Nutrient Composition of Autochthonous Beef from Southwest Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Slaughter of Animals and Sample Collection
2.3. Proximate Composition
2.4. Mineral Composition
2.5. Fatty Acid Profile
2.6. Volatile Compound
2.7. Statistical Analysis
3. Results
3.1. Discriminant Analysis
3.2. Carcass Weight and Proximate Composition
3.3. Mineral Composition of Meat
3.4. Fatty Acid Composition of Meat
3.5. Volatile Compounds Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. EU Agricultural Outlook Report. 2024. Available online: https://agriculture.ec.europa.eu/data-and-analysis (accessed on 10 July 2025).
- MAPA. Informe Resumen Caracterización Sector Vacuno de Carne. Año 2023. Ministerio de Agricultura. Pesca y Alimentación. Gobierno de España. 2024. Available online: https://www.mapa.gob.es/es/ (accessed on 25 May 2025).
- Scollan, N.D.; Hocquette, J.F.; Nuernberg, K.; Dannenberger, D.; Richardson, R.I.; Maloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.F.; McAuliffe, G.A.; Tweed, J.K.S.; Griffith, B.A.; Morgan, S.A.; Rivero, M.J.; Harris, P.; Takahashi, T.; Cardenas, L. Nutritional value of suckler beef from temperate pasture systems. Animal 2021, 15, e100257. [Google Scholar] [CrossRef]
- Sainsbury, J.; Schonfeldt, H.C.; Van Heerden, S.M. The nutrient composition of South African mutton. J. Food Compos. Anal. 2011, 24, 720–726. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Crops and Livestock Products. 2025. Available online: https://www.fao.org/statistics/es (accessed on 10 July 2025).
- MAPA. Logotipo Raza Autóctona. Ministerio de Agricultura. Pesca y Alimentación. Gobierno de España. 2025. Available online: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia (accessed on 25 May 2025).
- ARCA. Sistema Nacional de Información de Razas. Gobierno de España. 2021. Available online: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia/razas-ganaderas/default.aspx (accessed on 10 July 2025).
- Gaspar, P.; Escribano, M.; Meseías, F.J.; Rodríguez de Ledesma, A.; Pulido, F. Sheep farms in the Spanish rangelands (dehesas): Typologies according to livestock management and economic indicators. Small Rumin. Res. 2008, 74, 52–63. [Google Scholar] [CrossRef]
- Piedrafita, J.; Quintanilla, R.; Sañudo, C.; Olleta, J.L.; Campo, M.M.; Panea, B.; Renand, G.; Turin, F.; Jabet, S.; Osoro, K.; et al. Carcass quality of 10 beef cattle breeds of the Southwest of Europe in their typical production systems. Livest. Prod. Sci. 2003, 82, 1–13. [Google Scholar] [CrossRef]
- Rubio Lozano, M.S.; Ngapo, T.M.; Huerta-Leidenz, N. Tropical beef: Is there an axiomatic basis to define the concept? Foods 2021, 10, 1025. [Google Scholar] [CrossRef]
- Sevane, N.; Nute, G.; Sañudo, C.; Cortés, O.; Cañón, J.; Williams, J.L.; Dunner, S.; the GemQual Consortium. Muscle lipid composition in bulls from 15 European breeds. Livest. Sci. 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Campo, M.M.; Romero, J.V.; Guerrero, A.; Bouzaida, M.D.; Resconi, V.C.; Tesniere, G.; Santolaria, P.; Olleta, J.L. Nutrient composition of beef from the Pyrenees. J. Food Compos. Anal. 2024, 133, 106452. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Regulation 1099/2009 Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Off. J. Eur. Union 2009, 303, 1–30. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Horwitz, W., Latimer, G., Eds.; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Türkmen, M.; Ciminli, C. Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry. Food Chem. 2007, 103, 670–675. [Google Scholar] [CrossRef]
- Gutiérrez-Peña, R.; Avilés, C.; Galán-Soldevilla, H.; Polvillo, O.; Ruiz, P.; Guzmán, J.L.; Horcada, A.; Delgado-Pertíñez, M. Physicochemical Composition, Antioxidant Status, Fatty Acid Profile, and Volatile Compounds of Milk and Fresh and Ripened Ewes’ Cheese from a Sustainable Part-Time Grazing System. Foods 2021, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Peña, R.; García-Infante, M.; Delgado-Pertíñez, M.; Guzmán, J.L.; Zarazaga, L.A.; Simal, S.; Horcada, A. Organoleptic and nutritional traits of lambs from Spanish mediterranean Islands raised under a traditional production system. Foods 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Resconi, V.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples: Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Stashenko, E.; Martínez, J. Algunos aspectos prácticos para la identificación de analitos por cromatografía de gases acoplada a espectrometría de masas. Sci. Chromatogr. 2010, 2, 29–47. [Google Scholar]
- Statgraphics Centurion, Version 18; Statgraphics Technologies, Inc.: The Plains, VA, USA, 2017.
