Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mango Peel Powder
2.2. Extraction of Mango Peel Pectin Using Microwave-Assisted Technique
2.3. Scanning Electron Microscope
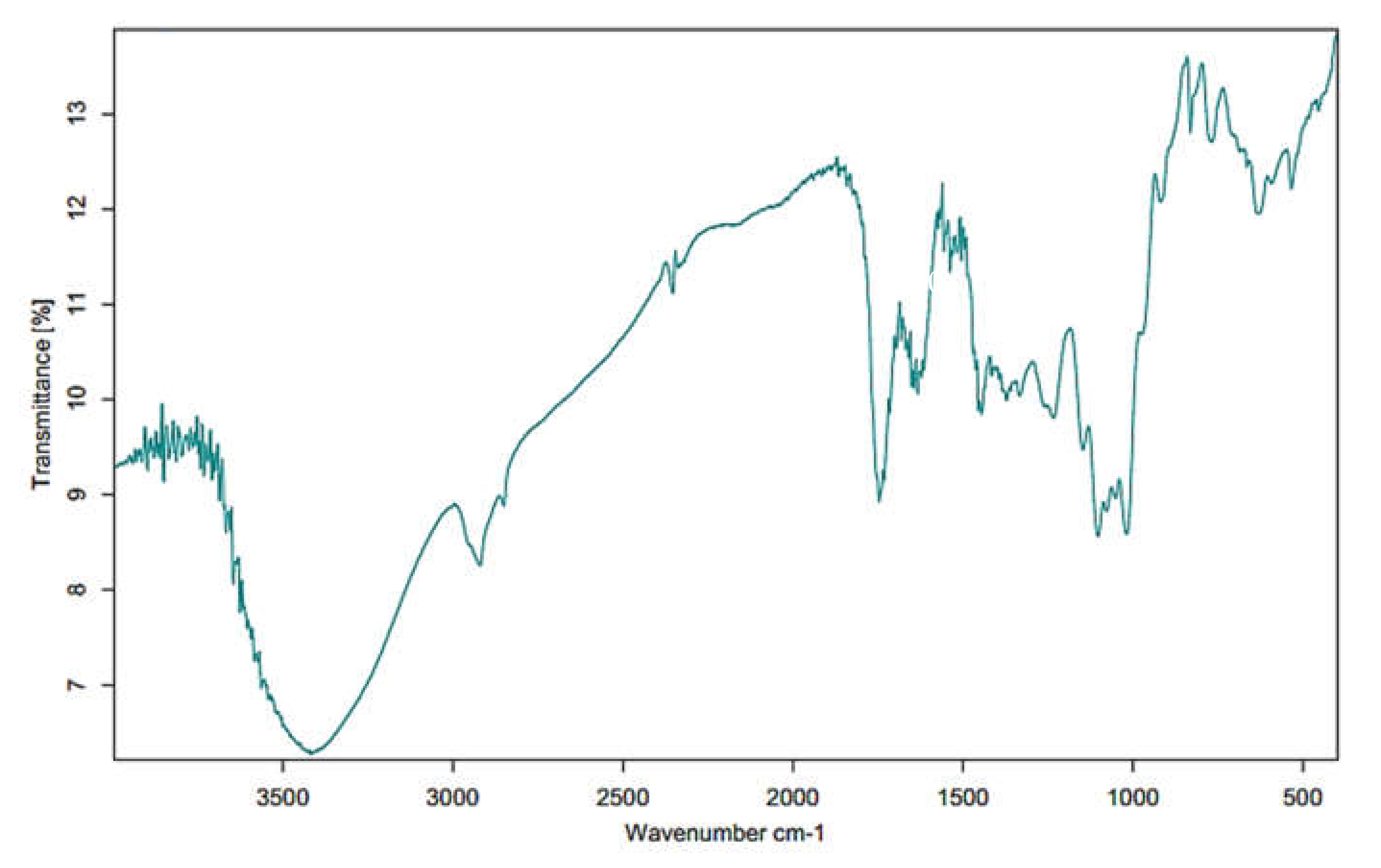
2.4. Fourier Transform Infrared Spectrophotometer (FT-IR)
2.5. Mango Peel Pectin Characterisations
2.6. Processing and Evaluation of Dried Chinese Sausage with Added Mango Peel Pectin
2.6.1. Dried Chinese Sausage Formulation
2.6.2. Colour Evaluation
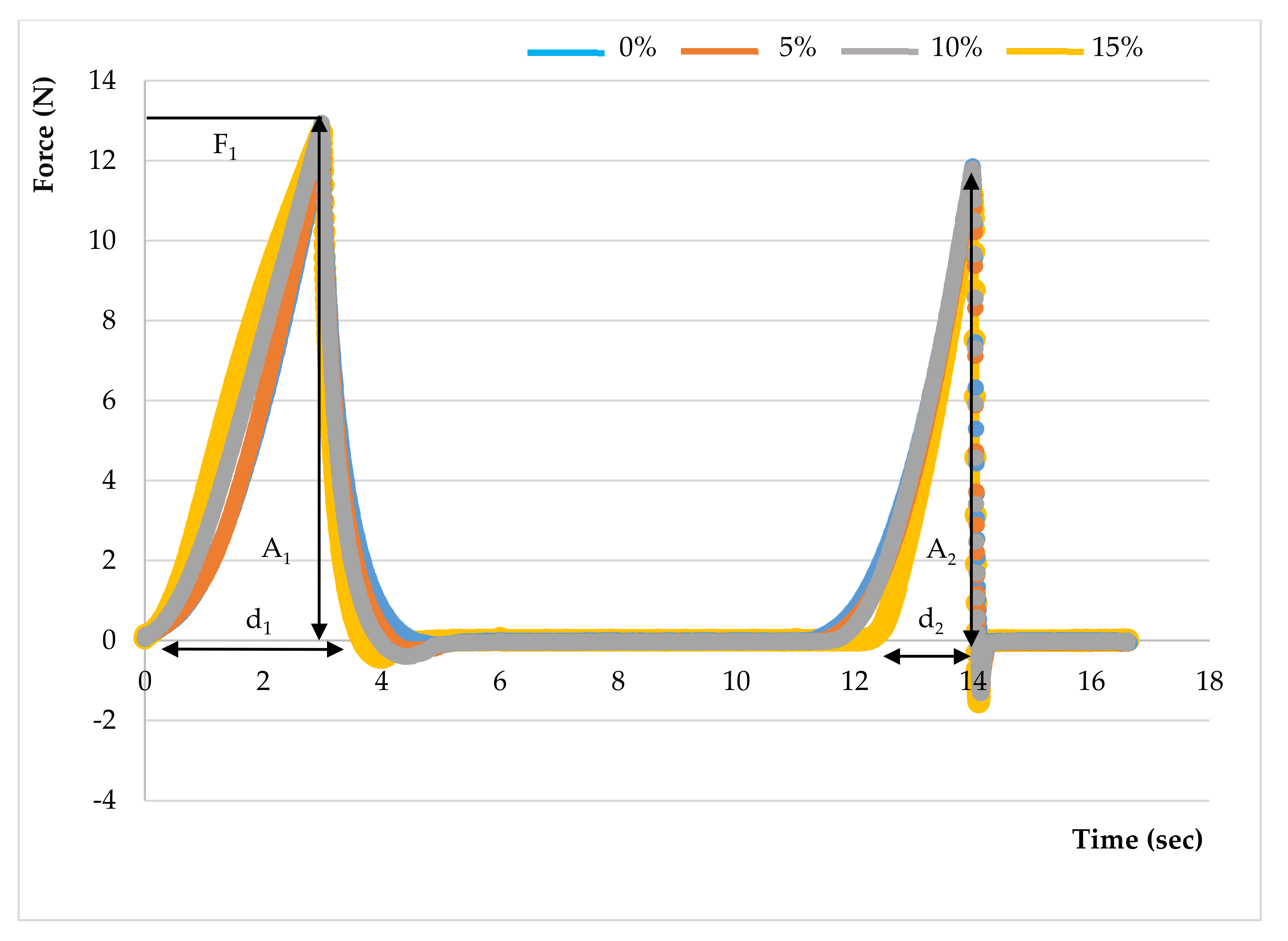
2.6.3. Texture Profile Analysis
2.6.4. Sensory Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscope
3.2. FT-IR Analysis of MPP
3.3. Characterisation of Mango Peel Pectin
3.4. Physical Quality Assessments of Formulated Dried Chinese Sausage
3.4.1. Colour
3.4.2. Texture
3.4.3. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Colmenero, F.; Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Pintado, T.; Solas, M.T. Technological and sensory characteristics of reduced/low-fat, low-salt frankfurters as affected by the addition of konjac and seaweed. Meat Sci. 2010, 84, 356–363. [Google Scholar] [CrossRef]
- Ritzoulis, C.; Petridis, D.; Derlikis, E.M.; Fytianos, K.; Asteriou, P. Utilization of inverse water-in-oil emulsions as fat replacers in frankfurter model sausages: Influence of fat emulsion content on the organoleptic and mechanical properties. J. Texture Stud. 2010, 41, 62–74. [Google Scholar] [CrossRef]
- Carrapiso, A. Effect of fat content on flavor release from sausages. Food Chem. 2007, 103, 396–403. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Ren, Y.; Fan, E.; Chang, H.; Wu, H. Effects of high pressure treatment and temperature on lipid oxidation and fatty acid composition of yak (Poephagus grunniens) body fat. Meat Sci. 2013, 94, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Mendes, R.; Nunes, M. Development of a healthy low-fat fish sausage containing dietary fibre. Int. J. Food Sci. Technol. 2008, 43, 276–283. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, H.-W.; Hwang, K.E.; Song, D.H.; Choi, J.-H.; Lee, M.-A.; Chung, H.-J.; Kim, C.-J. Physicochemical properties and sensory characteristics of reduced-fat frankfurters with pork back fat replaced by dietary fiber extracted from makgeolli lees. Meat Sci. 2014, 96, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Rezende, N.V.; Benassi, M.T.; Vissotto, F.Z.; Augusto, P.P.C.; Grossmann, M.V.E. Mixture design applied for the partial replacement of fat with fibre in sucrose-free chocolates. LWT-Food Sci. Technol. 2015, 62, 598–604. [Google Scholar] [CrossRef]
