Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Sample Preparation
2.3.1. Whole Seed Flour Extracts
2.3.2. Soaking Water
2.3.3. Coats and Cotyledons Extracts
2.4. Determination of the Phenolic Compounds Content
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Total Proanthocyanidin Content (TPAC)
2.5. In Vitro Antioxidant Activity
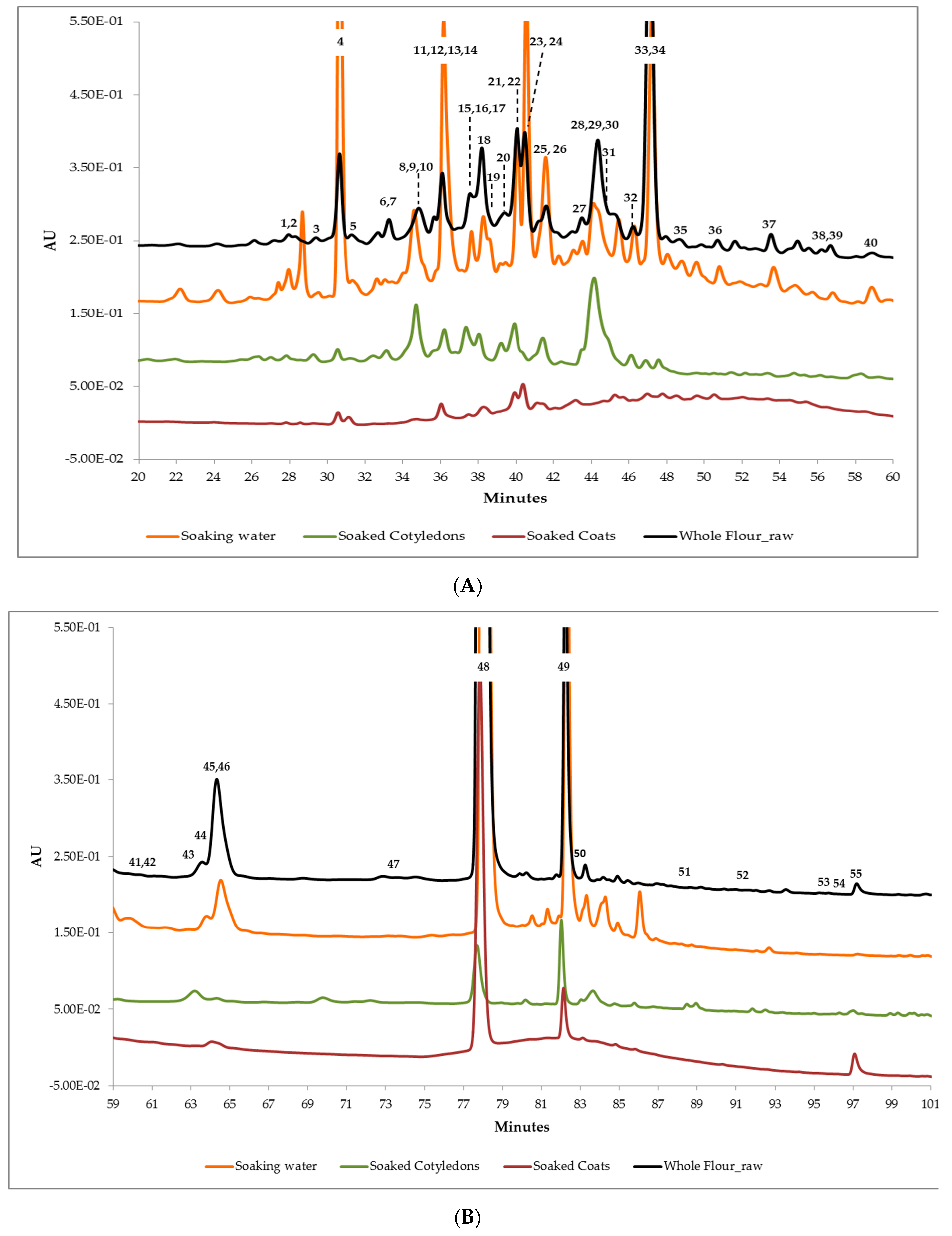
2.6. Phenolic Compounds Identification and Relative Quantification
Analysis by UPLC-TripleTOF 6600 Mass Spectrometer
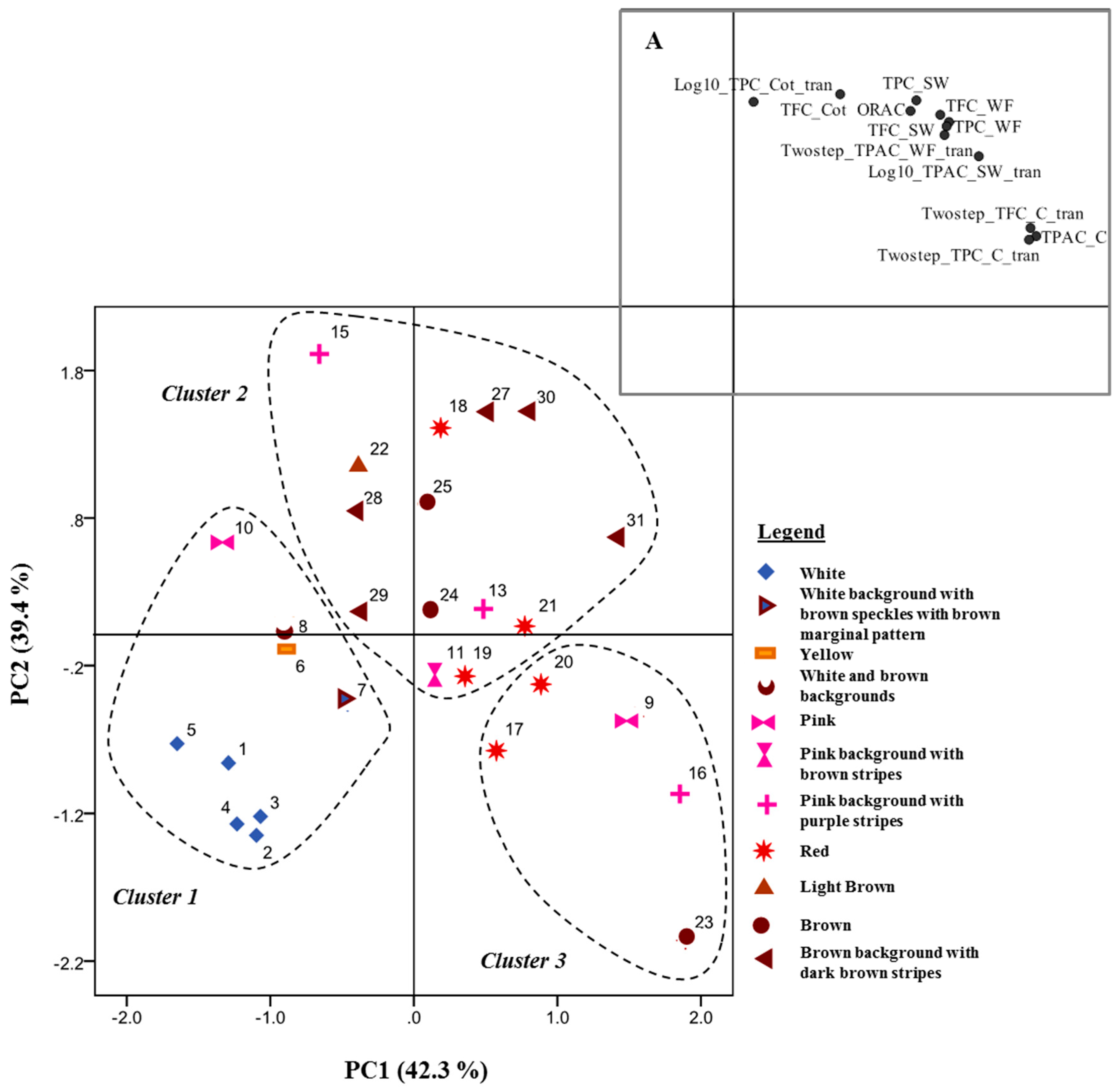
2.7. Data Analysis
3. Results and Discussion
3.1. Phenolic Content in Common Beans’ Whole Flour, Before Soaking
3.2. Phenolic Content in Soaking Water, Soaked Coats and Soaked Cotyledons
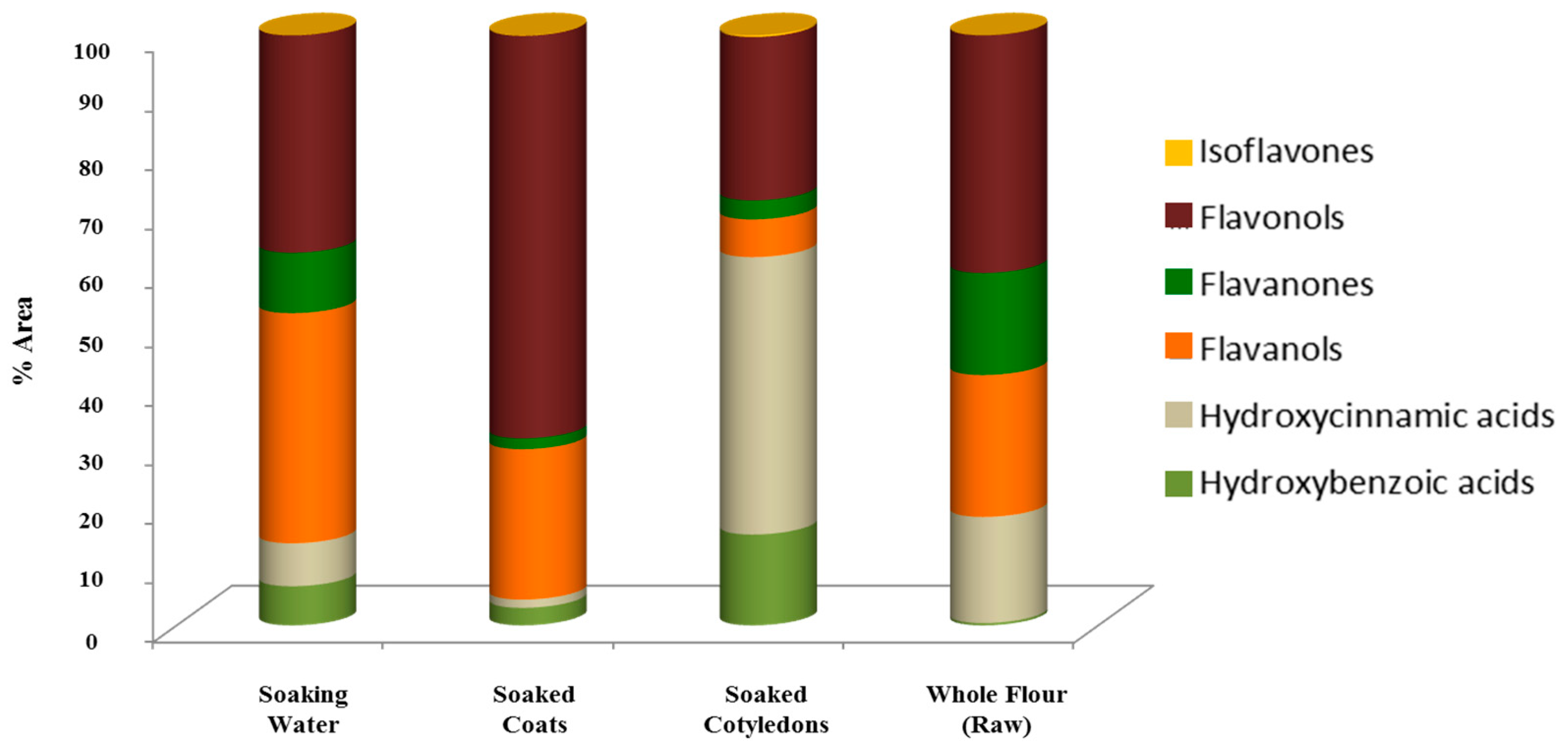
3.3. Phenolic Compounds’ Characterization
3.3.1. Hydroxybenzoic Acids
3.3.2. Hydroxycinnamic Acids
3.3.3. Flavan-3-ols
3.3.4. Flavanones
3.3.5. Flavonols
3.3.6. Isoflavones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Navazio, J.; Colley, M.; Dillon, M. Principles and Practices of Organic Bean Seed Production in the Pacific Northwest; Organic Seed Alliance: Port Townsend, WA, USA, 2007; pp. 1–12. [Google Scholar]
- The National Academies of Sciences and Medicine. Dietary Reference Intakes Tables. Available online: http://nationalacademies.org/HMD/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx (accessed on 29 April 2019).
- DGS. A Nova Roda Dos Alimentos. Um Guia Para a Escolha Alimentar Diária! Available online: https://www.dgs.pt/promocao-da-saude/educacao-para-a-saude/areas-de-intervencao/alimentacao.aspx (accessed on 9 May 2019).
- USDA. Food Composition Database. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 9 May 2019).
- Vaz Patto, M.C. Grain legume protein quality: A hot subject. Arbor 2016, 192, a314. [Google Scholar] [CrossRef]
- Patto, M.C.V.; Araújo, S.S. Positioning Portugal into the context of world production and research in grain legumes. Rev. Ciências Agrárias 2016, 39, 471–489. [Google Scholar] [CrossRef]
- Leitão, S.T.; Dinis, M.; Veloso, M.M.; Šatović, Z.; Vaz Patto, M.C. Establishing the bases for introducing the unexplored Portuguese common bean germplasm into the breeding world. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- INE. Estatísticas Agrícolas 2014. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=224773630&PUBLICACOESmodo=2 (accessed on 9 May 2019).
