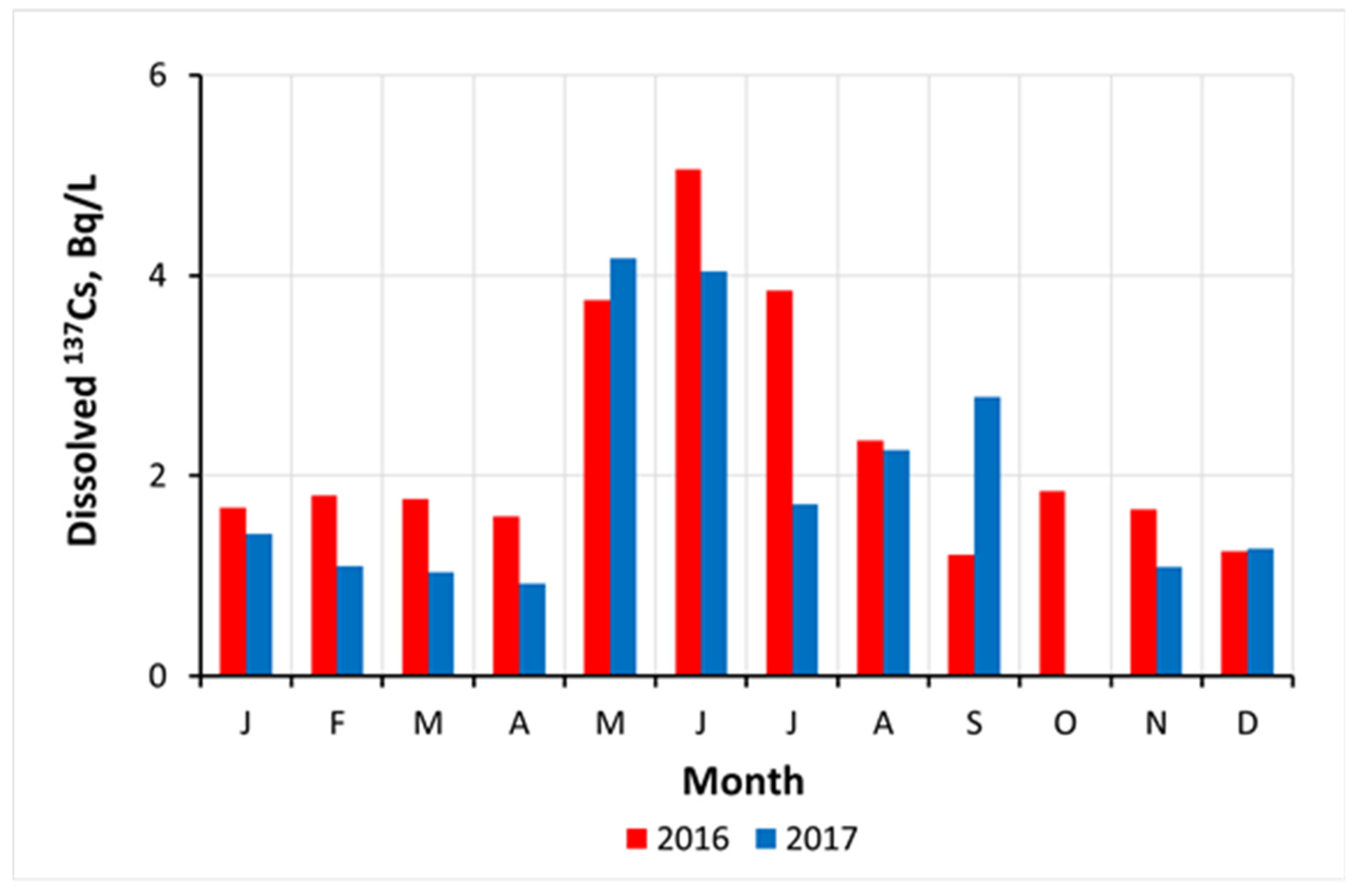
4.3. Seasonal Variation and Temperature Dependence of Radiocesium in Freshwaters
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legasov, V.A. (Ed.) Information on the accident at the Chernobyl nuclear power plant and its consequences prepared for IAEA. Sov. At. Energy 1986, 61, 845–868. [Google Scholar] [CrossRef]
- IAEA. The Fukushima Daiichi Accident; Technical Volume 4/5: Radiological Consequences; IAEA: Vienna, Austria, 2015; 250p. [Google Scholar]
- Alexakhin, R.M.; Buldakov, A.A.; Gubanov, V.A.; Drozhko, E.G.; Il’in, L.A.; Kryshev, I.I.; Linge, I.I.; Romanov, G.N.; Savkin, M.N.; Saurov, M.M.; et al. Severe Nuclear Accidents: Consequences and Protective Measures; IzdAT: Moscow, Russia, 2001; 752p. (In Russian) [Google Scholar]
- Hirose, K. 2011 Fukushima Dai-ichi nuclear power plant accident: Summary of regional radioactive deposition monitoring results. J. Environ. Radioact. 2012, 111, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.V.; Bulgakov, A.A.; Popov, V.E.; Bobovnikova, T.I. Behaviour of Long-lived Chernobyl Radionuclides in a Soil-Water System. Analyst 1992, 117, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Beresford, N.A. (Eds.) Chernobyl Catastrophe and Consequences; Springer-Praxis: Berlin/Heidelberg, Germany, 2005; 310p. [Google Scholar]
- Beresford, N.; Fesenko, S.; Konoplev, A.; Skuterud, L.; Smith, J.T.; Voigt, G. Thirty years after the Chernobyl accident: What lessons have we learnt? J. Environ. Radioact. 2016, 157, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.; Golosov, V.; Laptev, G.; Nanba, K.; Onda, Y.; Takase, T.; Wakiyama, Y.; Yoshimura, K. Behavior of accidentally released radiocesium in soil-water environment: Looking at Fukushima from a Chernobyl perspective. J. Environ. Radioact. 2016, 151, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.; Kato, K.; Kalmykov, S.N. (Eds.) Behavior of Radionuclides in the Environment II: Chernobyl; Springer Nature: Singapore, 2020; 295p, ISBN 978-981-15-3567-3. [Google Scholar]
- Evrard, O.; Laceby, J.P.; Lepage, H.; Onda, Y.; Cerdan, O.; Ayrault, S. Radiocesium transfer from hillslopes to the Pacific Ocean after the Fukushima Nuclear Power Plant accident: A review. J. Environ. Radioact. 2015, 148, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Laceby, J.P.; Chartin, C.; Evrard, O.; Onda, Y.; Garcia-Sanchez, L.; Cerdan, O. Rainfall erosivity in catchments contaminated with fallout from the Fukushima Daiichi nuclear power plant accident. Hydrol. Earth Syst. Sci. 2016, 20, 2467–2482. [Google Scholar] [CrossRef]
- Nagao, S.; Kanamori, M.; Ochiai, S.; Fukushi, K.; Yamamoto, M. Export of 134Cs and 137Cs in the Fukushima River systems at heavy rains by Typhoon Roke in September 2011. Biogeosciences 2013, 10, 6215–6223. [Google Scholar] [CrossRef]
- Evrard, O.; Chartin, C.; Onda, Y.; Lepage, H.; Cerdan, O.; Lefevre, I.; Ayrault, S. Renewed soil erosion and remobilization of radioactive sediment in Fukushima coastal rivers after the 2013 typhoons. Sci. Rep. 2014, 4, 4574. [Google Scholar] [CrossRef] [PubMed]
- Yamashiki, Y.; Onda, Y.; Smith, H.G.; Blake, W.H.; Wakahara, T.; Igarashi, Y.; Matsuura, Y.; Yoshimura, K. Initial flux of sediment-associated radiocesium to the ocean from largest river impacted by Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2014, 4, 3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konoplev, A.; Golosov, V.; Wakiyama, Y.; Takase, T.; Yoschenko, V.; Yoshihara, T.; Parenyuk, O.; Cresswell, A.; Ivanov, M.; Carradine, M.; et al. Natural attenuation of Fukushima-derived radiocesium in soils due to its vertical and lateral migration. J. Environ. Radioact. 2018, 186, 23–33. [Google Scholar] [CrossRef]
- Takata, H.; Wakiyama, Y.; Niida, T.; Igarashi, Y.; Konoplev, A.; Inatomi, N. Importance of desorption process from Abukuma River’s suspended particles in increasing dissolved 137Cs in coastal water during river-flood caused by typhoons. Chemosphere 2021, 281, 130751. [Google Scholar] [CrossRef]
- Nakao, A.; Ogasawara, S.; Sano, O.; Ito, T.; Yanai, J. Radiocesium sorption in relation to clay mineralogy of paddy soils in Fukushima, Japan. Sci. Total Environ. 2014, 468–469, 523–529. [Google Scholar] [CrossRef]
- Konoplev, A.; Kanivets, V.; Zhukova, O.; Germenchuk, M.; Derkach, G. Mid- to long-term radiocesium wash-off from contaminated catchments at Chernobyl and Fukushima. Water Res. 2021, 188, 116514. [Google Scholar] [CrossRef]
- Nanba, K.; Konoplev, A.; Wada, T. (Eds.) Behavior of Radionuclides in the Environment III: Fukushima; Springer Nature: Singapore, 2022; 510p. [Google Scholar]
- Bogatov, S.A.; Borovoi, A.A.; Dubasov, Y.V.; Lomonosov, V.V. Form and parameters of the particles of the fuel ejection in the Chernobyl reactor accident. Sov. At. Energy 1990, 69, 595–601. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Bobovnikova, T.I. Comparative analysis of chemical Forms of long-lived radionuclides and their migration and transformation in the environment following the Kyshtym and Chernobyl accidents. In Proceedings Report EUR 13574, Proceedings of the Seminar on Comparative Assessment of the Environmental Impact of Radionuclides Released during Three Major Nuclear Accidents: Kyshtym, Windscale, Chernobyl, Luxembourg, 1–5 October 1990; CEC: Luxembourg, 1991; Volume 1, pp. 371–396. [Google Scholar]
- Victorova, N.V.; Garger, E.K. Investigation of the deposition and spread of radioactive aerosol particles in the Chernobyl zone based on biological monitoring. In Proceedings Report EUR 13574, Proceedings of the CEC Seminar on Comparative Assessment of the Environmental Impact of Radionuclides Released during Three Major Nuclear Accidents: Kyshtym, Windscale and Chernobyl, Luxembourg, 1–5 October 1990; CEC: Luxembourg, 1991; Volume 1, pp. 223–236. [Google Scholar]
- Sandalls, F.J.; Segal, M.J.; Victorova, N. Hot Particles from Chernobyl: A Review. J. Environ. Radioact. 1993, 18, 5–22. [Google Scholar] [CrossRef]
- Salbu, B.; Krekling, T.; Oughton, D.H.; Ostby, G.; Kashparov, V.A.; Brand, T.L.; Day, J.P. Hot particles in accidental releases from Chernobyl and Windscale nuclear installations. Analyst 1994, 119, 125–130. [Google Scholar] [CrossRef]