- Koutsopoulos, D.A.; Koutsimanis, G.E.; Bloukas, J.G. Effect of carrageenan level and packaging during ripening on processing and quality characteristics of low-fat fermented sausages produced with olive oil. Meat Sci. 2008, 79, 188–197. [Google Scholar] [CrossRef]
- Heinz, G.; Hautzinger, P. Meat Processing Technology for Small to Medium Scale Producers; Food and Agricultural Organization of the United Nations: Bangkok, Thailand, 2007. [Google Scholar]
- Tan, F.-J.; Liao, F.-Y.; Jhan, Y.-J.; Liu, D.-C. Effect of replacing pork backfat with yams (Dioscorea alata) on quality characteristics of Chinese sausage. J. Food Eng. 2007, 79, 858–863. [Google Scholar] [CrossRef]
- Lin, K.-W.; Huang, C.-Y. Physicochemical and textural properties of ultrasound-degraded konjac flour and their influences on the quality of low-fat Chinese-style sausage. Meat Sci. 2008, 79, 615–622. [Google Scholar] [CrossRef]
- Biswas, A.; Kumar, V.; Bhosle, S.; Sahoo, J.; Chatli, M.K. Dietary fibers as functional ingredients in meat products and their role in human health. Int. J. Livest. Prod. 2011, 2, 45–54. [Google Scholar]
- Villegas, D.; Handford, M.; Alcalde, J.A.; Perez-Donoso, A. Exogenous application of pectin-derived oligosaccharides to grape berries modifies anthocyanin accumulation, composition and gene expression. Plant Physiol. Biochem. 2016, 104, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Macagnan, F.; Santos, L.; Roberto, B.; Moura, F.; Bizzani, M.; Silva, L. Biological properties of apple pomace, orange bagasse and passion fruit peel as alternative source of dietary fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Méndez-Zamora, G.; García-Macías, J.A.; Santellano-Estrada, E.; Chávez-Martínez, A.; Durán-Meléndez, L.A.; Silva-Vázquez, R.; Quintero-Ramos, A. Fat reduction in the formulation of frankfurter sausages using inulin and pectin. Food Sci. Technol. 2015, 35, 25–31. [Google Scholar] [CrossRef]
- Verma, A.; Banerjee, R. Dietary fibre as functional ingredient in meat products: A novel approach for healthy living—A review. J. Food Sci. Technol. 2010, 47, 247–257. [Google Scholar] [CrossRef]
- Silva-Vazquez, R.; Flores-Giron, E.; Quintero-Ramos, A.; Hume, M.E.; Méndez-Zamora, G. Effect of inulin and pectin on physicochemical characteristics and emulsion stability of meat batters. CyTA-J. Food 2018, 16, 306–310. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665. [Google Scholar]
