Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation and Characterization of NADES
2.2.1. NADES Preparation
2.2.2. pH and Density
2.2.3. Viscosity
2.2.4. Polarity
2.3. Production and Characterization of NADES–Annatto Seed Extract
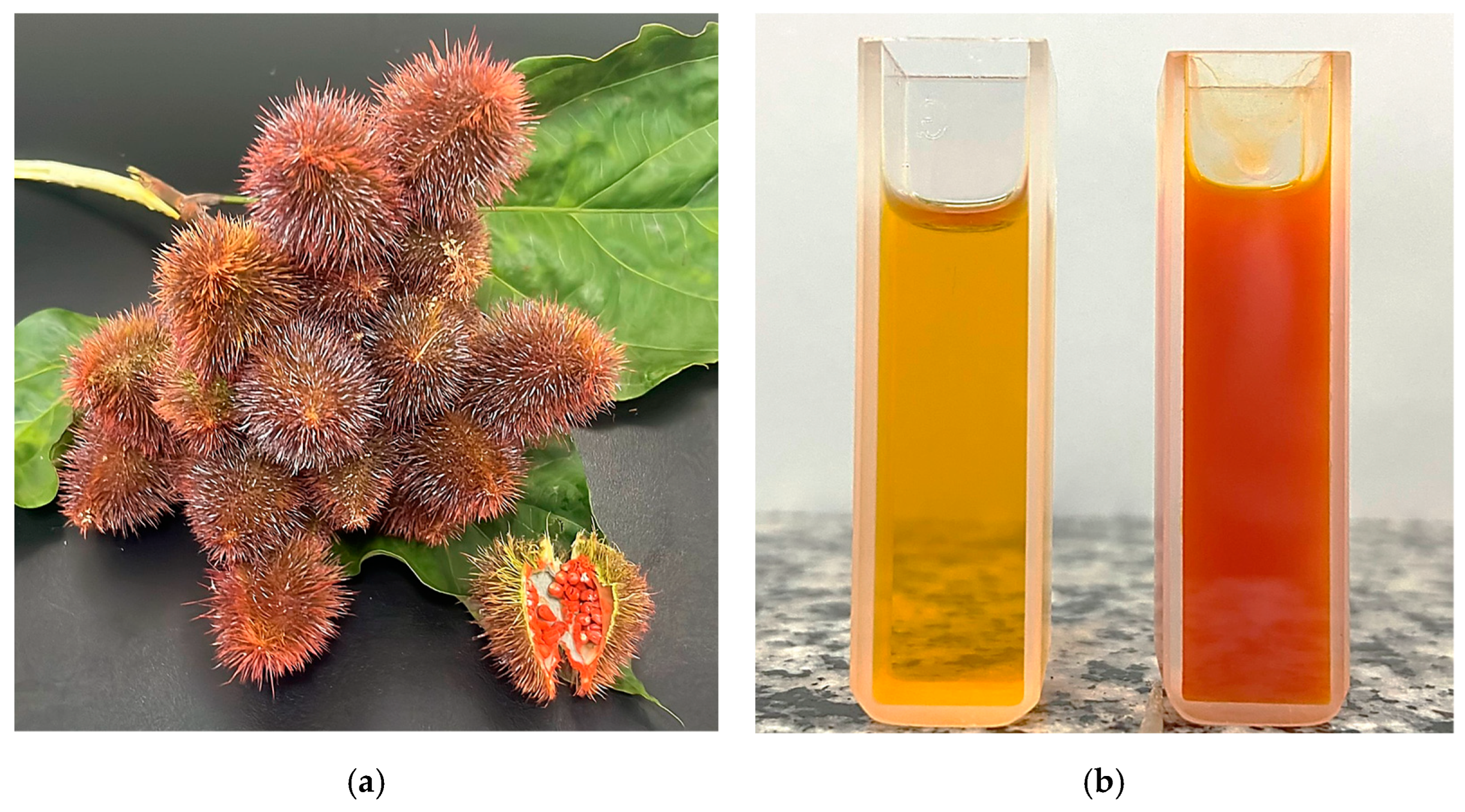
2.3.1. Production of NADES–Annatto Seed Extract
2.3.2. pH, Viscosity, and Color
2.3.3. Bixin Quantification by High-Performance Liquid Chromatography (HPLC)
2.3.4. Total Carotenoids and Norbixin Content
2.3.5. Antioxidant Activity
2.4. Production and Characterization of Gelatin- and Starch-Based Inks
2.4.1. Production of Gelatin- and Starch-Based Inks
2.4.2. Firmness and Adhesiveness
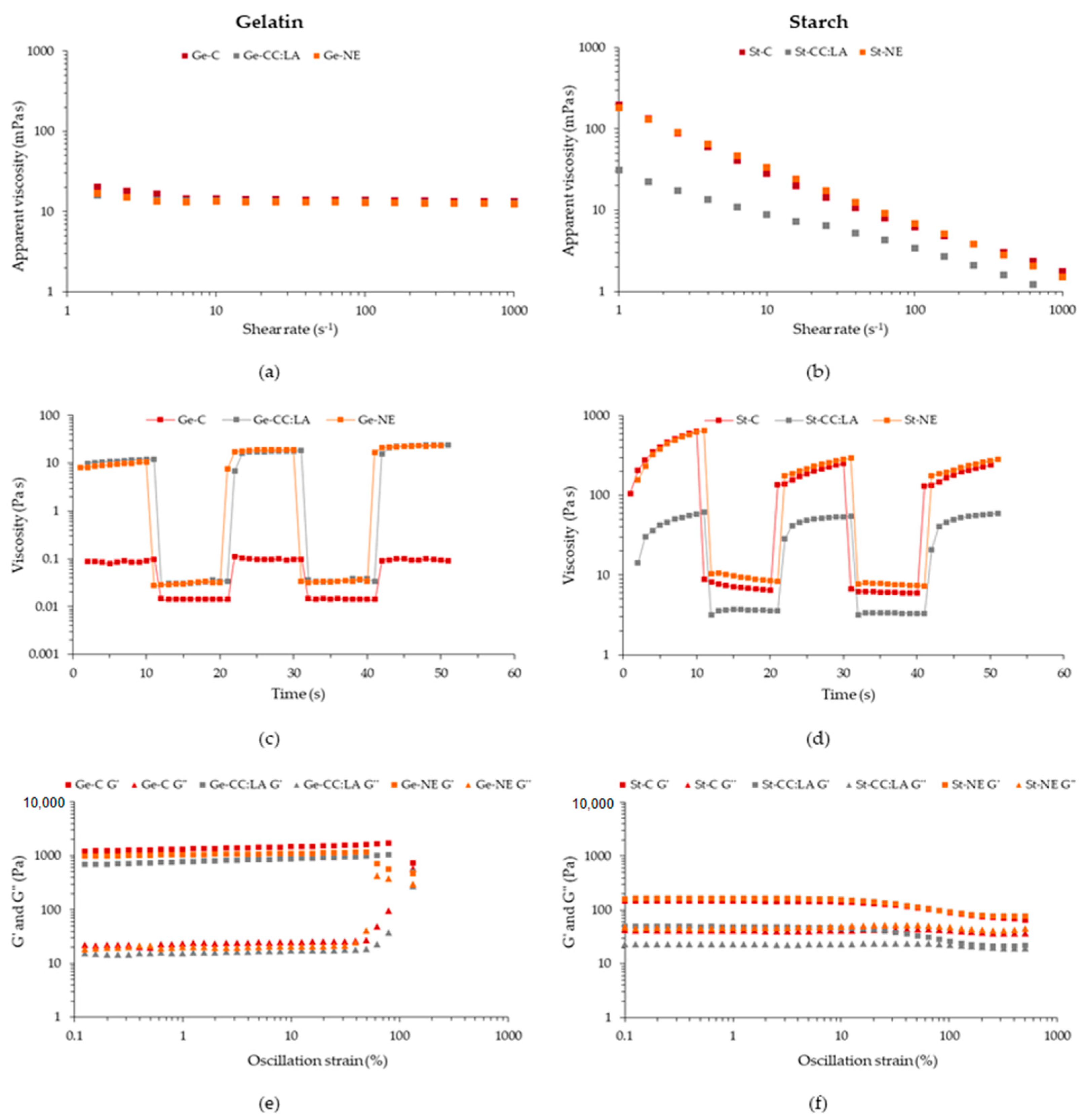
2.4.3. Rheological Behavior
2.5. Production and Characterization of 3D Printed Gummy Based on Gelatin and Starch
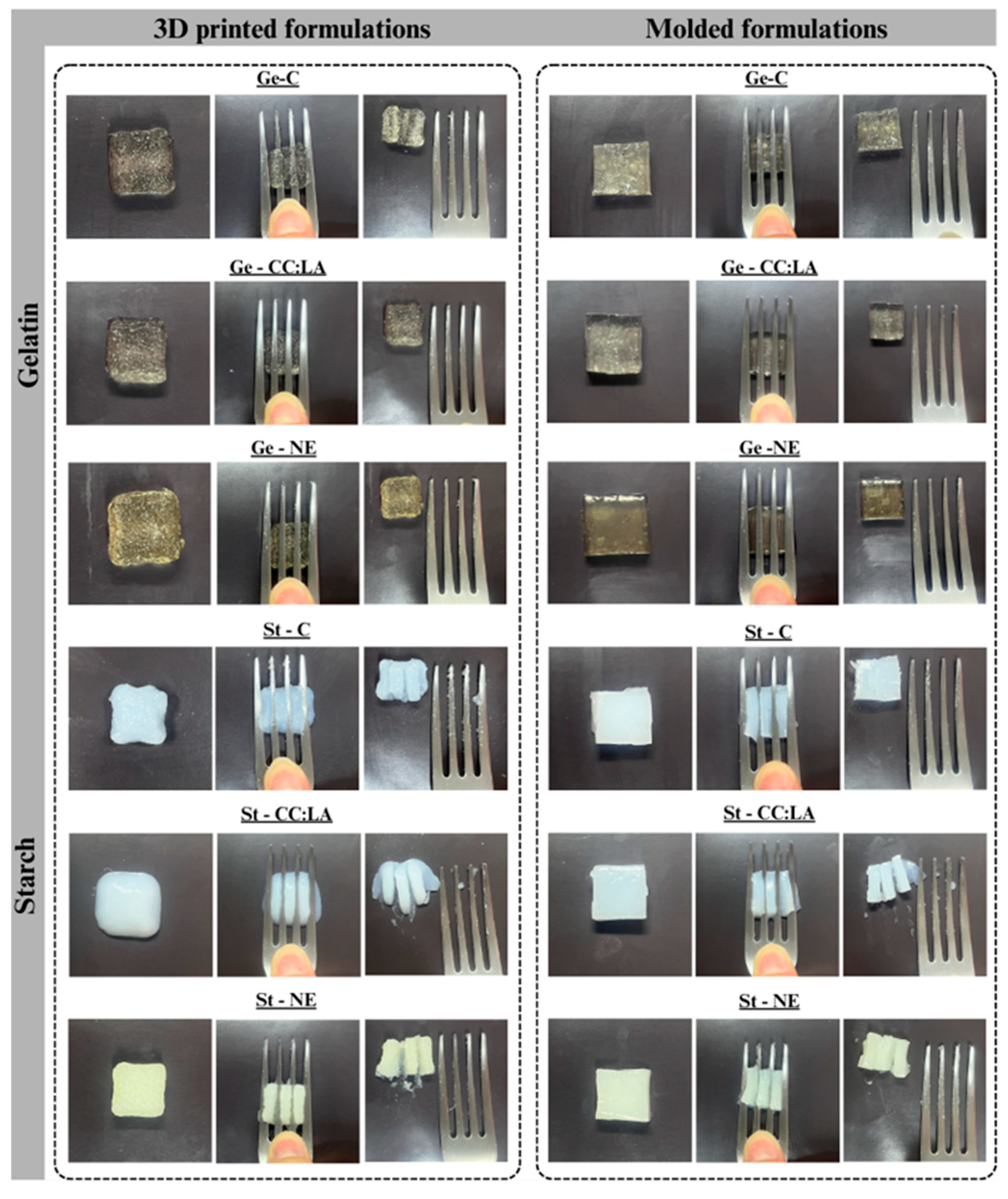
2.5.1. Production of 3D Printed Gummies
2.5.2. Printability and Reproducibility
2.5.3. Color and Antioxidant Activity
2.5.4. Texture Profile Analysis
2.5.5. Fork Pressure Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of NADES
3.2. Characterization of NADES–Annatto Seed Extract
3.3. Characterization of Gelatin- and Starch-Based Inks
3.3.1. Firmness and Adhesiveness
3.3.2. Rheological Behavior
3.4. Characterization of 3D Printed Gummies Based on Gelatin and Starch
3.4.1. Printability and Reproducibility
3.4.2. Color
3.4.3. Texture Profile Analysis
3.4.4. Fork Pressure Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bitencourt, B.S.; Guedes, J.S.; Saliba, A.S.M.C.; Sartori, A.G.O.; Torres, L.C.R.; Amaral, J.E.P.G.; Alencar, S.M.; Maniglia, B.C.; Augusto, P.E.D. Mineral Bioaccessibility in 3D Printed Gels Based on Milk/Starch/ĸ-Carrageenan for Dysphagic People. Food Res. Int. 2023, 170, 113010. [Google Scholar] [CrossRef] [PubMed]
