Koumiss (Fermented Mare’s Milk) as a Functional Food: Bioactive Proteins, Peptides, and Future Perspectives
Abstract
1. Introduction
Literature Search Strategy and Review Framework
2. Composition and Native Bioactive Proteins of Raw Mare’s Milk
2.1. Casein and Whey Fractions
2.2. Protein Composition and Key Bioactive Components
2.3. Lipid Profile
2.4. Carbohydrates and Oligosaccharides
2.5. Enzymes and Micronutrients
2.6. Functional Significance
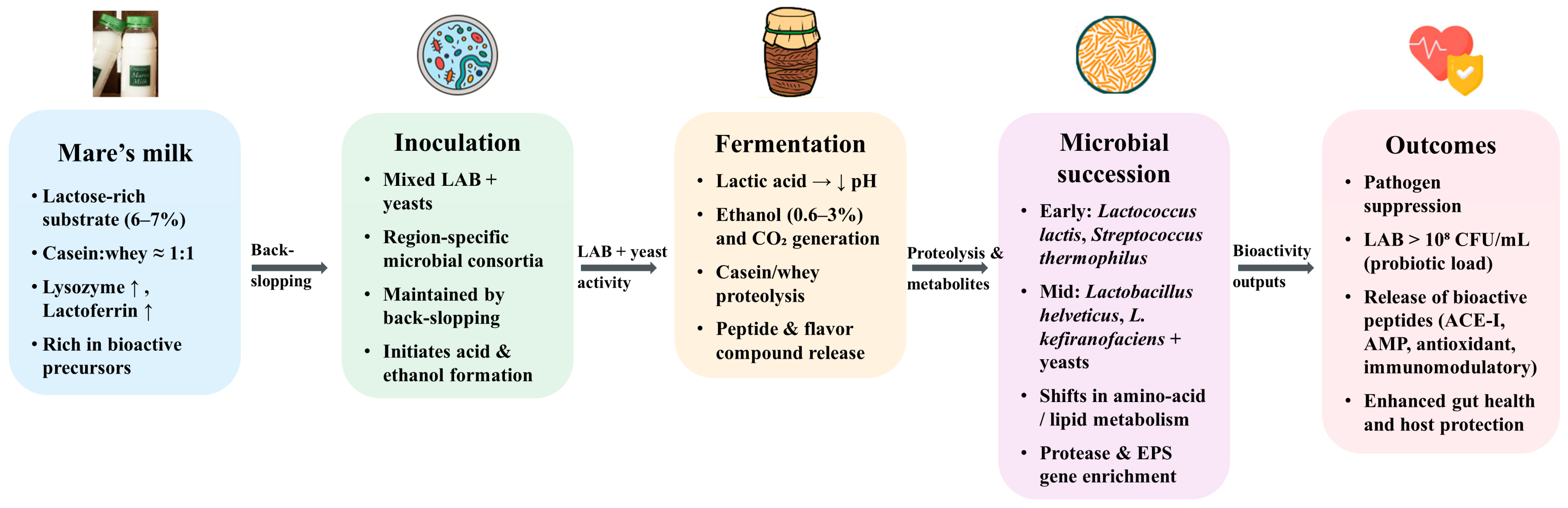
3. Fermentation Ecology and Microbial Transformation in Koumiss
3.1. Core Microbial Groups
3.2. Effects on Safety and Probiotic Enrichment
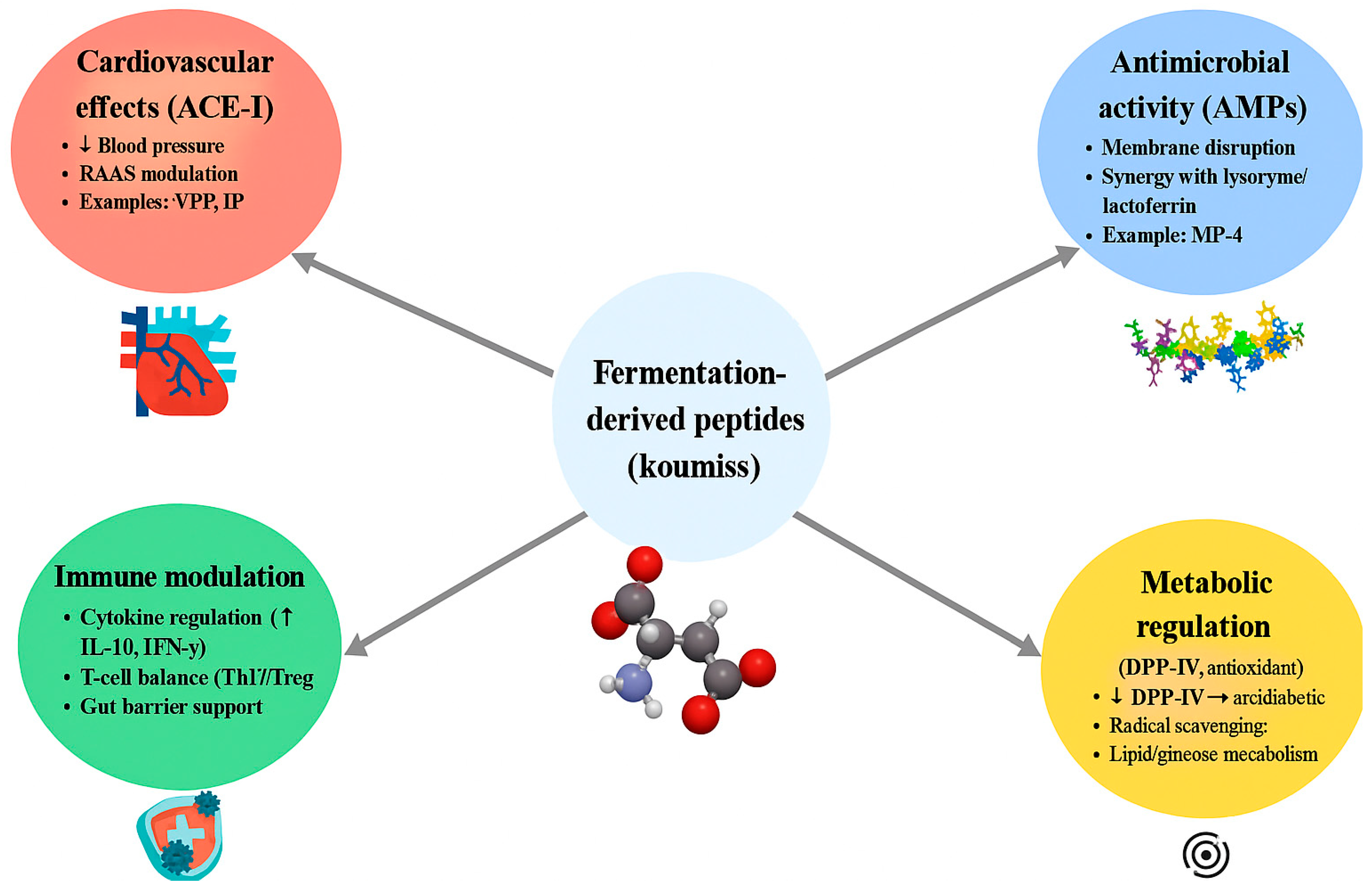
4. Native Bioactive Proteins in Koumiss
4.1. Lysozyme
4.2. Lactoferrin
4.3. Immunoglobulins
4.4. α-Lactalbumin
4.5. β-Lactoglobulin and Allergenicity
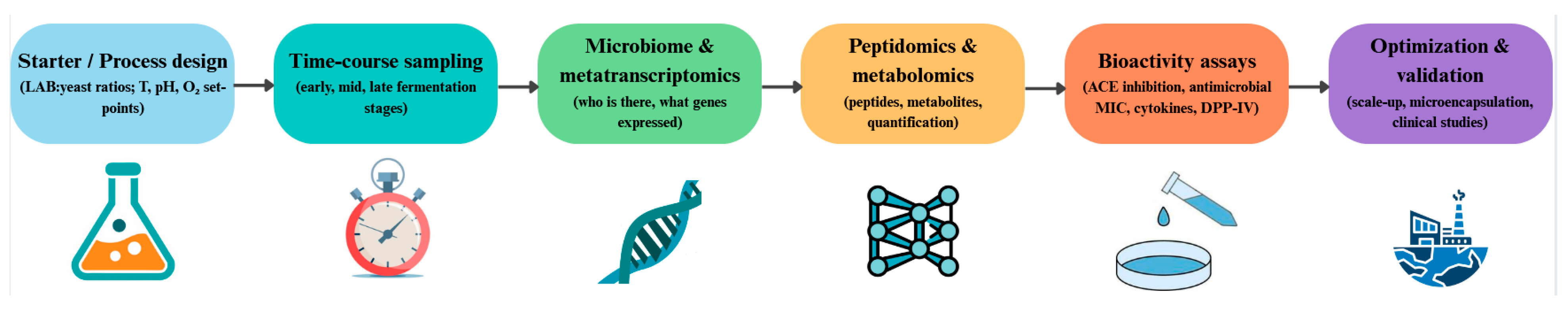
5. Multi-Omics Insights and Technological Advances
5.1. Metabolomics: System-Level Shifts During Fermentation
5.2. Proteomics and Peptidomics: Mapping the Peptide Repertoire
5.3. Microbiome and Metatranscriptomics: Who Is There and What They Do
5.4. Microbiome and Metatranscriptomics: Who Is There and What They Do
5.5. Technological Strategies to Steer Peptide Functionality
5.6. Gaps, Standards, and Scale-Up Challenges
6. Evidence in Animals and Humans
6.1. Insights from Animal Studies
6.2. Evidence from Human Studies
6.3. Mechanistic Considerations and Knowledge Gaps
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| CMPA | Cow’s Milk Protein Allergy |
| CO2 | Carbon Dioxide |
| DPP-IV | Dipeptidyl Peptidase-IV |
| EPS | Exopolysaccharides |
| FA | Fatty Acids |
| GST | Glutathione S-Transferase |
| HAMLET | Human α-Lactalbumin Made Lethal to Tumor Cells |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HMOs | Human Milk Oligosaccharides |
| IPP | Isoleucine-Proline-Proline |
| LAB | Lactic Acid Bacteria |
| LC-PUFA | Long-Chain Polyunsaturated Fatty Acids |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MMOs | Mare’s Milk Oligosaccharides |
| Neu5Ac | N-Acetylneuraminic Acid |
| PepT1 | Peptide Transporter 1 |
| PUFA | Polyunsaturated Fatty Acids |
| SCFA | Short-Chain Fatty Acids |
| TNF-α | Tumor Necrosis Factor Alpha |
| UPLC-Q-TOF-MS | Ultra-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry |
| VPP | Valine-Proline-Proline |
References
- Outram, A.K.; Stear, N.A.; Bendrey, R.; Olsen, S.; Kasparov, A.; Zaibert, V.; Thorpe, N.; Evershed, R.P. The earliest horse harnessing and milking. Science 2009, 323, 1332–1335. [Google Scholar] [CrossRef]
- Sheng, Q.; Fang, X. Bioactive components in mare milk. In Bioactive Components in Milk and Dairy Products; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 195–213. [Google Scholar]
- Blanco-Doval, A.; Barron, L.J.R.; Aldai, N. Nutritional Quality and Socio-Ecological Benefits of Mare Milk Produced under Grazing Management. Foods 2024, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, E.; Wadas, E.; Daszkiewicz, T.; Pietrzak-Fiećko, E. Nutritional Value and Health-Promoting Properties of Mare’s Milk—A Review. Czech J. Anim. Sci. 2017, 62, 511–518. [Google Scholar] [CrossRef]
- Lozovich, S. Medical uses of whole and fermented mare milk in Russia. Cult. Dairy Prod. J. 1995, 30, 18–21. [Google Scholar]
- Wang, J.; Chen, X.; Liu, W.; Yang, M.; Airidengcaicike; Zhang, H. Identification of Lactobacillus from koumiss by conventional and molecular methods. Eur. Food Res. Technol. 2008, 227, 1555–1561. [Google Scholar] [CrossRef]
- Navrátilová, P.; Pospíšil, J.; Borkovcová, I.; Kaniová, L.; Dluhošová, S.; Horáková, S. Content of nutritionally important components in mare milk fat. Dairy/Mljekarstvo 2018, 68, 282–294. [Google Scholar] [CrossRef]
- Guo, L.; Ya, M.; Guo, Y.-S.; Xu, W.-L.; Li, C.-D.; Sun, J.-P.; Zhu, J.-J.; Qian, J.-P. Study of bacterial and fungal community structures in traditional koumiss from Inner Mongolia. J. Dairy Sci. 2019, 102, 1972–1984. [Google Scholar] [CrossRef]
- Kondybayev, A.; Loiseau, G.; Achir, N.; Mestres, C.; Konuspayeva, G. Fermented mare milk product (Qymyz, Koumiss). Int. Dairy J. 2021, 119, 105065. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Fox, P.F. Equid milk: Chemistry, biochemistry and processing. Food Biochem. Food Process. 2012, 26, 491–530. [Google Scholar]
- Li, H.; Wang, Y.; Zhang, T.; Li, J.; Zhou, Y.; Li, H.; Yu, J. Comparison of backslopping and two-stage fermentation methods for koumiss powder production based on chemical composition and nutritional properties. J. Sci. Food Agric. 2020, 100, 1822–1826. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, J.; Miao, W.; Shuang, Q. A UPLC-Q-TOF-MS-based metabolomics approach for the evaluation of fermented mare’s milk to koumiss. Food Chem. 2020, 320, 126619. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Ganzorig, K.; Urashima, T.; Fukuda, K. Exploring potential bioactive peptides in fermented bactrian camel’s milk and mare’s milk made by mongolian nomads. Foods 2020, 9, 1817. [Google Scholar] [CrossRef]
- Miranda, G.; Mahé, M.F.; Leroux, C.; Martin, P. Proteomic tools to characterize the protein fraction of Equidae milk. Proteomics 2004, 4, 2496–2509. [Google Scholar] [CrossRef]
