Bioprocessing of Grape Pomace for the Development of a Nutraceutical Formulation: Bridging Winemaking By-Products and Functional Innovation
Abstract
1. Introduction
2. Materials and Methods
2.1. By-Products and Waste Management: Survey Design for Wine Industry Stakeholders
2.2. Materials
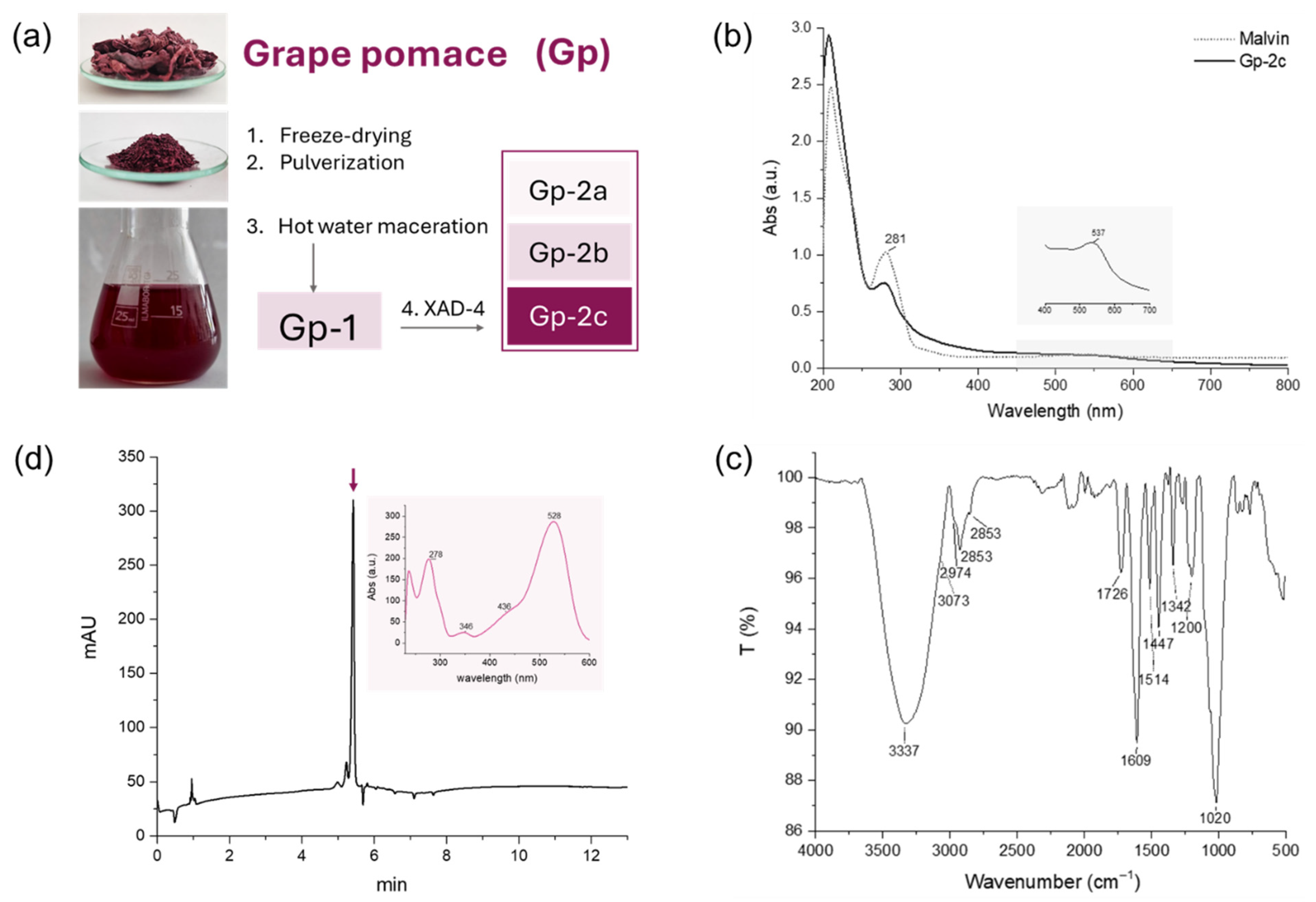
2.3. Grape Pomace Collection and Extraction/Fractionation Procedure
2.4. Spectroscopic Investigation
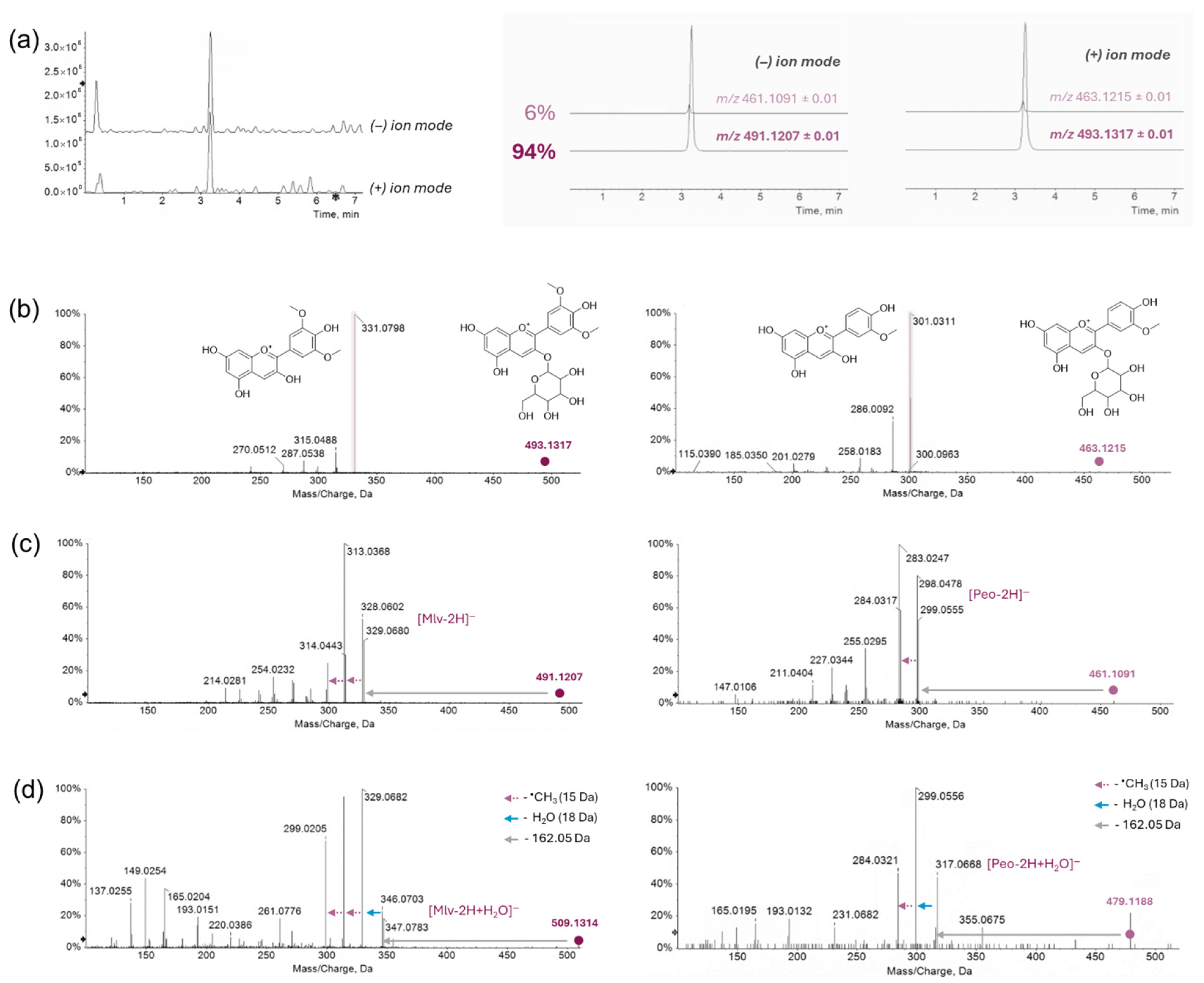
2.5. HPLC-UV-DAD and UHPLC-HRMS Analysis
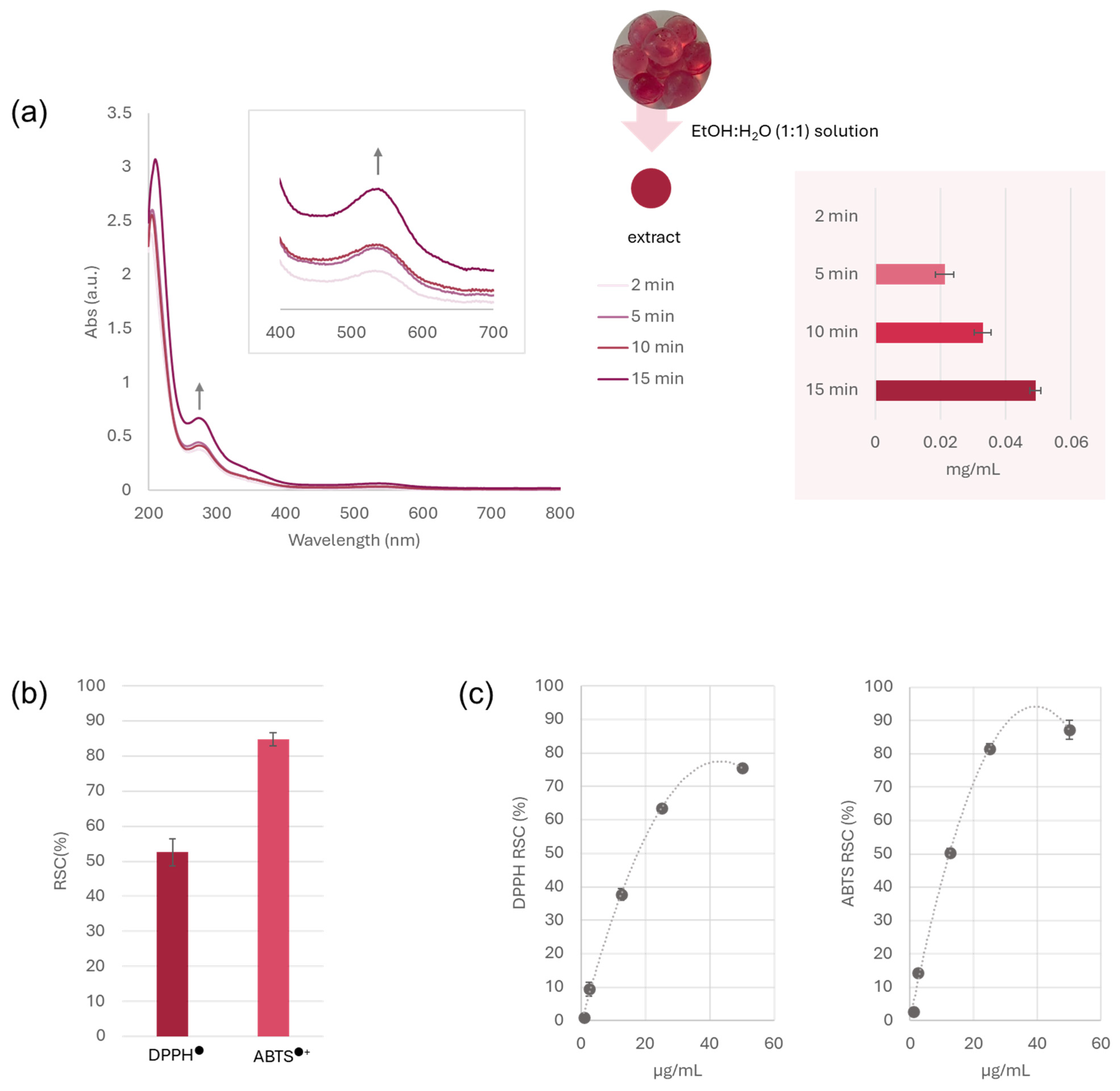
2.6. Antiradical Capacity Assessment
2.7. Gp-2c Based Jelly Candies
2.7.1. Preparation
2.7.2. Color Measurement
2.7.3. Bioactive Compound Release and Antiradical Capacity
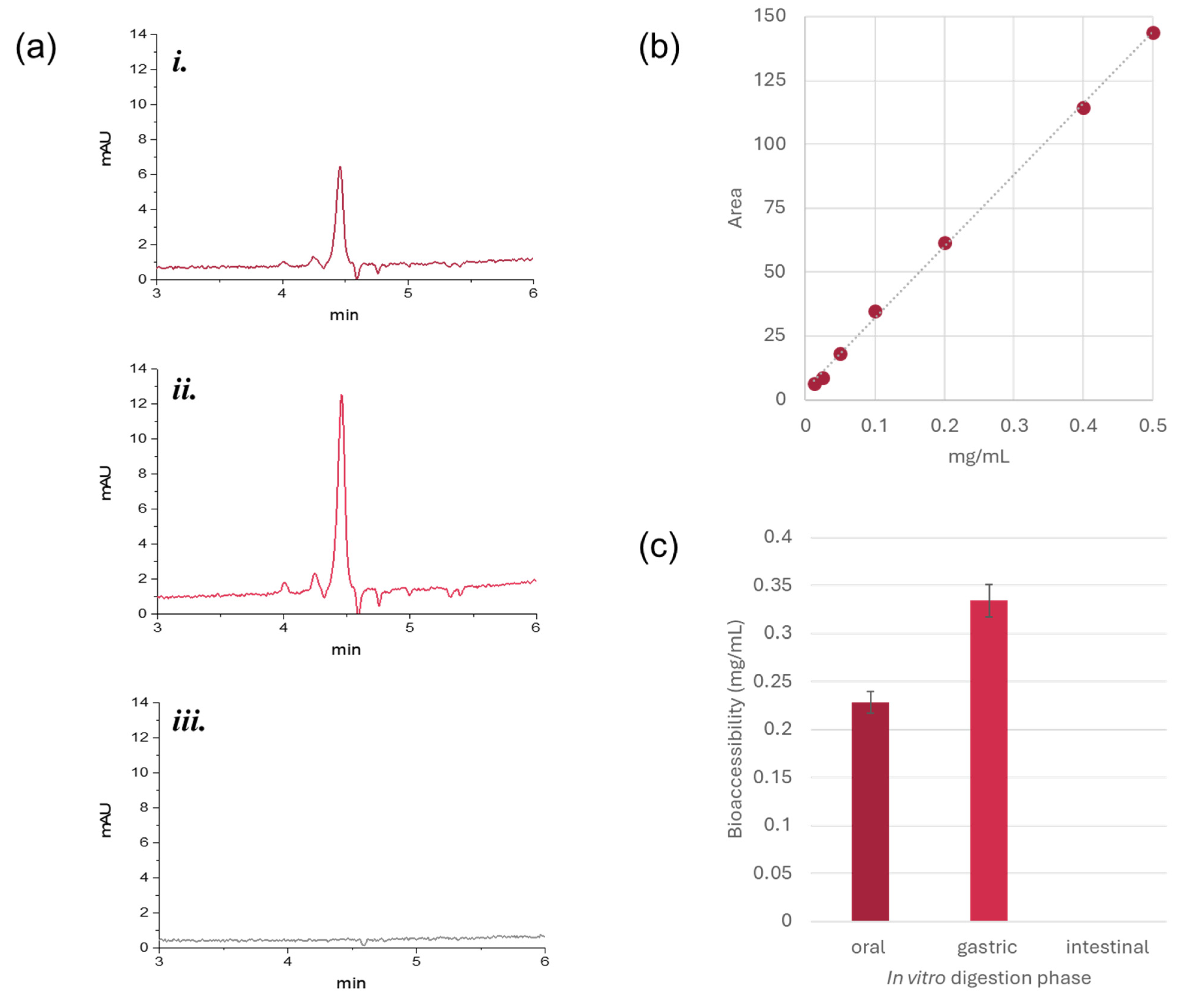
2.7.4. Bioaccessibility Evaluation
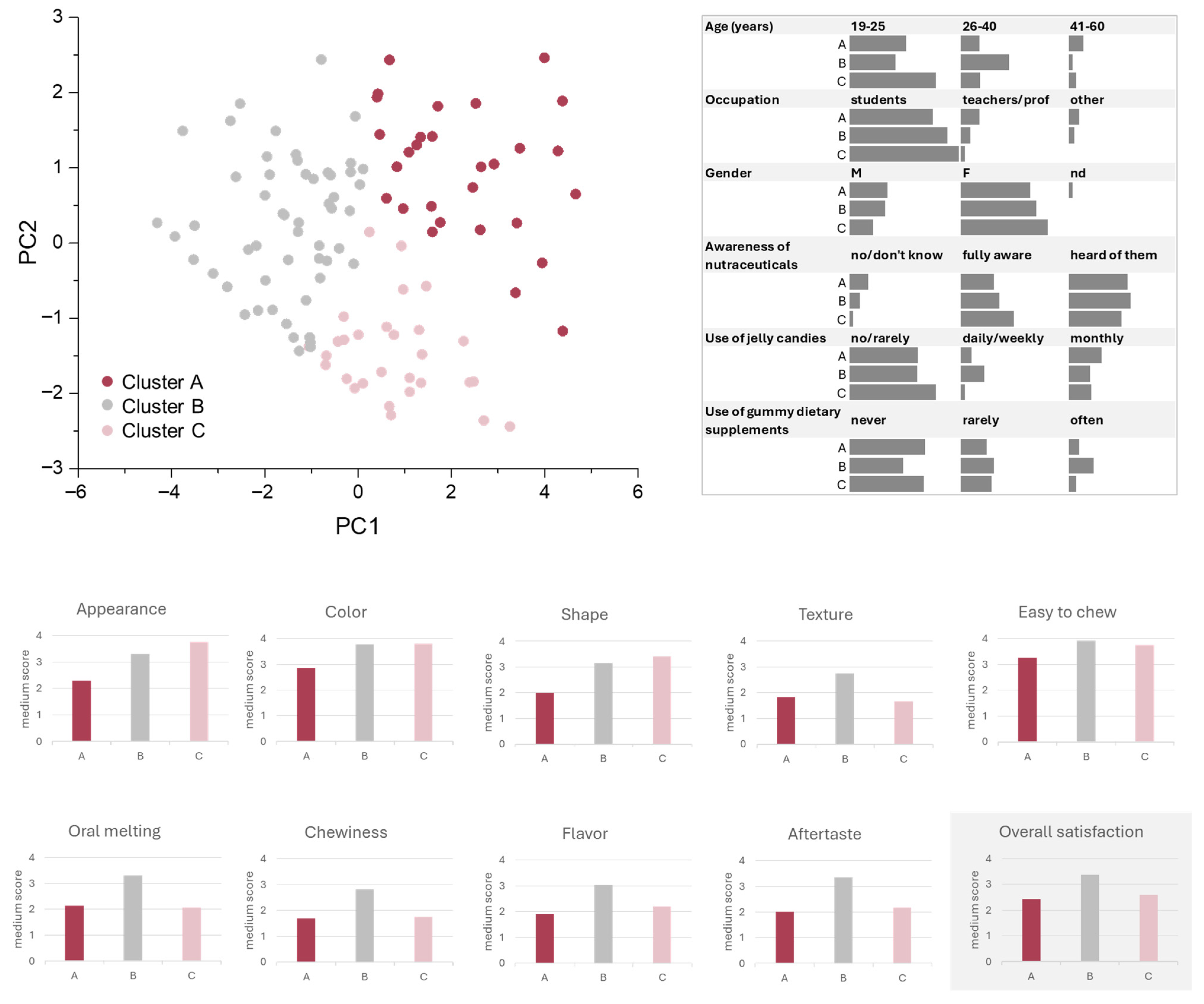
2.7.5. Consumer Perception: Sensory Panel Test
3. Results and Discussion
3.1. Preface to the Experimental Setup
3.2. From Raw Grape Pomace to Purified Bioactives: Chemical and Nutraceutical Insights
