Pumpkin Seed Proteins: The Potentially Alternative Protein Supplements for Food Applications
Abstract
1. Introduction
2. Composition of Pumpkin Seed Protein
2.1. Protein Fractions
2.2. Amino Acid Composition
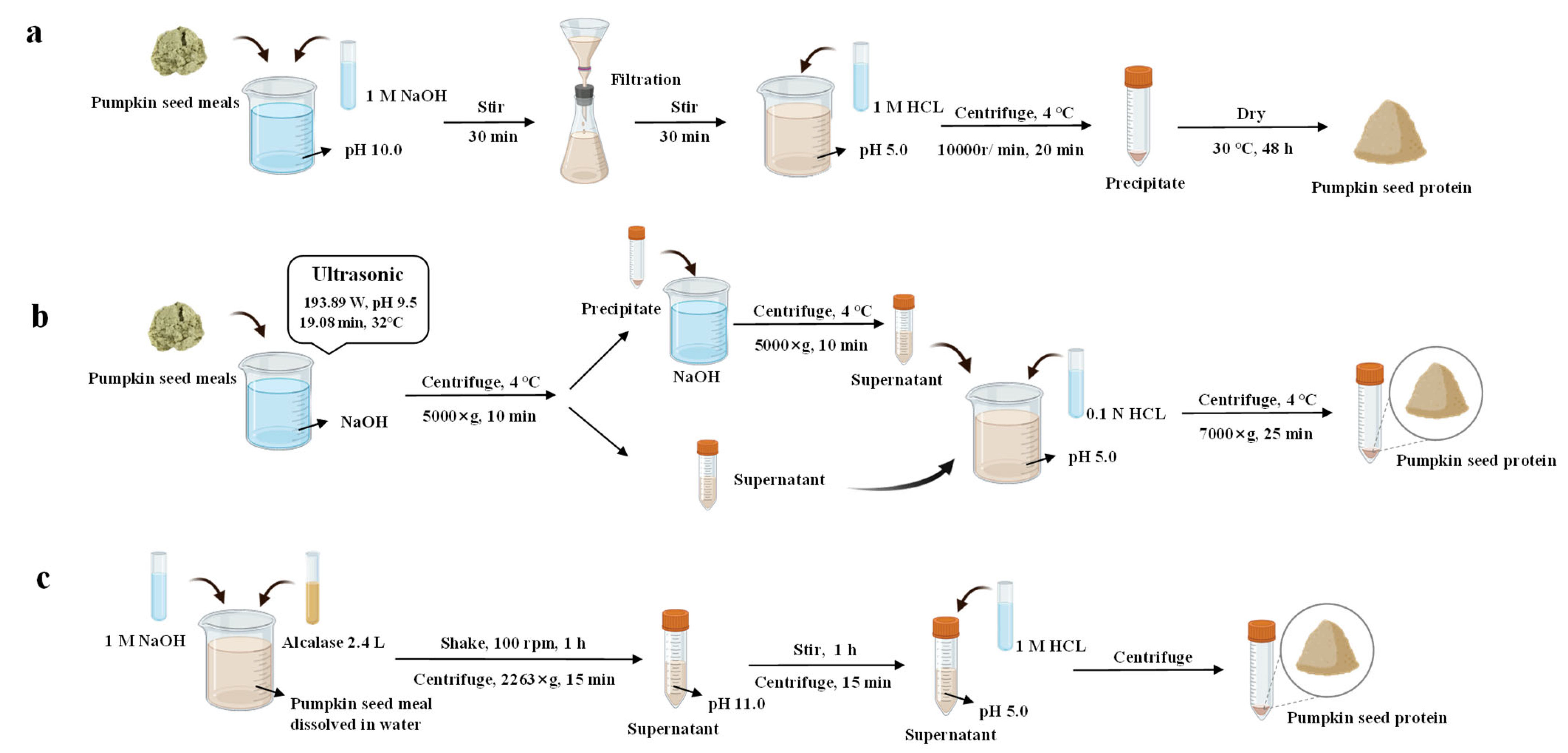
3. Extraction Methods
3.1. Alkaline Extraction
3.2. Ultrasonic-Assisted Extraction
3.3. Enzymatic-Assisted Extraction
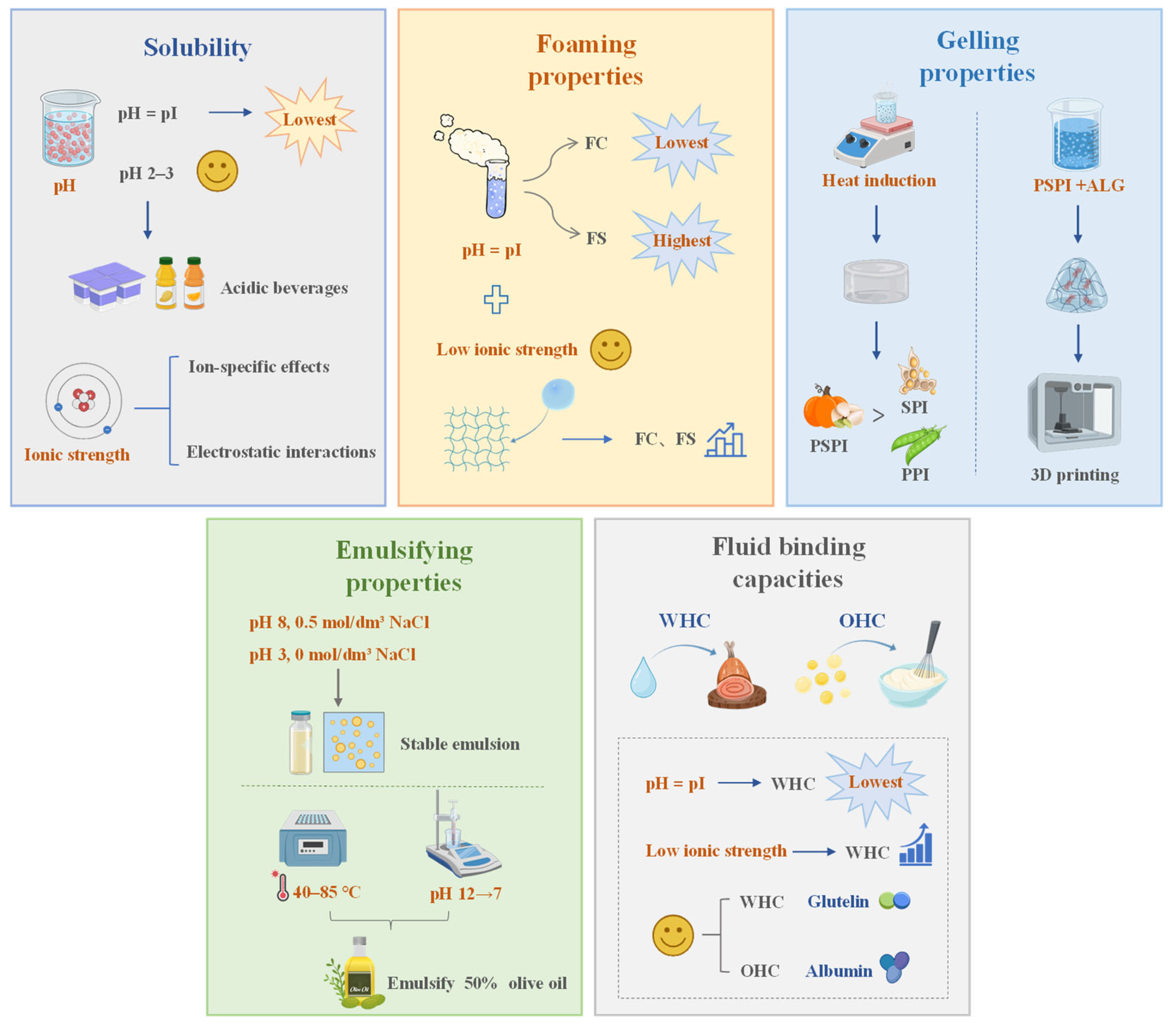
4. Functional Properties
4.1. Solubility
4.2. Foaming Properties
4.3. Gelling Properties
4.4. Emulsifying Properties
4.5. Fluid Binding Capacities
5. Modification Methods
5.1. Enzymatic Modification
5.2. Physical Modification
5.2.1. Conventional Thermal Technology
5.2.2. Non-Thermal Technology
5.3. Chemical Modification
5.4. Combined Modification
6. Biological Activities
6.1. Antioxidant Activity
6.2. Antihypertensive and Antidiabetic Activities
6.3. Antibacterial and Anticancer Activities
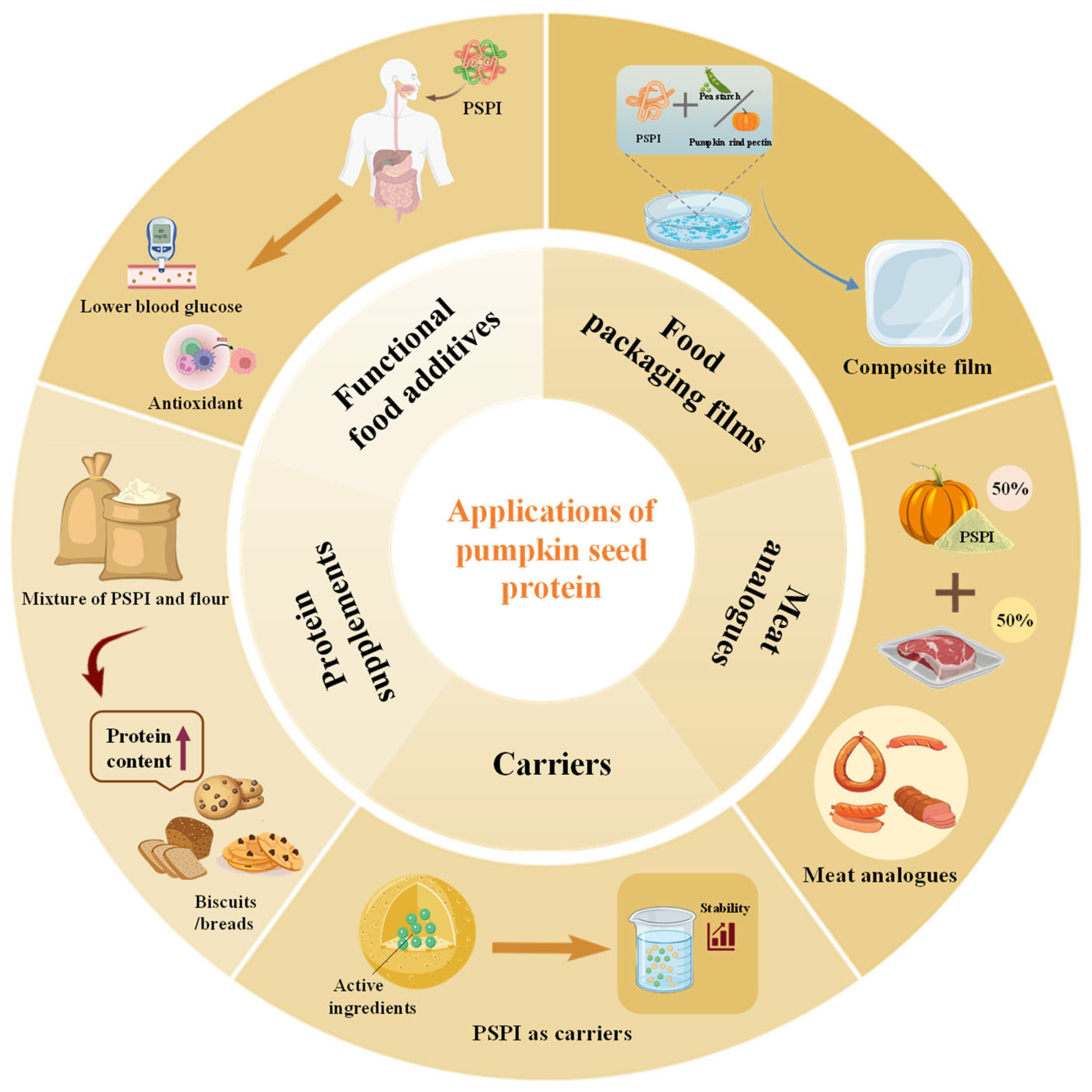
7. Food Applications
7.1. Food Packaging Films
7.2. Meat Analogs
7.3. Carriers for Active Ingredients
7.4. Protein Supplements
7.5. Functional Food Additives
8. Challenges and Solutions
8.1. Limitations of Pumpkin Seed Protein in Digestibility
8.2. Limitations of Pumpkin Seed Protein in Sensory Acceptability
8.3. Economic Limitations of Pumpkin Seed Protein Extraction
9. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Zuo, S.; Gao, X.; Li, Z.; Wang, S.; Chen, B.; Li, X.; Zhu, L.; Zhang, Y. Extraction and functional properties of pigment from pumpkin peels by a novel green deep eutectic alcohol two-phase system. Sustain. Chem. Pharm. 2023, 33, 101067. [Google Scholar] [CrossRef]
- Achu, M.B.; Fokou, E.; Kansci, G.; Fotso, M. Chemical evaluation of protein quality and phenolic compound levels of some Cucurbitaceae oilseeds from Cameroon. Afr. J. Biotechnol. 2013, 12, 735–743. [Google Scholar]
- Singh, A.; Kumar, V. Pumpkin seeds as nutraceutical and functional food ingredient for future: A review. Grain Oil Sci. Technol. 2024, 7, 12–29. [Google Scholar] [CrossRef]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita maxima) seed proteins: Sequential extraction processing and fraction characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Lalnunthari, C.; Devi, L.M.; Amami, E.; Badwaik, L.S. Valorisation of pumpkin seeds and peels into biodegradable packaging films. Food Bioprod. Process. 2019, 118, 58–66. [Google Scholar] [CrossRef]
- Nooshi Manjili, Z.; Sadeghi Mahoonak, A.; Ghorbani, M.; Shahiri Tabarestani, H. Multi-layer encapsulation of pumpkin (Cucurbita maxima L.) seed protein hydrolysate and investigating its release and antioxidant activity in simulated gastrointestinal digestion. Heliyon 2024, 10, e29669. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, Y.; Liu, X. Research on the factors affecting digestibility of protein. Grain Sci. Technol. Econ. 2015, 40, 43–46. [Google Scholar]
- Sá, A.G.A.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Processing effects on the protein quality and functional properties of cold-pressed pumpkin seed meal. Food Res. Int. 2023, 169, 112876. [Google Scholar] [CrossRef]
- Vinayashree, S.; Vasu, P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021, 340, 128177. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Cai, L.H.; Cai, X.L.; Wang, Y.J.; Li, Y.Q. Amino acid profiles and quality from lotus seed proteins. J. Sci. Food Agric. 2013, 93, 1070–1075. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Yang, C.; Wang, B.; Wang, J.; Xia, S.; Wu, Y. Effect of pyrogallic acid (1,2,3-benzenetriol) polyphenol-protein covalent conjugation reaction degree on structure and antioxidant properties of pumpkin (Cucurbita sp.) seed protein isolate. LWT 2019, 109, 443–449. [Google Scholar] [CrossRef]
- Choi, H.W.; Hahn, J.; Kim, H.-S.; Choi, Y.J. Thermorheological properties and structural characteristics of soy and pumpkin seed protein blends for high-moisture meat analogs. Food Chem. 2024, 464, 8141768. [Google Scholar] [CrossRef] [PubMed]
- Tomić, J.; Škrobot, D.; Popović, L.; Dapčević-Hadnađev, T.; Čakarević, J.; Maravić, N.; Hadnađev, M. Gluten-Free Crackers Based on Chickpea and Pumpkin Seed Press Cake Flour: Nutritional, Functional and Sensory Properties. Food Technol. Biotechnol. 2022, 60, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Yildiz, G. From seed to solution: Enhancing techno-functionality and digestibility of pumpkin seed protein isolate through high-intensity ultrasound, high-pressure processing, and pH-shifting. Food Chem. 2025, 474, 143222. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed proteins—Properties and application as a food ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Popović, L.; Vaštag, Ž.; Petrović, L.; Vučinić–Vasić, M. Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. LWT—Food Sci. Technol. 2015, 64, 609–615. [Google Scholar] [CrossRef]