- Beriain, M.J.; Horcada, A.; Lizaso, G.; Insausti, K.; Purroy, A. Meat quality from fighting bulls in Spain. Rev. Cient. 2011, 21, 88–95. [Google Scholar]
- Lomillos, J.M.; de la Vega, M.A. Characteristics, treatment and commercialization of lidia cattle meat. Rev. Complut. Cienc. Vet. 2016, 10, 94–111. [Google Scholar] [CrossRef]
- Toldra, F. Lawrie’s Meat Science, 9th ed.; Woodhead Publishing, Elsevier: Oxford, UK, 2023; p. 173. [Google Scholar]
- Cafferky, J.; Hamill, R.; Allen, P.; O’Doherty, J.V.; Cromie, A.; Sweeney, T. Effect of Breed and Gender on Meat Quality of M. longissimus thoracis et lumborum Muscle from Crossbred Beef Bulls and Steers. Foods 2019, 8, 173. [Google Scholar] [CrossRef]
- Nian, Y.; Allen, P.; Harrison, S.M.; Kerry, J.P. Effect of castration and carcass suspension method on the quality and fatty acid profile of beef from male dairy cattle. J. Sci. Food Agric. 2018, 98, 4339–4350. [Google Scholar] [CrossRef]
- Nogales, S.; Bressan, M.C.; Delgado, J.V.; da Gama, L.T.; Barba, C.; Camacho, M.E. Fatty acid profile of feral cattle meat. Ital. J. Anim. Sci. 2016, 16, 172–184. [Google Scholar] [CrossRef]
- Humada, M.J.; Serrano, E.; Sañudo, C.; Rolland, D.C.; Dugan, M.E.R. Production system and slaughter age effects on intramuscular fatty acids from young Tudanca Bulls. Meat Sci. 2012, 90, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.; Alves, S.; Martins, S.; Costa, A.; Fontes, C.; Lemos, J.; Bessa, R.; Prates, J. Effect of the feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Mao, Y.; Hopkins, D.; Zhang, Y.; Li, P.; Zhu, L.; Dong, P.; Liang, R.; Dai, J.; Wang, W.; Luo, X. Beef quality with different intramuscular fat content and proteomic analysis using isobaric tag for relative and absolute quantitation of differentially expressed proteins. Meat Sci. 2016, 118, 96–102. [Google Scholar] [CrossRef]
- Horcada, A.; Polvillo, O.; Juárez, M.; Avilés, C.; Martínez, A.L.; Peña, F. Influence of feeding system (concentrate and total mixed ration) on fatty acid profiles of beef from three lean cattle breeds. J. Food Compos. Anal. 2016, 49, 110–116. [Google Scholar] [CrossRef]