- Giura, L.; Urtasun, L.; Belarra, A.; Ansorena, D.; Astiasarán, I. Exploring Tools for Designing Dysphagia-Friendly Foods: A Review. Foods 2021, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Chitrakar, B.; Hati, S.; Xie, S.; Li, H.; Li, C.; Liu, Z.; Mo, H. Development of Black Fungus-Based 3D Printed Foods as Dysphagia Diet: Effect of Gums Incorporation. Food Hydrocoll. 2022, 123, 107173. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Zhao, R.; Liu, Q.; Hu, H. Effect of Twin-Screw Extrusion Pretreatment on Starch Structure, Rheological Properties and 3D Printing Accuracy of Whole Potato Flour and Its Application in Dysphagia Diets. Int. J. Biol. Macromol. 2024, 278, 134796. [Google Scholar] [CrossRef]
- Bolívar-Prados, M.; Baixauli, R.; Ismael-Mohammed, K.; Ortega, O.; Clavé, P.; Laguna, L. Texture-Modified Foods for Patients with Swallowing and/or Mastication Impairments. In A Multidisciplinary Approach to Managing Swallowing Dysfunction in Older People; Academic Press: Cambridge, MA, USA, 2024; pp. 223–231. [Google Scholar] [CrossRef]
- Sponchiado, P.A.I.; de Melo, M.T.; Bitencourt, B.S.; Guedes, J.S.; Tapia-Blácido, D.R.; Augusto, P.E.D.; Ramos, A.P.; Maniglia, B.C. Clean Modification of Potato Starch to Improve 3D Printing of Potential Bone Bio-Scaffolds. Emergent Mater. 2024, 5, 1–14. [Google Scholar] [CrossRef]
- Montoya, J.; Medina, J.; Molina, A.; Gutiérrez, J.; Rodríguez, B.; Marín, R. Impact of Viscoelastic and Structural Properties from Starch-Mango and Starch-Arabinoxylans Hydrocolloids in 3D Food Printing. Addit. Manuf. 2021, 39, 101891. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Z.; Hu, L.; Cheng, Y.; Zhu, J.; Ma, L.; Zhang, Y. Self-Assembly of Gelatin and Phycocyanin for Stabilizing Thixotropic Emulsions and Its Effect on 3D Printing. Food Chem. 2022, 397, 133725. [Google Scholar] [CrossRef]
- Yap, K.L.; Kong, I.; Abdul Kalam Saleena, L.; Pui, L.P. 3D Printed Gelatin Film with Garcinia atroviridis Extract. J. Food Sci. Technol. 2022, 59, 4341–4351. [Google Scholar] [CrossRef]