- Garcia-Magana Mde, L.; Garcia, H.S.; Bello-Perez, L.A.; Sayago-Ayerdi, S.G.; de Oca, M.M. Functional properties and dietary fiber characterization of mango processing by-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of beta-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–108230. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Berardini, N.; Carle, R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef]
- Nagel, A.; Sirisakulwat, S.; Carle, R.; Neidhart, S. An acetate-hydroxide gradient for the quantitation of the neutral sugar and uronic acid profile of pectins by HPAEC-PAD without postcolumn pH adjustment. J. Agric. Food Chem. 2014, 62, 2037–2048. [Google Scholar] [CrossRef]
- Panouillé, M.; Ralet, M.C.; Bonnin, E.; Thibault, J.F. Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 417–447. [Google Scholar]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Guolin, H.; Jeffrey, S.; Kai, Z.; Xiaolan, H. Application of ionic liquids in the microwave-assisted extraction of pectin from lemon peels. J. Anal. Methods Chem. 2012. [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef]
- Matharu, A.; Houghton, J.; Lucas-Torres, C.; Moreno, A. Acid-free microwave-assisted hydrothermal extraction of pectin and porous cellulose from mango peel waste—Towards a zero waste mango biorefinery. Green Chem. 2016, 18, 5280–5287. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S. Bioactive functionality of pectin from peels of seven Thai mango cultivars. Acta Hortic. 2018. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Proces. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.-H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S.G. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT-Food Sci. Technol. 2015, 64, 1010–1014. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, Y.; Zhu, X.; Xiong, H.; Woo, M.W.; Hu, J. Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohydr. Polym. 2012, 87, 1663–1669. [Google Scholar] [CrossRef]
- Ranganna, S. Hand Book of Analysis and Quality Control for Fruits and Vegetable Products, 2nd ed.; McGraw Hill Publishing Co., Ltd.: New Delhi, India, 1995. [Google Scholar]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata Mac Graw Hill: New Delhi, India, 1986. [Google Scholar]
- Pinheiro, E.S.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration properties of dietary fibre and resistant starch: A european collaborative study. LWT-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Bolumar, T.; Toepfl, S.; Heinz, V. Fat reduction and replacement in dry-cured fermented sausage by using high pressure processing meat as fat replacer and olive oil. Pol. J. Food Nutr. Sci. 2015, 65, 175–182. [Google Scholar] [CrossRef]