- Kashparov, V.A.; Ivanov, Y.A.; Zvarich, S.I.; Protsak, V.P.; Khomutinin, Y.V.; Kurepin, A.D.; Pazukhin, E.M. Formation of hot particles during the Chernobyl nuclear power plant accident. Nucl. Technol. 1996, 114, 246–253. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Viktorova, N.V.; Virchenko, E.P.; Popov, V.E.; Bulgakov, A.A.; Desmet, G.M. Influence of agricultural countermeasures on the ratio of different chemical forms of radionuclides in soil and soil solution. Sci. Total Environ. 1993, 137, 147–162. [Google Scholar] [CrossRef]
- Konoplev, A. Mobility and bioavailability of Chernobyl-derived radionuclides in soil-water environment: Review. In Behavior of Radionuclides in the Environment II: Chernobyl; Konoplev, A., Kato, K., Kalmykov, S.N., Eds.; Springer Nature: Singapore, 2020; pp. 157–193. [Google Scholar]
- Bobovnikova, T.I.; Makhon’ko, K.P.; Siverina, A.A.; Rabotnova, F.A.; Gutareva, V.P.; Volokitin, A.A. Physical-chemical forms of radionuclides in atmospheric fallout, and their transformations in soil, after the accident at the Chernobyl Atomic Energy Plant. Sov. At. Energy 1991, 71, 932–936. [Google Scholar] [CrossRef]
- Hilton, J.; Cambray, R.S.; Green, N. Fractionation of radioactive caesium in airborne particles containing bomb fallout, Chernobyl fallout and atmospheric material from the Sellafield site. J. Environ. Radioact. 1992, 15, 103–108. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Borzilov, V.A.; Bobovnikova, T.I.; Virchenko, E.P.; Popov, V.E.; Kutnyakov, I.V.; Chumichev, V.B. Distribution of radionuclides in the soil-water system due to fallout after the Chernobyl disaster. Russ. Meteorol. Hydrol. 1988, 12, 42–49. [Google Scholar]
- Krouglov, S.V.; Kurinov, A.D.; Alexakhin, R.M. Chemical fractionation of 90Sr, 106Ru, 137Cs, and 144Ce in Chernobyl-contaminated soils: An evolution in the course of time. J. Environ. Radioact. 1998, 38, 59–76. [Google Scholar] [CrossRef]
- Smith, J.T.; Clarke, R.T.; Saxen, R. Time-dependent behaviour of radiocaesium: A new method to compare the mobility of weapons test and Chernobyl derived fallout. J. Environ. Radioact. 2000, 49, 65–83. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Bulgakov, A.A. Kinetics of the leaching of 90Sr from fuel particles in soil in the near zone of the Chernobyl nuclear power plant. At. Energy 1999, 86, 136–141. [Google Scholar] [CrossRef]
- Kashparov, V.A.; Oughton, D.H.; Zvarich, S.I.; Protsak, V.P.; Levchuk, S.E. Kinetics of fuel particles weathering and 90Sr mobility in 30-km exclusion zone. Health Phys. 1999, 76, 251–259. [Google Scholar] [CrossRef]
- Bulgakov, A.; Konoplev, A.; Smith, J.; Laptev, G.; Voitsekhovich, O. Fuel particles in the Chernobyl cooling pond: Current state and prediction for remediation options. J. Environ. Radioact. 2009, 100, 329–332. [Google Scholar] [CrossRef]
- Kashparov, V.; Salbu, B.; Simonucci, C.; Levchuk, S.; Reinoso-Maset, E.; Lind, O.C.; Maloshtan, I.; Protsak, V.; Courbet, C.; Nguyen, H. Validation of a fuel particle dissolution model with samples from Red Forest within the Chernobyl exclusion zone. J. Environ. Radioact. 2020, 223–224, 106387. [Google Scholar] [CrossRef] [PubMed]
- Vdovenko, V.M. Chemistry of Uranium and Trans-Uranium Elements; Russian Academy of Science: Moscow, Russia; Saint Petersburg, Russia, 1960; 700p. (In Russian) [Google Scholar]
- Kaneyasu, N.; Ohashi, H.; Suzuki, F.; Okuda, T.; Ikemori, F. Sulfate aerosol as a potential transport medium of radiocesium from the Fukushima nuclear accident. Environ. Sci. Technol. 2012, 46, 5720–5726. [Google Scholar] [CrossRef]
- Adachi, K.; Kajino, M.; Zaizen, Y.; Igarashi, Y. Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci. Rep. 2013, 3, 2554. [Google Scholar] [CrossRef]
- Abe, Y.; Iizawa, Y.; Terada, Y.; Adachi, K.; Igarashi, Y.; Nakai, I. Detection of uranium and chemical state analysis of individual radioactive microparticles emitted from the Fukushima nuclear accident using multiple synchrotron radiation X-ray analyses. Anal. Chem. 2015, 88, 8521–8525. [Google Scholar] [CrossRef] [PubMed]
- Niimura, N.; Kikuchi, K.; Tuyen, N.D.; Komatsuzaki, M.; Motohashi, Y. Physical properties, structure and shape of radioactive Cs from the Fukushima Daiichi Nuclear Power Plant accident derived from soil, bamboo and shiitake mushroom measurements. J. Environ. Radioact. 2015, 139, 234–239. [Google Scholar] [CrossRef]
- Igarashi, Y.; Kogure, T.; Kurihara, Y.; Miura, H.; Okumura, T.; Satou, Y.; Takahashi, Y.; Yamaguchi, N. A review of Cs-bearing microparticles in the environment emitted by the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2019, 205–206, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Sueki, K.; Sasa, K.; Yoshikawa, H.; Nakama, S.; Minowa, H.; Abe, Y.; Naklai, I.; Ono, T.; Adachi, K.; et al. Analysis of two forms of radioactive particles emitted during the early stages of the Fukushima Dai-ichi Nuclear Power Station accident. Geochem. J. 2018, 52, 137–143. [Google Scholar] [CrossRef]
- Miura, H.; Kurihara, Y.; Sakaguchi, A.; Tanaka, K.; Yamaguchi, N.; Higaki, S.; Takahashi, Y. Discovery of radiocesium-bearing microparticles in river water and their influence on the solid-water distribution coefficient (Kd) of radiocesium in the Kuchibuto River in Fukushima. Geochem. J. 2018, 52, 145–154. [Google Scholar] [CrossRef]
- Ikehara, R.; Suetake, M.; Komiya, T.; Furuki, G.; Ochiai, A.; Yamasaki, S.; Bower, W.R.; Law, G.T.W.; Ohnuki, T.; Grambow, B.; et al. Novel method of quantifying cesium-rich microparticles (CsMPs) in the environment from the Fukushima Daiichi nuclear power plant. Environ. Sci. Technol. 2018, 52, 6390–6398. [Google Scholar] [CrossRef]
- Ikehara, R.; Morooka, K.; Suetake, M.; Komiya, T.; Kurihara, E.; Takehara, M.; Takami, R.; Kino, C.; Horie, K.; Takehara, M.; et al. Abundance and distribution of radioactive cesium-rich microparticles released from the Fukushima Daiichi Nuclear Power Plant into the environment. Chemosphere 2020, 241, 125019. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.V. Distribution of radiocesium of accidental origin between the suspended alluvium and solution in rivers: Comparison of Fukushima and Chernobyl. Radiochemistry 2015, 57, 552–556. [Google Scholar] [CrossRef]
