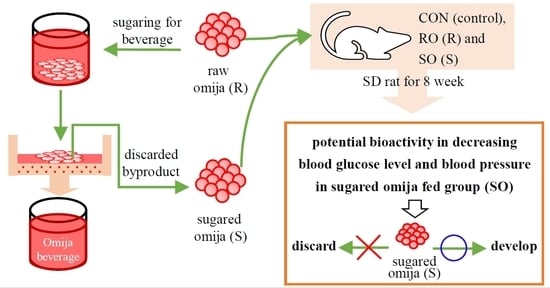
Evaluation of Lignan Compound Content and Bioactivity of Raw Omija and Sugared Omija in Serum of Sprague Dawley Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Analysis of Lignan Compounds
2.3. Animal Experiments
2.4. Serum Analysis
2.5. Statistical Analysis
3. Results
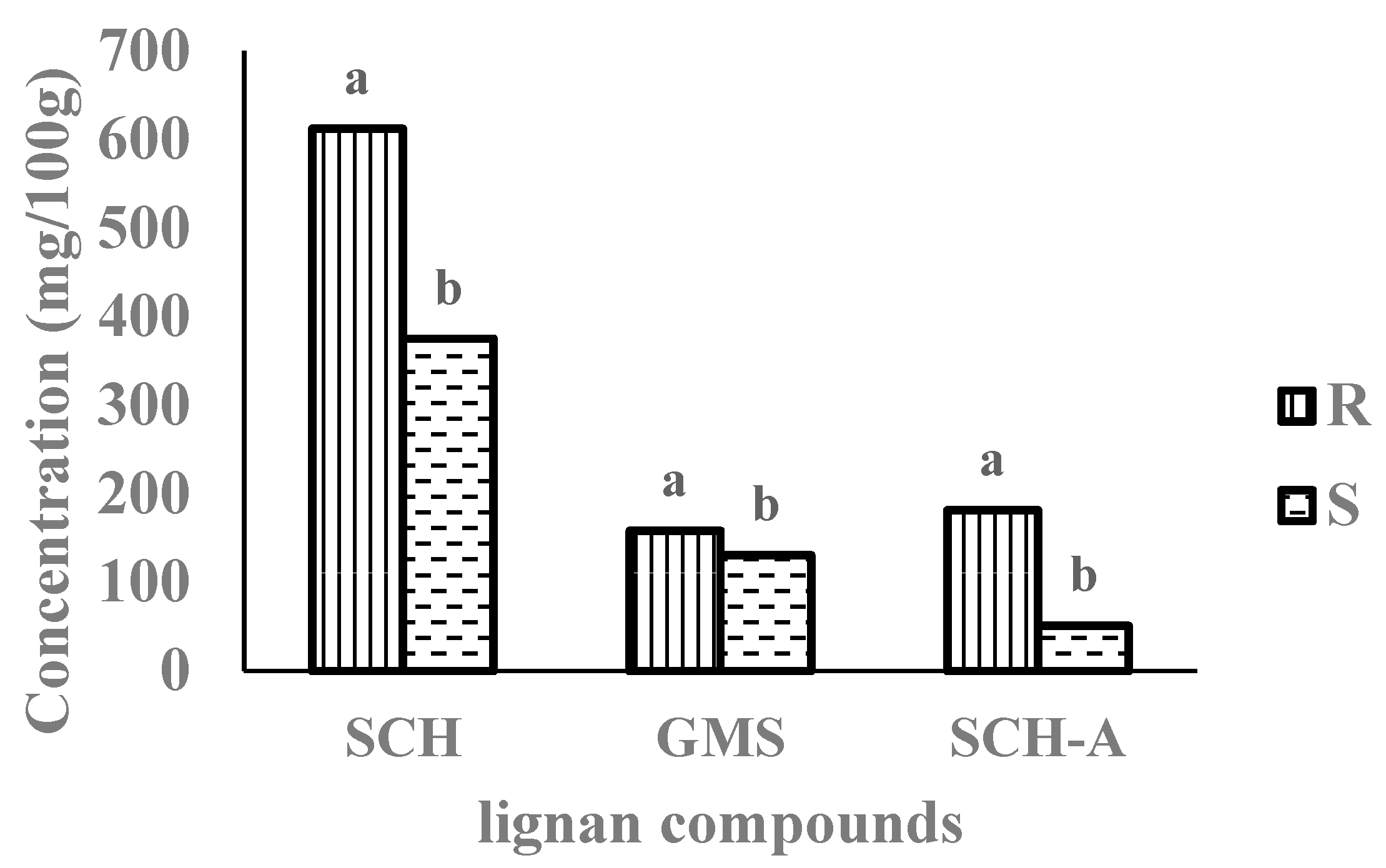
3.1. Composition of Lignan Compounds
3.2. Animal Experiments
3.2.1. Final Body Weight, Body Weight Gain, and Total Feed Intake
3.2.2. Weight of Organs
3.2.3. Serum Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.-H.; Joo, M.H.; Yoo, S.H. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J. Food Sci. 2009, 74, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, J.S.; Lee, Y.M.; Choi, J.Y.; Sung, J.H.; Chung, H.S.; Moon, K.D. Quality characteristic of Omija (Schizandra chinensis Baillon) seed oils by roasting conditions and extraction methods. Korean J. Food Preserv. 2015, 22, 845–850. [Google Scholar] [CrossRef]
- Jung, G.-T.; Ju, I.-O.; Choi, J.-S.; Hong, J.-S. The antioxidative, antimicrobial and nitrite scavenging effects of Schizandra chinensis RUPRECHT(Omija) seed. Korean J. Food Sci. Technol. 2000, 32, 928–935. [Google Scholar]
- Lee, K.S.; Lee, B.H.; Seong, B.J.; Kim, S.I.; Han, S.H.; Kim, G.H.; Park, S.B.; Kim, H.H.; Choi, T.Y. Chemical components composition on different parts of fruit in Schisandra chinensis baillon. J. Korean Soc. Food Sci. Nutr. 2016, 45, 851–858. [Google Scholar] [CrossRef]
- Chae, H.J.; Hwang, H.I.; Lee, I.S.; Moon, H.Y. Comparison of on rat intestinal digestive enzyme inhibitory activity and antioxidant enzyme activity of Korean and Chinese Schizandra chinensis. J. Exp. Biomed. Sci. 2005, 11, 517–523. [Google Scholar]
- Park, S.Y.; Choung, S.Y. Inhibitory effect of schizandrin on nephrotoxicity of cisplatin. Korean J. Environ. Toxicol. 1998, 13, 125–131. [Google Scholar]
- Heo, J.H.; Park, J.G.; Cheon, H.J.; Kim, Y.S.; Kang, S.S.; Hung, T.M.; Bae, K.H.; Lee, S.M. Hepatoprotective activities of gomisin A and gomisin N. Korean J. Pharmacogn. 2006, 37, 294–301. [Google Scholar]