- Alichanidis, E.; Moatsou, G.; Polychroniadou, A. Composition and properties of non-cow milk and products. In Non-Bovine Milk and Milk Products; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–116. [Google Scholar]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Złotkowska, D.; Stachurska, E.; Fuc, E.; Wróblewska, B.; Mikołajczyk, A.; Wasilewska, E. Differences in regulatory mechanisms induced by β-lactoglobulin and κ-casein in cow’s milk allergy mouse model–in vivo and ex vivo studies. Nutrients 2021, 13, 349. [Google Scholar] [CrossRef]
- Wszolek, M.; Kupiec-Teahan, B.; Skov Guldager, H.; Tamime, A. Production of kefir, koumiss and other related products. In Fermented Milks; Wiley: Hoboken, NJ, USA, 2006; pp. 174–216. [Google Scholar]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- van der Strate, B.W.A. Anti-Cytomegalovirus Applications of the Intrinsically Active Drug Carrier Lactoferrin; University of Groningen: Groningen, The Netherlands, 2001. [Google Scholar]
- Afzaal, M.; Saeed, F.; Anjum, F.; Waris, N.; Husaain, M.; Ikram, A.; Ateeq, H.; Muhammad Anjum, F.; Suleria, H. Nutritional and ethnomedicinal scenario of koumiss: A concurrent review. Food Sci. Nutr. 2021, 9, 6421–6428. [Google Scholar] [CrossRef]
- Tang, H.; Ma, H.; Hou, Q.; Li, W.; Xu, H.; Liu, W.; Sun, Z.; Haobisi, H.; Menghe, B. Profiling of koumiss microbiota and organic acids and their effects on koumiss taste. BMC Microbiol. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Martuzzi, F.; Franceschi, P.; Formaggioni, P. Fermented mare milk and its microorganisms for human consumption and health. Foods 2024, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yuan, X.; Ji, Z.; Li, H.; Yao, Y. Nutritional ingredients and prevention of chronic diseases by fermented koumiss: A comprehensive review. Front. Nutr. 2023, 10, 1270920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, S.; Zhao, J.; Shuang, Q.; Xia, Y.; Zhang, F. A novel endogenous antimicrobial peptide MP-4 derived from koumiss of Inner Mongolia by peptidomics, and effects on Staphylococcus aureus. LWT 2024, 191, 115595. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Miyamoto, M.; Seto, Y.; Nakajima, H.; Burenjargal, S.; Gombojav, A.; Demberel, S.; Miyamoto, T. Denaturing gradient gel electrophoresis analysis of lactic acid bacteria and yeasts in traditional Mongolian fermented milk. Food Sci. Technol. Res. 2010, 16, 319–326. [Google Scholar] [CrossRef]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Feng, M.; Zhang, H.; Xu, J.; Ding, J.; Cheng, Z.; Qian, L. Peptidomics Analysis Reveals Peptide PDCryab1 Inhibits Doxorubicin-Induced Cardiotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 7182428. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Chen, X.; Liu, Y.; Zhang, H.; Sun, T. Identification of angiotensin I-converting enzyme inhibitory peptides from koumiss, a traditional fermented mare’s milk. J. Dairy Sci. 2010, 93, 884–892. [Google Scholar] [CrossRef]
- Kusumaningtyas, E.; Widiastuti, R.; Kusumaningrum, H.; Suhartono, M. Bioactivities and analysis of peptides from Sumbawa horse milk generated by bacillus thuringiensis protease. J. Ilmu Ternak Vet. 2018, 21, 244–254. [Google Scholar] [CrossRef]
- Rubak, Y.T.; Nuraida, L.; Iswantini, D.; Prangdimurti, E. Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria. Vet. World 2020, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Iroyukifujita, H.; Eiichiyokoyama, K.; Yoshikawa, M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Du, M.; Ji, X.; Mao, X. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Zhang, J.-X.; Li, G.; Xu, Y.-Y.; Lu, K.; Wang, Z.-G.; Liu, J.-P. Antimicrobial activity and mechanism of peptide CM4 against Pseudomonas aeruginosa. Food Funct. 2020, 11, 7245–7254. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Bilige, M.; Wu, R.; Wang, L.; Yang, X.; Xu, J.; Dong, Y.; Sun, Z.; Zang, H. Isolation and identification of Lactobacillus from koumiss collected from Inner Mongolia and the People’s Republic of Mongolia. China Dairy Ind. 2004, 32, 6–11. [Google Scholar]
- Rong, J.; Zheng, H.; Liu, M.; Hu, X.; Wang, T.; Zhang, X.; Jin, F.; Wang, L. Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol. 2015, 15, 196. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Wang, J.; Li, H.; Menghe, B.; Wu, J.; Guo, M.; Zhang, H. Isolation and preliminary probiotic selection of lactobacilli from koumiss in Inner Mongolia. J. Basic Microbiol. 2009, 49, 318–326. [Google Scholar] [CrossRef]
- Li, C.K.; Hou, Q.C.; Laga, W.; Liu, H.X.; Sun, B.Y.; Kwok, L.-Y.; Zhang, H.P.; Menghe, B. Koumiss consumption alleviates symptoms of patients with chronic atrophic gastritis: A Possible link to modulation of gut microbiota. J. Nutr. Oncol. 2017, 2, 36–51. [Google Scholar]
- Musaev, A.; Sadykova, S.; Anambayeva, A.; Saizhanova, M.; Balkanay, G.; Kolbaev, M. Mare’s milk: Composition, properties, and application in medicine. Arch. Razi Inst. 2021, 76, 1125. [Google Scholar]
- Cais-Sokolińska, D.; Danków, R.; Bierzuńska, P.; Kaczyński, Ł.; Chudy, S.; Teichert, J.; Dobek, A.; Skotarczak, E.; Pikul, J. Freezing point and other technological properties of milk of the Polish Coldblood horse breed. J. Dairy Sci. 2018, 101, 9637–9646. [Google Scholar] [CrossRef]