- Fang, E.F.; Wong, J.H.; Lin, P.; Ng, T.B. Biochemical characterization of the RNA-hydrolytic activity of a pumpkin 2S albumin. FEBS Lett. 2010, 584, 4089–4096. [Google Scholar] [CrossRef]
- Dhiman, A.K.; Vidiya, N.; Surekha, A.; Preethi, R. Studies on development and storage stability of dehydrated pumpkin based instant soup mix. J. Appl. Nat. Sci. 2017, 9, 1815–1820. [Google Scholar] [CrossRef]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Alfawaz, M.A. Chemical composition and oil characteristics of pumpkin (Cucurbita maxima) seed kernels. Agric. Food Sci 2004, 2, 5–18. [Google Scholar]
- Nourmohammadi, E.; SadeghiMahoonak, A.; Alami, M.; Ghorbani, M. Amino acid composition and antioxidative properties of hydrolysed pumpkin (Cucurbita pepo L.) oil cake protein. Int. J. Food Prop. 2017, 20, 3244–3255. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Silva, D.C.d.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Oilseed by-products as plant-based protein sources: Amino acid profile and digestibility. Future Foods 2021, 3, 100023. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeniran, O.H.; Jimoh, T.O.; Oboh, G.; Boligon, A.A. Fluted pumpkin (Telfairia occidentalis) seed modulates some markers of erectile function in isolated rat’s corpus cavernosum: Influence of polyphenol and amino acid constituents. J. Food Biochem. 2019, 43, e13037. [Google Scholar] [CrossRef]
- FAO; WHO. Protein and Amino acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series No. 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Bučko, S.; Katona, J.; Popović, L.; Petrović, L.; Milinković, J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016, 60, 271–278. [Google Scholar] [CrossRef]
- Das, M.; Devi, L.M.; Badwaik, L.S. Ultrasound-assisted extraction of pumpkin seeds protein and its physicochemical and functional characterization. Appl. Food Res. 2022, 2, 100121. [Google Scholar] [CrossRef]
- Helikh, A.O.; Gao, D. Optimization of Ultrasound-Assisted Alkaline Extraction of Pumpkin Seed Meal Protein Isolate by Response Surface Methodology. Sci. Notes Taurida Natl. V.I. Vernadsky University. Ser. Tech. Sci. 2020, 2, 44–48. [Google Scholar]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Liu, R.-L.; Yu, P.; Ge, X.-L.; Bai, X.-F.; Li, X.-Q.; Fu, Q. Establishment of an Aqueous PEG 200-Based Deep Eutectic Solvent Extraction and Enrichment Method for Pumpkin (Cucurbita moschata) Seed Protein. Food Anal. Methods 2017, 10, 1669–1680. [Google Scholar] [CrossRef]
- Tas, O.; Sumnu, S.G.; Oztop, M.H. Effect of Extraction Methods and Preheat Treatments on the Functional Properties of Pumpkin Seed Protein Concentrate. Food Sci. Technol. 2025, 5, 105–117. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Kalpanadevi, C.; Muthukumar, S.P.; Govindaraju, K.; Subramanian, R. Rice bran protein: An alternative plant-based protein to ameliorate protein malnourishment. J. Cereal Sci. 2021, 97, 103154. [Google Scholar] [CrossRef]
- Alexandrino, T.D.; Ferrari, R.A.; de Oliveira, L.M.; Rita de Cássia, S.; Pacheco, M.T.B. Fractioning of the sunflower flour components: Physical, chemical and nutritional evaluation of the fractions. LWT 2017, 84, 426–432. [Google Scholar] [CrossRef]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Esclapez, M.; García-Pérez, J.V.; Mulet, A.; Cárcel, J. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Sert, D.; Rohm, H.; Struck, S. Ultrasound-assisted extraction of protein from pumpkin seed press cake: Impact on protein yield and techno-functionality. Foods 2022, 11, 4029. [Google Scholar] [CrossRef]
- Tu, G.L.; Bui, T.H.N.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (Cucurbita pepo) seed powder. Food Technol. Biotechnol. 2015, 53, 479–487. [Google Scholar] [CrossRef]
- Liu, R.-L.; Song, S.-H.; Wu, M.; He, T.; Zhang, Z.-Q. Rapid analysis of fatty acid profiles in raw nuts and seeds by microwave–ultrasonic synergistic in situ extraction–derivatisation and gas chromatography–mass spectrometry. Food Chem. 2013, 141, 4269–4277. [Google Scholar] [CrossRef]