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume Consumption and Risk of Coronary Heart Disease in US Men and Women. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Pérez, L.A.; Álvarez-Parrilla, E.; De La Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Subuola, F.; Widodo, Y.; Kehinde, T. Processing and utilization of legumes in the tropics. In Trends in Vital Food and Control Engineering, 1st ed.; Eissa, A., Ed.; IntechOpen: London, UK, 2012; pp. 71–84. [Google Scholar]
- Bellido, G.; Arntfield, S.; Cenkowski, S.; Scanlon, M. Effects of micronization pretreatments on the physicochemical properties of navy and black beans (Phaseolus vulgaris L.). LWT 2006, 39, 779–787. [Google Scholar] [CrossRef]
- Zamindar, N.; Baghekhandan, M.S.; Nasirpour, A.; Sheikhzeinoddin, M. Effect of line, soaking and cooking time on water absorption, texture and splitting of red kidney beans. J. Food Sci. Technol. 2013, 50, 108–114. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Nishida, W.; Proença, R.P.D.C. Influence of soaking on the nutritional quality of common beans (Phaseolus vulgaris L.) cooked with or without the soaking water: A review. Int. J. Food Sci. Technol. 2010, 45, 2209–2218. [Google Scholar] [CrossRef]
- Huma, N.; Anjum, M.; Sehar, S.; Hussain, S.; Khan, M.I. Effect of soaking and cooking on nutritional quality and safety of legumes. Nutr. Food Sci. 2008, 38, 570–577. [Google Scholar] [CrossRef]
- Soetan, K.O.; Oyewole, O.E. The need for adequate processing to reduce the anti- nutritional factors in plants used as human foods and animal feeds: A review. Afr. J. Food Sci. 2009, 3, 223–232. [Google Scholar]
- Krupa, U. Main nutritional and antinutritional compounds of bean seeds—A review. Pol. J. Food Nutr. Sci. 2008, 58, 149–155. [Google Scholar]
- Fabbri, A.D.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastron. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Eshraq, B.; Mona, A.; Sayed, A.; Emam, A. Effect of soaking, cooking and germination on chemical constituents and bioactive compounds as well as their cytotoxic activities of black bean extracts. Nat. Prod. Chem. Res. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Moreira, P.M.; Carvalho, V.; Pego, S. Collecting maize (Zea mays L. convar. mays) with potential technological ability for bread making in Portugal. Genet. Resour. Crop Evol. 2007, 54, 1555–1563. [Google Scholar] [CrossRef]
- International Board for Plant Genetic Resources. Phaseolus Vulgaris Descriptors. Available online: http://www.bioversityinternational.org/uploads/tx_news/Phaseolus_vulgaris_descriptors_160.pdf (accessed on 9 May 2019).