- Femenia, A.; Lefebvre, A.C.; Thebaudin, J.; Robertson, J.A.; Bourgeois, C.M. Physical and sensory properties of model foods supplemented with cauliflower fiber. J. Food Sci. 2006, 62, 635–639. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Farsi, S.A. Instrumental texture profile analysis (TPA) of date flesh as a function of moisture content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
- Siddaiah, D.; Sagar Reddy, G.V.; Raju, C.V.; Chandrasekhar, T.C. Changes in lipids, proteins and kamaboko forming ability of silver carp (Hypophthalmichthys molitrix) mince during frozen storage. Food Res. Int. 2001, 34, 47–53. [Google Scholar] [CrossRef]
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: Effects of drying. Int. J. Food Prop. 2017, 20, S190–S201. [Google Scholar] [CrossRef]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Muhammad, K. Physicochemical properties, rheological behavior and morphology of pectin-pea protein isolate mixtures and conjugates in aqueous system and oil in water emulsion. Food Hydrocoll. 2016, 56, 405–416. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The Effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101. [Google Scholar] [CrossRef]
- Kacŭráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.R.; Mercado, J.A.; Morris, V.J.; Quesada, M.A. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydr. Polym. 2012, 88, 882–890. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.; Sylvie, B.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2016, 215, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Čopíková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Baississe, S.; Ghannem, H.; Fahloul, D.; Lekbir, A. Comparison of structure and emulsifying activity of pectin extracted from apple pomace and apricot pulp. World J. Dairy Food Sci. 2010, 5, 79–84. [Google Scholar]
- Nguyen, B.M.N.; Pirak, T.; Yildiz, F. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Vaclavik, V.A.; Christian, E.W. Essentials in Food Science, 3rd ed.; Springer Science and Business: New York, NY, USA, 2008. [Google Scholar]
- Azad, M.A.K.; Ali, M.; Akter, M.; Rahman, M.J. Isolation and characterization of pectin extracted from lemon pomace during ripening. J. Food Nutr. Sci. 2014, 2, 30–35. [Google Scholar] [CrossRef]
- Ranajit, K.S.; Yoga, N.A.P.P.; Asrul, A. Opimized extraction condition and characterization of pectin from kaffir lime (Citrus hystrix). Res. J. Agric. For. Sci. 2013, 1, 1–11. [Google Scholar]