- Venuste, M.; Zhang, X.; Shoemaker, C.F.; Karangwa, E.; Abbas, S.; Kamdem, P.E. Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food Funct. 2013, 4, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ghoshal, G. Sunflower protein isolates-composition, extraction and functional properties. Adv. Colloid Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Effects of pH and salt concentration on functional properties of pumpkin seed protein fractions. J. Food Process. Preserv. 2017, 41, e13073. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Maurer, R.W.; Sandler, S.I.; Lenhoff, A.M. Salting-in characteristics of globular proteins. Biophys. Chem. 2011, 156, 72–78. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Tang, Z.X.; Ying, R.F.; Shi, L.E. Physicochemical and functional characteristics of proteins treated by a pH-shift process: A review. Int. J. Food Sci. Technol. 2020, 56, 515–529. [Google Scholar] [CrossRef]
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT 2022, 155, 112952. [Google Scholar] [CrossRef]
- Brückner-Gühmann, M.; Kratzsch, A.; Sozer, N.; Drusch, S. Oat protein as plant-derived gelling agent: Properties and potential of modification. Future Foods 2021, 4, 100053. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Physicochemical and gel properties of pumpkin seed protein: A comparative study. Int. J. Food Sci. Technol. 2023, 58, 1639–1651. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Tian, X.; Liu, J.; Ye, H.; Shen, X. Effect of ultrasound pretreatment on structural, physicochemical, rheological and gelation properties of transglutaminase cross-linked whey protein soluble aggregates. Ultrason. Sonochem. 2021, 74, 105553. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Lin, S.; Chen, S.; Jing, L.; Liu, Z.; Wu, M.; Yu, X.; Fu, C.; Wang, J.; Huang, D. Pumpkin seed protein-based hydrogel as gelatin mimics and edible inks in 3D-Printed food. Food Hydrocolloids 2025, 162. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Li, C.; Wang, Y.; Mao, Y.; Yang, C.; Li, Y. Preparation of pumpkin seed protein isolate nanoparticles by heat-assisted pH-shifting: Enhanced emulsification performance and dispersibility. J. Food Eng. 2024, 377, 112087. [Google Scholar] [CrossRef]
- Rezig, L.; Riaublanc, A.; Chouaibi, M.; Guéguen, J.; Hamdi, S. Functional properties of protein fractions obtained from pumpkin (Cucurbita maxima) seed. Int. J. Food Prop. 2016, 19, 172–186. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Petrović, L.; Milinković, J.; Spasojević, L.; Mucić, N.; Miller, R. The Influence of Enzymatic Hydrolysis on Adsorption and Interfacial Dilatational Properties of Pumpkin (Cucurbita pepo) Seed Protein Isolate. Food Biophys. 2018, 13, 217–225. [Google Scholar] [CrossRef]
- Rong, L.; Ouyang, K.; Liu, M.; Xiao, F.; Chen, Y.; Woo, M.W.; Zhao, Q. Valorizing pumpkin (Cucurbita moschata) seed meal into protein hydrolysates: Impact of different proteases on the structural, physicochemical, and functional properties. Food Biosci. 2025, 68, 106366. [Google Scholar] [CrossRef]
- Mazloomi-Kiyapey, S.N.; Sadeghi-Mahoonak, A.; Ranjbar-Nedamani, E.; Nourmohammadi, E. Production of antioxidant peptides through hydrolysis of medicinal pumpkin seed protein using pepsin enzyme and the evaluation of their functional and nutritional properties. Arya Atheroscler. 2019, 15, 218. [Google Scholar]
- Zhang, Q.; Ouyang, K.; Huang, F.; Yan, Z.; Li, G.; Liu, M.; Xiong, H.; Zhao, Q. Evaluating the effects of protein-glutaminase treatment on the structural and functional properties of pumpkin (Cucurbita moschata) seed protein. Int. J. Biol. Macromol. 2025, 309 Pt 2, 142989. [Google Scholar] [CrossRef] [PubMed]
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Production of enzymatic hydrolysates with antioxidant and angiotensin-I converting enzyme inhibitory activity from pumpkin oil cake protein isolate. Food Chem. 2011, 124, 1316–1321. [Google Scholar] [CrossRef]
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Hydrolysis of pumpkin oil cake protein isolate and free radical scavenging activity of hydrolysates: Influence of temperature, enzyme/substrate ratio and time. Food Bioprod. Process. 2010, 88, 277–282. [Google Scholar] [CrossRef]
- Lei, F.; Chen, Y.; Chen, L.; Zhang, L.; Zheng, J. An arginine aminopeptidase from marine Bacillus axarquiensis SWJSX8 and its application in improving pumpkin seed protein hydrolysis. Int. J. Food Sci. Technol. 2021, 56, 4680–4689. [Google Scholar] [CrossRef]