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [Green Version]
- AACC. AACC International Approved Methods of Analysis. Method 56-Method for Determining Water Hydration Capacity and Percentage of Unhydrated Seeds of Pulses; AACC: Washington, DC, USA, 2007. [Google Scholar]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and Antioxidant Capacity of Seed Coat and Cotyledon from Brazilian and Peruvian Bean Cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef]
- Serrano, C.; Carbas, B.; Castanho, A.; Soares, A.; Patto, M.C.V.; Brites, C. Characterisation of nutritional quality traits of a chickpea (Cicer arietinum) germplasm collection exploited in chickpea breeding in Europe. Crop. Pasture Sci. 2017, 68, 1031–1040. [Google Scholar] [CrossRef]
- Stamatakis, G.; Tsantila, N.; Samiotaki, M.; Panayotou, G.N.; Dimopoulos, A.C.; Halvadakis, C.P.; Demopoulos, C.A. Detection and Isolation of Antiatherogenic and Antioxidant Substances Present in Olive Mill Wastes by a Novel Filtration System. J. Agric. Food Chem. 2009, 57, 10554–10564. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 41–59. [Google Scholar] [CrossRef]
- Hair, J.F.J.; Black, W.C.; Babin, B.J.; Anderson, R.E. Factor Analysis. In Multivariate Data Analysis—A Global Prespective, 7th ed.; Partridge, J., Ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 91–151. ISBN 13:978-0-13-515309-3. [Google Scholar]
- Padhi, E.M.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Screening different Zambian market classes of common beans (Phaseolus vulgaris) for antioxidant properties and total phenolic profiles. J. Food Nutr. Res. 2016, 4, 230–236. [Google Scholar] [CrossRef]
- Chutipanyaporn, P.; Kruawan, K.; Chupeerach, C.; Santivarangkna, C.; Suttisansanee, U. The effect of cooking process on antioxidant activities and total phenolic compounds of five colored beans. FABJ 2014, 2, 183–191. [Google Scholar]
- Xu, B.; Chang, S. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.; Willett, J.; Lesch, W.; Tangsrud, R.; Biswas, A. Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res. Int. 2010, 43, 516–519. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Yuan, S.; Chang, S. Comparative Analyses of Phenolic Composition, Antioxidant Capacity, and Color of Cool Season Legumes and Other Selected Food Legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef]
- Chen, P.X.; Bozzo, G.G.; Freixas-Coutin, J.A.; Marcone, M.F.; Pauls, P.K.; Tang, Y.; Zhang, B.; Liu, R.; Tsao, R. Free and conjugated phenolic compounds and their antioxidant activities in regular and non-darkening cranberry bean (Phaseolus vulgaris L.) seed coats. J. Funct. Foods 2015, 18, 1047–1056. [Google Scholar] [CrossRef]
- Ross, K.A.; Zhang, L.; Arntfield, S.D. Understanding Water Uptake from the Induced Changes Occurred During Processing: Chemistry of Pinto and Navy Bean Seed Coats. Int. J. Food Prop. 2010, 13, 631–647. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 1–19. [Google Scholar]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm during Seed Germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef]
- Silva, M.O.; Brigide, P.; de Toledo, N.M.V.; Canniatti-Brazaca, S.G. Phenolic compounds and antioxidant activity of two bean cultivars (Phaseolus vulgaris L.) submitted to cooking. Braz. J. Food Technol. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Faller, A.; Fialho, E. From the market to the plate: Fate of bioactive compounds during the production of feijoada meal and the impact on antioxidant capacity. Food Res. Int. 2012, 49, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Harborne, J.B.; Becana, M. Characterization of phenolic glucosides from soybean root nodules by ion-exchange high performance liquid chromatography, ultraviolet spectroscopy and electrospray mass spectrometry. Phytochem. Anal. 1998, 9, 171–176. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical Components with Health Implications in Wild and Cultivated Mexican Common Bean Seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef]
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Bioactive phenolic compounds and functional properties of dehydrated bean flours. Food Res. Int. 2011, 44, 774–780. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Total Phenolic, Phenolic Acid, Anthocyanin, Flavan-3-ol, and Flavonol Profiles and Antioxidant Properties of Pinto and Black Beans (Phaseolus vulgaris L.) as Affected by Thermal Processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gomez-Serranillos, M.P.; Palomino, O.M.; Carretero, M.E.; Duenas-Paton, M. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; Martínez-Villaluenga, C.; Limón, R.I.; Peñas, E.; Frías, J. Effect of germination and elicitation on phenolic composition and bioactivity of kidney beans. Food Res. Int. 2015, 70, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef]
- And, T.M.; Shahidi, F. Antioxidant Potential of Pea Beans (Phaseolus vulgaris L.). J. Food Sci. 2005, 70, S85–S90. [Google Scholar] [CrossRef]
- Ombra, M.N.; D’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxidative Med. Cell. Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Dimeric Procyanidins: Screening for B1 to B8 and Semisynthetic Preparation of B3, B4, B6, and B8 from a Polymeric Procyanidin Fraction of White Willow Bark (Salix alba). J. Agric. Food Chem. 2010, 58, 7820–7830. [Google Scholar] [CrossRef]
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Galardi, C.; Mulinacci, N.; Benedettelli, S.; Heimler, D. Germplasm Characterization of Zolfino Landraces (Phaseolus vulgaris L.) by Flavonoid Content. J. Agric. Food Chem. 2004, 52, 3838–3842. [Google Scholar] [CrossRef]