- Reinoso-Maset, E.; Brown, J.; Pettersen, M.N.; Steenhuisen, F.; Tetteh, A.; Wada, T.; Hinton, T.G.; Salbu, B.; Lind, O.C. Linking heterogeneous distribution of radiocaesium in soils and pond sediments in the Fukushima Daiichi exclusion zone to mobility and potential bioavailability. J. Environ. Radioact. 2020, 211, 106080. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Kurihara, Y.; Yamamoto, M.; Sakaguchi, A.; Yamaguchi, N.; Sekizawa, O.; Nitta, K.; Higaki, S.; Tsumune, D.; Itai, T.; et al. Characterization of two types of cesium-bearing microparticles emitted from the Fukushima accident via multiple synchrotron radiation analyses. Sci. Rep. 2020, 10, 11421. [Google Scholar] [CrossRef]
- Salbu, B.; Lind, O.C.; Skipperud, L. Radionuclide speciation and its relevance in environmental impact assessments. J. Environ. Radioact. 2004, 74, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Salbu, B.; Kashparov, V.; Lind, O.C.; Garcia-Tenorio, R.; Johansen, M.P.; Child, D.P.; Roos, P.; Sancho, C. Challenges associated with the behaviour of radioactive particles in the environment. J. Environ. Radioact. 2018, 186, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.; Wakiyama, Y.; Wada, T.; Golosov, V.; Nanba, K.; Takase, T. Radiocesium in ponds in the near zone of Fukushima Dai-ichi NPP. Water Resour. 2018, 45, 589–597. [Google Scholar] [CrossRef]
- Konoplev, A.; Wakiyama, Y.; Wada, T.; Udy, C.; Kanivets, V.; Ivanov, V.; Komissarov, M.; Goto, A.; Nanba, K. Radiocesium distribution and mid-term dynamics in the ponds of the Fukushima Dai-ichi nuclear power plant exclusion zone. Chemosphere 2021, 265, 129058. [Google Scholar] [CrossRef]
- Okumura, T.; Yamaguchi, N.; Dohi, T.; Iijima, K.; Kogure, T. Dissolution behavior of radiocesium-bearing microparticles released from the Fukushima nuclear plant. Sci. Rep. 2019, 9, 3520. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, B.L. Selective sorption and fixation of cations by clay minerals: A review. Clays Clay Miner. 1972, 20, 93–100. [Google Scholar] [CrossRef]
- Cremers, A.; Elsen, A.; De Preter, P.; Maes, A. Quantitative analysis of radiocesium retention in soils. Nature 1988, 335, 247–249. [Google Scholar] [CrossRef]
- Comans, R.N.J.; Haller, M.; De Preter, P. Sorption of caesium on illite: Nonequilibrium behaviour and reversibility. Geochim. Cosmochim. Acta 1991, 55, 433–440. [Google Scholar] [CrossRef]
- Comans, R.N.J.; Hockley, D.E. Kinetics of caesium sorption on illite. Geochim. Cosmochim. Acta 1992, 56, 1157–1164. [Google Scholar] [CrossRef]
- Bots, P.; Comarmond, M.J.; Payne, T.E.; Gückel, K.; Lunn, R.J.; Rizzo, L.; Schellenger, A.E.P.; Renshaw, J.C. Emerging investigator series: A holistic approach to multicomponent EXAFS: Sr and Cs complexation in clayey soils. Environ. Sci. Process. Impacts 2021, 23, 1101–1115. [Google Scholar] [CrossRef]
- Fuller, A.J.; Shaw, S.; Peacock, C.L.; Trivedi, D.; Small, J.S.; Abrahamsen, L.G.; Burke, I.T. Ionic strength and pH dependent multi-site sorption of Cs onto a micaceous aquifer sediment. Appl. Geochem. 2014, 40, 32–42. [Google Scholar] [CrossRef]
- Hwang, J.; Choung, S.; Shin, W.; Han, W.S.; Chon, C.-M. A Batch Experiment of Cesium Uptake Using Illitic Clays with Different Degrees of Crystallinity. Water 2021, 13, 409. [Google Scholar] [CrossRef]
- Kerisit, S.; Okumura, M.; Rosso, K.M.; Machida, M. Molecular simulation of cesium adsorption at the basal surface of phyllosilicate minerals. Clays Clay Miner. 2016, 64, 389–400. [Google Scholar] [CrossRef]
- Vasconcelos, I.F.; Bunker, B.A.; Cygan, R.T. Molecular dynamics modeling of ion adsorption to the basal surfaces of kaolinite. J. Phys. Chem. C 2007, 111, 6753–6762. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Bulgakov, A.A.; Popov, V.E.; Hilton, J.; Comans, R.N.J. Long-term investigation of 137Cs fixation by soils. Radiat. Prot. Dosim. 1996, 64, 15–18. [Google Scholar] [CrossRef]
- Fuller, A.J.; Shaw, S.; Ward, M.B.; Haigh, S.J.; Mosselmans, J.F.W.; Peacock, C.L.; Stackhouse, S.; Dent, A.J.; Trivedi, D.; Burke, I.T. Caesium incorporation and retention in illite interlayers. Appl. Clay Sci. 2015, 108, 128–134. [Google Scholar] [CrossRef]
- Pavlotskaya, F.I. Migration of Radioactive Products of Global Fallout in Soils; Atomizdat: Moscow, Russia, 1974; 270p. (In Russian) [Google Scholar]
- Smith, J.T.; Comans, R.N.J. Modelling the diffusive transport and remobilisation of 137Cs in sediments: The effect of sorption kinetics and reversibility. Geokhim. Cosmochim. Acta 1996, 60, 995–1004. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Bulgakov, A.A. Transformation of the forms of 90Sr and 137Cs in soil and bottom deposits. At. Energy 2000, 88, 56–60. [Google Scholar] [CrossRef]
- IAEA. Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments; Technical reports series No. 472; IAEA: Vienna, Austria, 2010; 194p. [Google Scholar]
- Konoplev, A.V.; Bulgakov, A.A. 90Sr and 137Cs exchangeable distribution coefficient in soil-water systems. At. Energy 2000, 88, 158–163. [Google Scholar] [CrossRef]
- Smith, J.T.; Comans, R.N.J.; Ireland, D.G.; Nolan, L.; Hilton, J. Experimental and in situ study of radiocesium transfer across the sediment-water interface and mobility in lake sediments. Appl. Geochem. 2000, 15, 833–848. [Google Scholar] [CrossRef]
- Vandebroek, L.; Van Hees, M.; Delvaux, B.; Spaargaren, O.; Thiry, Y. Relevance of radiocesium interception potential (RIP) on a worldwide scale to assess soil vulnerability to 137Cs contamination. J. Environ. Radioact. 2012, 104, 87–93. [Google Scholar] [CrossRef]
- Wauters, J.; Madruga, M.J.; Vidal, M.; Cremers, A. Solid phase speciation of radiocesium in bottom sediments. Sci. Total Environ. 1996, 187, 121–130. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Kaminski, S.; Klemt, E.; Konopleva, I.; Miller, R.; Zibold, G. Comparative study of 137Cs partitioning between solid and liquid phases in Lakes Constance, Lugano and Vorsee. J. Environ. Radioact. 2002, 58, 1–11. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Konopleva, I.V. Characteristics of steady-state selective sorption of radiocesium on soils and bottom sediments. Geochem. Internat. 1999, 37, 177–183. [Google Scholar]
- Ries, T.; Putyrskaya, V.; Klemt, E. Long-term distribution and migration of 137Cs in a small lake ecosystem with organic rich catchment: A caser study of Lake Vorsee (Southern Germany). J. Environ. Radioact. 2019, 198, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A.V.; Bulgakov, A.A. Modelling the transformation processes of Chernobyl origin Cs-137 and Sr-90 speciation in soil and bottom sediments. In Environmental Impact of Radioactive Releases, Proceedings of the International Symposium, Vienna, Austria, 8–12 May 1995; IAEA: Vienna, Austria, 1996; pp. 311–324. [Google Scholar]