- Ramondo, A.; Marulo, S.; Sorrentino, A.; Masi, P.; Di Pierro, P. Modification of Physicochemical and Functional Properties of Pumpkin Seeds Protein Isolate (PsPI) by High-Intensity Ultrasound: Effect of Treatment Time. ACS Food Sci. Technol. 2024, 4, 40–48. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; de Souza, L.B.; Paiva, P.H.C.; Lelis, C.A.; Vieira, E.N.R.; Tribst, A.A.L.; Leite Júnior, B.R.d.C. Impact of ultrasound on pumpkin seed protein concentrate hydrolysis: Effects on Alcalase, protein, and assisted reaction. Appl. Food Res. 2023, 3, 100281. [Google Scholar] [CrossRef]
- Sert, D.; Rohm, H.; Struck, S. High-pressure-assisted protein isolation from pumpkin seed press cake. Int. J. Food Sci. Technol. 2024, 59, 368–379. [Google Scholar] [CrossRef]
- Gao, D.; Helikh, A.; Duan, Z.; Xie, Q. Thermal, structural, and emulsifying properties of pumpkin seed protein isolate subjected to pH-shifting treatment. J. Food Meas. Charact. 2023, 17, 2301–2312. [Google Scholar] [CrossRef]
- Miedzianka, J.; Zambrowicz, A.; Zielinska-Dawidziak, M.; Drozdz, W.; Nems, A. Effect of Acetylation on Physicochemical and Functional Properties of Commercial Pumpkin Protein Concentrate. Molecules 2021, 26, 1575. [Google Scholar] [CrossRef]
- Lovatto, N.d.M.; Loureiro, B.B.; Bender, A.B.B.; Loureiro, C.B.; Goulart, F.R.; Speroni, C.S.; Macagnan, F.T.; Piana, M.; Silva, L.P.d. Phosphorylated protein concentrate pumpkin seed (Cucurbita moschata): Optimization by response surface methodology and nutritional characterization. Ciência Rural 2020, 50, e20190093. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Lu, Y.; Huang, Y.; Yan, S.; Li, Y. Characterization of the fibrillation of pumpkin seed protein: Kinetics, structure, and fibril-forming regions. Food Hydrocoll. 2025, 165, 111254. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Han, Y.; Wang, B.; Liu, Z.; Hu, H.; Guan, Z.; Yang, Y.; Wang, J. Fabrication of polyphenol-pumpkin seed protein isolate (PSPI) covalent conjugate microparticles to protect free radical scavenging activity of polyphenol. Food Biosci. 2023, 55, 102982. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, M.; Zhang, C.; Meng, K.; Liang, F.; Shi, J. Covalent conjugation of pumpkin seed protein induction by epigallocatechin-3-gallate: Structural formation, functional and in vitro digestion properties. LWT 2025, 222, 117636. [Google Scholar] [CrossRef]
- Liang, F.; Shi, Y.; Shi, J.; Zhang, T.; Zhang, R. A novel Angiotensin-I-converting enzyme (ACE) inhibitory peptide IAF (Ile-Ala-Phe) from pumpkin seed proteins: In silico screening, inhibitory activity, and molecular mechanisms. Eur. Food Res. Technol. 2021, 247, 2227–2237. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Pereira, G.Z.; Paiva, P.H.C.; Lelis, C.A.; Tribst, A.A.L.; Leite Júnior, B.R.d.C. Structural changes induced by ultrasound in proteases and their consequences on the hydrolysis of pumpkin seed proteins and the multifunctional properties of hydrolysates. Food Bioprod. Process. 2024, 144, 13–21. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Nalon, G.A.; Cunha, J.S.; Andressa, I.; Costa Paiva, P.H.; Tribst, A.A.L.; Leite Júnior, B.R.d.C. Impact of ultrasonic pretreatment on pumpkin seed protein: Effect on protease activities, protein structure, hydrolysis kinetics and functional properties. Food Res. Int. 2025, 201, 115538. [Google Scholar] [CrossRef]
- Sadeghi Mahoonak, A.; Erfani Moghadam, V.; Ghorbani, M.; Shahiri Tabarestani, H. Optimizing enzymatic hydrolysis of pumpkin seeds protein (Cucurbita maxima L.) by pancreatin with using microwave pretreatment. J. Food Sci. Technol. 2023, 20, 78–90. [Google Scholar]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Vaštag, Ž. Characterization of bioactive protein products obtained by enzymatic modifications of pumpkin oil cake protein isolate. J. Biotechnol. 2010, 150, 304. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA-J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Kapoor, R.; Karabulut, G.; Mundada, V.; Feng, H. Non-thermal ultrasonic contact drying of pea protein isolate suspensions: Effects on physicochemical and functional properties. Int. J. Biol. Macromol. 2023, 253, 126816. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Bai, R.; Lu, F.; Zhang, T.; Gao, Z.-S.; Zhao, S.-M.; Zhu, M.-M.; Ma, H.-J. Effects of high-pressure homogenization on the solubility, foaming, and gel properties of soy 11S globulin. Food Hydrocoll. 2022, 124, 107261. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Fang, Y.; Zhang, W.; Zhang, B. DSC study on the thermal properties of soybean protein isolates/corn starch mixture. J. Therm. Anal. Calorim. 2014, 115, 1633–1638. [Google Scholar] [CrossRef]
- Tarahi, M.; Ahmed, J. Recent advances in legume protein-based colloidal systems. Legume Sci. 2023, 5, e185. [Google Scholar] [CrossRef]