- Brugiapaglia, A.; Lussiana, C.; Destefanis, G. Fatty acid profile and cholesterol content of beef at retail of Piemontese, Limousin and Friesian breeds. Meat Sci. 2014, 96, 568–573. [Google Scholar] [CrossRef]
- Panea, B.; Ripoll, G.; Sañudo, C.; Olleta, J.L.; Albertí, P. Calidad instrumental de la carne de terneros procedentes del cruce industrial de la raza Retinta. Inf. Tec. Econ. Agrar. 2011, 112, 286–300. [Google Scholar]
- Moreno, T.; Varela, A.; Portela, C.; Pérez, N.; Carballo, J.A.; Monserrat, L. The effect of grazing on the fatty acid profiles of longissimus thoracis muscle in Galician Blond calves. Animal 2007, 1, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Suman, S.P.; Canto, A.; da Costa-Lima, B.; Viana, F.; Monteiro, M.; Silva, T.; Conte, C. Influence of muscle source on proximate composition, texture profile and protein oxidation of beef from grain-finished Bos indicus cattle. Cienc. Rural 2019, 49, e20180996. [Google Scholar] [CrossRef]
- Daza, A.; Rey, A.I.; Lopez-Carrasco, C.; Lopez-Bote, C.J. Effect of gender on growth performance, carcass characteristics and meat and fat quality of calves of avileña-negra ibérica breed fattened under free-range conditions. Span. J. Agric. Res. 2014, 12, 683–693. [Google Scholar] [CrossRef]
- Silva, F.L.; Oliveira-Júnior, E.S.; Silva, M.H.M.; Lopez-Alonso, M.; Pierangeli, M.A.P. Trace elements in beef cattle: A review of the scientific approach from one health perspective. Animals 2022, 12, 2254. [Google Scholar] [CrossRef]
- Taylor, J.B.; Marchello, M.J.; Finley, J.W.; Neville, T.L.; Combs, G.F.; Caton, J.S. Nutritive value and display-life attributes of selenium-enriched beef-muscle foods. J. Food Compos. Anal. 2008, 21, 183–186. [Google Scholar] [CrossRef]
- Reiné, R.; Ascaso, J.; Barrantes, O. Nutritional quality of plant species in Pyrenean hay meadows of high diversity. Agronomy 2020, 10, 883. [Google Scholar] [CrossRef]
- European Food Safety Authority. Available online: https://multimedia.efsa.europa.eu/drvs/index.htm (accessed on 10 November 2025).
- Campo, M.M.; Silva, A.; Guerrero, A.; Castro, G.; Olleta, J.L.; Martín, N.; Fernández, C.; López, F. Nutrient composition of Spanish small ruminant. J. Food Compos. Anal. 2021, 102, 104019. [Google Scholar] [CrossRef]