- Barłowska, J.; Polak, G.; Janczarek, I.; Tkaczyk, E. The influence of selected factors on the nutritional value of the milk of cold-blooded mares: The example of the Sokólski breed. Animals 2023, 13, 1152. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-related aspects of milk proteins. Iran. J. Pharm. Res. 2016, 15, 573. [Google Scholar]
- Talbert, J.A.; Townsend, S.D. Human milk as a complex natural product. Nat. Prod. Rep. 2025, 42, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.S.; Reed, S.A. Lactation in horses. Anim. Front. 2023, 13, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, K.; Gantner, V.; Kuterovac, K.; Cividini, A. Mare’s milk: Composition and protein fraction in comparison with different milk species. Mljekarstvo Časopis Unaprjeđenje Proizv. I Prerade Mlijeka 2011, 61, 107–113. [Google Scholar]
- Barreto, Í.M.L.G.; Rangel, A.H.d.N.; Urbano, S.A.; Bezerra, J.d.S.; Oliveira, C.A.d.A. Equine milk and its potential use in the human diet. Food Sci. Technol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Pieszka, M.; Luszczynski, J.; Zamachowska, M.; Augustyn, R.; Dlugosz, B.; Hedrzak, M. Is mare milk an appropriate food for people?—A review. Ann. Anim. Sci. 2016, 16, 33. [Google Scholar] [CrossRef]
- Matéos, A.; Miclo, L.; Mollé, D.; Dary, A.; Girardet, J.-M.; Gaillard, J.-L. Equine αS1-casein: Characterization of alternative splicing isoforms and determination of phosphorylation levels. J. Dairy Sci. 2009, 92, 3604–3615. [Google Scholar] [CrossRef]
- Matéos, A.; Girardet, J.M.; Mollé, D.; Corbier, C.; Gaillard, J.L.; Miclo, L. Identification of phosphorylation sites of equine β-casein isoforms. Rapid Commun. Mass Spectrom. 2010, 24, 1533–1542. [Google Scholar] [CrossRef]
- Kossaliyeva, G.; Rysbekuly, K.; Zhaparkulova, K.; Kozykan, S.; Li, J.; Serikbayeva, A.; Shynykul, Z.; Zhaparkulova, M.; Yessimsiitova, Z. Chemical composition, physical properties, and immunomodulating study of mare’s milk of the Adaev horse breed from Kazakhstan. Front. Nutr. 2025, 12, 1443031. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Iametti, S.; Tedeschi, G.; Oungre, E.; Bonomi, F. Primary structure of κ-casein isolated from mares’ milk. J. Dairy Res. 2001, 68, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Cammertoni, N.; Santini, G.; Klimanova, Y.; Zhang, J.; Vincenzetti, S. Nutritional properties of camelids and equids fresh and fermented milk. Dairy 2021, 2, 288–302. [Google Scholar] [CrossRef]
- Lv, K.; Yang, Y.; Li, Q.; Chen, R.; Deng, L.; Zhang, Y.; Jiang, N. Identification and comparison of milk fat globule membrane and whey proteins from Selle Français, Welsh pony, and Tieling Draft horse mare’s milk. Food Chem. 2024, 437, 137915. [Google Scholar] [CrossRef]
- Barnard, J.; Roberts, S.; Kelly, M.; Lastella, M.; Aisbett, B.; Condo, D. Alpha-lactalbumin and sleep: A systematic review. J. Sleep Res. 2024, 33, e14141. [Google Scholar] [CrossRef]
- Gao, P.; Morita, N.; Shinkura, R. Role of mucosal IgA antibodies as novel therapies to enhance mucosal barriers. In Proceedings of the Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2025; p. 1. [Google Scholar]
- Rusconi, B.; Bard, A.K.; McDonough, R.; Kindsvogel, A.M.; Wang, J.D.; Udayan, S.; McDonald, K.G.; Newberry, R.D.; Tarr, P.I. Intergenerational protective anti-gut commensal immunoglobulin G originates in early life. Proc. Natl. Acad. Sci. USA 2024, 121, e2309994121. [Google Scholar] [CrossRef]
- Peres Fabbri, L.; Cavallero, A.; Vidotto, F.; Gabriele, M. Bioactive peptides from fermented foods: Production approaches, sources, and potential health benefits. Foods 2024, 13, 3369. [Google Scholar] [CrossRef]
- Narmuratova, M.; Berillo, D.; Narmuratova, Z.; Tarlykov, P.; Serikbayeva, A.; Kanayat, S. Antihypertensive, Anti-Inflammatory, and Antiangiogenic In Silico Activity of Lactoferrin-Derived Peptides of Equine Milk Hydrolysate. Biomedicines 2024, 12, 2715. [Google Scholar] [CrossRef]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Li Volti, G.; Leonardi, S. Lactoferrin: Cytokine modulation and application in clinical practice. J. Clin. Med. 2021, 10, 5482. [Google Scholar] [CrossRef] [PubMed]
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The immunomodulatory effects of lactoferrin and its derived peptides on NF-κB signaling pathway: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e972. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.S.; Choi, Y.J.; Kim, C. The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation. Biomolecules 2025, 15, 1174. [Google Scholar] [CrossRef] [PubMed]
- Kazimierska, K.; Kalinowska-Lis, U. Milk proteins—Their biological activities and use in cosmetics and dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef]
- Turner, J.; Arns, M.; Minton, J. Effects of non-specific immunostimulation of prepartum mares on colostral quality and foal immune function. Prof. Anim. Sci. 2003, 19, 62–67. [Google Scholar] [CrossRef]
- Wodas, L.; Mackowski, M.; Borowska, A.; Puppel, K.; Kuczynska, B.; Cieslak, J. Genes encoding equine β-lactoglobulin (LGB1 and LGB2): Polymorphism, expression, and impact on milk composition. PLoS ONE 2020, 15, e0232066. [Google Scholar] [CrossRef]