- Soares Ad, S.; Leite Júnior, B.R.d.C.; Tribst, A.A.L.; Augusto, P.E.D.; Ramos, A.M. Effect of ultrasound on goat cream hydrolysis by lipase: Evaluation on enzyme, substrate and assisted reaction. LWT 2020, 130, 109636. [Google Scholar] [CrossRef]
- Gong, X.; Sui, L.; Morton, J.; Brennan, M.A.; Brennan, C.S. Investigation of Nutritional and Functional Effects of Rice Bran Protein Hydrolysates by Using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Guidelines: A Review. Trends Food Sci. Technol. 2021, 110, 798–811. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Cunha, J.S.; Santos, F.R.d.; Pacheco, J.C.C.; Correa, K.d.P.; Orlando Junior, W.d.A.; Paiva, P.H.C.; Leite Junior, B.R.d.C. Bibliometric analysis of pumpkin seed proteins: A review of the multifunctional properties of their hydrolysates and future perspectives. Food Biosci. 2024, 59, 104269. [Google Scholar] [CrossRef]
- Vargas, M.A.; Bernal, C.; Martínez, R. Protease-assisted process for tryptophan release from pumpkin (Cucurbita maxima) seed protein extracts. J. Food Process. Preserv. 2022, 46, e16290. [Google Scholar] [CrossRef]
- Manjili, Z.N.; Mahoonak, A.S.; Ghorbani, M.; Tabarestani, H.S.; Moghadam, V.E. Composite alginate-based hydrogel delivery of antioxidant pumpkin protein hydrolysate in simulated gastrointestinal condition. Curr. Res. Food Sci. 2024, 8, 100739. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.; Zhang, Q.; Shen, Q. Recent Research in Antihypertensive Activity of Food Protein-derived Hydrolyzates and Peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 760–787. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the Mechanisms of ACE Inhibitory Peptides from Food Proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Popović, L.; Stolić, Ž.; Čakarević, J.; Torbica, A.; Tomić, J.; Šijački, M. Biologically Active Digests from Pumpkin Oil Cake Protein: Effect of Cross-linking by Transglutaminase. J. Am. Oil Chem. Soc. 2017, 94, 1245–1251. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Adelerin, R.O.; Awolu, O.O.; Ifesan, B.O.T.; Nwaogu, M.U. Pumpkin-based cookies formulated from optimized pumpkin flour blends: Nutritional and antidiabetic potentials. Food Humanit. 2024, 2, 100215. [Google Scholar] [CrossRef]
- Čakarević, J.; Torbica, A.; Belović, M.; Tomić, J.; Sedlar, T.; Popović, L. Pumpkin oil cake protein as a new carrier for encapsulation incorporated in food matrix: Effect of processing, storage and in vitro digestion on bioactivity. Int. J. Food Sci. Technol. 2021, 56, 3400–3408. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.B.; Abd El-Kalek, H.H. Antimicrobial proteins and oil seeds from pumpkin (Cucurbita moschata). Nat. Sci. 2011, 9, 105–119. [Google Scholar]
- Nahla, M.A.; Abdel Azim, A.M.; Aisha, S.M.F. The Nutritive and Functional Properties of Dry Bean (Phaseolus vulgaris) as Affected by Gamma Irradiation. Pak. J. Nutr. 2009, 8, 1739–1742. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown Rice versus White Rice: Nutritional Quality, Potential Health Benefits, Development of Food Products, and Preservation Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef]
- Tomar, P.P.S.; Nikhil, K.; Singh, A.; Selvakumar, P.; Roy, P.; Sharma, A.K. Characterization of anticancer, DNase and antifungal activity of pumpkin 2S albumin. Biochem. Biophys. Res. Commun. 2014, 448, 349–354. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.-y.; Ji, H.; Ran, Y.; Li, S.-h.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Popović, S.; Peričin, D.; Vaštag, Ž.; Lazić, V.; Popović, L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J. Food Eng. 2012, 110, 374–379. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of protein and pectin from pumpkin industry by-products and their utilization for developing edible film. J. Food Sci. Technol. 2020, 57, 1807–1816. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Duan, S.; Liu, X.; Zhang, K.; Tu, J. A novel pumpkin seeds protein-pea starch edible film: Mechanical, moisture distribution, surface hydrophobicity, UV-barrier properties and potential application. Mater. Res. Express 2020, 6, 125355. [Google Scholar] [CrossRef]
- Hromiš, N.; Lazić, V.; Popović, S.; Šuput, D.; Bulut, S.; Kravić, S.; Romanić, R. The possible application of edible pumpkin oil cake film as pouches for flaxseed oil protection. Food Chem. 2022, 371, 131197. [Google Scholar] [CrossRef]
- İşçimen, E.M.; Hayta, M. Optimisation of ultrasound assisted extraction of rice bran proteins: Effects on antioxidant and antiproliferative properties. Qual. Assur. Saf. Crops Foods 2018, 10, 165–174. [Google Scholar] [CrossRef]