- Arenas de Moreno, L.; Jerez-Timaure, N.; Huerta-Leidenz, N.; Giuffrida-Mendoza, M.; Mendoza-Vera, E.; Uzcategui-Brach, S. Multivariate relationships among carcass traits and proximate composition, lipid profile, and mineral content of longissimus lumborum of grass-fed male cattle produced under tropical conditions. Foods 2021, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.; Wilkinson, B.; Carruthers, F.; Jackson, F. A comparison of the nutrient content of uncooked and cooked lean from New Zealand beef and lamb. J. Food Compos. Anal. 2014, 35, 75–82. [Google Scholar] [CrossRef]
- Poveda-Arteaga, A.; Krell, J.; Gibis, M.; Heinz, V.; Terjung, N.; Tomasevic, I. Intrinsic and extrinsic factors affecting the color of fresh beef meat—Comprehensive review. Appl. Sci. 2023, 13, 4382. [Google Scholar] [CrossRef]
- Haase, H.; Ellinger, S.; Linseisen, J.; Neuhäuser-Berthold, M.; Richter, M. German Nutrition Society. Revised DA-CH-reference values for the intake of zinc. J. Trace Elem. Med. Biol. 2020, 61, 126536. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Adachi, H.; Toyomasu, K.; Yoshida, N.; Enomoto, M.; Hiratsuka, A.; Hirai, Y.; Satoh, A.; Imaizumi, T. Very long chain N-3 fatty acids intake and carotid atherosclerosis: An epidemiological study evaluated by ultrasonography. Atherosclerosis 2004, 176, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.; Gibbs, R. Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them. Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Lock, A.L.; Shingfield, K.J.; Bauman, D.E. Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv. Food Nutr. Res. 2005, 50, 179–217. [Google Scholar] [CrossRef]
- McAfee, A.J.; Mc Sorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Rodero, E.; González, A.; Avilés, C.; Luque, M. Conservation of Endangered Spanish Cattle Breeds Using Markers of Candidate Genes for Meat Quality. Anim. Biotechnol. 2013, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.F.; Camacho Vallejo, M.E.; González Ariza, A.; León Jurado, J.M.; Delgado Bermejo, J.V.; Marín Navas, C.; Navas González, F.J. Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk. Animals 2024, 14, 1131. [Google Scholar] [CrossRef] [PubMed]