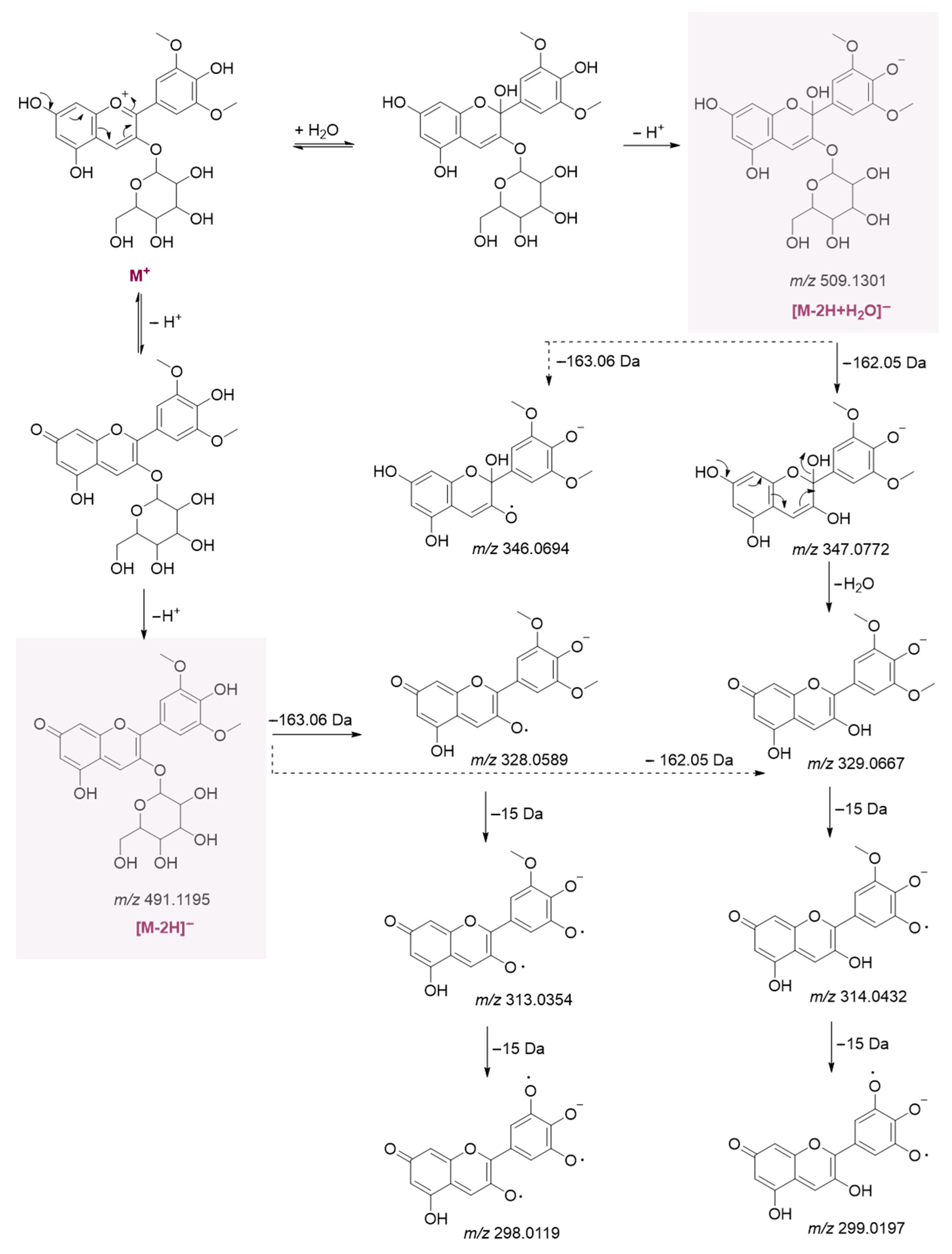
Spectroscopic and Mass Spectrometric Characterization
3.3. Gp-2c as the Bioactive Ingredient of Functional Jelly Candies
3.3.1. Bioactive Compound Release from Jelly Candies and Antiradical Capacity
3.3.2. Bioaccessibility of Gp-2c from Jelly Candies by In Vitro Digestion
3.4. Gp-2c-Based Jelly Candies Consumer Perception: Sensory Panel Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Yücetepe, M.; Özaslan, Z.T.; Karakuş, M.Ş.; Akalan, M.; Karaaslan, A.; Karaaslan, M.; Başyiğit, B. Unveiling the multifaceted world of anthocyanins: Biosynthesis pathway, natural sources, extraction methods, copigmentation, encapsulation techniques, and future food applications. Food Res. Int. 2024, 187, 114437. [Google Scholar] [CrossRef]
- Xu, M.; Fang, D.; Kimatu, B.M.; Lyu, L.; Wu, W.; Cao, F.; Li, W. Recent advances in anthocyanin-based films and its application in sustainable intelligent food packaging: A review. Food Control 2024, 162, 110431. [Google Scholar] [CrossRef]