- Wauters, J.; Elsen, A.; Cremers, A.; Konoplev, A.; Bulgakov, A.; Comans, R.N.J. Prediction of solid/liquid distribution coefficients of radiocesium in soils and sediments. Part one: A simplified procedure for the solid phase characterization. Appl. Geochem. 1996, 11, 589–594. [Google Scholar] [CrossRef]
- Konoplev, A.; Wakiyama, Y.; Wada, T.; Igarashi, Y.; Kanivets, V.; Nanba, K. Behavior of Fukushima-derived radiocesium in the soil–water environment: Review. In Behavior of Radionuclides in the Environment III: Fukushima; Nanba, K., Konoplev, A., Wada, T., Eds.; Springer Nature: Singapore, 2022; pp. 33–68. [Google Scholar]
- Nakao, A.; Nakao, A.; Ryoji, T.; Osagawara, S.; Yanai, J. Aeolian-dust-derived micaceous minerals control radiocesium retention in andosols in Japan. Soil Sci. Soc. Am. J. 2015, 79, 1590–1600. [Google Scholar] [CrossRef]
- Nakao, A.; Thiry, Y.; Funakawa, S.; Kosaki, T. Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soil Sci. Plant Nutr. 2008, 54, 479–489. [Google Scholar] [CrossRef]
- De Koning, A.; Konoplev, A.; Comans, R. Measuring the specific caesium sorption capacity of soils, sediments and clay minerals. Appl. Geochem. 2007, 22, 219–229. [Google Scholar] [CrossRef]
- Kogure, T.; Morimoto, K.; Tamura, K.; Sato, H.; Yamagishi, A. XRD and HRTEM evidence for fixation of cesium ions in vermiculite clay. Chem. Lett. 2012, 41, 380–382. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tanaka, M.; Tanaka, K.; Sakaguchi, A.; Takahashi, Y. An EXAFS study on the effects of natural organic matter and expandability of clay minerals on cesium adsorption and mobility. Geochim. Cosmochim. Acta 2014, 135, 49–65. [Google Scholar] [CrossRef]
- Mukai, H.; Hatta, T.; Kitazawa, H.; Yamada, H.; Yaita, T.; Kogure, T. Speciation of radioactive soil particles in the Fukushima contaminated area by IP autoradiography and microanalysis. Environ. Sci. Technol. 2014, 48, 13053–13059. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Takeda, A.; Ogasawara, S.; Yanai, J.; Sano, O.; Ito, T. Relationship between paddy soil radiocesium interception potentials and physicochemical properties in Fukushima, Japan. J. Environ. Qual. 2015, 44, 780–788. [Google Scholar] [CrossRef]
- Mukai, H.; Motai, S.; Yaita, T.; Kogure, T. Identification of the actual cesium-adsorbing materials in the contaminated Fukushima soil. Appl. Clay Sci. 2016, 121–122, 188–193. [Google Scholar] [CrossRef]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef]
- Okumura, M.; Kerisit, S.; Bourg, I.C.; Lammers, L.N.; Ikeda, T.; Sassi, M.; Rosso, K.M.; Machida, M. Radiocesium interaction with clay minerals: Theory and simulation advances Post-Fukushima. J. Environ. Radioact. 2018, 189, 135–145. [Google Scholar] [CrossRef]
- Nakao, A.; Tomita, M.; Wagai, R.; Tanaka, R.; Yanai, J.; Kosaki, T. Asian dust increases radiocesium retention ability of serpentine soils in Japan. J. Environ. Radioact. 2019, 204, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Yamaguchi, N.; Imamura, N.; Kobayashi, M.; Kaneko, S.; Takahashi, M. Effects of radiocesium fixation potentials on 137Cs retention in volcanic soil profiles of Fukushima forests. J. Environ. Radioact. 2019, 198, 126–134. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Tsukada, H.; Kohyama, K.; Takata, Y.; Takeda, A.; Isono, S.; Taniyama, I. Radiocesium interception potential of agricultural soils in northeast Japan. Soil Sci. Plant Nutr. 2017, 63, 119–126. [Google Scholar] [CrossRef]
- Yoshimura, K.; Onda, Y.; Sakaguchi, A.; Yamamoto, M.; Matsuura, Y. An extensive study of the concentrations of particulate/dissolved radiocaesium derived from the Fukushima Dai-ichi Nuclear Power Plant accident in various river systems and their relationship with catchment inventory. J. Environ. Radioact. 2015, 139, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Tanaka, K.; Iwatani, H.; Chiga, H.; Fan, Q.; Onda, Y.; Takahashi, Y. Size distribution studies of 137Cs in river water in the Abukuma Riverine system following the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2015, 139, 379–389. [Google Scholar] [CrossRef]
- Taniguchi, K.; Onda, Y.; Smith, H.G.; Blake, W.; Yoshimura, K.; Yamashiki, Y.; Kuramoto, T.; Saito, K. Transport and redistribution of radiocesium in Fukushima fallout through rivers. Environ. Sci. Technol. 2019, 53, 12339–12347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi Nuclear Power Plant in terrestrial systems. Nat. Rev. Earth Environ. 2020, 1, 644–660. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sakuma, K. Trend of 137Cs concentration in river water in the medium term and future following the Fukushima nuclear accident. Chemosphere 2019, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Funaki, H.; Sakuma, K.; Nakanishi, T.; Yoshimura, K.; Katengeza, E.W. Reservoir sediments as a long-term source of dissolved radiocesium in water system: A mass balance case study of an artificial reservoir in Fukushima, Japan. Sci. Total Environ. 2020, 743, 140668. [Google Scholar] [CrossRef]
- Igarashi, Y.; Nanba, K.; Wada, T.; Wakiyama, Y.; Onda, Y.; Moritaka, S.; Konoplev, A. Factors controlling the dissolved 137Cs seasonal fluctuations in Abukuma River under the influence of the Fukushima Nuclear Power Plant Accident. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006591. [Google Scholar] [CrossRef]
- Ueda, S.; Hasegawa, H.; Ohtsuka, Y.; Ochiai, S.; Tani, T. Ten-year radiocesium fluvial discharge patterns from watersheds contaminated by the Fukushima nuclear power plant accident. J. Environ. Radioact. 2021, 240, 106759. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, A. Comparative analysis of radiocesium wash-off from contaminated watersheds as a result of the accidents at Fukushima Dai-ichi and Chernobyl NPPs. Geochem. Internat. 2016, 54, 522–528. [Google Scholar] [CrossRef]
- Konoplev, A.; Kanivets, V.; Laptev, G.; Voitsekhovich, O.; Zhukova, O.; Germenchuk, M. Long-term dynamics of the Chernobyl-derived radionuclides in rivers and lakes. In Behavior of Radionuclides in the Environment II: Chernobyl; Konoplev, A., Kato, K., Kalmykov, S.N., Eds.; Springer Nature: Singapore, 2020; pp. 323–348. [Google Scholar]