- García-Infante, M.; Castro-Valdecantos, P.; Delgado-Pertíñez, M.; Teixeira, A.; Guzmán, J.L.; Horcada, A. Effectiveness of machine learning algorithms as a tool to meat traceability system. A case study to classify Spanish Mediterranean lamb carcasses. Food Control 2024, 164, 110604. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F. Quality of meat and meat products produced from Southwest European pig breeds. Meat Sci. 2012, 90, 511–518. [Google Scholar] [CrossRef]
- Chambers, E., 4th; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Gorráiz, C.; Beriain, M.J.; Chasco, J.; Insausti, K. Effect of Aging Time on Volatile Compounds. Odor and Flavor of Cooked Beef from Pirenaica and Friesian bulls and heifers. J. Food Sci. 2002, 67, 916–922. [Google Scholar] [CrossRef]
- Park, M.K.; Shin, D.M.; Choi, Y.S. Comparison of volatile compound profiles derived from various livestock protein alternatives including edible-insect and plant-based proteins. Food Chem. X 2024, 23, 101570. [Google Scholar] [CrossRef] [PubMed]
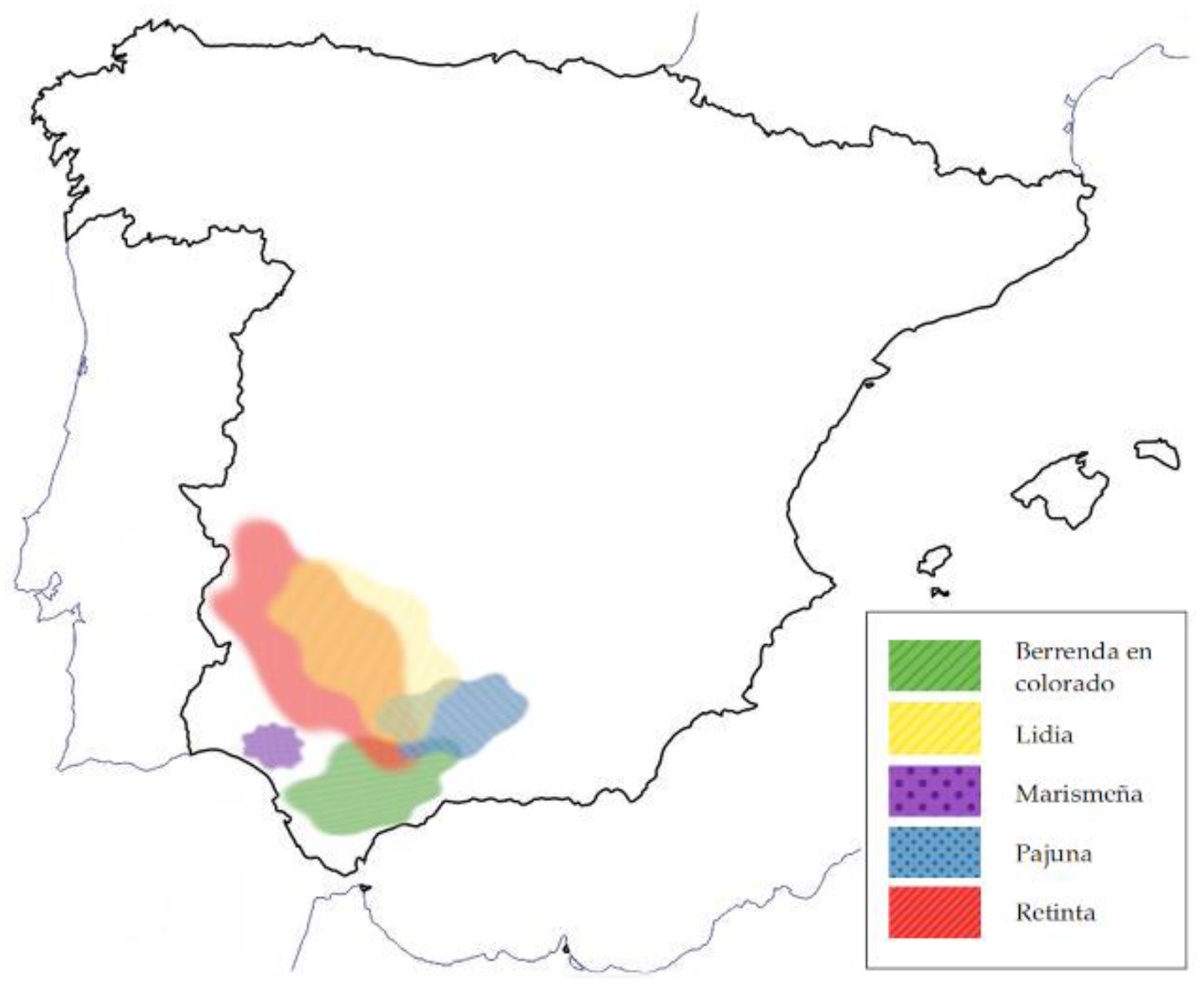
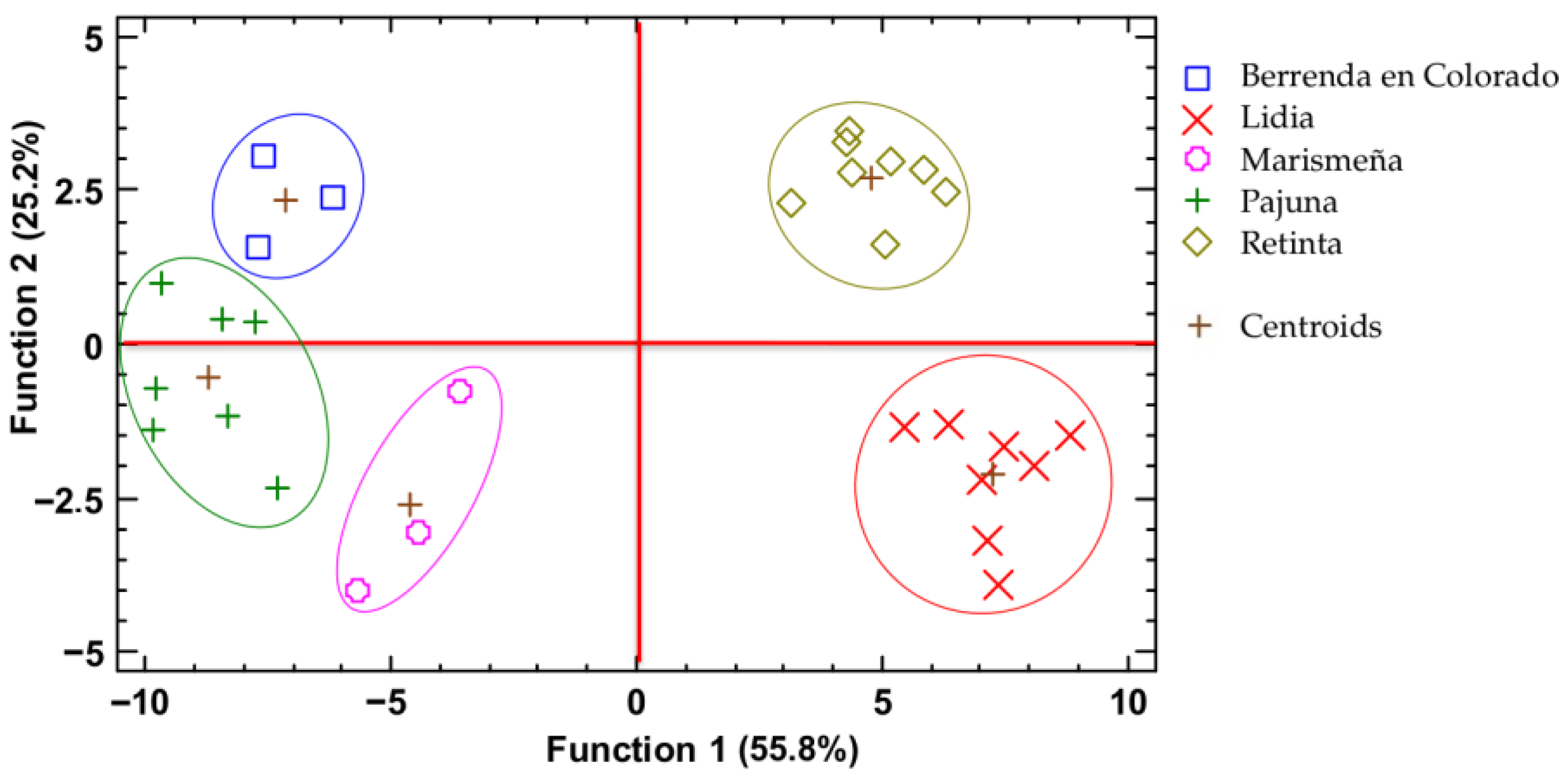
| Retinta | Pajuna | Marismeña | Berrenda en Colorado | Lidia | |
|---|---|---|---|---|---|
| n | 8 | 7 | 3 | 3 | 8 |
| Sex | Male | Castrated | Male | Male | Female |
| System of feeding | F + C | P + C | P + C | P + C | P + C |
| Age at slaughter (days) | 546 ± 165 | 722 ± 157 | 892 ± 27 | 1201 ± 396 | 946 ± 204 |
| Cold carcass weight (kg) | 301.40 ± 37.29 | 276.49 ± 30.64 | 327.67 ± 19.22 | 235.40 ± 39.30 | 108.22 ± 19.10 |
| Breed | SE | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Retinta | Pajuna | Marismeña | Berrenda en Colorado | Lidia | |||
| Cold carcass weight (kg) | 301.40 a | 276.49 ab | 327.67 a | 235.40 b | 108.22 c | 16.58 | ≤0.001 |
| pH24h | 5.56 | 5.66 | 5.75 | 5.65 | 5.71 | 0.03 | 0.676 |
| Fat (%) | 3.90 bc | 8.41 a | 4.62 bc | 5.56 b | 2.98 c | 0.44 | ≤0.001 |
| Moisture (%) | 71.20 ab | 69.19 c | 70.55 bc | 69.47 bc | 72.62 a | 0.30 | ≤0.001 |
| Protein (%) | 23.51 a | 20.98 b | 23.70 a | 23.82 a | 22.61 a | 0.24 | ≤0.001 |
| Ash (%) | 1.05 | 1.09 | 1.06 | 1.03 | 1.17 | 0.02 | 0.433 |
| Breed | SE | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Retinta | Pajuna | Marismeña | Berrenda en Colorado | Lidia | |||