- Businco, L.; Giampietro, P.G.; Lucenti, P.; Lucaroni, F.; Pini, C.; Di Felice, G.; Iacovacci, P.; Curadi, C.; Orlandi, M. Allergenicity of mare’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2000, 105, 1031–1034. [Google Scholar] [CrossRef]
- Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cavagni, G.; Fiocchi, A.; Poiesi, C.; Velonà, T.; Ugazio, A.G.; Galli, C.L. Cross-reactivity between milk proteins from different animal species. Clin. Exp. Allergy 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Chilliard, Y.; Doreau, M. Characterization of lipase in mare milk. J. Dairy Sci. 1985, 68, 37–39. [Google Scholar] [CrossRef]
- Kushugulova, A.; Kozhakhmetov, S.; Sattybayeva, R.; Nurgozhina, A.; Ziyat, A.; Yadav, H.; Marotta, F. Mare’s milk as a prospective functional product. Funct. Foods Health Dis. 2018, 8, 548–554. [Google Scholar] [CrossRef][Green Version]
- Deng, L.; Yang, Y.; Li, Z.; Li, J.; Zhu, Y.; Meng, Q.; Liu, J.; Wang, X. Impact of different dietary regimens on the lipidomic profile of mare’s milk. Food Res. Int. 2022, 156, 111305. [Google Scholar] [CrossRef] [PubMed]
- Gregić, M.; Mijić, P.; Baban, M.; Aladrović, J.; Pađen, L.; Gantner, V.; Bobić, T. Changes in the fatty acid composition of milk of Lipizzaner mares during the lactation period. Metabolites 2022, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Kęszycka, M.; Wójtowski, J.; Czyżak-Runowska, G.; Kuczyńska, B.; Puppel, K.; Krzyżewski, J.; Strzałkowska, N.; Jóźwik, A.; Bagnicka, E. Concentration of selected fatty acids, fat-soluble vitamins and β-carotene in late lactation mares’ milk. Int. Dairy J. 2014, 38, 31–36. [Google Scholar] [CrossRef]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative proteomics of milk fat globule membrane (MFGM) proteome across species and lactation stages and the potentials of MFGM fractions in infant formula preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Tomczyński, R.; Smoczyński, S.S. Effect of lactation period on the fatty acid composition in mares’ milk from different breeds. Arch. Anim. Breed. 2013, 56, 335–343. [Google Scholar] [CrossRef]
- Guo, L.; Xu, W.L.; Li, C.D.; Ya, M.; Guo, Y.S.; Qian, J.P.; Zhu, J.J. Production technology, nutritional, and microbiological investigation of traditionally fermented mare milk (Chigee) from Xilin Gol in China. Food Sci. Nutr. 2020, 8, 257–264. [Google Scholar] [CrossRef]
- Orlandi, M.; Curadi, M.C.; Monti, L. Sialylated oligosaccharides in mare and ass milk: Preliminary results. Prog. Nutr. 2016, 18, 283–287. [Google Scholar]
- Difilippo, E.; Willems, H.; Vendrig, J.; Fink-Gremmels, J.; Gruppen, H.; Schols, H. Comparison of milk oligosaccharides pattern in colostrum of different horse breeds. J. Agric. Food Chem. 2015, 63, 4805–4814. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S. Health promoting aspects of milk oligosaccharides. Int. Dairy J. 2006, 16, 1341–1346. [Google Scholar] [CrossRef]
- Durham, S.D.; Wei, Z.; Lemay, D.G.; Lange, M.C.; Barile, D. Creation of a milk oligosaccharide database, MilkOligoDB, reveals common structural motifs and extensive diversity across mammals. Sci. Rep. 2023, 13, 10345. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; Van Neerven, R.J. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Gormley, A.; Garavito-Duarte, Y.; Kim, S.W. The role of milk oligosaccharides in enhancing intestinal microbiota, intestinal integrity, and immune function in pigs: A comparative review. Biology 2024, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Auer, F.; Jarvas, G.; Guttman, A. Recent advances in the analysis of human milk oligosaccharides by liquid phase separation methods. J. Chromatogr. B 2021, 1162, 122497. [Google Scholar] [CrossRef] [PubMed]
- Rieland, E.; Hatzipanagiotou, A.; Jahnecke, S.; Enbergs, H. Activities of the enzymes LDH, gamma-GT, GOT, GPT and lactoperoxidase in the milk of breeding mares during the course of lactation. Berl. Munch. Tierarztl. Wochenschr. 1998, 111, 81–89. [Google Scholar]
- Kocyigit, E.; Abdurakhmanov, R.; Kocyigit, B.F. Potential role of camel, mare milk, and their products in inflammatory rheumatic diseases. Rheumatol. Int. 2024, 44, 425–434. [Google Scholar] [CrossRef]
- Richards, N.; Choct, M.; Hinch, G.N.; Rowe, J.B. Examination of the use of exogenous α-amylase and amyloglucosidase to enhance starch digestion in the small intestine of the horse. Anim. Feed. Sci. Technol. 2004, 114, 295–305. [Google Scholar] [CrossRef][Green Version]
- Awol, K.; Taye, M.; Kassa, B. Activation of lactoperoxidase system and its potential for microbial inhibition and preservation of milk in the Great African Rift Valley climate. Cogent Food Agric. 2023, 9, 2247691. [Google Scholar] [CrossRef]
- Alipour, A.; Akrami Mohajeri, F.; Javdan, G.; Pourramezani, F.; Fallahzadeh, H.; Khalili Sadrabad, E. Concentration of mineral and heavy metals in raw mare (horse) milk consumed in Yazd, Iran: A risk assessment study. Vet. Med. Sci. 2023, 9, 1592–1598. [Google Scholar] [CrossRef]
- Aldai, N.; Blanco-Doval, A.; Barron, L.J.R. Mineral Content of Mare Milk Produced in Semi-Extensive Rural Farms: Effect of Lactation. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4424645 (accessed on 12 February 2025).