- Renaldi, G.; Junsara, K.; Jannu, T.; Sirinupong, N.; Samakradhamrongthai, R.S. Physicochemical, Textural, and Sensory Qualities of Pectin/Gelatin Gummy Jelly Incorporated with Garcinia Atroviridis and Its Consumer Acceptability. Int. J. Gastron. Food Sci. 2022, 28, 100505. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of Rheological Properties of Potato, Rice and Corn Starches on Their Hot-Extrusion 3D Printing Behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Kokol, V.; Pottathara, Y.B.; Mihelčič, M.; Perše, L.S. Rheological Properties of Gelatine Hydrogels Affected by Flow- and Horizontally-Induced Cooling Rates during 3D Cryo-Printing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126356. [Google Scholar] [CrossRef]
- Cheng, Y.; Liang, K.; Chen, Y.; Gao, W.; Kang, X.; Li, T.; Cui, B. Effect of Molecular Structure Changes during Starch Gelatinization on Its Rheological and 3D Printing Properties. Food Hydrocoll. 2023, 137, 108364. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Preparation of Cassava Starch Hydrogels for Application in 3D Printing Using Dry Heating Treatment (DHT): A Prospective Study on the Effects of DHT and Gelatinization Conditions. Food Res. Int. 2020, 128, 108803. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Sartori, A.G.; Saliba, A.S.M.C.; Bitencourt, B.S.; Guedes, J.S.; Torres, L.C.R.; de Alencar, S.M.; Augusto, P.E.D. Anthocyanin Bioaccessibility and Anti-Inflammatory Activity of a Grape-Based 3D Printed Food for Dysphagia. Innov. Food Sci. Emerg. Technol. 2023, 84, 103289. [Google Scholar] [CrossRef]
- Strieder, M.M.; Vardanega, R.; Moraes, M.N.; Silva, E.K.; Meireles, M.A.A. One-Step Ultrasound-Assisted Recovery of Yellow-Orange-Red Natural Coloring from Defatted Annatto Seeds: A Cleaner Processing Alternative. Ultrason. Sonochem. 2024, 107, 106906. [Google Scholar] [CrossRef]