| Calcium | 11.58 a | 8.87 ab | 8.66 ab | 4.83 b | 7.90 ab | 0.65 | 0.035 |
| Sodium | 57.41 ab | 63.26 ab | 72.06 a | 53.72 ab | 52.31 b | 1.82 | 0.012 |
| Phosphorus | 235.15 | 220.67 | 234.17 | 222.19 | 235.47 | 3.02 | 0.311 |
| Iron | 1.81 | 2.17 | 2.55 | 1.64 | 2.71 | 0.14 | 0.083 |
| Potassium | 278.13 | 263.55 | 286.18 | 273.75 | 265.00 | 4.14 | 0.485 |
| Magnesium | 24.64 | 21.97 | 24.12 | 23.76 | 23.15 | 0.34 | 0.057 |
| Zinc | 5.01 | 6.03 | 6.02 | 4.79 | 6.76 | 0.24 | 0.059 |
| Selenium | traces | traces | traces | traces | traces | - | - |
| Breed | SE | p-Values | |||||
|---|---|---|---|---|---|---|---|
| Retinta | Pajuna | Marismeña | Berrenda en Colorado | Lidia | |||
| Total SFA | 4.243 b,c | 5.451 b | 3.021 c | 6.379 a | 5.620 b | 0.27 | 0.005 |
| C8:0 | 0.037 b,c | 0.050 a,b | 0.048 a,b | 0.060 a | 0.047 a,b | 0.00 | 0.016 |
| C10:0 | 0.054 | 0.060 | 0.056 | 0.075 | 0.057 | 0.00 | 0.212 |
| C11:0 | 0.008 b | 0.011 a,b | 0.016 a | 0.010 a,b | 0.007 b | 0.00 | 0.003 |
| C12:0 | 0.065 b | 0.069 a,b | 0.061 b | 0.104 a | 0.065 b | 0.00 | 0.005 |
| C13:0 | 0.013 b | 0.019 a | 0.011 b | 0.013 a,b | 0.016 a,b | 0.00 | 0.011 |
| C14:0 | 0.236 a,b | 0.294 a,b | 0.140 c | 0.359 a | 0.206 b,c | 0.02 | 0.005 |
| C15:0 | 0.051 | 0.050 | 0.047 | 0.065 | 0.067 | 0.00 | 0.117 |
| C16:0 | 2.084 b | 2.735 a,b | 1.424 b | 3.460 a | 2.518 a,b | 0.15 | 0.006 |
| C17:0 | 0.075 a,b | 0.100 a,b | 0.065 b | 0.117 a,b | 0.106 a | 0.01 | 0.010 |
| C18:0 | 1.553 b | 1.922 a,b | 1.089 b | 2.002 a,b | 2.430 a | 0.11 | 0.001 |
| C20:0 | 0.024 b | 0.062 a | 0.016 b | 0.038 a,b | 0.052 a | 0.00 | 0.000 |
| C21:0 | 0.008 c | 0.019 b | 0.011 b,c | 0.030 a | 0.015 b | 0.00 | 0.000 |
| C22:0 | 0.010 b,c | 0.012 a,b | 0.012 a,b,c | 0.016 a | 0.008 c | 0.00 | 0.000 |
| C23:0 | 0.013 b | 0.035 a | 0.019 a,b | 0.020 a,b | 0.010 b | 0.00 | 0.001 |
| C24:0 | 0.004 b,c | 0.006 a,b | 0.003 | 0.009 a | 0.014 a | 0.00 | 0.000 |
| Total MUFA | 2.862 c | 4.480 b | 2.162 c | 5.124 a | 4.220 b | 0.25 | 0.002 |
| C14:1 | 0.034 b | 0.025 b | 0.020 b | 0.069 a | 0.035 b | 0.00 | 0.000 |
| C15:1 | 0.023 | 0.025 b | 0.031 | 0.032 | 0.028 | 0.00 | 0.658 |