- Narmuratova, M.K.; Cakir-Kiefer, C.; Narmuratova, Z.B. Isolation and purification of lactoferrin from Kazakhstan mare milk. Int. J. Biol. Chem. 2019, 12, 64–69. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 550441. [Google Scholar] [CrossRef]
- Liu, B.; Liang, Y.-h.; He, Y.-Z.; Ye, W.; Deng, Z.-Y.; Li, J.; Guo, S. Differences in fat digestion from milk of different Species: In vitro gastrointestinal digestion model for infants. Food Res. Int. 2023, 174, 113571. [Google Scholar] [CrossRef] [PubMed]
- Lajnaf, R.; Feki, S.; Ameur, S.B.; Attia, H.; Kammoun, T.; Ayadi, M.A.; Masmoudi, H. Cow’s milk alternatives for children with cow’s milk protein allergy-Review of health benefits and risks of allergic reaction. Int. Dairy J. 2023, 141, 105624. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Zhang, B.; Li, D.; Yan, J.; Yang, J.; Sun, J.; Cao, H.; Wang, Y.; Zhang, F. Effects of different n-6/n-3 polyunsaturated fatty acids ratios on lipid metabolism in patients with hyperlipidemia: A randomized controlled clinical trial. Front. Nutr. 2023, 10, 1166702. [Google Scholar] [CrossRef]
- Raz, C.; Paramonov, M.M.; Shemesh, M.; Argov-Argaman, N. The milk fat globule size governs a physiological switch for biofilm formation by Bacillus subtilis. Front. Nutr. 2022, 9, 844587. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Weimann, C.; Erhardt, G. Mare’s milk as a source of ACE-inhibitory peptides for human nutrition. Zuchtungskunde 2018, 90, 168–178. [Google Scholar]
- Karav, S.; Salcedo, J.; Frese, S.; Barile, D. Thoroughbred mare’s milk exhibits a unique and diverse free oligosaccharide profile. FEBS Open Bio 2018, 8, 1219–1229. [Google Scholar] [CrossRef]
- Mady, E.A.; Osuga, H.; Toyama, H.; El-Husseiny, H.M.; Inoue, R.; Murase, H.; Yamamoto, Y.; Nagaoka, K. Relationship between the components of mare breast milk and foal gut microbiome: Shaping gut microbiome development after birth. Vet. Q. 2024, 44, 1–9. [Google Scholar] [CrossRef]
- Hauser, J.; Pisa, E.; Arias Vásquez, A.; Tomasi, F.; Traversa, A.; Chiodi, V.; Martin, F.-P.; Sprenger, N.; Lukjancenko, O.; Zollinger, A. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol. Psychiatry 2021, 26, 2854–2871. [Google Scholar] [CrossRef]
- Dedola, S.; Ahmadipour, S.; de Andrade, P.; Baker, A.N.; Boshra, A.N.; Chessa, S.; Gibson, M.I.; Hernando, P.J.; Ivanova, I.M.; Lloyd, J.E. Sialic acids in infection and their potential use in detection and protection against pathogens. RSC Chem. Biol. 2024, 5, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yue, X.; Ren, X.; Pang, Y.; Wang, T.; Huangfu, B.; Mikhailovich, Z.A.; Vasilievich, K.V.; Zhang, M.; Luan, Y. Mare milk and fermented mare milk alleviate dextran sulfate sodium salt–induced ulcerative colitis in mice by reducing inflammation and modulating intestinal flora. J. Dairy Sci. 2025, 108, 2182–2198. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xie, Q.; Chen, Q.; Evivie, S.E.; Liu, D.; Dong, J.; Huo, G.; Li, B. Cow, goat, and mare milk diets differentially modulated the immune system and gut microbiota of mice colonized by healthy infant feces. J. Agric. Food Chem. 2020, 68, 15345–15357. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Xilin, T.; Ji, M.; Meng, X.; Zhao, Y.; Siqin, B.; Zhang, N.; Li, M. Effects of koumiss on intestinal immune modulation in immunosuppressed rats. Front. Nutr. 2022, 9, 765499. [Google Scholar] [CrossRef]
- McGuire, G. Cultural histories of kumiss: Tuberculosis, heritage and national health in post-Soviet Kazakhstan. Central Asian Survey 2017, 36, 493–510. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, J.; Liu, H.; Feng, C.; Shuang, Q. Novel insight into physicochemical and flavor formation in koumiss based on microbial metabolic network. Food Res. Int. 2021, 149, 110659. [Google Scholar] [CrossRef]
- Istanbullugil, F.R.; Risvanli, A.; Salikov, R.; Bayraktar, M.; Zhunushova, A.; Acaroz, U.; Acaroz, D.A.; Yilmaz, O.; Yuksel, B.F.; Turanli, M. Koumiss and immunity: A thorough investigation of fermentation parameters and their impact on health benefits. J. Dairy Sci. 2024, 107, 6451–6459. [Google Scholar] [CrossRef]
- Zhao, X.; Song, L.; Han, D.; Han, P.; Bai, F. Microbiological and physicochemical dynamics in traditional and industrial fermentation processes of koumiss. Fermentation 2024, 10, 66. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The evolution of fermented milks, from artisanal to industrial products: A critical review. Fermentation 2022, 8, 679. [Google Scholar] [CrossRef]
- Zhang, M.; Dang, N.; Ren, D.; Zhao, F.; Lv, R.; Ma, T.; Bao, Q.; Menghe, B.; Liu, W. Comparison of bacterial microbiota in raw mare’s milk and koumiss using PacBio single molecule real-time sequencing technology. Front. Microbiol. 2020, 11, 581610. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, W.; Zhang, J.; Yu, J.; Zhang, W.; Cai, C.; Menghe, B.; Sun, T.; Zhang, H. Identification and characterization of the dominant lactobacilli isolated from koumiss in China. J. Gen. Appl. Microbiol. 2010, 56, 257–265. [Google Scholar] [CrossRef][Green Version]
- Bogoyavlenskiy, A.; Alexyuk, M.; Alexyuk, P.; Amanbayeva, M.; Anarkulova, E.; Imangazy, A.; Bektuganova, A.; Berezin, V. Metagenomic exploration of koumiss from Kazakhstan. Microbiol. Resour. Announc. 2022, 11, e01082-21. [Google Scholar] [CrossRef]
- Boranbayeva, T.; Karahan, A.G.; Toishimanov, M.; Zhalelov, D.; Bolat, A. Effects of seasonal and regional variations on the bacterial and fungal biodiversity of mares’ milk and koumiss in the Almaty and Zhambyl regions of Kazakhstan. Int. Dairy J. 2025, 169, 106331. [Google Scholar] [CrossRef]
- İstanbullugil, F.R.; Sanli, K.; Ozturk, T.; Keskin, B.C.; Düyşöbayeva, A.; Risvanli, A.; Acaröz, U.; Acaröz, D.A.; Salykov, R.; Sahin, M. Koumiss Microbiome: Investigation of the Microbial Composition and Functional Potential of a Unique Beverage of Fermented Milk Produced at Kyrgyz Mountains. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–17. [Google Scholar]
- Oki, K.; Dugersuren, J.; Demberel, S.; Watanabe, K. Pyrosequencing analysis of the microbial diversity of airag, khoormog and tarag, traditional fermented dairy products of Mongolia. Biosci. Microbiota Food Health 2014, 33, 53–64. [Google Scholar] [CrossRef]
- Teichert, J.; Cais-Sokolińska, D.; Bielska, P.; Danków, R.; Chudy, S.; Kaczyński, Ł.K.; Biegalski, J. Milk fermentation affects amino acid and fatty acid profile of mare milk from Polish Coldblood mares. Int. Dairy J. 2021, 121, 105137. [Google Scholar] [CrossRef]