- Kashparov, V.A.; Protsak, V.P.; Ahamdach, N.; Stammose, D.; Peres, J.M.; Yoschenko, V.I.; Zvarich, S.I. Dissolution kinetics of particles of irradiated Chernobyl nuclear fuel: Influence of pH and oxidation state on the release of radionuclides in contaminated soil of Chernobyl. J. Nucl. Mater. 2000, 279, 225–233. [Google Scholar] [CrossRef]
- Kashparov, V.A.; Ahamdach, N.; Zvarich, S.I.; Yoschenko, V.I.; Maloshtan, I.M.; Dewiere, L. Kinetics of dissolution of Chernobyl fuel particles in soil in natural conditions. J. Environ. Radioact. 2004, 72, 335–353. [Google Scholar] [CrossRef]
- Van der Stricht, E.; Kirchman, R. (Eds.) Radioecology: Radioactivity & Ecosystems; Fortemps: Liege, Belgium, 2001; pp. 92–98. [Google Scholar]
- Kanivets, V.; Laptev, G.; Konoplev, A.; Lisovyi, H.; Derkach, G.; Voitsekhovich, O. Distribution and dynamics of radionuclides in the Chernobyl cooling pond. In Behavior of Radionuclides in the Environment II: Chernobyl; Konoplev, A., Kato, K., Kalmykov, S.N., Eds.; Springer Nature: Singapore, 2020; pp. 349–405. [Google Scholar]
- Bobovnikova, T.I.; Virchenko, E.P.; Konoplev, A.V.; Siverina, A.A.; Shkuratova, I.G. Chemical forms of occurrence of long-lived radionuclides and their alteration in soils near the Chernobyl Nuclear Power Station. Sov. Soil Sci. 1991, 23, 52–57. [Google Scholar]
- Bulgakov, A.A.; Konoplev, A.V. Diffusional modelling of radiocaesium fixation by soils. Radiat. Prot. Dosim. 1996, 64, 11–13. [Google Scholar] [CrossRef]
- Bulgakov, A.A. Modeling of 137Cs fixation in soils. Eurasian Soil Sci. 2009, 42, 675–681. [Google Scholar] [CrossRef]
- Bulgakov, A.A.; Konoplev, A.V. Diffusional model for radionuclide fixation in soils: A comparison with experimental data and other models. Geochem. Intern. 2001, 39, 191–195. [Google Scholar]
- Bulgakov, A.A.; Konoplev, A.V. Parameters of a diffusional model of 137Cs and 90Sr fixation in soils. Eurasian Soil Sci. 2002, 35, 417–420. [Google Scholar]
- Prokhorov, V.M. Migration of Radioactive Contaminants in Soil; Energoizdat: Moscow, Russia, 1981; 98p. (In Russian) [Google Scholar]
- Bulgakov, A.A.; Konoplev, A.V.; Popov, V.E.; Bobovnikova, T.I.; Siverina, A.A.; Shkuratova, I.G. Mechanisms of vertical migration of long-lived radionuclides in soils within 30 km of the Chernobyl nuclear power station. Sov. Soil Sci. 1991, 23, 46–51. [Google Scholar]
- Konoplev, A.V.; Golosov, V.N.; Yoschenko, V.I.; Nanba, K.; Onda, Y.; Takase, T.; Wakiyama, Y. Vertical distribution of radiocesium in soils of the area affected by the Fukushima Dai-ichi nuclear power plant accident. Eurasian Soil Sci. 2016, 49, 570–580. [Google Scholar] [CrossRef]
- Malins, A.; Kurikami, H.; Nakama, S.; Saito, T.; Okumura, M.; Machida, M.; Kitamura, A. Evaluation of ambient dose equivalent rates influenced by vertical and horizontal distribution of radioactive cesium in soil in Fukushima. J. Environ. Radioact. 2016, 151, 38–49. [Google Scholar] [CrossRef]
- Bulgakov, A.A.; Konoplev, A.V.; Shkuratova, I.G. Distribution of 137Cs in the topmost soil layer within a 30-km zone around the Chernobyl nuclear power plant. Pochvovedenie 2000, 9, 1149–1152. (In Russian) [Google Scholar]
- Van Genuchten, M.T.; Wierenga, P.J. Solute Dispersion Coefficients and Retardation Factors. In Methods of Soil Analysis. Part 1 Physical and Mineralogical Methods; ASA & SSSA Publisher: Madison, WI, USA, 1986; pp. 1025–1054. [Google Scholar]
- Bulgakov, A.A.; Konoplev, A.V. Modelling of 137Cs vertical soil transfer by a tree root system. Radiat. Biol. Radioecol. 2002, 42, 556–560. (In Russian) [Google Scholar]
- Ivanov, Y.A.; Lewyckyj, N.; Levchuk, S.E.; Prister, B.S.; Firsakova, S.K.; Arkhipov, N.P.; Kruglov, S.V.; Alexakhin, R.M.; Sandalls, J.; Askbrant, S. Migration of 137Cs and 90Sr from Chernobyl fallout in Ukrainian, Belarussian and Russian soils. J. Environ. Radioact. 1997, 35, 1–21. [Google Scholar] [CrossRef]
- Smith, J.T.; Hilton, J.; Comans, R.N.J. Application of two simple models to the transport of 137Cs in an upland organic catchment. Sci. Total Environ. 1995, 168, 57–61. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Golubenkov, A.V. Modeling of the vertical radionuclide migration in soil (as a result of a nuclear accident). Meteorol. Gidrol. 1991, 10, 62–68. (In Russian) [Google Scholar]
- Bossew, P.; Kirchner, G. Modelling the vertical distribution of radionuclides in soil. Part 1: The convection-dispersion equation revisited. J. Environ. Radioact. 2004, 73, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Onda, Y.; Teramage, M. Depth distribution of 137Cs, 134Cs and 131I in soil profile after Fukushima Dai-ichi Nuclear Power Plant Accident. J. Environ. Radioact. 2012, 111, 59–64. [Google Scholar] [CrossRef]
- Vakulovsky, S.M.; Nikitin, A.I.; Chumichev, V.B.; Katrich, I.Y.; Voitsekhovich, O.V.; Medinets, V.I.; Pisarev, V.V.; Bovkun, L.A.; Khersonsky, E.S. Cesium-137 and strontium-90 contamination of water bodies in the areas affected by releases from the Chernobyl nuclear power plant accident: An overview. J. Environ. Radioact. 1994, 23, 103–122. [Google Scholar] [CrossRef]
- Sansone, U.; Belli, M.; Voitsekhovitch, O.; Kanivets, V. 137Cs and 90Sr in water and suspended particulate matter of the Dneper River-Reservoirs System. Sci. Total Environ. 1996, 186, 257–271. [Google Scholar] [CrossRef]
- IAEA. Radiological Conditions in the Dnieper River Basin: Assessment by an International Expert Team and Recommendations for an Action Plan; IAEA: Vienna, Austria, 2006; 185p. [Google Scholar]
- Ueda, S.; Hasegawa, H.; Kakiuchi, H.; Akata, N.; Ohtsuka, Y. Fluvial Discharges of Radiocesium from Watersheds Contaminated by Fukushima Dai-ichi Nuclear Plant Accident, Japan. J. Environ. Radioact. 2013, 118, 96–104. [Google Scholar] [CrossRef]
- Nagao, S.; Kanamori, M.; Ochiai, S.; Suzuki, K.; Yamamoto, M. Dispersion of Cs-134 and Cs-137 in river waters from Fukushima and Gunma prefectures at nine months after the Fukushima Daiichi NPP accident. Prog. Nucl. Sci. Technol. 2014, 4, 9–13. [Google Scholar] [CrossRef]
- Environmental Consequences of the Chernobyl Accident and Their Remediation: Twenty Years of Experience. Report of the Chernobyl Forum Expert Group ‘Environment’; IAEA: Vienna, Austria, 2006; 164p.