| C16:1 | 0.201 a,b | 0.338 a | 0.151 b | 0.409 a | 0.217 b | 0.02 | 0.000 |
| C17:1 | 0.047 c | 0.083 a,b | 0.050 b,c | 0.113 a | 0.073 b,c | 0.01 | 0.000 |
| C18:1n-9t | 0.080 b | 0.185 a | 0.044 b | 0.096 b | 0.082 b | 0.01 | 0.000 |
| C18:1n-11t | 0.194 a,b | 0.190 a,b | 0.138 b | 0.251 a,b | 0.253 a | 0.01 | 0.033 |
| C18:1n-9c | 2.240 b,c | 3.577 a | 1.702 c | 4.107 a | 3.479 a,b | 0.21 | 0.002 |
| C20:1n-9 | 0.025 | 0.036 | 0.016 | 0.017 | 0.030 | 0.00 | 0.075 |
| C22:1n-9 | 0.016 a,b | 0.016 a,b | 0.013 b | 0.021 a,b | 0.021 a | 0.00 | 0.011 |
| C24:1 | 0.003 b | 0.004 b | 0.002 a,b | 0.007 a,b | 0.018 a | 0.00 | 0.014 |
| Total PUFA | 0.712 b | 0.913 a,b | 0.562 c | 0.779 b | 1.161 a | 0.05 | 0.001 |
| C18:2n-6t | 0.020 b,c | 0.042 a | 0.010 c | 0.033 a,b | 0.049 a | 0.00 | 0.000 |
| C18:2n-6c | 0.428 a,b | 0.459 a,b | 0.323 b,c | 0.281 c | 0.508 a | 0.02 | 0.002 |
| C18:3n-6 | 0.011 a,b | 0.014 a | 0.006 b | 0.015 a | 0.012 a,b | 0.00 | 0.032 |
| C18:3n-3 | 0.022 | 0.038 | 0.018 | 0.036 | 0.217 | 0.03 | 0.090 |
| 9c-11t CLA | 0.023 b | 0.048 a | 0.022 b | 0.051 a | 0.039 a,b | 0.00 | 0.001 |
| 9c-11c CLA | 0.008 b,c | 0.013 a | 0.004 c | 0.013 a,b | 0.011 a,b | 0.00 | 0.000 |
| 10t12c CLA | 0.006 a,b | 0.009 a | 0.003 b | 0.009 a | 0.009 a | 0.00 | 0.001 |
| C20:2 | 0.012 b | 0.019 a | 0.009 b | 0.013 a,b | 0.012 b | 0.00 | 0.000 |
| C20:3n-6 | 0.030 c,d | 0.038 b,c | 0.018 | 0.072 a | 0.050 a,b | 0.00 | 0.000 |
| C20:4n-6 | 0.071 b | 0.087 a,b | 0.076 a,b | 0.113 a,b | 0.116 a | 0.01 | 0.003 |
| C20:3n-3 | 0.008 a | 0.004 b | 0.005 b | 0.008 a | 0.008 a | 0.00 | 0.000 |
| C20:5n-3 (EPA) | 0.010 c | 0.024 b | 0.022 a,b | 0.037 a | 0.026 a,b | 0.00 | 0.000 |
| C22:2 | 0.008 | 0.014 | 0.008 | 0.010 | 0.012 | 0.00 | 0.377 |
| C22:5n-3 (DPA) | 0.043 b | 0.079 a | 0.034 b | 0.075 a | 0.081 a | 0.00 | 0.000 |
| C22:6n-3 (DHA) | 0.011 b | 0.022 a | 0.006 b | 0.015 a,b | 0.016 a | 0.00 | 0.000 |
| n-6 PUFA | 0.55 b | 0.63 a | 0.43 b | 0.50 b | 0.72 a | 0.03 | 0.001 |
| n-3 PUFA | 0.09 b | 0.17 a | 0.08 b | 0.17 a | 0.35 a | 0.03 | 0.027 |
| PUFA/SFA | 0.17 a | 0.17 a | 0.19 a | 0.13 b | 0.21 a | 0.01 | 0.012 |
| n-6/n-3 | 6.09 a | 3.74 b | 5.23 a | 2.96 b | 2.27 b | 0.29 | 0.000 |