- Israel, K.A.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.; Dizon, E.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana (Musa ‘saba’(Musa acuminata × Musa balbisiana)) peel wastes: A preliminary study. Int. Food Res. J. 2015, 22, 202–207. [Google Scholar]
- Constenla, D.; Lozano, J. Kinetic model of pectin demethylation. Lat. Am. Appl. Res. 2003, 33, 91–95. [Google Scholar]
- Rouse, A.H.; Atkins, C.D.; Moore, E.L. The occurrence and evaluation of pectin in component parts of valencia oranges during maturation. Fla. State Hortic. Soc. 1962, 75, 307–311. [Google Scholar]
- Sudhir, D.Y.; Namrata, S.B.; Namrata, N.W.; Deepali, C.S. Extraction and Characterization of Pectin from sweet lime. In Proceedings of the 4th International Conference on Multidisciplinary Research & Practice, Ahmedabad, India, 22 December 2017; pp. 58–63. [Google Scholar]
- Barrera, A.M.; Ramírez, J.A.; González-Cabriales, J.J.; Vázquez, M. Effect of pectins on the gelling properties of surimi from silver carp. Food Hydrocoll. 2002, 16, 441–447. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res. Int. 2013, 51, 756–763. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Lan, G.; Chen, H.; Chen, S.; Tian, J. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res. Int. 2012, 49, 406–410. [Google Scholar] [CrossRef]
- Chirinang, P.; Oonsivilai, R. Physicochemical properties, in vitro binding capacities for lard, cholesterol, bile acids and assessment of prebiotic potential of dietary fiber from cassava pulp. Int. Food Res. J. 2018, 25, S63–S74. [Google Scholar]
- Jacometti, G.A.; Mello, L.R.P.F.; Nascimento, P.H.A.; Sueiro, A.C.; Yamashita, F.; Mali, S. The physicochemical properties of fibrous residues from the agro industry. LWT-Food Sci. Technol. 2015, 62, 138–143. [Google Scholar] [CrossRef]
- Boulos, N.N.; Greenfield, H.; Wills, R.B.H. Water holding capacity of selected soluble and insoluble dietary fibre. Int. J. Food Prop. 2000, 3, 217–231. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sendra, E.; Navarro, C.; Sayas, E.; Viuda-Martos, M.; Pérez-Álvarez, J. Storage stability of a high dietary fibre powder from orange by-products. Int. J. Food Sci. Technol. 2009, 44, 748–756. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J.A. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Femenia, A.; Sastre-Serrano, G.; Simal, S.; Garau, M.C.; Eim, V.S.; Rosselló, C. Effects of air-drying temperature on the cell walls of kiwifruit processed at different stages of ripening. LWT-Food Sci. Technol. 2009, 42, 106–112. [Google Scholar] [CrossRef]