- Ebert, S.; Jungblut, F.; Herrmann, K.; Maier, B.; Terjung, N.; Gibis, M.; Weiss, J. Influence of wet extrudates from pumpkin seed proteins on drying, texture, and appearance of dry-cured hybrid sausages. Eur. Food Res. Technol. 2022, 248, 1469–1484. [Google Scholar] [CrossRef]
- Gao, D.; Helikh, A.; Duan, Z.; Liu, Y.; Shang, F. Study on Application of Pumpkin Seed Protein Isolate in Sausage Production Process. Technol. Audit. Prod. Reserves 2022, 2, 31–35. [Google Scholar] [CrossRef]
- Baig, M.A.; Ajayi, F.F.; Mostafa, H.; Sivapragasam, N.; Maqsood, S. Mungbean and pumpkin protein isolates as novel ingredients for the development of meat analogs using heat-induced gelation technique. Front. Sustain. Food Syst. 2023, 7, 1243183. [Google Scholar] [CrossRef]
- Čakarević, J.; Šeregelj, V.; Tumbas Šaponjac, V.; Ćetković, G.; Čanadanović Brunet, J.; Popović, S.; Kostić, M.H.; Popović, L. Encapsulation of beetroot juice: A study on the application of pumpkin oil cake protein as new carrier agent. J. Microencapsul. 2020, 37, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Lelis, C.A.; Andrade, J.C.; Lima, R.C.; Ribeiro, R.A.P.; Pacheco, A.F.C.; Pacheco, F.C.; Leite-Junior, B.R.C.; Paiva, P.H.C.; Alvares, T.S.; Conte-Junior, C.A. Pumpkin seed protein as a carrier for Astaxanthin: Molecular characterization of interactions and implications for stability. Food Chem. 2025, 468, 142452. [Google Scholar] [CrossRef]
- Harman, C.L.G.; Patel, M.A.; Guldin, S.; Davies, G.-L. Recent developments in Pickering emulsions for biomedical applications. Curr. Opin. Colloid Interface Sci. 2019, 39, 173–189. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Ding, J.; Ni, X.; Li, C.; Wang, J.; Yang, C. High ethanol tolerance of oil-in-water Pickering emulsions stabilized by protein nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127777. [Google Scholar] [CrossRef]
- Kong, Y.; Huang, D. Pumpkin seed proteins rival animal gelatin in increasing the cytoaffinity of edible microbeads for cell-based meat culture. Food Res. Int. 2023, 168, 112750. [Google Scholar] [CrossRef]
- Fatima, H.; Hussain, A.; Ambreen; Kabir, K.; Arshad, F.; Ayesha, A.; Bibi, B.; Ahmed, A.; Najam, A.; Firdous, N.; et al. Pumpkin seeds; an alternate and sustainable source of bioactive compounds and nutritional food formulations. J. Food Compost. Anal. 2025, 137. [Google Scholar] [CrossRef]
- Galenko, O.; Shevchenko, A.; Ceccanti, C.; Mignani, C.; Litvynchuk, S. Transformative shifts in dough and bread structure with pumpkin seed protein concentrate enrichment. Eur. Food Res. Technol. 2024, 250, 1177–1188. [Google Scholar] [CrossRef]
- Juhasz, R.; Hajas, L.; Csajbokne Csobod, E.; Palinkas, Z.; Szilagyi-Utczas, M.; Benedek, C. Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies. Foods 2025, 14, 1518. [Google Scholar] [CrossRef]
- Voučko, B.; Novotni, D.; Balbino, S.; Mustač, N.Č.; Drakula, S.; Dujmić, F.; Habuš, M.; Jarni, K.; Ćurić, D. Utilization of pumpkin seed oil cake and proso millet flour in enhancing gluten-free bread quality. J. Food Process. Preserv. 2022, 46, e17070. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Shah, A.; Gani, A.; Gani, A. Effect of enzymatic hydrolysis of pulse protein macromolecules to tailor structure for enhanced nutraceutical properties. LWT 2024, 205, 116502. [Google Scholar] [CrossRef]
- Qian, S.; Lan, T.; Zhao, X.; Song, T.; Cao, Y.; Zhang, H.; Liu, J. Mechanism of ultrasonic combined with different fields on protein complex system and its effect on its functional characteristics and application: A review. Ultrason. Sonochem. 2023, 98, 106532. [Google Scholar] [CrossRef] [PubMed]
- Giami, S.Y. Effect of fermentation on the seed proteins, nitrogenous constituents, antinutrients and nutritional quality of fluted pumpkin (Telfairia occidentalis Hook). Food Chem. 2004, 88, 397–404. [Google Scholar] [CrossRef]
- Hadidi, M.; Tarahi, M.; Gunther Innerhofer, M.; Pitscheider, I.; Loscher, A.; Pignitter, M. Pumpkin seed as a sustainable source of plant-based protein for novel food applications. Crit. Rev. Food Sci. Nutr. 2025, 65, 8566–8591. [Google Scholar] [CrossRef]
- Aziz, A.; Noreen, S.; Khalid, W.; Ejaz, A.; Faiz ul Rasool, I.; Maham; Munir, A.; Farwa; Javed, M.; Ercisli, S.; et al. Pumpkin and Pumpkin Byproducts: Phytochemical Constitutes, Food Application and Health Benefits. ACS Omega 2023, 8, 23346–23357. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrason. Sonochem. 2020, 65, 105063. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolates and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef]