- Leonarski, E.; Kuasnei, M.; Cesca, K.; de Oliveira, D.; Zielinski, A.A.F. Black rice and its by-products: Anthocyanin rich extracts and their biological potential. Crit. Rev. Food Sci. Nutr. 2024, 64, 9261–9279. [Google Scholar] [CrossRef]
- Ochoa, S.; Durango-Zuleta, M.M.; Osorio-Tobón, J.F. Integrated process for obtaining anthocyanins rich-extract by ultrasound-assisted extraction and starch recovery from purple yam (Dioscorea alata): A techno-economic evaluation. Biomass Convers. Biorefin. 2023, 13, 10605–10614. [Google Scholar] [CrossRef]
- Jansen, E.T.; da Cruz, E.P.; Fonseca, L.M.; Hackbart, H.C.S.; Radünz, M.; Siebeneichler, T.J.; Gandra, E.A.; Rombaldi, C.V.; Dias, A.R.G.; Zavareze, E.R. Anthocyanin-rich grape pomace extract encapsulated in protein fibers: Colorimetric profile, in vitro release, thermal resistance, and biological activities. Food Res. Int. 2024, 196, 115081. [Google Scholar] [CrossRef] [PubMed]
- Allison, B.J.; Simmons, C.W. Obtaining multiple coproducts from red grape pomace via anthocyanin extraction and biogas production. J. Agric. Food Chem. 2018, 66, 8045–8053. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, M.; Esposito, G.; Labanca, F.; Scognamiglio, P.; Sinisgalli, C.; Milella, L.; Faraone, I. Aglianico grape pomace as a source of antioxidant and antiproliferative biomolecules: Eco-friendly extraction and HRMS/MS-based molecular networking. Food Chem. 2025, 469, 142573. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The high-value and sustainable utilization of grape pomace: A review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Mathews, A.; Arbal, A.V.; Kaarunya, A.; Jha, P.K.; Le Bail, A.; Rawson, A. Conventional vs modern extraction techniques in the food industry. In Extraction Processes in the Food Industry: Unit Operation and Processing Equipment in the Food Industry; Jafari, S.M., Akhavan-Mahdavi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 97–146. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Choo, W.S. Hot water extraction, ultrasound, microwave and pectinase-assisted extraction of anthocyanins from blue pea flower. Food Chem. Adv. 2023, 2, 100209. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing green methods for pectin extraction from waste orange peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef] [PubMed]
- Ijod, G.; Musa, F.N.; Anwar, F.; Suleiman, N.; Mohd Adzahan, N.; Mohamad Azman, E. Thermal and nonthermal pretreatment methods for the extraction of anthocyanins: A review. J. Food Process. Preserv. 2022, 46, e17255. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Formisano, C.; Piccolella, S.; Fiorentino, A.; Tenore, G.C.; Izzo, A.A.; Rigano, D.; Pacifico, S. Hyssopus officinalis subsp. aristatus: An unexploited wild-growing crop for new disclosed bioactives. Ind. Crops Prod. 2019, 140, 111594. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Olivieri, A.; Aleixandre, J.L.; du Toit, W. Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control 2018, 85, 11–22. [Google Scholar] [CrossRef]
- Dong, W.; Yang, X.; Zhang, N.; Chen, P.; Sun, J.; Harnly, J.M.; Zhang, M. Study of UV–Vis molar absorptivity variation and quantitation of anthocyanins using molar relative response factor. Food Chem. 2024, 444, 138653. [Google Scholar] [CrossRef]