- Konoplev, A.V.; Bulgakov, A.A.; Popov, V.E.; Popov, O.F.; Scherbak, A.V.; Shveikin, Y.V.; Hoffman, F.O. Model testing using Chernobyl data: I. Wash-off of Sr-90 and Cs-137 from two experimental plots established in the vicinity of Chernobyl reactor. Health Phys. 1996, 70, 8–12. [Google Scholar] [CrossRef]
- Bulgakov, A.A.; Konoplev, A.V.; Popov, V.E.; Scherbak, A.V. Removal of long-lived radionuclides from soil by surface runoff near the Chernobyl nuclear power station. Sov. Soil Sci. 1991, 23, 124–131. [Google Scholar]
- Garcia-Sanchez, L.; Konoplev, A.V. Watershed wash-off of atmospherically deposited radionuclides: A review of normalized entrainment coefficients. J. Environ. Radioact. 2009, 100, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J. Aquatic radioecology post Chernobyl—A review of the past and look to the future. In Freshwater and Estuarine Radioecology; Desmet, G., Blust, R.J., Comans, R.N.J., Fernandez, J.A., Hilton, J., De Bettencourt, A., Eds.; Elsevier: Amsterdam, The Netherland, 1997; pp. 47–74. [Google Scholar]
- Monte, L. A collective model for predicting the long-term behavior of radionuclides in rivers. Sci. Total Environ. 1997, 201, 227–237. [Google Scholar] [CrossRef]
- Smith, J.T.; Belova, N.V.; Bulgakov, A.A.; Comans, R.N.J.; Konoplev, A.V.; Kudelsky, A.V.; Madruga, M.J.; Voitsekhovich, O.V.; Zibold, G. The “AQUASCOPE” simplified model for predicting 89,90Sr, and 134,137Cs in surface waters after a large-scale radioactive fallout. Health Phys. 2005, 89, 628–644. [Google Scholar] [CrossRef]
- Borzilov, V.A.; Konoplev, A.V.; Revina, S.K.; Bobovnikova, T.I.; Lyutik, P.M.; Shveikin, Y.V.; Shcherbak, A.V. An experimental study of the washout of radionuclides fallen on soil in consequence of the Chernobyl failure. Sov. Meteorol. Hydrol. 1988, 11, 27–33. [Google Scholar]
- Borzilov, V.A.; Sedunov, Y.S.; Novitskii, M.A.; Vozzhennikov, O.I.; Konoplev, A.V.; Dragolyubova, I.V. Physico-mathematical modeling of washout of long-lived radionuclides from drainage basins in the 30-km zone around the Chernobyl Nuclear Power Station. Sov. Meteorol. Hydrol. 1989, 1, 1–8. [Google Scholar]
- Borzilov, V.A.; Novitsky, M.A.; Konoplev, A.V.; Vozzhennikov, O.I.; Gerasimenko, A.C. A model for prediction and assessment of surface water contamination in emergency situations and methodology of determining its parameters. Rad. Prot. Dosim. 1993, 50, 349–351.e134. [Google Scholar] [CrossRef]