| Amino Acid Composition | Pumpkin Seed Species | FAO/WHO | |||||
|---|---|---|---|---|---|---|---|
| C. maxima | C. moschata var. Kashi Harit | C. pepo L. | C. moschata | C. maxima Linn | For Children | For Adult | |
| Alanine | 5.12 | 4.84 | 3.14 | 3.63 ± 0.05 | 5.07 | - | - |
| Arginine | 15.8 | 16.04 | 10.65 | 14.00 ± 1.00 | 9.06 | - | - |
| Aspartic acid | 9.56 | 7.12 | 7.22 | 11.94 ± 0.03 | 8.73 | - | - |
| Cysteine | - | 0.45 | - | - | 9.39 | - | - |
| Glutamic acid | 23.23 | 20.61 | 14.28 | 20.40 ± 0.10 | 3.37 | - | - |
| Glycine | 6.01 | 5.17 | 4.23 | 7.30 ± 0.70 | 5.63 | - | - |
| Histidine | 2.66 | 1.52 | 1.83 | 1.48 ± 0.06 | 2.16 | 1.90 | 1.60 |
| Isoleucine | 3.59 | 4.14 | 2.88 | 4.05 ± 0.03 | 3.51 | 2.80 | 1.30 |
| Leucine | 7.25 | 7.82 | 5.33 | 6.60 ± 0.03 | 7.24 | 6.60 | 1.90 |
| Lysine | 3.71 | 3.38 | 2.65 | 4.66 ± 0.02 | 2.78 | 5.80 | 1.60 |
| Methionine | 1.83 | 2.57 | 2.42 | - | 1.20 | - | - |
| Phenylalanine | 5.29 | 5.32 | 4.22 | - | 3.05 | - | - |
| Proline | - | 3.82 | - | 3.65 ± 0.01 | 2.32 | - | - |
| Serine | 5.85 | 4.43 | 3.97 | 4.90 ± 0.20 | 4.90 | - | - |
| Threonine | 3.04 | 2.19 | 1.88 | 1.39 ± 0.08 | 2.96 | 3.40 | 0.90 |
| Tryptophan | 1.10 | 2.10 | - | - | - | 1.10 | 0.50 |
| Tyrosine | 3.26 | 2.90 | 2.32 | - | 2.78 | - | - |
| Valine | 4.45 | 5.60 | 3.35 | 4.69 ± 0.03 | 2.40 | 3.50 | 1.30 |
| References | [24] | [10] | [25] | [26] | [27] | [28] | [28] |
| Amino Acid Composition | Preschool-Aged Children (FAO/WHO) | Adults (FAO/WHO) | |||
|---|---|---|---|---|---|
| PSPI | WF | SF | AF | PSPI | |
| Histidine | 80.00 | 85.79 | 33.16 | 120.53 | 154.2 ± 1.9 |
| Threonine | 64.41 | 76.47 | 25.88 | 67.94 | 129.3 ± 1.0 |
| Valine | 160.00 | 133.43 | 64.57 | 156.57 | 125.6 ± 0.1 |
| Methionine + Cysteine | 120.80 | 102.80 | 442.00 | 130.80 | 119.9 ± 0.1 |
| Isoleucine | 147.86 | 91.43 | 74.29 | 147.50 | 142.6 ± 1.7 |
| Leucine | 118.48 | 88.79 | 84.70 | 119.09 | 109.2 ± 0.5 |
| Phenylalanine + Tyrosine | 130.48 | 82.22 | 69.84 | 147.46 | 217.5 ± 1.4 |
| Lysine | 58.28 | 88.97 | 31.38 | 54.66 | 86.5 ± 2.2 |
| Tryptophan | 190.90 | 105.45 | 143.64 | 206.36 | 211.1 ± 0.5 |
| References | [10] | [10] | [10] | [10] | [9] |
| Extraction Methods | Extraction Conditions | Protein Content (%) | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Alkaline extraction | At room temperature, pH 10.0 for 30 min, then the pH was adjusted to 5.0 | 94.3 | Simple, low cost, and easy to extract | Long time, requires a large amount of buffer solution, and may also cause the loss of essential amino acids | [20] |
| At room temperature, pH 11.0 for 30 min, then the pH was adjusted to 5.0 | 84.87 | [29] | |||
| Ultrasonic-assisted extraction | 20–25 kHz, 193.89 W, 19.08 min, 32 °C, pH 9.5 | 86.07 | Simple operation and environmental friendliness | High cost and unsuitable for industrial scale | [30] |
| 456 W, 22 min, solid–liquid ratio 27 mg/L | 81.86 | [31] | |||
| 25 kHz, 60 min, 600 W/cm2, 50% amplitude | 94.04 ± 0.77 | [32] | |||
| PEG 200-based DES concentration: 28% (w/w), solid–liquid ratio 28 mg/L, 43 °C, 140 W | 93.95 ± 0.23 | [33] | |||
| Enzymatic-assisted extraction | 2% Alcalase, 2.4 L, pH 8.0, 50 °C | 57.13 ± 0.65 | Mild conditions, improved protein functional properties, and enhanced biological activities | High price and low protein extraction rate | [34] |
| H1: Alcalase, 50 °C, enzyme to substrate ratio 0.5 mL/g. H2: Pepin, 37 °C, enzyme to substrate ratio 0.02 g/g | H1: 89.9 H2: 92.13 | [29] |
| Modification Type | Modification Method | Structural Change | Effect | References |
|---|---|---|---|---|
| Enzymatic modification | Alcalase: 50 °C, 0.5 mL/g, 30 min Pepsin: 37 °C, 0.02 g/g, 90 min | Production of small peptides and free amino acids. Alterations in secondary and tertiary structures | Enhanced solubility, emulsification, surface hydrophobicity, and thermal stability | [20,60] |
| Neutrase: 8000 U/g, 6 h | Improved solubility and antioxidant activity | [61] | ||
| Pepsin:30 °C, 1%, 2 h | Enhanced emulsifying capacity | [62] | ||
| Glutaminase: 45 °C, 1:100, 12 h | Improved solubility, foaming capacity, and OHC | [63] | ||
| Alcalase: 60 min, 1:250 Flavourzyme: 120 min, 1:385 | Improved antioxidant activity | [64] | ||
| Aspergillus niger pepsin: 40 °C, 4.38 HUT/mg, 85 min | Improved antioxidant activity | [65] | ||
| BAAP: 40 LAP, 12 h | Increased the small peptide content and antioxidant activity | [66] | ||