- Zhao, H.-Z.; Song, Q.-J.; Guo, H.; Liu, C.-Y.; Yang, C.; Li, X.; Wang, Y.-X.; Ma, Z.-P.; Wang, F.-X.; Wen, Y.-J. Characterization of a Potential Probiotic Strain in Koumiss. Fermentation 2023, 9, 87. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Lee, K.-H.; Kang, H.-B.; Kim, J.-E.; Oh, M.-H.; Ham, J.-S. Probiogenomic in-silico analysis and safety assessment of Lactiplantibacillus plantarum DJF10 strain isolated from Korean raw milk. Int. J. Mol. Sci. 2022, 23, 14494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, E.-S.; Kim, B.-M.; Ham, J.-S.; Oh, M.-H. Application of Lactic Acid Bacteria to Inhibit Fungal Contamination of Cured Cheeses. J. Dairy Sci. Biotechnol. 2022, 40, 103–109. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Xue, J.; Kwok, L.-y.; Yang, J.; Zhang, H.; Menghe, B. Characterization of angiotensin-converting enzyme inhibitory activity of fermented milk produced by Lactobacillus helveticus. J. Dairy Sci. 2015, 98, 5113–5124. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, X.; Yuan, H. Detection and identification of wild yeast in Koumiss. Food Microbiol. 2012, 31, 301–308. [Google Scholar] [CrossRef]
- Gethins, L.; Guneser, O.; Demirkol, A.; Rea, M.C.; Stanton, C.; Ross, R.P.; Yuceer, Y.; Morrissey, J.P. Influence of carbon and nitrogen source on production of volatile fragrance and flavour metabolites by the yeast Kluyveromyces marxianus. Yeast 2015, 32, 67–76. [Google Scholar]
- Wang, X.; Zhao, Z.; Zhao, F.; Li, Y.; Liang, Y.; Zhou, R.; Shen, S.; Yu, J.; Liu, W.; Menghe, B. Dual-omics strategy for selecting optimal fermentation strains in traditional koumiss production. Food Chem. X 2025, 27, 102407. [Google Scholar] [CrossRef]
- Reina-Posso, D.; Gonzales-Zubiate, F.A. Expanding Horizons: The Untapped Potential of Kluyveromyces marxianus in Biotechnological Applications. Fermentation 2025, 11, 98. [Google Scholar] [CrossRef]
- Gumustop, I.; Ortakci, F. Comparative genomics of Leuconostoc lactis strains isolated from human gastrointestinal system and fermented foods microbiomes. BMC Genom. Data 2022, 23, 61. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dioso, C.M.; Liong, M.-T.; Nero, L.A.; Khosravi-Darani, K.; Ivanova, I.V. Beneficial features of pediococcus: From starter cultures and inhibitory activities to probiotic benefits. World J. Microbiol. Biotechnol. 2023, 39, 4. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Kochetkova, T.V.; Grabarnik, I.P.; Klyukina, A.A.; Zayulina, K.S.; Elizarov, I.M.; Shestakova, O.O.; Gavirova, L.A.; Malysheva, A.D.; Shcherbakova, P.A.; Barkhutova, D.D. Microbial communities of artisanal fermented milk products from Russia. Microorganisms 2022, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H. Characterization of airag collected in Ulaanbaatar, Mongolia with emphasis on isolated lactic acid bacteria. J. Anim. Sci. Technol. 2016, 58, 10. [Google Scholar] [CrossRef]
- Xia, Y.; Miao, W.; Zhao, J.; Chen, Y. Fermentation characteristics of Lactobacillus helveticus 3–4 and Kazachstania unispora A-3 complex starter: Enhancing the fermentation performance and flavor of koumiss. J. Dairy Sci. 2025, 108, 3455–3467. [Google Scholar] [CrossRef]
- Rakhmanova, A.; Wang, T.; Xing, G.; Ma, L.; Hong, Y.; Lu, Y.; Xin, L.; Xin, W.; Zhu, Q.; Lü, X. Isolation and identification of microorganisms in Kazakhstan koumiss and their application in preparing cow-milk koumiss. J. Dairy Sci. 2021, 104, 151–166. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. Health-promoting properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, B.D.; Kosmerl, E.; Jiménez-Flores, R.; Alvarez, V.B. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus bdgo-ym6. Front. Microbiol. 2023, 14, 1236634. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, C.; Ma, K.; Wang, X.; Xiao, L.; Zhang, X.; Rui, X.; Li, W. Advanced structural characterization and in vitro fermentation prebiotic properties of cell wall polysaccharide from Kluyveromyces marxianus. Int. J. Biol. Macromol. 2023, 241, 124420. [Google Scholar] [CrossRef]
- Siesto, G.; Pietrafesa, R.; Infantino, V.; Thanh, C.; Pappalardo, I.; Romano, P.; Capece, A. In vitro study of probiotic, antioxidant and anti-inflammatory activities among indigenous Saccharomyces cerevisiae strains. Foods 2022, 11, 1342. [Google Scholar] [CrossRef]
- Rizwan, D.; Masoodi, F.; Wani, S.M.; Mir, S.A. Bioactive peptides from fermented foods and their relevance in COVID-19 mitigation. Food Prod. Process. Nutr. 2023, 5, 53. [Google Scholar] [CrossRef]
- Kirdar, S.S. Therapeutics effects and health benefits of the Caucasus koumiss: A review. Annu. Res. Rev. Biol. 2021, 36, 47–56. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Antimicrobial lactoferrin peptides: The hidden players in the protective function of a multifunctional protein. Int. J. Pept. 2013, 2013, 390230. [Google Scholar] [CrossRef]
- Kashung, P.; Karuthapandian, D. Milk-derived bioactive peptides. Food Prod. Process. Nutr. 2025, 7, 6. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Praženicová, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to kill and time to heal: The multifaceted role of lactoferrin and lactoferricin in host defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef] [PubMed]
- Narmuratova, M.; Orazova, S.; Serikbayeva, A.; Narmuratova, Z.; Zhardamalieva, A. Mare’s milk as a source of biologically active immunoglobulins: A review of scientific data. Int. J. Biol. Chem. 2025, 18, 81. [Google Scholar] [CrossRef]
- Di Cagno, R.; Tamborrino, A.; Gallo, G.; Leone, C.; De Angelis, M.; Faccia, M.; Amirante, P.; Gobbetti, M. Uses of mares’ milk in manufacture of fermented milks. Int. Dairy J. 2004, 14, 767–775. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An overview of antimicrobial activity of lysozyme and its functionality in cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Xu, S.; Shi, J.; Dong, Y.; Li, Z.; Wu, X.; Lin, Y.; Che, L.; Li, J.; Feng, B.; Fang, Z. Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci. Rep. 2020, 10, 3210. [Google Scholar] [CrossRef]
- Yang, W.; Xi, C.; Yao, H.; Yuan, Q.; Zhang, J.; Chen, Q.; Wu, G.; Hu, J. Oral administration of lysozyme protects against injury of ileum via modulating gut microbiota dysbiosis after severe traumatic brain injury. Front. Cell. Infect. Microbiol. 2024, 14, 1304218. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Callahan, M.; De La Torre, U.; Maga, E.A. Comparing microbiotas of foals and their mares’ milk in the first two weeks after birth. BMC Vet. Res. 2024, 20, 17. [Google Scholar] [CrossRef]
- Czyżak-Runowska, G.; Wójtowski, J.; Niewiadomska, A.; Markiewicz-Keszycka, M. Quality of fresh and stored mares’ milk. Mljekarstvo Časopis Unaprjeđenje Proizv. I Prerade Mlijeka 2018, 68, 108–115. [Google Scholar] [CrossRef]
- Akal, H.C.; Ozturkoglu-Budak, S.; Bereli, N.; Cimen, D.; Akgonullu, S. Effect of donkey milk lactoferrin and lysozyme on yoghurt properties. Mljekarstvo Časopis Unaprjeđenje Proizv. I Prerade Mlijeka 2022, 72, 77–87. [Google Scholar]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef] [PubMed]
- Narmuratova, Z.; Hentati, F.; Girardet, J.-M.; Narmuratova, M.; Cakir-Kiefer, C. Equine lactoferrin: Antioxidant properties related to divalent metal chelation. LWT 2022, 161, 113426. [Google Scholar] [CrossRef]