- Hirko, B.; Getu, A. Bixa orellana (Annatto Bixa): A Review on Use, Structure, Extraction Methods and Analysis. J. Agron. Technol. Eng. Manag. 2022, 5, 687–696. [Google Scholar]
- Shridar, B.; Paramadhas, S.; Palanisamy, P.; Murugesan, B.; Kalyanasundaram, K.; Jayakumar, J.; Pandiselvam, R. Development and Optimization of Temperature and Pressure-Assisted Mechanical Extraction System for Enhancing the Bixin Yield from Annatto Seeds. Biomass Convers. Biorefin. 2025, 15, 985–997. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; da Costa Lima, J.L.F.; Bragagnolo, N. In Vitro Scavenging Capacity of Annatto Seed Extracts against Reactive Oxygen and Nitrogen Species. Food Chem. 2011, 127, 419–426. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Sivapragasam, N.; Ali Redha, A.; Maqsood, S. Sustainable Green Extraction of Anthocyanins and Carotenoids Using Deep Eutectic Solvents (DES): A Review of Recent Developments. Food Chem. 2024, 448, 139061. [Google Scholar] [CrossRef]
- Silveira, T.M.G.; Tapia-Blácido, D.R. Is Isolating Starch from the Residue of Annatto Pigment Extraction Feasible? Food Hydrocoll. 2018, 77, 117–125. [Google Scholar] [CrossRef]
- Paramadhas, S.; Selvi, P.; Shridar, B.; Palanisamy, P.; Baburaj, N.S.; Govindarajan, N.; Pandiselvam, R. Optimization and Extraction of Annatto Pigments Obtained from Bixa orellana L. Using Supercritical Fluid Extraction. Microchem. J. 2024, 206, 111494. [Google Scholar] [CrossRef]
- Jayakumar, J.; Sudha, P.; Rajkumar, P.; Pandiselvam, R.; Gurusamy, K.; Kumaran, K.; Subramanian, P. Comparative Study on the Effect of Solvents on Extraction of Bixin from Annatto Seed (Bixa orellana L.) and Optimization of Process Parameters Using Box–Behnken Design. Biomass Convers. Biorefin. 2024, 14, 19863–19874. [Google Scholar] [CrossRef]