- Pitura, K. Evaluation of the Antioxidant and Anti-Inflammatory Activity of Extracts and Flavonol Glycosides Isolated from the Seed Coats of Coloured Beans (Phaseolus vulgaris L.). Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2011. [Google Scholar]
- Juurlink, B.H.; Azouz, H.J.; Aldalati, A.M.; AlTinawi, B.M.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Miyakawa, M.E.F. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118. [Google Scholar] [CrossRef] [Green Version]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front. Nutr. 2017, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef]
- Khandelwal, S.; Udipi, S.A.; Ghugre, P. Polyphenols and tannins in Indian pulses: Effect of soaking, germination and pressure cooking. Food Res. Int. 2010, 43, 526–530. [Google Scholar] [CrossRef]
- Mokdad-Bzeouich, I.; Mustapha, N.; Sassi, A.; Bedoui, A.; Ghoul, M.; Ghedira, K.; Chekir-Ghedira, L. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular anti-oxidant activity. Cell Stress Chaperones 2016, 21, 773–781. [Google Scholar] [CrossRef] [Green Version]
- HMDB. Available online: http://www.hmdb.ca/metabolites/HMDB0037433 (accessed on 9 May 2019).
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoğlu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Fernandez-Lopez, A.; Lamothe, V.; Delample, M.; Denayrolles, M.; Bennetau-Pelissero, C. Removing isoflavones from modern soyfood: Why and how? Food Chem. 2016, 210, 286–294. [Google Scholar] [CrossRef] [Green Version]
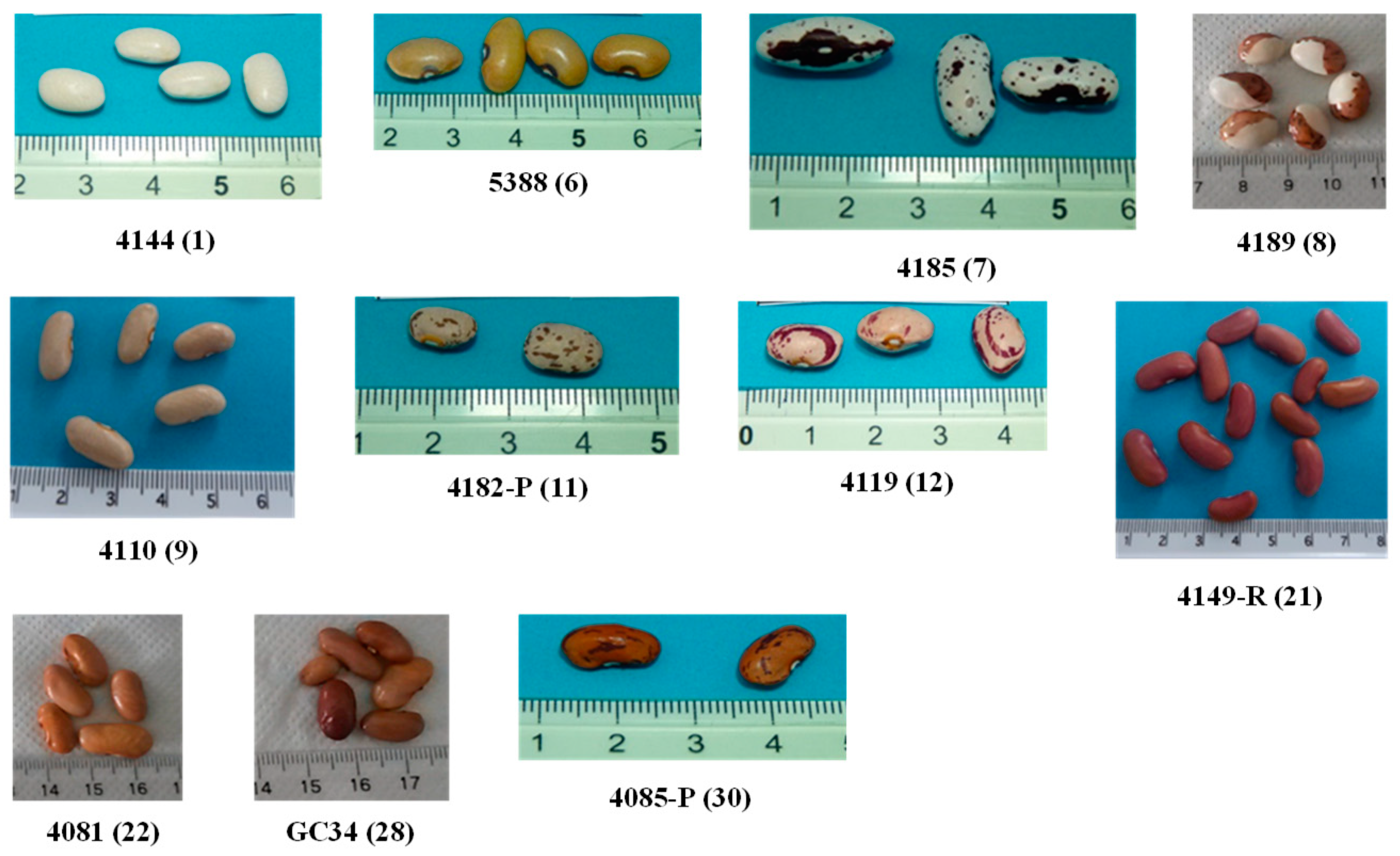
| Sample | PRT 005 Accession nº | Latitude | Longitude | Color | Pattern |
|---|---|---|---|---|---|
| 1 | 4144 | 40°51′0″ | 7°29′49″ | White | Plain |
| 2 | 5383 | 41°32′43″ | 8°25′35″ | White | Plain |
| 3 | 4088 | 40°41′51″ | 8°5′4″ | White | Plain |
| 4 | 1979 | 40°45′3″ | 7°32′14″ | White | Plain |
| 5 | 5249 | 40°39′24″ | 7°54′52″ | White | Plain |
| 6 | 5388 | 40°39′24″ | 7°54′52″ | Yellow | Plain |
| 7 | 4185 | 40°20′7″ | 7°9′48″ | White background with brown speckles and brown marginal pattern | Speckled with marginal color |
| 8 | 4189 | 40°20′7″ | 7°49′48″ | White and brown backgrounds | Spotted bicolor |
| 9 | 4110 | 40°37′60″ | 8°3′40″ | Pink | Plain |
| 10 | 4179 | 40°37′60″ | 7°23′34″ | Pink | Plain |
| 11 | 4182-P | 40°39′37″ | 7°24′38″ | Pink background with brown stripes | Stripes |
| 12 | 4119 | 40°39′24″ | 7°54′52″ | Pink background with purple stripes | Stripes |
| 13 | 4097 | 40°41′51″ | 8°5′4″ | Pink background with purple stripes | Stripes |
| 14 | 4038 | 40°53′43″ | 7°44′49″ | Pink background with purple stripes | Stripes |
| 15 | 4051 | 40°52′50″ | 7°48′16″ | Pink background with purple stripes | Stripes |
| 16 | 5389 | 40°39′24″ | 7°54′52″ | Pink background with purple stripes | Stripes |
| 17 | 4120 | 40°39′24″ | 7°54′52″ | Red | Plain |
| 18 | 5387 | 40°32′19″ | 7°16′3″ | Red | Plain |
| 19 | 4070 | 40°54′49″ | 7°58′32″ | Red | Plain |
| 20 | 5382 | 41°32′43″ | 8°25′35″ | Red | Plain |
| 21 | 4149-R | 40°19’33″ | 7°41′16″ | Red | Plain |
| 22 | 4081 | 41°11′50″ | 7°49′33″ | Light Brown | Plain |
| 23 | 4182-B | 40°39′37″ | 7°24′38″ | Brown | Plain |
| 24 | GC-34 | 40°32′19″ | 7°16′3″ | Brown | Plain |
| 25 | GC-35 | 40°32′19″ | 7°16′3″ | Brown | Plain |
| 26 | GC-17 | 40°12′7″ | 8°26′48″ | Brown | Plain |
| 27 | 4194 | 40°20′50″ | 7°51′26″ | Brown background with dark brown stripes | Stripes |
| 28 | GC-40 | 40°32′19″ | 7°16′3″ | Brown background with dark brown stripes | Stripes |
| 29 | 5384 | 39°14′12″ | 8°41′9″ | Brown background with dark brown stripes | Stripes |
| 30 | 4085 | 40°41′51″ | 8°5′4″ | Brown background with dark brown stripes | Stripes |
| 31 | 4071 | 40°54′49″ | 7°58′32″ | Brown background with dark brown stripes | Stripes |
| Parameter | Analyzed Fraction | White (n = 5) | White and Brown (n = 2) | Pink (n = 8) | Red (n = 5) | Brown (n = 10) | Described in Literature |
|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g DW) | WF (raw) | 1.38 ± 0.22 (a) | 2.73 ± 0.23 (ab) | 4.58 ± 0.67 (bc) | 4.62 ± 0.70 (bc) | 5.12 ± 1.22 (c) | 1.59 ± 0.08 (navy [34]); 0.37 ± 0.026 (white [35]); 0.45 ± 0.23 (navy [36]); 3.11 ± 0.14 (dark red kidney [34]); 1.24 ± 0.043 (red [35]); 2.15 ± 0.94 (red kidney [36]); 2.25 ± 0.05 (red kidney [37]); 0.60 ± 0.038 (Brown [35]) |
| SW | 0.12 ± 0.05 (a) | 1.20 ± 0.14 (ab) | 1.98 ± 0.77 (b) | 1.92 ± 0.66 (b) | 2.18 ± 0.78 (c) | n.d | |
| Coats | 0.04 ± 0.00 (a) | 2.38 ± 0.82 (ab) | 3.42 ± 0.93 (b) | 3.77 ± 0.31 (b) | 3.71 ± 0.62 (b) | 1.20 ± 0.02 (navy); 1.16 ± 0.01 (great northern); 1.88 ± 0.00 (pink); 1.44 ± 0.00 (dark red kidney); 5.53 ± 0.87 (light red kidney); 3.79 ± 0.04 (small red) [38] | |
| Cotyledons | 0.87 ± 0.06 (a) | 1.01 ± 0.14 (a) | 1.02 ± 0.09 (a) | 0.96 ± 0.11 (a) | 1.06 ± 0.16 (a) | 2.00 ± 0.01 (navy); 1.86 ± 0.04 (great northern); 1.97 ± 0.01 (pink); 2.11 ± 0.06 (dark red kidney); 2.15 ± 0.04 (light red kidney); 2.02 ± 0.08 (small red) [38] | |
| TFC (mg CE/g DW) | WF (raw) | 0.14 ± 0.04 (a) | 0.77 ± 0.00 (b) | 2.01 ± 0.70 (c) | 1.45 ± 0.24 (c) | 1.85 ± 0.64 (c) | 0.85 ± 0.03 (red kidney [37]) |
| SW | 0.01 ± 0.00 (a) | 0.59 ± 0.35 (ab) | 1.33 ± 0.49 (b) | 1.13 ± 0.22 (b) | 1.33 ± 0.55 (b) | n.d | |
| Coats | 0.01 ± 0.00 (a) | 1.67 ± 0.61 (ab) | 2.26 ± 0.68 (b) | 2.36 ± 0.13 (b) | 2.31 ± 0.38 (b) | n.d | |
| Cotyledons | 0.15 ± 0.01 (a) | 0.17 ± 0.01 (ab) | 0.21 ± 0.03 (bc) | 0.23 ± 0.04 (bc) | 0.22 ± 0.04 (c) | n.d | |