- Angulo-Zamudio, U.A.; Vidal, J.E.; Nazmi, K.; Bolscher, J.G.; Leon-Sicairos, C.; Antezana, B.S.; Canizalez-Roman, A.; León-Sicairos, N. Lactoferrin disaggregates pneumococcal biofilms and inhibits acquisition of resistance through its DNase activity. Front. Microbiol. 2019, 10, 2386. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mazón, L.; Ramírez-Rico, G.; de la Garza, M. Lactoferrin: A secret weapon in the war against pathogenic bacteria. Explor. Drug Sci. 2024, 2, 734–743. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.-S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef]
- Fedorka, C.; Scoggin, K.; Boakari, Y.; Hoppe, N.; Squires, E.; Ball, B.; Troedsson, M. The anti-inflammatory effect of exogenous lactoferrin on breeding-induced endometritis when administered post-breeding in susceptible mares. Theriogenology 2018, 114, 63–69. [Google Scholar] [CrossRef]
- Ellison, R., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Ha, G.E.; Chang, O.K.; Jo, S.-M.; Han, G.-S.; Park, B.-Y.; Ham, J.-S.; Jeong, S.-G. Identification of antihypertensive peptides derived from low molecular weight casein hydrolysates generated during fermentation by Bifidobacterium longum KACC 91563. Korean J. Food Sci. Anim. Resour. 2015, 35, 738. [Google Scholar] [CrossRef]
- Chang, O.; Seol, K.-H.; Jeong, S.-G.; Oh, M.-H.; Park, B.-Y.; Perrin, C.; Ham, J.-S. Casein hydrolysis by Bifidobacterium longum KACC91563 and antioxidant activities of peptides derived therefrom. J. Dairy Sci. 2013, 96, 5544–5555. [Google Scholar] [CrossRef]
- Jenvey, C.; Caraguel, C.; Howarth, G.; Riley, C. Identification of periparturient mare and foal associated predictors of post parturient immunoglobulin A concentrations in T horoughbred foals. Equine Vet. J. 2012, 44, 73–77. [Google Scholar] [CrossRef]
- Rivero, M.J.; Cooke, A.S.; Gandarillas, M.; Leon, R.; Merino, V.M.; Velásquez, A. Nutritional composition, fatty acids profile and immunoglobulin G concentrations of mare milk of the Chilean Corralero horse breed. PLoS ONE 2024, 19, e0310693. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Narayan, K.S.; Vij, S. Evaluation of the antimicrobial attribute of bioactive peptides derived from colostrum whey fermented by Lactobacillus against diarrheagenic E. coli strains. J. Food Sci. Technol. 2023, 60, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A. α-Lactalbumin, amazing calcium-binding protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef]
- Markiewicz-Kęszycka, M.; Wójtowski, J.; Kuczyńska, B.; Puppel, K.; Czyżak-Runowska, G.; Bagnicka, E.; Strzałkowska, N.; Jóźwik, A.; Krzyżewski, J. Chemical composition and whey protein fraction of late lactation mares’ milk. Int. Dairy J. 2013, 31, 62–64. [Google Scholar] [CrossRef]
- Gratwicke, M.; Miles, K.; Clark, B.; Pumpa, K. The effect of α-lactalbumin consumption on sleep quality and quantity in female rugby union athletes: A field-based study. Biol. Sport 2022, 40, 449–455. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Mariotti, F.o.; Simbelie, K.L.; Makarios-Lahham, L.; Huneau, J.-F.o.; Tomé, D.; Even, P.C.; Laplaize, B. Acute ingestion of dietary proteins improves post-exercise liver glutathione in rats in a dose-dependent relationship with their cysteine content. J. Nutr. 2004, 134, 128–131. [Google Scholar] [CrossRef]
- Mossberg, A.K.; Hun Mok, K.; Morozova-Roche, L.A.; Svanborg, C. Structure and function of human α-lactalbumin made lethal to tumor cells (HAMLET)-type complexes. FEBS J. 2010, 277, 4614–4625. [Google Scholar] [CrossRef]
- Nakamura, T.; Aizawa, T.; Kariya, R.; Okada, S.; Demura, M.; Kawano, K.; Makabe, K.; Kuwajima, K. Molecular mechanisms of the cytotoxicity of human α-lactalbumin made lethal to tumor cells (HAMLET) and other protein-oleic acid complexes. J. Biol. Chem. 2013, 288, 14408–14416. [Google Scholar] [CrossRef]
- Lalonde, R. Anxiolytic-like effects of milk proteins. Pharmacol. Biochem. Behav. 2024, 240, 173789. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Han, S.; Ma, S.; Zou, Z.; Ding, F.; Li, X.; Li, L.; Tang, B.; Wang, H. Production of hypoallergenic milk from DNA-free beta-lactoglobulin (BLG) gene knockout cow using zinc-finger nucleases mRNA. Sci. Rep. 2018, 8, 15430. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Vincenzetti, S. Use of donkey milk in children with cow’s milk protein allergy. Foods 2013, 2, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; An, F.; Lin, H.; Li, M.; Wu, J.; Wu, R. Advances in fermented foods revealed by multi-omics: A new direction toward precisely clarifying the roles of microorganisms. Front. Microbiol. 2022, 13, 1044820. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Park, W.-S.; Bae, I.-S.; Yoo, J.; Yun, J.; Hoa, V.-B.; Ham, J.-S. HRMAS-NMR-Based Metabolomics Approach to Discover Key Differences in Cow and Goat Milk Yoghurt Metabolomes. Foods 2024, 13, 3483. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Ham, J.-S. Rapid discrimination and authentication of Korean farmstead mozzarella cheese through MALDI-TOF and multivariate statistical analysis. Metabolites 2021, 11, 333. [Google Scholar] [CrossRef]
- Xia, Y.; Oyunsuren, E.; Yang, Y.; Shuang, Q. Comparative metabolomics and microbial communities associated network analysis of black and white horse-sourced koumiss. Food Chem. 2022, 370, 130996. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.Á.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef]
- Banić, M.; Butorac, K.; Čuljak, N.; Butorac, A.; Novak, J.; Pavunc, A.L.; Rušanac, A.; Stanečić, Ž.; Lovrić, M.; Šušković, J. An Integrated Comprehensive Peptidomics and In Silico Analysis of Bioactive Peptide-Rich Milk Fermented by Three Autochthonous Cocci Strains. Int. J. Mol. Sci. 2024, 25, 2431. [Google Scholar] [CrossRef]
- Rangel, A.H.d.N.; Bezerra, D.A.F.V.d.A.; Sales, D.C.; Araújo, E.d.O.M.; Lucena, L.M.d.; Porto, A.L.F.; Véras, Í.V.U.M.; Lacerda, A.F.; Ribeiro, C.V.D.M.; Anaya, K. An overview of the occurrence of bioactive peptides in different types of cheeses. Foods 2023, 12, 4261. [Google Scholar] [CrossRef]
- Şanli, T.; Akal, H.C.; Yetişemiyen, A.; Hayaloglu, A.A. Influence of adjunct cultures on angiotensin-converting enzyme (ACE)-inhibitory activity, organic acid content and peptide profile of kefir. Int. J. Dairy Technol. 2018, 71, 131–139. [Google Scholar] [CrossRef]