- Jang, J.T.; Seo, W.H.; Baek, H.H. Enzymatic hydrolysis optimization of a snow crab processing by-product. Korean J. Food Sci. Technol. 2009, 41, 622–627. [Google Scholar]
- Seo, G.U.; Choi, S.Y.; Kim, T.W.; Ryu, S.G.; Park, J.H.; Lee, S.C. Functional activity of Makgeolli by-products as cosmetic materials. J. Korean Soc. Food Sci. Nutr. 2013, 42, 505–511. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Baek, J.H.; Jeong, E.J.; Cha, Y.J. Optimal extraction conditions of flavonoids from onion peels via response surface methodology. J. Korean Soc. Food Sci. Nutr. 2012, 41, 695–699. [Google Scholar] [CrossRef]
- Park, G.J.; Lin, B.P.; NGU, M.C.; Jones, D.B.; Katelaris, P.H. Aspartate aminotransferase: Alanine aminotransferase ration in chronic hepatitis C infections: Is it a useful predictor of cirrhosis? J. Gastroen. Hepatol. 2000, 15, 386–390. [Google Scholar] [CrossRef]
- Burnett, J.C. Jr.; Kao, P.C.; Hu, D.C.; Heser, D.W.; Heublein, D.; Granger, J.P.; Opgenorth, T.J.; Reeder, G.S. Atrial natriuretic peptide elevation in congenstive heart failure in the human. Science 1986, 231, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Song, J.; Seong, N.S. Analysis of ACE inhibitory activity. Korean J. Crop. Sci. 2004, 49, 203–207. [Google Scholar]
- Jo, S.H.; Ha, K.S.; Moon, K.S.; Lee, O.H.; Jang, H.D.; Kwon, Y.I. In Vitro and in Vivo Anti-hyperglycemic effects of Omija (Schizandra chinesis) fruit. Int. J. Mol. Sci. 2011, 12, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.C.; Suh, W.S.; Baek, S.Y.; Shim, S.H. Quantitative determination of lignans from Schizandra chinensis by HPLC. Kor. J. Pharmacogn. 2011, 42, 233–239. [Google Scholar]
- Daehan Bio Link Co., Ltd. Available online: http://www.dbl.kr/bbs/board.php?tbl=animal&mode=VIEW&num=90&chr=&category=&findType=&findWord=&sort1=&sort2=&page=1 (accessed on 1 June 2019).