| TPAC (mg CE/g DW) | WF (raw) | 0.02 ± 0.01 (a) | 0.29 ± 0.25 (ab) | 0.70 ± 0.49 (b) | 0.65 ± 0.37 (b) | 0.65 ± 0.39 (b) | 0.30 ± 0.03 (red kidney [37]) |
| SW | 0.04 ± 0.01 (a) | 0.07 ± 0.03 (ab) | 0.62 ± 0.35 (c) | 0.46 ± 0.36 (bc) | 0.38 ± 0.27 (bc) | n.d | |
| Coats | 0.01 ± 0.00 (a) | 1.24 ± 0.21 (ab) | 2.49 ± 1.02 (bc) | 2.38 ± 0.70 (bc) | 2.60 ± 0.75 (c) | n.d | |
| ORAC (μmol TEAC/ g DW) | WF (raw) | 37.35 ± 4.77 (a) | 76.00 ± 2.59 (b) | 143.22 ± 35.39 (c) | 125.46 ± 31.50 (bc) | 154.83 ± 40.41 (c) | 59.41 ± 3.26 (red kidney [37]) |
| Soaking Water | Soaked Coats | Soaked Cotyledons | Whole Flour | References | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CF | Expected Mass Da | Fragments MS2 | RT Average ± SD (min) | Found at Mass Da [M-H]- | Error ppm | IS | Found at Mass Da [M-H]- | Error ppm | IS | Found at Mass Da [M-H]- | Error ppm | IS | Found at Mass Da [M-H]- | Error ppm | IS | |
| HBA | (1) Protocatechuic acid-4-O-Gl | C13H16O9 | 316.0794 | 108.0241/109.0319/152.0150/153.0229 | 27.8 ± 0.1 | 315.0722 | 0.0 | 0.8835 | 315.0724 | 0.8 | 0.8915 | 315.0716 | −1.8 | 0.8896 | 315.0701 | −6.5 | 0.7409 | [49] |
| (2) Vanillic acid | C8H8O4 | 168.0423 | 108.0240/123.0489/124.0157/152.0158 | 28.1 ± 0.1 | 167.0351 | 1.0 | 0.9916 | 167.0352 | 1.4 | 0.7308 | 167.0351 | 0.7 | 0.9901 | 167.0351 | 0.8 | 0.9683 | [50,51] | |
| (5) Protocatechuic acid* | C7H6O4 | 154.0266 | 108.0241/109.0325 | 31.4 ± 0.1 | 153.0196 | 1.7 | 0.9835 | 153.0200 | 4.4 | 0.9558 | 153.0195 | 0.9 | 0.9721 | 153.0194 | 0.5 | 0.9770 | [52,53] | |
| (12) p-Hydroxybenzoic acid-4-O-Gl | C13H16O8 | 300.0845 | 93.0372/137.0304 | 36.5 ± 0.1 | 299.0775 | 0.9 | 0.8742 | 299.0770 | −0.8 | 0.8896 | 299.0771 | −0.5 | 0.8573 | 299.0756 | −5.6 | 0.9116 | [49] | |
| (25) p-Hydroxybenzoic acid* | C7H6O3 | 138.0317 | 65.0413/93.0369 | 41.4 ± 0.1 | 137.0246 | 1.0 | 0.9898 | 137.0245 | 0.7 | 0.9804 | 137.0246 | 1.0 | 0.9797 | 137.0245 | 0.7 | 0.9880 | [51] | |
| (27) Gentisic acid* | C7H6O4 | 154.0266 | 108.0241/109.0322 | 43.4 ± 0.1 | 153.0199 | 3.8 | 0.9684 | 153.0195 | 1.2 | 0.9061 | 153.0194 | 0.6 | 0.9798 | 153.0193 | −0.4 | 0.8411 | [13,54] | |
| HCA | (3) p-Coumaroyl aldaric acid 1 | C15H16O10 | 356.0743 | 57.0358/59.0152/85.0312/89.0261/119.0370/129.0215/147.0313/191.0233/209.0339 | 29.5 ± 0.1 | 355.0673 | 0.6 | 0.9334 | 355.0653 | −4.9 | 0.9543 | 355.0745 | 20.8 | 0.9266 | 355.0714 | 12.3 | 0.9840 | [51] |
| (6) p-Coumaroyl aldaric acid 2 | C15H16O10 | 356.0743 | 57.0363/59.0156/85.0316/129.0216/147.0330/163.0428/191.0235/209.0343 | 32.7 ± 0.1 | 355.067 | −0.3 | 0.8553 | 355.0656 | −4.2 | 0.9724 | 355.0657 | −3.9 | 0.8463 | 355.0654 | −4.6 | 0.8660 | [51] | |
| (7) Feruloyl aldaric acid 1 | C16H18O11 | 386.0849 | 57.0362/59.0154/85.0315/129.0216/147.0327/191.0234/209.0339 | 33.4 ± 0.1 | 385.0772 | −1.1 | 0.8642 | 385.0755 | −5.5 | 0.9330 | 385.0761 | −4.0 | 0.8615 | 385.0761 | −3.9 | 0.8802 | [51,55] | |
| (8) p-Coumaroyl aldaric acid 3 | C15H16O10 | 356.0743 | 57.0364/59.0159/85.0320/129.0225/147.0336/ 191.0246/ 209.0357 | 34.9 ± 0.1 | 355.0672 | 0.3 | 0.8547 | 355.0659 | −3.4 | 0.9032 | 355.0659 | −3.3 | 0.8706 | 355.0655 | −4.4 | 0.8581 | ND | |
| HCA | (9) Sinapoyl aldaric acid 1 | C17H20O12 | 416.0955 | 57.0363/59.0155/85.0316/129.0220/147.0329/191.0230/209.0338 | 35.0 ± 0.1 | 415.0869 | −3.0 | 0.9592 | 415.0878 | −1.0 | 0.9388 | 415.0882 | 0.1 | 0.9354 | 415.0856 | −6.4 | 0.8778 | [55] |
| (10) Feruloyl aldaric acid 2 | C16H18O11 | 386.0849 | 57.0362/85.0312/147.0323/191.0227/209.0339/223.0495 | 35.3 ± 0.1 | 385.0761 | −4.0 | 0.9050 | 385.0755 | −5.4 | 0.9710 | 385.0761 | −3.9 | 0.8701 | 385.0756 | −5.3 | 0.8887 | [51,55] | |
| (13) Feruloyl aldaric acid 3 | C16H18O11 | 386.0849 | 57.0359/59.0154/85.0311/129.0217/147.0319/191.0228/193.0539/209.0331 | 36.6 ± 0.1 | 385.0764 | −3.3 | 0.9242 | 385.0753 | −6.0 | 0.9524 | 385.0763 | −3.4 | 0.8808 | 385.0758 | −4.8 | 0.8981 | [51,55] | |
| (14) p-Coumaroyl aldaric acid 4 | C15H16O10 | 356.0743 | 85.0315/147.0326/191.0233/209.0343 | 36.8 ± 0.2 | 355.0665 | −1.6 | 0.8922 | 355.0653 | −4.8 | 0.9581 | 355.0659 | −3.2 | 0.8631 | 355.065 | −5.7 | 0.8873 | ND | |
| (15) p-Coumaroyl aldaric acid 5 | C15H16O10 | 356.0743 | 57.0361/59.0153/85.0315/129.0216/147.0324/191.0234/209.0343 | 37.6 ± 0.1 | 355.0664 | −1.9 | 0.8651 | 355.0658 | −3.6 | 0.9319 | 355.0660 | −3.1 | 0.8480 | 355.0655 | −4.5 | 0.8628 | ND | |
| (16) Feruloyl aldaric acid 4 | C16H18O11 | 386.0849 | 57.0361/85.0313/129.0211/147.0322/191.0228/209.0333 | 38.2 ± 0.1 | 385.0775 | −0.5 | 0.8943 | 385.0762 | −3.7 | 0.9035 | 385.0766 | −2.6 | 0.8748 | 385.076 | −4.3 | 0.8851 | [55] | |
| (19) Sinapoyl aldaric acid 2 | C17H20O12 | 416.0955 | 57.0360/59.0154/85.0314/129.0214/147.0327/191.0234/209.0339/223.0661 | 38.7 ± 0.1 | 415.0865 | −4.0 | 0.9481 | 415.0856 | −6.2 | 0.9243 | 415.0855 | −6.4 | 0.9349 | 415.0851 | −7.5 | 0.9162 | [55] | |
| (20) Sinapoyl aldaric acid 3 | C17H20O12 | 416.0955 | 57.0363/85.0317/129.0220/147.0329/191.0237/209.0343 | 39.4 ± 0.1 | 415.0871 | −2.5 | 0.8585 | 415.0855 | −6.5 | 0.9589 | 415.0859 | −5.5 | 0.8748 | 415.0857 | −6.1 | 0.8622 | ND | |
| (22) Feruloyl aldaric acid 5 | C16H18O11 | 386.0849 | 57.0363/85.0315/129.0216/147.0326/191.0232/209.0342 | 40.2 ± 0.1 | 385.0774 | −0.7 | 0.8745 | 385.0758 | −4.8 | 0.9144 | 385.0767 | −2.5 | 0.8842 | 385.0762 | −3.6 | 0.8726 | [55] | |
| (23) Sinapoyl aldaric acid 4 | C17H20O12 | 416.0955 | 57.0362/59.0154/85.0315/129.0215/147.0324/191.0234/209.0343 | 40.8 ± 0.1 | 415.0861 | −5.0 | 0.9706 | 415.0853 | −7.1 | 0.9082 | 415.0861 | −4.9 | 0.8771 | 415.0856 | −6.3 | 0.8977 | ND | |