- Benvenutti, L.; del Pilar Sanchez-Camargo, A.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria cauliflora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Bogusz Junior, S.; Mitchell, A.E. Green Strategies for Recovery of Bioactive Phenolic Compounds from Agro-Industrial Wastes (Pomegranate Peels, Almond Hulls, and Elderberry Pomace) Using Natural Deep Eutectic Solvents. ACS Food Sci. Technol. 2023, 3, 2144–2156. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of Pomegranate Peel Waste: Natural Deep Eutectic Solvents as a Green Strategy to Recover Valuable Phenolic Compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Haghbakhsh, R.; Marques, R.; Paiva, A.; Carlyle, L.; Duarte, A.R.C. Evaluation of Deep Eutectic Systems as an Alternative to Solvents in Painting Conservation. ACS Sustain. Chem. Eng. 2021, 9, 15451–15460. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Fernandes, J.; Gonçalves, R.F.S.; Martins, J.T.; Berni, P.; Ribeiro, A.P.B.; Vicente, A.A.; Pinheiro, A.C. Performance of Β-carotene-loaded Nanostructured Lipid Carriers under Dynamic in Vitro Digestion System: Influence of the Emulsifier Type. J. Food Sci. 2024, 89, 3290–3305. [Google Scholar] [CrossRef]
- Smith, J. Annatto extracts-chemical and technical assessment. Chem Tech Assess Manual. 2006, 1–21. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Moraes, I.C.F.; Carvalho, R.A.; Bittante, A.M.Q.B.; Solorza-Feria, J.; Sobral, P.J.A. Film Forming Solutions Based on Gelatin and Poly(Vinyl Alcohol) Blends: Thermal and Rheological Characterizations. J. Food Eng. 2009, 95, 588–596. [Google Scholar] [CrossRef]
- IDDSI International Dysphagia Diet Standardization Initiative Framework Testing Methods 2.0. Available online: https://www.iddsi.org/images/Publications-Resources/DetailedDefnTestMethods/English/V2TestingMethodsEnglish31july2019.pdf (accessed on 6 February 2025).