- Moura, F.A.; Macagnan, F.T.; Santos, L.R.; Bizzani, M.; Petkowicz, C.L.O.; Silva, L.P. Characterization and physicochemical properties of pectins extracted from agroindustrial by-products. J. Food Sci. Technol. 2017, 54, 3111–3117. [Google Scholar] [CrossRef]
- Mielnik, J.A.N.; Slinde, E. Sausage color measured by integrating sphere reflectance spectrophotometry when whole blood or blood cured by nitrite is added to sausages. J. Food Sci. 2006, 48, 1723–1725. [Google Scholar] [CrossRef]
- Sarıçoban, C.; Özalp, B.; Yılmaz, M.T.; Özen, G.; Karakaya, M.; Akbulut, M. Characteristics of meat emulsion systems as influenced by different levels of lemon albedo. Meat Sci. 2008, 80, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Wagner, R.; Mascarin, L.; Zepka, L.; Campagnol, P. Production of low-fat emulsified cooked sausages using amorphous cellulose gel. J. Food Qual. 2014, 37, 437–443. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Z.; Li, G.; Zhao, X.; Wu, H.; Ren, Y. Tomato peel powder as fat replacement in low-fat sausages: Formulations with mechanically crushed powder exhibit higher stability than those with airflow ultra-micro crushed powder: Tomato particles as fat replacement on quality of sausages. Eur. J. Lipid Sci. Technol. 2015, 118, 175–184. [Google Scholar] [CrossRef]
- Zapata, J.I.H.; Pava, G.C. Physicochemical analysis of frankfurter type sausages made with red tilapia fillet waste (Oreochromis sp.) and quinoa flour (Chenopodium quinoa W.). Braz. J. Food Technol. 2017, 21. [Google Scholar] [CrossRef]
- Garcia-Santos, M.D.S.L.; ConceiÇÃO, F.S.; Villas Boas, F.; Salotti De Souza, B.M.; Barretto, A.C.D.S. Effect of the addition of resistant starch in sausage with fat reduction on the physicochemical and sensory properties. Food Sci. Technol. 2019, 39, 491–497. [Google Scholar] [CrossRef]
- Karanjalker, G.; Kodthalu, S.K.R.; Dinesh, M.R.; Geetha, G.; Pavithra, K.; Ravishankar, K. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566–4577. [Google Scholar]
- Ajila, C.; Naidu, A.; Bhat, S.G.; Prasada Rao, U. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Szczesniak, A. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Cierach, M.; Modzelewska-Kapitula, M.; Szacilo, K. The influence of carrageenan on the properties of low-fat frankfurters. Meat Sci. 2009, 82, 295–299. [Google Scholar] [CrossRef]