| HCA | (26) p-Coumaroyl aldaric acid 6 | C15H16O10 | 356.0743 | 57.0361/59.0154/85.0313/ 129.0212/147.0326/163.0420/191.0232/209.0339 | 41.8 ± 0.1 | NF | - | - | 355.0654 | −4.7 | 0.7331 | 355.0658 | −3.6 | 0.8664 | 355.0654 | −4.7 | 0.8717 | ND |
| (30) Feruloyl aldaric acid 6 | C16H18O11 | 386.0849 | 57.0364/59.0157/85.0317/111.0106/129.0218/147.0333/191.0241/209.0348 | 44.5 ± 0.1 | 385.0772 | −1.0 | 0.8900 | 385.0760 | −4.1 | 0.8934 | 385.0766 | −2.8 | 0.9271 | 385.0764 | −3.2 | 0.8801 | ND | |
| (31) Sinapoyl aldaric acid 5 | C17H20O12 | 416.0955 | 57.0361/59.0152/85.0314/129.0219/147.0326/191.0232/209.0339 | 44.8 ± 0.1 | 415.0871 | −2.7 | 0.8610 | 415.0857 | −6.1 | 0.9493 | 415.0863 | −4.7 | 0.8794 | 415.0858 | −5.9 | 0.8865 | ND | |
| (40) p-Coumaric acid* | C9H8O3 | 164.0473 | 93.0364/117.0369/119.0529 | 58.9 ± 0.2 | 163.0407 | 4.2 | 0.9776 | 163.0404 | 1.9 | 0.9503 | NF | - | - | 163.0401 | 0.2 | 0.8870 | [25] | |
| (43) Sinapic acid* | C11H12O5 | 224.0685 | 93.0361/121.0308/149.0272/163.0423/164.05077/165.0227/193.0175/208.0417 | 63.1 ± 0.2 | 223.0613 | 0.6 | 0.9604 | 223.0610 | −1.0 | 0.7187 | 223.0611 | −0.5 | 0.9814 | 223.0605 | −3.1 | 0.9721 | [25] | |
| (44) Ferulic acid* | C10H10O4 | 194.0579 | 102.9351/133.0316/134.0400/149.0636/178.0305 | 63.8 ± 0.2 | 193.0510 | 1.9 | 0.9545 | 193.0506 | −0.3 | 0.8344 | NF | - | - | 193.0507 | 0.3 | 0.9355 | [25,51] | |
| Flavanol | (4) (+)-Catechin 3′-O-glucose | C21H24O11 | 452.1319 | 137.0279/245.0874/289.0836/299.0835 | 30.8 ± 0.1 | 451.1234 | −2.5 | 0.8674 | 451.1227 | −4.2 | 0.8103 | 451.1220 | −5.8 | 0.8440 | 451.1218 | −6.1 | 0.8412 | [51] |
| (11) Procyanidin B1 | C30H26O12 | 578.1424 | 125.0272/161.0276/287.0613/289.0771/407.0836/425.0935/451.1095 | 36.3 ± 0.1 | 577.1338 | −2.4 | 0.8707 | 577.1322 | −5.1 | 0.8217 | NF | - | - | 577.1308 | −7.6 | 0.8528 | [51] | |
| (17) (+)-Catechin 7-O-β-D-Gl | C21H24O11 | 452.1319 | 245.0860/289.0766 | 37.7 ± 0.1 | 451.124 | −1.3 | 0.8408 | 451.1226 | −4.5 | 0.8522 | 451.1216 | −6.6 | 0.9377 | 451.1214 | −7.1 | 0.8410 | [56] | |
| (18) Procyanidin B2* | C30H26O12 | 578.1424 | 125.0270/289.0768/407.0831/425.0936/451.1091 | 38.3 ± 0.1 | 577.1334 | −3.0 | 0.8429 | 577.1320 | −5.5 | 0.7773 | 577.1306 | −7.8 | 0.7106 | 577.1306 | −7.8 | 0.8349 | [51] | |
| (21) Procyanidin C1 | C45H38O18 | 866.2058 | 575.1276/577.1431/695.1510/713.1622 | 40.2 ± 0.1 | 865.1957 | −3.3 | 0.7994 | 865.1925 | −7.0 | 0.7427 | 865.1950 | −4.1 | 0.5898 | 865.1907 | −9.0 | 0.8559 | [43,57] | |
| Flavanol | (24) (+)-Catechin* | C15H14O6 | 290.079 | 109.0321/123.0479/125.0272/137.0274/151.0433/179.0388/203.0753/205.0546/221.0865/245.0866 | 40.6 ± 0.1 | 289.0719 | 0.4 | 0.8594 | 289.0718 | 0.0 | 0.8649 | 289.0711 | −2.1 | 0.9019 | 289.0714 | −1.1 | 0.8714 | [51] |
| (28) Procyanidin B3 | C30H26O12 | 578.1424 | 125.0267/287.0609/289.0762/407.0829/425.0932/451.1094 | 43.7 ± 0.1 | 577.1331 | −3.5 | 0.8371 | 577.1312 | −6.9 | 0.8396 | NF | - | - | 577.1290 | −10.7 | 0.9183 | [51] | |
| (32) Procyanidin B4 | C30H26O12 | 578.1424 | 125.0266/161.0267/287.0598/289.0756/407.0813/425.0923/451.1092 | 46.4 ± 0.1 | 577.1327 | −4.2 | 0.8305 | 577.1310 | −7.2 | 0.8814 | NF | - | - | 577.1294 | −9.9 | 0.7277 | [53] | |
| (33) (-)-Epicatechin* | C15H14O6 | 290.079 | 109.0317/123.0476/125.0267/151.0425/203.0749/205.0537/245.0860 | 46.8 ± 0.1 | 289.0719 | 0.6 | 0.8671 | 289.0716 | −0.4 | 0.8691 | 289.0705 | −4.2 | 0.9701 | 289.0712 | −1.8 | 0.8682 | [58] | |
| (35) Procyanidin C2 | C45H38O18 | 866.2058 | 575.1269 | 48.1 ± 0.2 | 865.1952 | −3.9 | 0.7947 | 865.1920 | −7.6 | 0.7222 | 865.1909 | −8.8 | 0.8242 | 865.1908 | −8.9 | 0.7730 | [57] | |
| (36) Procyanidin B5 | C30H26O12 | 578.1424 | 125.0266/287.0603/289.0760/407.0825/425.0924/451.1082 | 50.8 ± 0.2 | 577.1334 | −3.0 | 0.8527 | 577.1316 | −6.2 | 0.8447 | 577.1296 | −9.6 | 0.7375 | 577.1308 | −7.6 | 0.8452 | [59] | |
| Flavanones | (29) Eriodictyol-hexoside 1 | C21H22O11 | 450.1162 | 125.0267/179.0017/243.0698/259.0662/283.0657/287.0615/301.0764/421.1202 | 43.7 ± 0.1 | 449.1074 | −3.4 | 0.8475 | 449.1054 | −8.0 | 0.8682 | 449.1052 | −8.3 | 0.8673 | 449.1052 | −8.4 | 0.8317 | [51,55] |
| (34) Eriodictyol-hexoside 2 | C21H22O11 | 450.1162 | 125.0273/259.0672/269.0510/287.0618 | 47.2 ± 0.1 | 449.108 | −2.0 | 0.8735 | 449.1068 | −4.6 | 0.8607 | 449.1070 | −4.3 | 0.8465 | 449.1061 | −6.2 | 0.8569 | [55] | |
| (37) Eriodictyol-hexoside 3 | C21H22O11 | 450.1162 | 259.0651/287.0607 | 53.6 ± 0.1 | 449.1077 | −2.7 | 0.8574 | 449.1060 | −6.6 | 0.9051 | 449.1057 | −7.3 | 0.8843 | 449.1064 | −5.7 | 0.8703 | ND | |
| Flavanones | (38) Eriodictyol-hexoside 4 | C21H22O11 | 450.1162 | 125.0262/151.0061/152.0143/179.0014/180.0094/269.0497/287.0590 | 56.7 ± 0.2 | 449.1078 | −2.6 | 0.8554 | 449.1056 | −7.5 | 0.9268 | 449.1056 | −7.4 | 0.9693 | 449.1062 | −6.1 | 0.8633 | ND |
| (42) Naringenin-7-Gl | C21H22O10 | 434.1213 | 119.0521/151.0057/271.0647/313.0589 | 60.4 ± 0.2 | 433.1119 | −4.8 | 0.9503 | 433.1107 | −7.6 | 0.8883 | 433.1088 | −12.0 | 0.7686 | 433.1097 | −10.0 | 0.9045 | [53] | |
| (50) Eriodictyol | C15H12O6 | 288.0634 | 125.0269/243.0703/259.0659 | 82.7 ± 0.2 | 287.0561 | −0.1 | 0.9315 | 287.0557 | −1.5 | 0.8979 | 287.0547 | −4.8 | 0.9471 | 287.0553 | −2.7 | 0.9044 | [51] | |
| (53) Naringenin | C15H12O5 | 272.0685 | 107.0160/119.0524/151.0058/177.0228 | 95.4 ± 0.1 | 271.0610 | −0.7 | 0.9873 | 271.0605 | −2.6 | 0.9748 | 271.0605 | -2.7 | 0.9126 | 271.0602 | −3.8 | 0.9438 | [51] | |