- Fanali, C.; Gallo, V.; Della Posta, S.; Dugo, L.; Mazzeo, L.; Cocchi, M.; Piemonte, V.; De Gara, L. Choline Chloride–Lactic Acid-Based NADES As an Extraction Medium in a Response Surface Methodology-Optimized Method for the Extraction of Phenolic Compounds from Hazelnut Skin. Molecules 2021, 26, 2652. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction: Green Approaches for Extraction of Wine Lees Anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef]
- Alcalde, R.; Gutiérrez, A.; Atilhan, M.; Aparicio, S. An Experimental and Theoretical Investigation of the Physicochemical Properties on Choline Chloride–Lactic Acid Based Natural Deep Eutectic Solvent (NADES). J. Mol. Liq. 2019, 290, 110916. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Paiva, A.; Haghbakhsh, R.; Rita, C.; Duarte, A. Is It Possible to Correlate Various Physicochemical Properties of Natural Deep Eutectic Systems in Order to Predict Their Behaviours as Solvents? J. Mol. Liq. 2023, 384, 122280. [Google Scholar] [CrossRef]
- Cardarelli, C.R.; de Toledo Benassi, M.; Mercadante, A.Z. Characterization of Different Annatto Extracts Based on Antioxidant and Colour Properties. LWT-Food Sci. Technol. 2008, 41, 1689–1693. [Google Scholar] [CrossRef]
- Deka, B.; Chakravorty, P.; Das, A.B. Impact of Different Natural Deep Eutectic Solvents on Dissolution Behaviour and Eutectogel Structure of Jackfruit Seed Starch. J. Polym. Environ. 2024, 32, 632–640. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Skowrońska, D. Evaluation of Starch Plasticization Efficiency by Deep Eutectic Solvents Based on Choline Chloride. J. Mol. Liq. 2023, 384, 122210. [Google Scholar] [CrossRef]
- Chen, G.; Hu, J.; Liang, Y.; Huang, X.; Seah, G.L.; Li, J.; Liu, D.; Tan, C. Protein Gel with Designed Network and Texture Regulated via Building Blocks to Study Dysphagia Diet Classifications. Food Hydrocoll. 2023, 140, 108640. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural Properties of Films and Rheology of Film-Forming Solutions Based on Chitosan and Chitosan-Starch Blend Enriched with Murta Leaf Extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.-S.; Girard, M.; Therriault, D.; Heuzey, M.-C. 3D Printed Protein/Polysaccharide Food Simulant for Dysphagia Diet: Impact of Cellulose Nanocrystals. Food Hydrocoll. 2024, 148, 109455. [Google Scholar] [CrossRef]



| Properties | CC:LA | etOH-60% |
|---|---|---|
| pH at 25 °C | 1.28 ± 0.02 b | 6.27 ± 0.02 a |
| Density at 25 °C (g cm−3) | 1.145 ± 0.007 a | 0.873 ± 0.006 b |
| Viscosity at 25 °C (mPa s) | 19.4 ± 0.3 | - |
| ENR (kcal mol−1) | 48.1 ± 0.1 b | 50.3 ± 0.1 a |
| Properties | NE | EE |
|---|---|---|
| pH at 25 °C | 1.40 ± 0.03 b | 5.34 ± 0.10 a |
| Viscosity at 25 °C (mPa s) | 15.5 ± 0.8 | - |
| L* | 48.9 ± 0.8 a | 42.5 ± 0.8 b |
| a* | 15.3 ± 0.1 a | 15.6 ± 0.8 a |
| b* | 18.8 ± 2.3 a | 7.5 ± 1.1 b |
| C* | 24.3 ± 1.7 a | 17.4 ± 0.9 b |
| h* (°) | 50.7 ± 3.7 a | 25.6 ± 3.4 b |
| Bixin content by HPLC (μg g−1 annatto seed) | 20.56 ± 1.04 b | 448 ± 59 a |
| Total carotenoid content (μg β-carotene g−1 annatto seed) | 45.66 ± 2.34 b | 632 ± 38 a |
| Norbixin (%) | 1.08 ± 0.02 b | 3.14 ± 0.26 a |