- Chandra, M.; Aswathnaryan, S. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2014, 18, 572–584. [Google Scholar] [CrossRef]
- Campagnol, P.; Dos Santos, B.; Wagner, R.; Terra, N.; Pollonio, M. Amorphous cellulose gel as a fat substitute in fermented sausages. Meat Sci. 2011, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Al-Mahrouqi, A. Instrumental texture profile analysis of gelatin gel extracted from grouper skin and commercial (bovine and porcine) gelatin gels. Int. J. Food Sci. Nutr. 2009, 7, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Radocaj, O.; Dimic, E.; Vujasinovic, V. Optimization of the texture of fat-based spread containing hull-less pumpkin (Cucurbita pepo L.) seed press-cake. Acta Period. Technol. 2011, 42, 131–143. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, H.Y.; Lee, J.M.; Kim, Y.J.; Kim, C.J. Quality of frankfurter-type sausages with added pig skin and wheat fiber mixture as fat replacers. Meat Sci. 2013, 93, 849–854. [Google Scholar] [CrossRef]
- Troutt, E.S.; Hunt, M.C.; Johnson, D.E.; Claus, J.R.; Kastner, C.L.; Kropf, D.H. Characteristics of low-fat ground beef containing texture-modifying ingredients. J. Food Sci. 1992, 57, 19–24. [Google Scholar] [CrossRef]
- Cardoso, C.M.L.; Mendes, R.; Nunes, M.L. Instrumental texture and sensory characteristics of cod frankfurter sausages. Int. J. Food Prop. 2009, 12, 625–643. [Google Scholar] [CrossRef]
- Feng, T.; Ye, R.; Zhuang, H.; Rong, Z.; Fang, Z.; Wang, Y.; Gu, Z.; Jin, Z. Physicochemical properties and sensory evaluation of Mesona Blumes gum/rice starch mixed gels as fat-substitutes in Chinese Cantonese-style sausage. Food Res. Int. 2013, 50, 85–93. [Google Scholar] [CrossRef]
- Rahman, M.; Al-Waili, H.; Guizani, N.; Kasapis, S. Instrumental-sensory evaluation of texture for fish sausage and its storage stability. Fish. Sci. 2007, 73, 1166–1176. [Google Scholar] [CrossRef]
- Lin, K.-W.; Huang, H.-Y. Konjac/gellan gum mixed gels improve the quality of reduced-fat frankfurters. Meat Sci. 2003, 65, 749–755. [Google Scholar] [CrossRef]

| Extraction Techniques | Qualities of Pectin | Functionalities of Pectin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pectin Yield (%) | L* | a* | b* | Eq.W (mg/mol) | Mox (%) | DE (%) | SWC (mL/g sample) | WHC (g water/g sample) | OHC (g oil/g sample) | |
| MAE 700 | 13.85 ± 0.51 | 36.33 ± 1.11 | 5.25 ± 1.05 | 11.26 ± 2.13 | 1485.78 ± 0.74 | 19.33 ± 0.04 | 77.19 ± 0.72 | 24.16 ± 0.22 | 9.60 ± 0.46 | 0.81 ± 0.04 |