- Konshin, O.V. Mathematical model of 137Cs migration in soil: Analysis of observations following the Chernobyl accident. Health Phys. 1992, 63, 301–306. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.K.; Bossew, P.; Sorimachi, A.; Tokonami, S. Vertical migration of radio-caesium derived from the Fukushima Dai-ichi Nuclear Power Plant accident in undisturbed soils of grassland and forest. J. Geochem. Explor. 2016, 169, 163–186. [Google Scholar] [CrossRef]
- Yoshimura, K.; Onda, Y.; Kato, H. Evaluation of radiocesium wash-off by soil erosion from various land uses using USLE plots. J. Environ. Radioact. 2015, 139, 362–369. [Google Scholar] [CrossRef]
- Wakiyama, Y.; Onda, Y.; Yoshimura, K.; Igarashi, Y.; Kato, H. Land use types control solid wash-off rate and entrainment coefficient of Fukushima-derived 137Cs, and their time dependence. J. Environ. Radioact. 2019, 210, 105990. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Onda, Y.; Wakiyama, Y.; Konoplev, A.; Zheleznyak, M.; Lisovyi, H.; Laptev, G.; Demiyanovich, V.; Samoilov, D.; Nanba, K.; et al. Impact of wildfire on 137Cs and 90Sr wash-off in heavily contaminated forests in the Chernobyl exclusion zone. Environ. Pollut. 2020, 259, 113764. [Google Scholar] [CrossRef]
- Konoplev, A.V.; Kanivets, V.I.; Zhukova, O.M.; Germenchuk, M.G.; Derkach, G.A. Semi-empirical diffusional model of radionuclide wash-off from contaminated watersheds ant its testing using monitoring data for Fukushima and Chernobyl rivers. Geochem. Intern. 2021, 59, 607–617. [Google Scholar] [CrossRef]
- Tsuji, H.; Nishikiori, T.; Yasutaka, T.; Watanabe, M.; Ito, S.; Hayashi, S. Behavior of dissolved radiocesium in river water in a forested watershed in Fukushima Prefecture. J. Geophys. Res.–Biogeosci. 2016, 121, 2588–2599. [Google Scholar] [CrossRef]
- Nanba, K.; Moritaka, S.; Igarashi, Y. Dynamics of radiocesium in urban river in Fukushima city. In Behavior of Radionuclides in the Environment III: Fukushima; Nanba, K., Konoplev, A., Wada, T., Eds.; Springer Nature: Singapore, 2022; pp. 137–152. [Google Scholar]