| ABTS method (mg Trolox equivalent g−1 annatto seed) | 4.99 ± 0.62 a | 5.76 ± 0.79 a |
| FRAP method (mg Trolox equivalent g−1 annatto seed) | 53.28 ± 4.67 a | 67.50 ± 10.83 a |
| Hydrogel-Based Ink | Power-Law Model | Apparent Viscosity at 100 s−1 (Pa s) | Shear Recovery Rate (%) | tan δ (G″/G′) | ||
|---|---|---|---|---|---|---|
| K | N | R² | ||||
| Ge-C | - | - | - | (13.36 ± 0.4) d × 10−3 | 103.5 ± 1.0 c | 0.0170 ± 0.0004 b |
| Ge-CC:LA | - | - | - | (13.24 ± 0.1) d × 10−3 | 208.3 ± 15.3 b | 0.0197 ± 0.0006 a |
| Ge-NE | - | - | - | (12.95 ± 0.3) d × 10−3 | 242.4 ± 9.7 a | 0.0192 ± 0.0007 a |
| St-C | 151.8 ± 4.1 b | 0.672 ± 0.004 b | 0.991 | 6.29 ± 0.08 b | 37.7 ± 1.2 d | 0.2828 ± 0.0035 b |
| St-CC:LA | 28.1 ± 0.7 c | 0.481 ± 0.006 c | 0.997 | 3.32 ± 0.08 c | 93.5 ± 5.8 c | 0.4325± 0.0197 a |
| St-NE | 169.9 ± 1.2 a | 0.691 ± 0.002 a | 0.999 | 6.83 ± 0.04 a | 43.5 ± 0.4 d | 0.2805 ± 0.0069 b |
| 3D Printed Cuboids |  |  |  |  |  |  |  |
| Formulation | Model | Ge-C | Ge-CC:LA | Ge-NE | St-C | St-CC:LA | St-NE |
| Area (cm2) | 2.25 | 2.26 ± 0.02 b | 2.34 ± 0.18 b | 2.22 ± 0.01 b | 3.00 ± 0.08 a | 3.26 ± 0.37 a | 2.50 ± 0.12 b |
| Geometric fidelity (%) | - | 100 ± 1 b | 104 ± 8 b | 98 ± 1 b | 133 ± 4 a | 144 ± 16 a | 111 ± 5 b |
| Smoothly and continuously extruded |  |  |  |  |  |  |  |
| Keeps the structure after printing |  |  |  |  |  |  |  |
| Smooth and continuous lines |  |  |  |  |  |  |  |
| Without separation of lines |  |  |  |  |  |  |  |
| Higher resolution (well-defined shape) |  |  |  |  |  |  |  |
| Broken deposited lines |  |  |  |  |  |  |  |
| 3D printed stars |  |  |  |  |  |  |  |
| Formulation | Model | Ge-C | Ge-CC:LA | Ge-NE | St-C | St-CC:LA | St-NE |
| Star angles (°) | 52 | 62 ± 3 cd | 65 ± 3 bc | 69 ± 1 b | 77 ± 0.8 a | 64 ± 2 bc | 56 ± 3 d |
| Geometric fidelity (%) | - | 119 ± 6 c | 125 ± 6 bc | 132 ± 2 b | 148 ± 1 a | 123 ± 4 bc | 108 ± 6 d |
| Similar shape to the predesigned star geometry with well-defined angles |  |  |  |  |  |  |  |
| Continuous lines |  |  |  |  |  |  |  |
| Retains its shape after printing |  |  |  |  |  |  |  |
| Smoothly extruded |  |  |  |  |  |  |  |
| Formulation | Method | L* | a* | b* | C* | h* |
|---|---|---|---|---|---|---|
| Ge-C | 3D printed | 6.4 ± 0.3 cA | −1.0 ± 0.1 cB | 3.4 ± 0.8 cA | 2.0 ± 0.8 dB | 104.6 ± 4.4 cA |
| Molded | 7.9 ± 1.4 A | 2.4 ± 0.6 A | 4.4 ± 0.9 A | 5.4 ± 1.0 A | 63.1 ± 5.0 B | |
| Ge-CC:LA | 3D printed | 11.0 ± 0.1 aB | 1.0 ± 0.2 aA | 4.0 ± 0.1 cA | 4.1 ± 0.2 cA | 103.3 ± 3.7 cA |
| Molded | 12.8 ± 1.1 A | −1.1 ± 0.4 B | 4.0 ± 0.5 A | 4.2 ± 0.6 A | 104.9 ± 4.2 A | |
| Ge-NE | 3D printed | 10.0 ± 0.6 bA | −0.7 ± 0.1 bcB | 7.8 ± 0.5 cA | 8.0 ± 0.8 bA | 94.7 ± 1.6 dB |
| Molded | 11.4 ± 1.2 A | −2.0 ± 0.3 A | 5.2 ± 0.6 B | 5.4 ± 0.8 B | 111.1 ± 5.4 A | |
| St-C | 3D printed | 3.1 ± 0.3 dA | −0.4 ± 0.0 bB | −10.3 ± 0.5 eB | 10.4 ± 0.5 aA | 92.7 ± 0.7 dB |
| Molded | 0.4 ± 0.1 B | 2.1 ± 0.5 A | −4.0 ± 0.7 A | 4.2 ± 0.6 B | 149.6 ± 11.1 A | |
| St-CC:LA | 3D printed | 0.3 ± 0.1 eB | 1.5 ± 0.0 aA | −4.6 ± 0.5 dA | 5.3 ± 1.1 cA | 161.1 ± 1.4 bB |
| Molded | 2.3 ± 0.2 A | −0.6 ± 0.0 B | −5.7 ± 0.2 B | 5.7 ± 0.2 A | 174.6 ± 1.1 A | |