- Han, S.H.; Shin, M.K.; Chung, Y.H. Effects of the Omija (Schizandra chinensis Baillon) extract on the metabolism and renal Cadmium contents in Cadmium administered rats. J. Korean Soc. Food Sci. Nutr. 2002, 31, 1102–1106. [Google Scholar]
- Cho, Y.J.; Ju, I.S.; Kim, B.C.; Lee, W.S.; Kim, M.J.; Lee, B.G.; An, B.J.; Kim, J.H.; Kwon, O.J. Biological activity of Omija (Schizandra chinensis Baillon) extracts. J. Korean Soc. Appl. Biol. Chem. 2007, 50, 198–203. [Google Scholar]
- Song, Y.O.; Lee, S.J.; Park, H.J.; Jang, S.H.; Chung, B.Y.; Song, Y.M.; Kim, G.S.; Cho, J.H. Hepatoprotective effect of Schisandra chinensis on high-fat diet-induced fatty liver in rats. Korean J. Vet. Serv. 2013, 36, 45–52. [Google Scholar] [CrossRef]
- Jang, M.K.; Nam, J.S.; Kim, J.H.; Yun, Y.R.; Han, C.W.; Kim, B.J.; Jeong, H.S.; Ha, K.T.; Jung, M.H. Schisandra chinensis extract ameliorates nonalcoholic fatty liver via inhibition of endoplasmic reticulum stress. J. Ethnopharmacol. 2016, 185, 96–104. [Google Scholar] [CrossRef]
- Park, S.H.; Hahm, T.S. Effects of Schizandriae fructus extract on the renal function by cardiovascular regulation. J. East. Asian Soc. Diet. Life 2005, 15, 558–565. [Google Scholar]
- Follenius, M.; Krieger, J.; Krauth, M.O.; Sforza, F.; Brandenberger, G. Obstructive sleep apnea treatment: Peripheral and central effects on plasma renin activity and aldosterone. Sleep 1991, 14, 211–217. [Google Scholar] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (g) | CON | RO | SO |
|---|---|---|---|
| Casein | 200.00 | 199.32 | 199.63 |
| Sucrose | 100.00 | 100.00 | 100.00 |
| Dextrose | 132.00 | 132.00 | 132.00 |
| Corn Starch | 397.49 | 394.14 | 393.39 |
| Cellulose | 50.00 | 50.00 | 50.00 |
| Soybean Oil | 70.00 | 69.03 | 69.49 |
| Raw omija powder | 5.00 | ||
| Sugared omija powder | 5.00 | ||
| TBHQ | 0.01 | 0.01 | 0.01 |
| Mineral | 35.00 | 35.00 | 35.00 |
| Vitamin mix | 10.00 | 10.00 | 10.00 |
| L-Cystine | 3.00 | 3.00 | 3.00 |
| Choline Bitartrate | 2.50 | 2.50 | 2.50 |
| Total (g) | 1000.00 | 1000.00 | 1000.00 |
| Kcal/kg | 4000.00 | 4000.00 | 4000.00 |
| CON | RO | SO | |