| Flavonols | (39) Luteolin 3′,7-di-O-Gl or kaempferol-3′,7-dihexoside | C27H30O16 | 610.1534 | 284.0373/285.0446 | 56.8 ± 0.2 | 609.1442 | −3.1 | 0.8322 | 609.1415 | −7.5 | 0.8720 | 609.1409 | −8.6 | 0.9374 | 609.1417 | −7.3 | 0.8715 | [60] |
| (41) Rutin* | C27H30O16 | 610.1534 | 284.0370/285.0453/300.0320/301.0386/327.0554/607.2499 | 59.9 ± 0.2 | 609.1441 | −3.2 | 0.8302 | 609.1421 | −6.6 | 0.8892 | 609.1418 | −7.1 | 0.8585 | 609.1410 | −8.3 | 0.8494 | [25,55] | |
| (45) Kaempferol-3-O-xylosyl-Gl | C26H28O15 | 580.1428 | 284.0377/285.0453/429.0891 | 64.7 ± 0.1 | 579.1335 | −3.5 | 0.8194 | NF | - | - | NF | - | - | 579.1316 | −6.9 | 0.8276 | [25,61] | |
| (46) Quercetin-3-O-Gl | C21H20O12 | 464.0955 | 300.0318/301.0392 | 65.0 ± 0.2 | 463.0867 | −3.2 | 0.8117 | 463.0855 | −5.9 | 0.8163 | NF | - | - | 463.0849 | −7.1 | 0.8265 | [25,61] | |
| (47) Quercetin-3-(6-O-acetyl-β-Gl) | C23H22O13 | 506.106 | 300.0319/301.0399/463.0923 | 73.2 ± 0.2 | 505.0970 | −3.5 | 0.8666 | 505.0942 | −9.1 | 0.9576 | NF | - | - | 505.0952 | −7.0 | 0.8700 | [55,62] | |
| (48) Kaempferol-3-O-Gl | C21H20O11 | 448.1006 | 227.0397/255.0363/284.0447/285.0476 | 78.1 ± 0.1 | 447.0934 | 0.3 | 0.8361 | 447.0918 | −3.4 | 0.8064 | 447.0904 | −6.4 | 0.8319 | 447.0909 | −5.2 | 0.8716 | [25,55,61] | |
| (49) Kaempferol-3-O-acetyl-Gl | C23H22O12 | 490.1111 | 255.0339/284.0386/285.0451 | 82.4 ± 0.1 | 489.1027 | −2.2 | 0.8634 | 489.1004 | −7.1 | 0.8248 | 489.1006 | −6.5 | 0.8577 | 489.1010 | −5.8 | 0.8644 | [61] | |
| (52) Quercetin* | C15H10O7 | 302.0427 | 107.0143/121.0312/151.0059/179.0017/255.2372/273.0434 | 91.2 ± 0.1 | 301.0347 | −2.1 | 0.9273 | 301.0353 | −0.2 | 0.8619 | 301.0341 | −4.3 | 0.9211 | 301.0336 | −5.9 | 0.9445 | [25,51] | |
| Flavonols | (55) Kaempferol* | C15H10O6 | 286.0477 | 93.0368/107.0159/151.0062/159.0479/185.0639/187.0428/211.0429/229.0545/239.0385/257.0493 | 97.4 ± 0.1 | 285.0407 | 1.0 | 0.8786 | 285.0403 | −0.5 | 0.8557 | 285.0402 | −1.0 | 0.8565 | 285.0400 | −1.6 | 0.8669 | [25,61] |
| Isoflavones | (51) Daidzein* | C15H10O4 | 254.0579 | 209.0643/224.0538/225.0617 | 88.5 ± 0.1 | 253.0505 | −0.4 | 0.9479 | 253.0503 | −1.2 | 0.8295 | 253.0502 | −1.7 | 0.9510 | 253.0495 | −4.4 | 0.7987 | [61] |
| (54) Genistein* | C15H10O5 | 270.0528 | 133.0309 | 96.3 ± 0.1 | 269.0452 | −1.2 | 0.7351 | 269.0447 | −3.1 | 0.9337 | 269.0449 | −2.5 | 0.8803 | NF | - | - | [61] | |
| Soaking Water | Soaked Coats | Soaked Cotyledons | Whole Flour (Raw) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Class | Name | Area (%, Area/Total Area) | %, Area/Total Compounds’ Class Area | Area (%, Area/Total Area) | %, Area/Total Compounds’ Class Area | Area (%, Area/Total Area) | %, Area/Total Compounds’ Class Area | Area (%, Area/Total Area) | %, Area/Total Compounds’ Class Area |
| HBA | (1) Protocatechuic acid-4-O-Gl | 1677120 (2.9) | 43.2 | 85308 (0.6) | 20.5 | 451623 (4.8) | 31.2 | 13153 (0.03) | 8.1 |
| (2) Vanillic acid | 67863 (0.1) | 1.7 | 4363 (0.03) | 1.0 | 15603 (0.2) | 1.1 | 29105 (0.1) | 17.9 | |
| (5) Protocatechuic acid | 130651 (0.2) | 3.4 | 137055 (1.0) | 33.0 | 9253 (0.1) | 0.6 | 25185 (0.1) | 15.5 | |
| (12) p-Hydroxybenzoic acid-4-O- Gl | 1841267 (3.2) | 47.4 | 163157 (1.2) | 39.3 | 949047 (10.1) | 65.6 | 23743 (0.1) | 14.6 | |
| (25) p-Hydroxybenzoic acid | 60267 (0.1) | 1.6 | 19416 (0.1) | 4.7 | 13706 (0.1) | 0.9 | 69860 (0.2) | 43.0 | |
| (27) Gentisic acid | 106891 (0.2) | 2.8 | 6345 (0.05) | 1.5 | 7149 (0.1) | 0.5 | 1446 (0.003) | 0.9 | |
| Total compounds’ class area | 3884059 (6.6) | 100.0 | 415644 (3.0) | 100.0 | 1446381 (14.7) | 100.0 | 162493 (0.4) | 100.0 | |
| HCA | (3) p-Coumaroyl aldaric acid 1 | 26590 (0.05) | 0.6 | 4318 (0.03) | 2.2 | 167283 (1.8) | 3.8 | 114749 (0.3) | 1.5 |
| (6) p-Coumaroyl aldaric acid 2 | 139230 (0.2) | 3.3 | 8798 (0.1) | 4.5 | 42992 (0.5) | 1.0 | 73863 (0.2) | 1.0 | |
| (7) Feruloyl aldaric acid 1 | 97092 (0.2) | 2.3 | 7766 (0.1) | 4.0 | 305601 (3.3) | 6.9 | 689964 (1.7) | 9.2 | |
| (8) p-Coumaroyl aldaric acid 3 | 1265546 (2.2) | 30.4 | 22910 (0.2) | 11.7 | 417109 (4.4) | 9.5 | 496423 (1.2) | 6.6 | |
| (9) Sinapoyl aldaric acid 1 | 35954 (0.1) | 0.9 | 1977 (0.01) | 1.0 | 65773 (0.7) | 1.5 | 183550 (0.4) | 2.5 | |
| (10) Feruloyl aldaric acid 2 | 12142 (0.02) | 0.3 | 3078 (0.02) | 1.6 | 137695 (1.5) | 3.1 | 130905 (0.3) | 1.7 | |
| (13) Feruloyl aldaric acid 3 | 66532 (0.1) | 1.6 | 3551 (0.03) | 1.8 | 49572 (0.5) | 1.1 | 139151 (0.3) | 1.9 | |
| (14) p-Coumaroyl aldaric acid 4 | 321109 (0.6) | 7.7 | 5263 (0.04) | 2.7 | 9651 (0.1) | 0.2 | 9516 (0.02) | 0.1 | |
| (15) p-Coumaroyl aldaric acid 5 | 150575 (0.3) | 3.6 | 17330 (0.1) | 8.9 | 396951 (4.2) | 9.0 | 339760 (0.8) | 4.5 | |
| (16) Feruloyl aldaric acid 4 | 139602 (0.2) | 3.3 | 3934 (0.03) | 2.0 | 213762 (2.3) | 4.9 | 125167 (0.3) | 1.7 | |
| (19) Sinapoyl aldaric acid 2 | 49814 (0.1) | 1.2 | 1705 (0.01) | 0.9 | 55150 (0.6) | 1.3 | 14544 (0.03) | 0.2 | |
| (20) Sinapoyl aldaric acid 3 | 172631 (0.3) | 4.1 | 5470 (0.04) | 2.8 | 242307 (2.6) | 5.5 | 677720 (1.6) | 9.0 | |
| (22) Feruloyl aldaric acid 5 | 470732 (0.8) | 11.3 | 44560 (0.3) | 22.8 | 757939 (8.1) | 17.2 | 1381715 (3.3) | 18.4 | |
| (23) Sinapoyl aldaric acid 4 | 171674 (0.3) | 4.1 | 8603 (0.06) | 4.4 | 291186 (3.1) | 6.6 | 747370 (1.8) | 10.0 | |
| (26) p-Coumaroyl aldaric acid 6 | NF | NF | 18685 (0.1) | 9.6 | 433166 (4.4) | 9.0 | 213995 (0.5) | 2.9 | |
| (30) Feruloyl aldaric acid 6 | 730915 (1.3) | 17.5 | 28548 (0.2) | 14.6 | 971927 (10.4) | 22.1 | 1644680 (4.0) | 22.0 | |