- Lee, S.S.; Fenter, P.; Nagy, K.L.; Sturchio, N.C. Real-time observation of cation exchange kinetics and dynamics at the muscovite-water interface. Nat. Commun. 2017, 8, 15826. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zachara, J.M.; Qafoku, O.; Smith, S. Effect of temperature on Cs+ sorption and desorption in subsurface sediments at the Hanford Site, USA. Environ. Sci. Technol. 2003, 37, 2640–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]

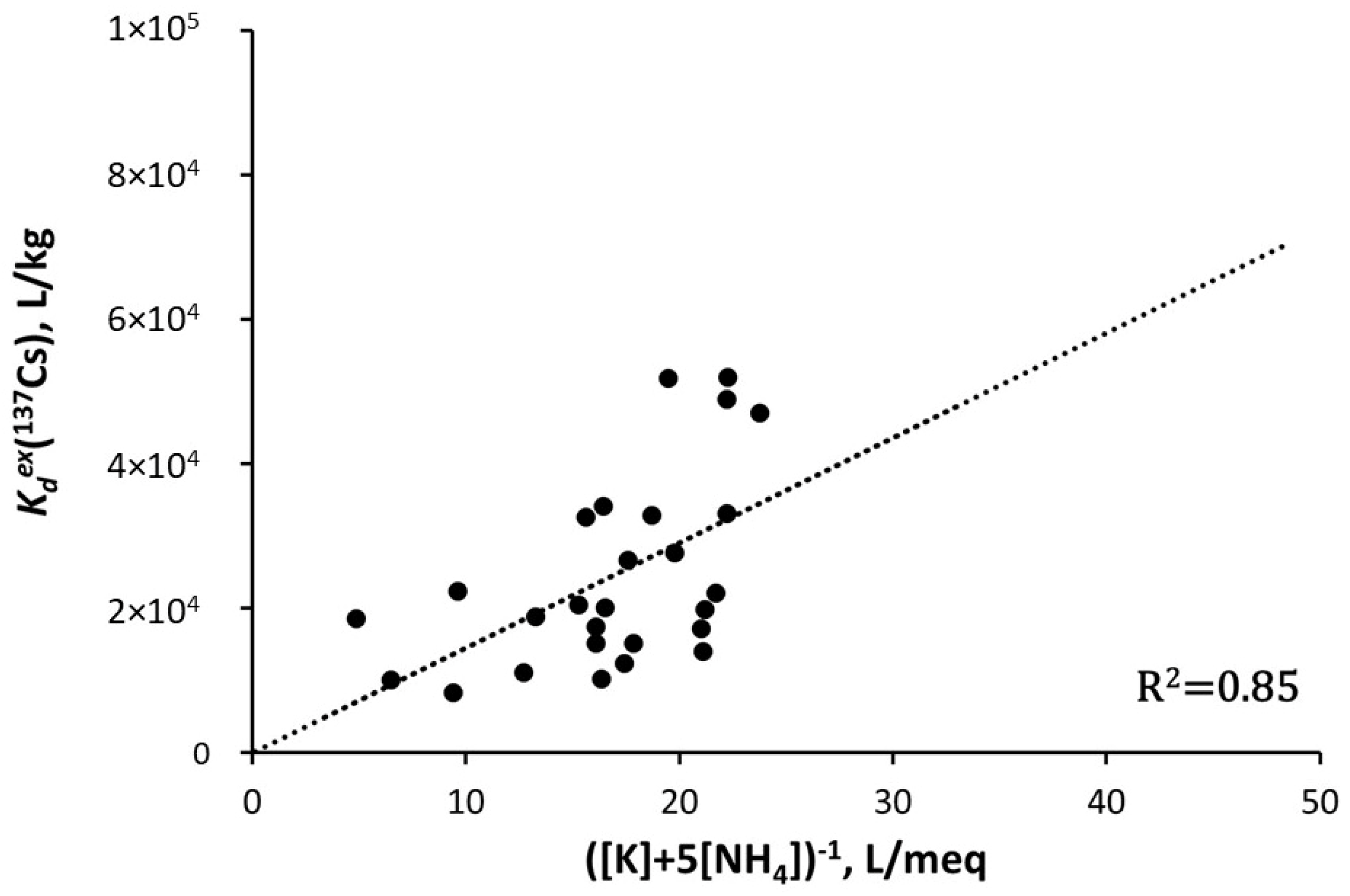



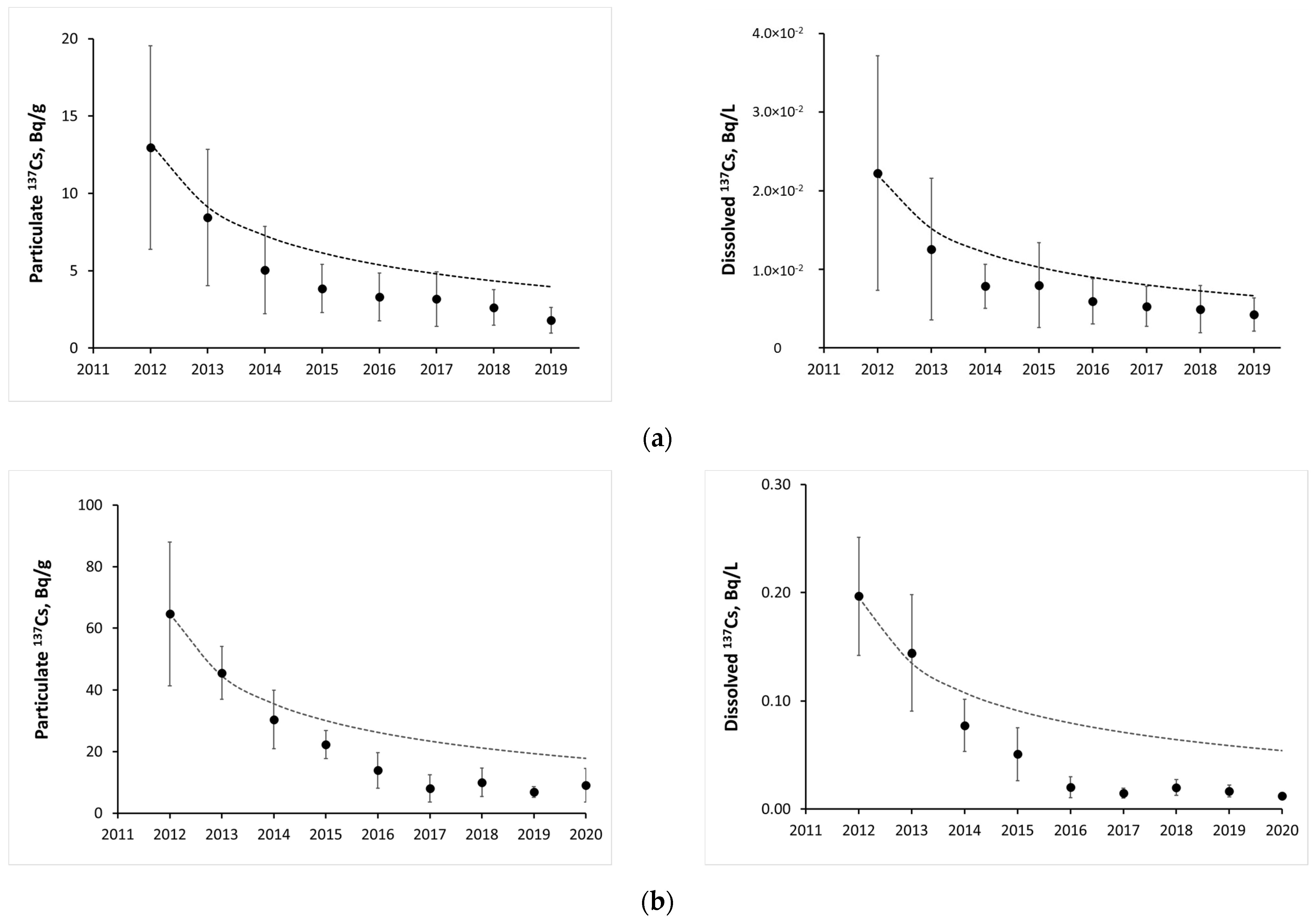

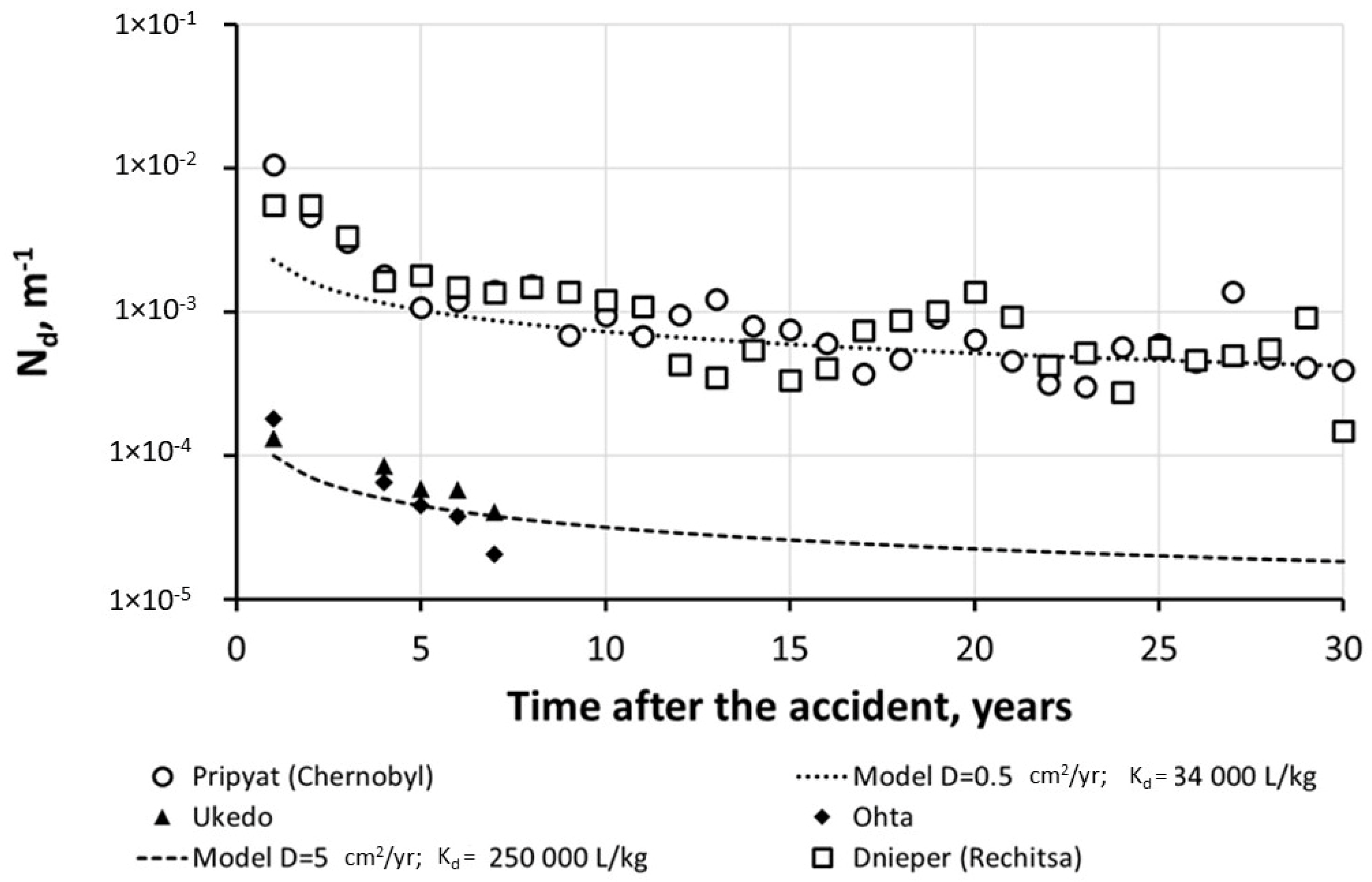
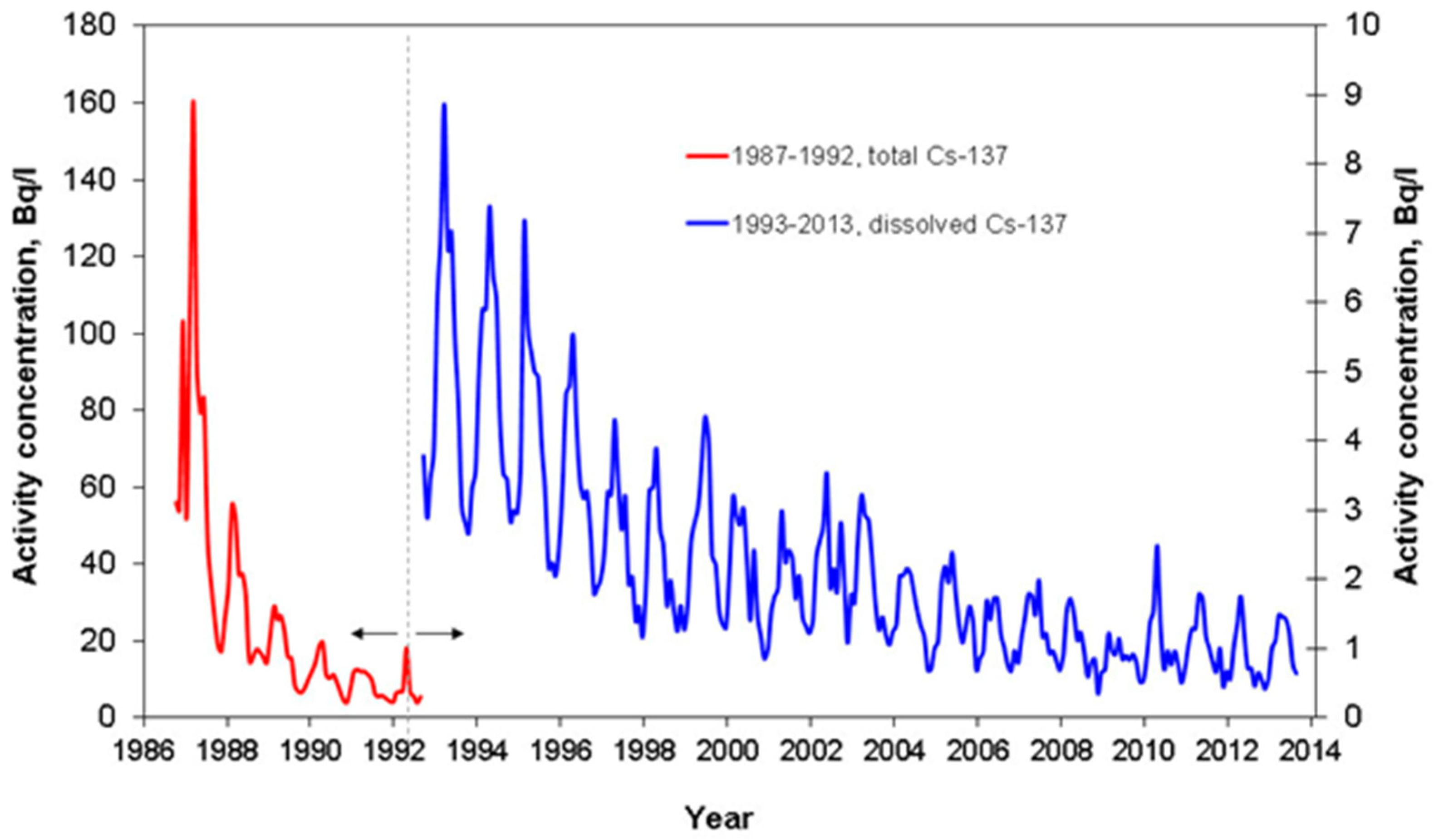
| River-Site | Observation Period | Mean Value | References |
|---|---|---|---|
| Chernobyl | |||
| Pripyat River-Chernobyl | 1990–2016 | (3.5 ± 0.6) × 104 | [18,102] |
| Dneper River-Nedanchichi | 1989–2012 | (6.4 ± 2.0) × 104 | [18,102] |
| Uzh River-Cherevach | 1987–1990 | (3.1 ± 2.0) × 104 | [47] |
| Fukushima | |||
| Abukuma River-Kuroiwa | 2012–2020 | (6.5 ± 3.0) × 105 | [99] |
| Ukedo River downstream | 2015–2018 | (2.2 ± 0.3) × 105 | [97] |
| Ukedo River at Ogaki dam inflow | 2014–2019 | (6.3 ± 2.0) × 105 | [98] |
| Kodeya River at Ogaki dam inflow | 2014–2019 | (8.6 ± 2.1) × 105 | [98] |
| Ukedo River at Ogaki dam outflow | 2014–2019 | (4.5 ± 1.8) × 105 | [98] |
| Ohta River downstream | 2015–2018 | (2.4 ± 0.6) × 105 | [97] |
| Hiso River (Niida River system) | 2011–2020 | (4.6 ± 3.0) × 105 | [100] |
| Wariki River (Niida River System) | 2011–2020 | (7.7 ± 6.3) × 105 | [100] |
| Group of Soil Types | (137Cs), % | δ(137Cs), Day1/2 |
|---|---|---|
| Sandy | 14 ± 5 | 3 ± 2 |
| Mineral | 12 ± 8 | 10 ± 4 |
| Turf | 6 ± 5 | 50 ± 30 |
| River (Cross-Section) | Two-Exponential Model (Equation (12)) | |||
|---|---|---|---|---|
| Bq·g−1 | Bq·g−1 | k1, Year−1 | k2, Year−1 | |
| Pripyat (Chernobyl) | 20 | 3.3 | 0.58 | 0.022 |
| Dneper (Nedanchichi) | 5.0 | 0.6 | 0.15 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konoplev, A. Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment. Toxics 2022, 10, 578. https://doi.org/10.3390/toxics10100578
Konoplev A. Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment. Toxics. 2022; 10(10):578. https://doi.org/10.3390/toxics10100578
Chicago/Turabian StyleKonoplev, Alexei. 2022. "Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment" Toxics 10, no. 10: 578. https://doi.org/10.3390/toxics10100578
APA StyleKonoplev, A. (2022). Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment. Toxics, 10(10), 578. https://doi.org/10.3390/toxics10100578