|---|---|---|---|
| Final body weight (g) | 400.00 ± 10.56 a | 385.33 ± 7.63 b | 384.00 ± 4.47 b |
| Body weight gain (g) | 234.17 ± 10.76 a | 217.67 ± 9.09 b | 217.33 ± 5.72 b |
| Feed intake (g) | 784.67 ± 4.08 NS | 781.65 ± 2.49 | 783.00 ± 0.00 |
| CON | RO | SO | |
|---|---|---|---|
| kidney (g) | 2.56 ± 0.19 NS | 2.67 ± 0.37 | 2.79 ± 0.08 |
| spleen (g) | 0.81 ± 0.10 NS | 0.78 ± 0.08 | 0.82 ± 0.10 |
| epididymal fat (g) | 5.67 ± 1.50 NS | 5.58 ± 2.42 | 5.29 ± 1.54 |
| retroperitoneal fat (g) | 8.12 ± 1.25 ab | 9.08 ± 2.61 a | 6.20 ± 0.92 b |
| CON | RO | SO | |
|---|---|---|---|
| Glucose (mg/dL) | 144.17 ± 38.32 a | 127.67 ± 24.34 ab | 104.83 ± 20.18 b |
| AST (U/L) | 185.50 ± 66.70 a | 148.33 ± 25.30 ab | 125.50 ± 18.11 b |
| ANP (pg/mL) | 75.35 ± 25.59 a | 42.18 ± 21.91 b | 67.01 ± 18.88 ab |
| Renin activity (ng/mL/h) | 34.18 ± 13.30 ab | 40.12 ± 11.14 a | 24.14 ± 8.49 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwa, S.-H.; Yeon, S.-J.; Hong, G.-E.; Cho, W.-Y.; Lee, H.-J.; Kim, J.-H.; Lee, C.-H. Evaluation of Lignan Compound Content and Bioactivity of Raw Omija and Sugared Omija in Serum of Sprague Dawley Rat. Foods 2019, 8, 373. https://doi.org/10.3390/foods8090373
Hwa S-H, Yeon S-J, Hong G-E, Cho W-Y, Lee H-J, Kim J-H, Lee C-H. Evaluation of Lignan Compound Content and Bioactivity of Raw Omija and Sugared Omija in Serum of Sprague Dawley Rat. Foods. 2019; 8(9):373. https://doi.org/10.3390/foods8090373
Chicago/Turabian StyleHwa, Sung-Hyun, Su-Jung Yeon, Go-Eun Hong, Won-Young Cho, Ha-Jung Lee, Ji-Han Kim, and Chi-Ho Lee. 2019. "Evaluation of Lignan Compound Content and Bioactivity of Raw Omija and Sugared Omija in Serum of Sprague Dawley Rat" Foods 8, no. 9: 373. https://doi.org/10.3390/foods8090373
APA StyleHwa, S.-H., Yeon, S.-J., Hong, G.-E., Cho, W.-Y., Lee, H.-J., Kim, J.-H., & Lee, C.-H. (2019). Evaluation of Lignan Compound Content and Bioactivity of Raw Omija and Sugared Omija in Serum of Sprague Dawley Rat. Foods, 8(9), 373. https://doi.org/10.3390/foods8090373