| Physical modification | Conventional thermal technology (87.8 °C, pH 8.0, 37 min) | Protein tertiary structure was disrupted, and hydrophobic groups were exposed; transitioned from natural conformation to semi-molten spheres | Enhanced FC (83.0%), WHC (1.90 g H2O/g), and OHC (0.90 g oil/g) | [9] |
| Ultrasonic treatment (20 kHz, at 100 W, 300 W, or 500 W for 30 min) | Effectively changed the secondary and tertiary structure of the PSPI | Improved solubility and emulsifying properties | [50] | |
| 150 W for 5 min | Improved foaming and emulsifying properties | [67] | ||
| 193.89 W for 19.08 min | Improved emulsifying properties and OHC | [30] | ||
| 40 kHz, 23.8 W/L, 25 °C, 120 min | Enhanced antioxidant capacity and improved color characteristics | [68] | ||
| High-pressure homogenization (100 MPa) | Disruption of protein tertiary and quaternary structures by hydrogen bond cleavage | Solubility increased to 30.21 ± 0.93%; effectively improved the emulsification index of PSPI; FC increased to 24.58 ± 5.57%, but FS showed a decreasing trend | [69] | |
| Chemical modification | pH-shifting treatment | At pH 2, 4, and 12, they possessed larger particle sizes and irregular morphology. At pH 6, 8, and 10, they exhibited a more uniform structure | Specific pH conditions maintained the thermal stability of the proteins and improved EAI. Extreme pH treatments more significantly improved surface activity and emulsion stability | [18,70] |
| Acylation | Appearance of acetyl groups | Enhanced WHC, OHC, and emulsification properties | [71] | |
| Phosphorylation | Phosphate groups occurrence | Reduced levels of anti-nutritional factors and fiber content; significant increase in protein digestibility and essential amino acid content | [72] | |
| Fibrillation | The process of protofibrillation showed different structures | PSPI contained abundant hydrophobic protofibril-forming regions | [73] | |
| Complexed with bioactive components (EGCG, chlorogenic acid, gallic acid, pyrogallic acid (1,2,3-benzenetriol), apigenin, hydrogel network) | The secondary structure of the coupling was more regular and stable than that of PSPI | Enhanced antioxidant capacity of the protein, which also led to an increase in thermal stability; 66.07% increase in solubility, with a maximum activity of 421.91 m2/g, and free radical scavenging activities of 82.73% and 90.70% against DPPH and ABTS, respectively. Surface hydrophobicity. Improved antioxidant activity and release behavior in the gastrointestinal environment | [7,13,74,75,76] | |
| Combined modification | pH + Ultrasonic treatment (20 kHz, 5 min) | Protein particles exhibited a narrower size distribution | Increased solubility, WHC, OHC, emulsification, and foaming properties; improved thermal stability and protein digestibility | [18] |
| Heat + pH shifting | Destruction of the primary, secondary, and tertiary structures of proteins | PSPI emulsification capacity was significantly improved, with internal oil phase volume fraction up to 80% | [57] | |
| Ultrasonication (40 kHz, 450 W) +enzymatic treatment (Brauzyn and Neutrase: 25 °C, 60 min; Flavourzyme; 25 °C, 90 min) | The tertiary structure of the protein was disrupted, and the hydrophobic groups were exposed | Improved antioxidant properties and solubility | [77] | |
| Ultrasonication (40 kHz, 23.8 W/L) +enzymatic treatment (Flavourzyme: 25 °C, 120 min. Neutrase: 40 °C, 60 min) | [78] | |||
| Microwave (500–900 W, 30–90 s) +enzymatic treatment (Trypsin: 1.5%, 105 min) | Secondary and tertiary structure disordered, hydrophobic groups exposed, surface hydrophobicity elevated | High iron chelating activity and DPPH radical scavenging capacity | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Wang, Y.; Jin, X.; Zhang, X.; Yang, R. Pumpkin Seed Proteins: The Potentially Alternative Protein Supplements for Food Applications. Foods 2025, 14, 3969. https://doi.org/10.3390/foods14223969
Xie Y, Wang Y, Jin X, Zhang X, Yang R. Pumpkin Seed Proteins: The Potentially Alternative Protein Supplements for Food Applications. Foods. 2025; 14(22):3969. https://doi.org/10.3390/foods14223969
Chicago/Turabian StyleXie, Yufeng, Yutong Wang, Xin Jin, Xinyi Zhang, and Rui Yang. 2025. "Pumpkin Seed Proteins: The Potentially Alternative Protein Supplements for Food Applications" Foods 14, no. 22: 3969. https://doi.org/10.3390/foods14223969
APA StyleXie, Y., Wang, Y., Jin, X., Zhang, X., & Yang, R. (2025). Pumpkin Seed Proteins: The Potentially Alternative Protein Supplements for Food Applications. Foods, 14(22), 3969. https://doi.org/10.3390/foods14223969