| Conventional | 0.80 ± 0.06 [34] | 36.88 ± 0.18 | 5.00 ± 0.20 | 11.39 ± 0.62 | 657.89 ± 47.33 [34] | 13.90 ± 2.10 [34] | 68.90 ± 3.70 [34] | 25.50 ± 0.61 | 11.10 ± 0.23 | 1.04 ± 0.05 |
| ANOVA Test | Ns | Ns | ns | ns | ns | ns | ||||
| Percentage of Pectin | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|
| 0 (CTRL) | 53.60 ± 7.44 a | 6.69 ± 2.40 c | 7.27 ± 1.32 c | - |  |
| 5 | 52.88 ± 2.87 a | 9.36 ± 0.80 b | 11.25 ± 0.62 b | 5.55 ± 1.02 a |  |
| 10 | 55.42 ± 1.82 a | 10.46 ± 0.69 ab | 13.31 ± 0.85 a | 7.59 ± 0.74 b |  |
| 15 | 50.37 ± 3.81 a | 12.16 ± 1.17 a | 14.14 ± 0.74 a | 9.91 ± 2.12 c |  |
| Texture Characteristics | Percentage of Mango Peel Pectin | |||
|---|---|---|---|---|
| 0 (CTRL) | 5 | 10 | 15 | |
| Hardness (N) | 15.87 ± 3.45 a | 13.15 ± 0.66 a | 12.89 ± 2.26 a | 12.70 ± 1.48 a |
| Springiness (mm) | 1.00 ± 0.01 a | 0.84 ± 0.07 b | 0.78 ± 0.06 b | 0.61 ± 0.04 c |
| Cohesiveness | 0.50 ± 0.01 a | 0.39 ± 0.06 b | 0.37 ± 0.03 b | 0.28 ± 0.02 c |
| Gumminess (N) | 7.92 ± 1.78 a | 5.15 ± 0.99 b | 4.78 ± 0.99 b | 3.54 ± 0.52 b |
| Chewiness (N.mm) | 7.94 ± 1.80 a | 4.40 ± 1.15 b | 3.78 ± 1.04 bc | 2.18 ± 0.41 c |
| Parameters | Percentage of Mango Peel Pectin (w/w) | |||
|---|---|---|---|---|
| 0% | 5% | 10% | 15% | |
| Appearance | 7.42 ± 2.15 a | 7.08 ± 1.08 a | 5.83 ± 1.53 ab | 4.92 ± 1.83 b |
| Juiciness | 8.33 ± 0.89 a | 6.92 ± 1.16 a | 6.83 ± 0.94 a | 5.42 ± 1.56 a |
| Springiness | 6.75 ± 1.66 a | 6.75 ± 1.82 a | 6.17 ± 1.53 ab | 4.00 ± 2.45 b |
| Firmness | 6.08 ± 2.02 a | 6.17 ± 1.70 a | 5.92 ± 1.56 a | 4.00 ± 2.00 b |
| Colour | 5.58 ± 2.23 a | 6.00 ± 2.00 b | 5.83 ± 1.70 b | 4.08 ± 2.68 c |
| Overall acceptability | 6.58 ± 1.68 a | 6.58 ± 1.56 a | 6.00 ± 1.41 a | 3.08 ± 1.88 b |
| Prepared sausage for sensory | 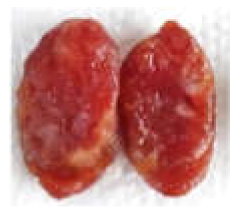 | 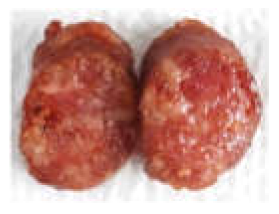 |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods 2020, 9, 450. https://doi.org/10.3390/foods9040450
Wongkaew M, Sommano SR, Tangpao T, Rachtanapun P, Jantanasakulwong K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods. 2020; 9(4):450. https://doi.org/10.3390/foods9040450
Chicago/Turabian StyleWongkaew, Malaiporn, Sarana Rose Sommano, Tibet Tangpao, Pornchai Rachtanapun, and Kittisak Jantanasakulwong. 2020. "Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage" Foods 9, no. 4: 450. https://doi.org/10.3390/foods9040450
APA StyleWongkaew, M., Sommano, S. R., Tangpao, T., Rachtanapun, P., & Jantanasakulwong, K. (2020). Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods, 9(4), 450. https://doi.org/10.3390/foods9040450