| St-NE | 3D printed | 9.3 ± 0.4 bB | −3.4 ± 0.4 dA | 5.9 ± 0.5 aB | 6.9 ± 0.5 bcA | 120.3 ± 4.1 aA |
| Molded | 10.8 ± 0.6 A | −3.0 ± 0.8 A | 4.1 ± 0.4 B | 4.6 ± 0.7 B | 114.6 ± 10.9 A |
| Formulation | Method | Hardness (N) | Adhesiveness (-) | Springiness (mm) | Cohesiveness (mJ) | Gumminess (N) |
|---|---|---|---|---|---|---|
| Ge-C | 3D printed | 15.81 ± 2.12 bB | * | 1.04 ± 0.03 aA | 0.92 ± 0.01 aA | 14.48 ± 1.86 aB |
| Molded | 24.59 ± 1.82 A | 18.91 ± 3.15 | 0.95 ± 0.01 B | 0.82 ± 0.04 B | 20.24 ± 1.05 A | |
| Ge-CC:LA | 3D printed | 21.04 ± 2.67 aB | * | 1.06 ± 0.05 aA | 0.88 ± 0.01 aA | 18.61 ± 2.38 aB |
| Molded | 77.61 ± 2.82 A | 20.63 ± 2.23 | 0.97 ± 0.02 B | 0.77 ± 0.06 B | 60.15 ± 5.33 A | |
| Ge-NE | 3D printed | 20.91 ± 2.47 aB | * | 1.09 ± 0.04 aA | 0.87 ± 0.01 aA | 18.24 ± 2.20 aB |
| Molded | 55.88 ± 8.91 A | 20.73 ± 3.91 | 0.95 ± 0.00 B | 0.69 ± 0.05 B | 38.59 ± 4.94 A | |
| St-C | 3D printed | 4.17 ± 0.37 cB | 1.94 ± 0.86 cB | 0.84 ± 0.08 aA | 0.48 ± 0.05 bA | 2.03 ± 0.28 bB |
| Molded | 15.82 ± 2.99 A | 50.41 ± 2.51 A | 0.87 ± 0.07 A | 0.49 ± 0.10 A | 7.01 ± 0.90 A | |
| St-CC:LA | 3D printed | 6.76 ± 1.07 cB | 7.48 ± 0.92 aB | 0.51 ± 0.09 aB | 0.36 ± 0.04 cB | 2.46 ± 0.42 bB |
| Molded | 17.00 ± 3.93 A | 28.64 ± 7.55 A | 0.79 ± 0.09 A | 0.48 ± 0.05 A | 7.52 ± 1.20 A | |
| St-NE | 3D printed | 4.51 ± 2.26 cB | 5.05 ± 1.20 bA | 0.60 ± 0.09 aB | 0.28 ± 0.01 dB | 1.29 ± 0.07 bB |
| Molded | 10.00 ± 0.88 A | 2.71 ± 1.6 A | 0.84 ± 0.07 A | 0.39 ± 0.04 A | 3.96 ± 0.52 A |
| Molded Samples | Level 5 Parameters | Ge-C | Ge-CC:LA | Ge-NE | St-C | St-CC:LA | St-NE |
| Easy to mash with a fork |  |  |  |  |  |  |  |
| Blanched white is noted |  |  |  |  |  |  |  |
| Shape recovery |  |  |  |  |  |  |  |
| Easily broken with a fork |  |  |  |  |  |  |  |
| Separate thin liquid |  |  |  |  |  |  |  |
| 3D printed samples | Level 5 parameters | Ge-C | Ge-CC:LA | Ge-NE | St-C | St-CC:LA | St-NE |
| Easy to mash with a fork |  |  |  |  |  |  |  |
| Blanched white is noted |  |  |  |  |  |  |  |
| Shape recovery |  |  |  |  |  |  |  |
| Easily broken with a fork |  |  |  |  |  |  |  |
| Separate thin liquid |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balabram, S.K.; Tessaro, L.; Astolfo, M.E.d.A.; Sponchiado, P.A.I.; Bogusz Junior, S.; Maniglia, B.C. Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients. Foods 2025, 14, 1604. https://doi.org/10.3390/foods14091604
Balabram SK, Tessaro L, Astolfo MEdA, Sponchiado PAI, Bogusz Junior S, Maniglia BC. Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients. Foods. 2025; 14(9):1604. https://doi.org/10.3390/foods14091604
Chicago/Turabian StyleBalabram, Sara Kierulff, Larissa Tessaro, Maria Eduarda de Almeida Astolfo, Pedro Augusto Invernizzi Sponchiado, Stanislau Bogusz Junior, and Bianca C. Maniglia. 2025. "Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients" Foods 14, no. 9: 1604. https://doi.org/10.3390/foods14091604
APA StyleBalabram, S. K., Tessaro, L., Astolfo, M. E. d. A., Sponchiado, P. A. I., Bogusz Junior, S., & Maniglia, B. C. (2025). Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients. Foods, 14(9), 1604. https://doi.org/10.3390/foods14091604