| (31) Sinapoyl aldaric acid 5 | 185506 (0.3) | 4.5 | 7616 (0.05) | 3.9 | 273562 (2.9) | 6.2 | 465873 (1.1) | 6.2 | |
| (40) p-Coumaric acid | 89291 (0.2) | 2.1 | 338 (0.002) | 0.2 | NF | NF | 11431 (0.03) | 0.2 | |
| (43) Sinapic acid | 2151 (0.004) | 0.1 | 418 (0.003) | 0.2 | 8055 (0.1) | 0.2 | 6305 (0.02) | 0.1 | |
| (44) Ferulic acid | 40918 (0.1) | 1.0 | 158 (0.001) | 0.1 | NF | NF | 23022 (0.1) | 0.3 | |
| Total compounds’ class area | 4168002 (7.1) | 100.0 | 195028 (1.4) | 100.0 | 4839680 (49.3) | 100.0 | 7489702 (18.0) | 100.0 | |
| Flavanols | (4) (+)-Catechin 3’-O-Gl | 7636898 (13.0) | 33.5 | 1165545 (8.3) | 32.7 | 518148 (5.3) | 86.3 | 3166476 (7.6) | 31.6 |
| (11) Procyanidin B1 | 4199734 (7.2) | 18.4 | 866524 (6.2) | 24.3 | NF | NF | 1885313 (4.5) | 18.8 | |
| (17) (+)-Catechin 7-O-Gl | 1214896 (2.1) | 5.3 | 70095 (0.5) | 2.0 | 10469 (0.1) | 1.7 | 172724 (0.4) | 1.7 | |
| (18) Procyanidin B2 | 926982 (1.6) | 4.1 | 197566 (1.4) | 5.5 | 2303 (0.02) | 0.4 | 468641 (1.1) | 4.7 | |
| (21) Procyanidin C1 | 1216935 (2.1) | 5.3 | 546543 (3.9) | 15.3 | 2018 (0.02) | 0.3 | 265008 (0.6) | 2.6 | |
| (24) (+)-Catechin | 5800883 (9.9) | 25.4 | 507760 (3.6) | 14.2 | 60537 (0.6) | 10.1 | 3025744 (7.3) | 30.2 | |
| (28) Procyanidin B3 | 205083 (0.4) | 0.9 | 21266 (0.2) | 0.6 | NF | NF | 38912 (0.1) | 0.4 | |
| (32) Procyanidin B4 | 60776 (0.1) | 0.3 | 8658 (0.1) | 0.2 | NF | NF | 28180 (0.1) | 0.3 | |
| (33) (-)-Epicatechin | 559522 (1.0) | 2.5 | 51252 (0.4) | 1.4 | 4432 (0.05) | 0.7 | 353630 (0.8) | 3.5 | |
| (35) Procyanidin C2 | 185749 (0.3) | 0.8 | 25218 (0.2) | 0.7 | 171 (0.002) | 0.0 | 85610 (0.2) | 0.9 | |
| (36) Procyanidin B5 | 820786 (1.4) | 3.6 | 104129 (0.7) | 2.9 | 2311 (0.02) | 0.4 | 522375 (1.3) | 5.2 | |
| Total compounds’ class area | 22828243 (39.0) | 100.0 | 3564555 (25.5) | 100.0 | 600389 (6.1) | 100.0 | 10012612 (24.0) | 100.0 | |
| Flavanones | (29) Eriodictyol-hexoside 1 | 98920 (0.2) | 1.7 | 4350 (0.03) | 1.7 | 4011 (0.04) | 1.3 | 197075 (0.5) | 2.7 |
| (34) Eriodictyol-hexoside 2 | 5166817 (8.8) | 86.4 | 178852 (1.3) | 70.6 | 272049 (2.8) | 90.9 | 5800730 (13.9) | 80.6 | |
| (37) Eriodictyol-hexoside 3 | 252875 (0.4) | 4.2 | 25333 (0.2) | 10.0 | 12016 (0.1) | 4.0 | 844769 (2.0) | 11.7 | |
| (38) Eriodictyol-hexoside 4 | 177730 (0.3) | 3.0 | 5960 (0.04) | 2.4 | 804 (0.01) | 0.3 | 165914 (0.4) | 2.3 | |
| (42) Naringenin-7-Gl | 10713 (0.02) | 0.2 | 585 (0.004) | 0.2 | 409 (0.004) | 0.1 | 5652 (0.01) | 0.1 | |
| (50) Eriodictyol | 254008 (0.4) | 4.2 | 28839 (0.2) | 11.4 | 1324 (0.01) | 0.4 | 161919 (0.4) | 2.3 | |
| (53) Naringenin | 15799 (0.03) | 0.3 | 9365 (0.1) | 3.7 | 8739 (0.1) | 2.9 | 17103 (0.04) | 0.2 | |
| Total compounds’ class area | 5976862 (10.2) | 100.0 | 253284 (1.8) | 100.0 | 299352 (3.0) | 100.0 | 7193164 (17.3) | 100.0 | |
| Flavonols | (39) Luteolin 3,7-di-O-Gl or Kaempferol-3′,7-dihexoside | 309103 (0.5) | 1.4 | 20371 (0.1) | 0.2 | 2187 (0.02) | 0.1 | 535830 (1.3) | 3.2 |
| (41) Rutin | 208461 (0.4) | 1.0 | 20628 (0.1) | 0.2 | 14381 (0.1) | 0.6 | 86221 (0.2) | 0.5 | |
| (45) Kaempferol-3-O-xylosyl-Gl | 2737477 (4.7) | 12.7 | NF | NF | NF | NF | 2217478 (5.3) | 13.2 | |
| (46) Quercetin-3-O-Gl | 1233782 (2.1) | 5.7 | 8850 (0.1) | 0.1 | NF | NF | 1485967 (3.6) | 8.9 | |
| (47) Quercetin-3-(6-O-acetyl-Gl) | 62179 (0.1) | 0.3 | 37 (0.003) | 0.0 | NF | NF | 63797 (0.2) | 0.4 | |
| (48) Kaempferol-3-O-Gl | 11475034 (19.6) | 53.2 | 6344589 (45.4) | 66.5 | 438101 (4.5) | 16.8 | 7437720 (17.9) | 44.3 | |
| (49) Kaempferol-3-O-acetyl-Gl | 5393293 (9.2) | 25.0 | 1228219 (8.8) | 12.9 | 1942204 (19.8) | 74.7 | 4250372 (10.2) | 25.3 | |
| (52) Quercetin | 8100 (0.01) | 0.0 | 12113 (0.1) | 0.1 | 10403 (0.1) | 0.4 | 17618 (0.04) | 0.1 | |
| Flavonols | (55) Kaempferol | 145232 (0.3) | 0.7 | 1903123 (13.6) | 20.0 | 194088 (2.0) | 7.5 | 682007 (1.6) | 4.1 |
| Total compounds’ class area | 21572661 (36.9) | 100.0 | 9537931 (68.2) | 100.0 | 2601364 (27.7) | 100.0 | 16777009 (40.3) | 100.0 | |
| Isoflavones | (51) Daidzein | 2243 (0.004) | 25.2 | 2434 (0.02) | 23.0 | 9867 (0.1) | 34.6 | 538 (0.001) | 100.0 |
| (54) Genistein | 6664 (0.01) | 74.8 | 8165 (0.1) | 77.0 | 18661 (0.2) | 65.4 | NF | NF | |
| Total compounds’ class area | 8907 (0.02) | 100.0 | 10599 (0.1) | 100.0 | 28528 (0.3) | 100.0 | 538 (0.001) | 100.0 | |
| Total area | 58438734 (100.0) | 13977041 (100.0) | 9815694 (100.0) | 41635518 (100.0) | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mecha, E.; Leitão, S.T.; Carbas, B.; Serra, A.T.; Moreira, P.M.; Veloso, M.M.; Gomes, R.; Figueira, M.E.; Brites, C.; Vaz Patto, M.C.; et al. Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm. Foods 2019, 8, 296. https://doi.org/10.3390/foods8080296
Mecha E, Leitão ST, Carbas B, Serra AT, Moreira PM, Veloso MM, Gomes R, Figueira ME, Brites C, Vaz Patto MC, et al. Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm. Foods. 2019; 8(8):296. https://doi.org/10.3390/foods8080296
Chicago/Turabian StyleMecha, Elsa, Susana T. Leitão, Bruna Carbas, Ana T. Serra, Pedro M. Moreira, Maria Manuela Veloso, Ricardo Gomes, Maria E. Figueira, Carla Brites, Maria C. Vaz Patto, and et al. 2019. "Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm" Foods 8, no. 8: 296. https://doi.org/10.3390/foods8080296
APA StyleMecha, E., Leitão, S. T., Carbas, B., Serra, A. T., Moreira, P. M., Veloso, M. M., Gomes, R., Figueira, M. E., Brites, C., Vaz Patto, M. C., & Bronze, M. R. (2019). Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm. Foods, 8(8), 296. https://doi.org/10.3390/foods8080296