- Bhushan, B.; Bibwe, B.; Pal, A.; Mahawar, M.K.; Dagla, M.C.; Yathish, K.R.; Jat, B.S.; Kumar, P.; Aggarwal, S.K.; Singh, A.; et al. FTIR spectra, antioxidant capacity and degradation kinetics of maize anthocyanin extract under variable process conditions. Appl. Food Res. 2023, 3, 100282. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin profiling using UV-Vis spectroscopy and liquid chromatography mass spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Sun, J.; Lin, L.-Z.; Chen, P. Study of the mass spectrometric behaviors of anthocyanins in negative ionization mode and its applications for characterization of anthocyanins and non-anthocyanin polyphenols. Rapid Commun. Mass Spectrom. 2012, 26, 1123–1133. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar] [CrossRef]
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Luo, M.R., Ed.; Springer: New York, NY, USA, 2016; pp. 207–212. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality parameters of Spanish lemons with commercial interest. Foods 2021, 10, 62. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crișan, G. Bioaccessibility and bioactive potential of different phytochemical classes from nutraceuticals and functional foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Dantas, A.M.; Fernandes, F.G.; Magnani, M.; Borges, G.S.C. Gastrointestinal digestion assays for evaluating the bioaccessibility of phenolic compounds in fruits and their derivates: An overview. Food Res. Int. 2023, 170, 112920. [Google Scholar] [CrossRef] [PubMed]
- Feraco, A.; Armani, A.; Amoah, I.; Guseva, E.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Lombardo, M. Assessing gender differences in food preferences and physical activity: A population-based survey. Front. Nutr. 2024, 11, 1348456. [Google Scholar] [CrossRef]
- Choi, W.-S.; Seo, H.-S. Effects of age group, gender, and consumption frequency on texture perception and liking of cooked rice or bread. Foods 2023, 12, 1793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolella, S.; Mucci, L.; Prato, F.; Pacifico, S. Bioprocessing of Grape Pomace for the Development of a Nutraceutical Formulation: Bridging Winemaking By-Products and Functional Innovation. Foods 2025, 14, 3967. https://doi.org/10.3390/foods14223967
Piccolella S, Mucci L, Prato F, Pacifico S. Bioprocessing of Grape Pomace for the Development of a Nutraceutical Formulation: Bridging Winemaking By-Products and Functional Innovation. Foods. 2025; 14(22):3967. https://doi.org/10.3390/foods14223967
Chicago/Turabian StylePiccolella, Simona, Lucia Mucci, Francesca Prato, and Severina Pacifico. 2025. "Bioprocessing of Grape Pomace for the Development of a Nutraceutical Formulation: Bridging Winemaking By-Products and Functional Innovation" Foods 14, no. 22: 3967. https://doi.org/10.3390/foods14223967
APA StylePiccolella, S., Mucci, L., Prato, F., & Pacifico, S. (2025). Bioprocessing of Grape Pomace for the Development of a Nutraceutical Formulation: Bridging Winemaking By-Products and Functional Innovation. Foods, 14(22), 3967. https://doi.org/10.3390/foods14223967