| Desirable fatty acids | 5.13 b | 7.31 a | 3.81 b | 7.91 a | 7.81 a | 0.39 | 0.002 |
| Breed | SE | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Retinta | Pajuna | Marismeña | Berrenda en Colorado | Lidia | |||
| Total carboxylic acid | 394.12 | 358.34 | 347.52 | 363.34 | 334.30 | 37.12 | 0.987 |
| Total alcohol | 413.18 | 567.09 | 803.54 | 610.23 | 489.23 | 58.90 | 0.467 |
| Total aldehydes | 629.11 | 411.33 | 598.95 | 426.82 | 212.50 | 62.89 | 0.138 |
| Total ketones | 86.08 b | 69.55 b | 196.92 a | 88.55 b | 40.04 c | 11.07 | 0.001 |
| Total sulfur compounds | 40.08 | 21.20 | 22.22 | 25.08 | 19.97 | 3.41 | 0.185 |
| Total furans | 32.55 | 21.93 | 50.42 | 33.85 | 21.58 | 4.14 | 0.332 |
| Total nitrogen compounds | 167.74 | 97.19 | 218.08 | 195.89 | 60.54 | 19.20 | 0.052 |
| Total pyrazines | 70.58 | 41.68 | 123.00 | 44.44 | 27.65 | 10.60 | 0.117 |
| Total pyrroles | 20.66 | 15.23 | 17.81 | 14.31 | 7.49 | 1.84 | 0.104 |
| Total esters | 36.68 b | 48.03 b | 63.66 a,b | 97.63 a | 29.73 b | 5.55 | 0.003 |
| Total aromatic hydrocarbons | 29.86 b | 76.21 a | 96.76 a | 75.68 a,b | 33.67 b | 6.29 | 0.000 |
| Total lactones | 27.76 | 28.29 | 24.40 | 55.26 | 25.49 | 3.52 | 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantarero-Aparicio, M.Á.; García-Infante, M.; Álvarez, C.; Polvillo, O.; Perea, J.M.; Horcada, A. Nutrient Composition of Autochthonous Beef from Southwest Spain. Foods 2025, 14, 3961. https://doi.org/10.3390/foods14223961
Cantarero-Aparicio MÁ, García-Infante M, Álvarez C, Polvillo O, Perea JM, Horcada A. Nutrient Composition of Autochthonous Beef from Southwest Spain. Foods. 2025; 14(22):3961. https://doi.org/10.3390/foods14223961
Chicago/Turabian StyleCantarero-Aparicio, Miguel Ángel, Manuel García-Infante, Carlos Álvarez, Oliva Polvillo, José Manuel Perea, and Alberto Horcada. 2025. "Nutrient Composition of Autochthonous Beef from Southwest Spain" Foods 14, no. 22: 3961. https://doi.org/10.3390/foods14223961
APA StyleCantarero-Aparicio, M. Á., García-Infante, M., Álvarez, C., Polvillo, O., Perea, J. M., & Horcada, A. (2025). Nutrient Composition of Autochthonous Beef from Southwest Spain. Foods, 14(22), 3961. https://doi.org/10.3390/foods14223961