- Ao, X.-L.; Liao, Y.-M.; Kang, H.-Y.; Li, H.-L.; He, T.; Zou, L.-K.; Liu, S.-L.; Chen, S.-J.; Yang, Y.; Liu, X.-Y. Untargeted metabolomics and physicochemical analysis revealed the quality formation mechanism in fermented milk inoculated with Lactobacillus brevis and Kluyveromyces marxianus isolated from traditional fermented milk. Foods 2023, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Butowski, C.F.; Dixit, Y.; Reis, M.M.; Mu, C. Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science. Metabolites 2025, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Elcheninov, A.G.; Zayulina, K.S.; Klyukina, A.A.; Kremneva, M.K.; Kublanov, I.V.; Kochetkova, T.V. Metagenomic insights into the taxonomic and functional features of traditional fermented milk products from Russia. Microorganisms 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Gesudu, Q.; Zhang, J.; Sun, Z.; Halatu, H.; Menghe, B.; Liu, W. Bacterial composition and function during fermentation of Mongolia koumiss. Food Sci. Nutr. 2021, 9, 4146–4155. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.; Wu, T.; Zhang, J.; Kwok, L.-Y.; Sun, Z.; Zhang, H.; Wang, J. Untargeted mass spectrometry-based metabolomics approach unveils biochemical changes in compound probiotic fermented milk during fermentation. npj Sci. Food 2023, 7, 21. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Liu, J.-L.; Jiang, T.-M.; Li, L.; Fang, G.-Z.; Liu, Y.-P.; Chen, L.-J. Influence of Kluyveromyces marxianus on proteins, peptides, and amino acids in Lactobacillus-fermented milk. Food Sci. Biotechnol. 2017, 26, 739–748. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, F.A.; Liu, T.; Chen, J.; He, G. Purification and identification of novel peptides with inhibitory effect against angiotensin I-converting enzyme and optimization of process conditions in milk fermented with the yeast Kluyveromyces marxianus. J. Funct. Foods 2015, 16, 278–288. [Google Scholar] [CrossRef]
- Nath, A.; Eren, B.A.; Zinia Zaukuu, J.-L.; Koris, A.; Pásztorné-Huszár, K.; Szerdahelyi, E.; Kovacs, Z. Detecting the bitterness of milk-protein-derived peptides using an electronic tongue. Chemosensors 2022, 10, 215. [Google Scholar] [CrossRef]
- Liu, B.; Li, N.; Chen, F.; Zhang, J.; Sun, X.; Xu, L.; Fang, F. Review on the release mechanism and debittering technology of bitter peptides from protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5153–5170. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive peptides in milk and dairy products: A review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, S.; Yun, S.; Zhang, M.; Peng, L.; Li, Y.; Zhou, Y. Microencapsulating alginate-based polymers for probiotics delivery systems and their application. Pharmaceuticals 2022, 15, 644. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Dhital, S.; Yu, A.; Chen, X.D. Impact of Freezing and Freeze Drying on Lactobacillus rhamnosus GG Survival: Mechanisms of Cell Damage and the Role of Pre-Freezing Conditions and Cryoprotectants. Foods 2025, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Han, J.; Sun, Q.; Ye, Z.; Zhou, Q.; Li, P.; Gu, Q. Optimization of cryoprotectants for improving the freeze-dried survival rate of potential probiotic Lactococcus lactis ZFM559 and evaluation of its storage stability. LWT 2024, 198, 116052. [Google Scholar] [CrossRef]
- Tlekbol, S.S.; Tolegen, S.A.; Saukymbek, K.S.; Monika, B.-P.; Pieszka, M. Milk productivity of Kazakh mare types Jabe and Novoaltay-Kazakh in the steppe zone of the North-East of Kazakhstan. Rocz. Nauk. Zootech. 2025, 52, 61–70. [Google Scholar] [CrossRef]
- Yan, X.; Sun, Y.; Zhang, G.; Han, W.; Gao, J.; Yu, X.; Jin, X. Study on the antagonistic effects of koumiss on Toxoplasma gondii infection in mice. Front. Nutr. 2022, 9, 1014344. [Google Scholar] [CrossRef]
- Abdel-Salam, A.M.; Al-Dekheil, A.; Babkr, A.; Farahna, M.; Mousa, H.M. High fiber probiotic fermented mare’s milk reduces the toxic effects of mercury in rats. N. Am. J. Med. Sci. 2010, 2, 569. [Google Scholar] [CrossRef]
- Ishii, S.; Samejima, K. Feeding rats with kumiss suppresses the serum cholesterol and triglyceride levels. Milk Sci. 2001, 50, 113–117. [Google Scholar]
- Yilmaz, S.; Kaya, E.; Yonar, H.; Mendil, A.S. Doxorubicin-induced oxidative stress injury: The protective effect of kumiss on cardiotoxicity. J. Hell. Vet. Med. Soc. 2022, 73, 4545–4558. [Google Scholar] [CrossRef]
- Zhang, H.; Nakamura, S.; Kitts, D.D. Antioxidant properties of casein phosphopeptides (CPP) and maillard-type conjugated products. Antioxidants 2020, 9, 648. [Google Scholar] [CrossRef]
- Du, M.; Liu, Y.; Cao, J.; Li, X.; Wang, N.; He, Q.; Zhang, L.; Zhao, B.; Dugarjaviin, M. Food from Equids—Commercial Fermented Mare’s Milk (Koumiss) Products: Protective Effects against Alcohol Intoxication. Foods 2024, 13, 2344. [Google Scholar] [CrossRef] [PubMed]
- Mukarromah, T.A.; Rustanti, N.; Mahati, E.; Suparmi; Ayustaningwarno, F. The Impact of Fermented Milk Products on Gut Microbiota-Derived Metabolites in Obesity: A Narrative Review. J. Food Sci. 2025, 90, e70301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Du, L.; Wang, Q.; Zhan, M.; Li, S.; Xiao, X. Daily koumiss has positive regulatory effects on blood lipids and immune system: A metabolomics study. Heliyon 2024, 10, e36429. [Google Scholar] [CrossRef]
- Hou, Q.; Li, C.; Liu, Y.; Li, W.; Chen, Y.; Bao, Y.; Zhang, H.; Menghe, B.; Sun, Z. Koumiss consumption modulates gut microbiota, increases plasma high density cholesterol, decreases immunoglobulin G and albumin. J. Funct. Foods 2019, 52, 469–478. [Google Scholar] [CrossRef]
- Usinger, L.; Reimer, C.; Ibsen, H. Fermented milk for hypertension. Cochrane Database Syst. Rev. 2012, 24, 678–683. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Blood Pressure Lowering Effect of Fermented Milk Products. Akad. Gıda 2018, 16, 67–77. [Google Scholar] [CrossRef]
- Okoniewski, A.; Dobrzyńska, M.; Kusyk, P.; Dziedzic, K.; Przysławski, J.; Drzymała-Czyż, S. The role of fermented dairy products on gut microbiota composition. Fermentation 2023, 9, 231. [Google Scholar] [CrossRef]
- Kudayarova, R.; Gilmutdinova, L.; Yamaletdinov, K.; Gilmutdinov, A.; Gabdelhakova, L.; Zinnatullin, R.K. Historical aspects of the use in medicine kumis. Bull. Sib. Med. 2010, 9, 186–189. [Google Scholar] [CrossRef]
- Barni, S.; Sarti, L.; Mori, F.; Muscas, G.; Belli, F.; Pucci, N.; Novembre, E. Tolerability and palatability of donkey’s milk in children with cow’s milk allergy. Pediatr. Allergy Immunol. 2018, 29, 329–331. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Chen, X.-M.; Kitts, D.D.; Li-Chan, E.C. Investigation into the bioavailability of milk protein-derived peptides with dipeptidyl-peptidase IV inhibitory activity using Caco-2 cell monolayers. Food Funct. 2017, 8, 701–709. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, P.; Deng, Z. Dose-dependent effect of intake of fermented dairy foods on the risk of diabetes: Results from a meta-analysis. Can. J. Diabetes 2022, 46, 307–312. [Google Scholar] [CrossRef] [PubMed]
- van der Pijl, P.C.; Kies, A.K.; Ten Have, G.A.; Duchateau, G.S.; Deutz, N.E. Pharmacokinetics of proline-rich tripeptides in the pig. Peptides 2008, 29, 2196–2202. [Google Scholar] [CrossRef]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S. Exploring the Functionality of Microbes in Fermented Foods: Technological Advancements and Future Directions. Fermentation 2025, 11, 300. [Google Scholar] [CrossRef]
- Ferrocino, I.; Cocolin, L. Current perspectives in food-based studies exploiting multi-omics approaches. Curr. Opin. Food Sci. 2017, 13, 10–15. [Google Scholar] [CrossRef]
- Kushugulova, A.; Löber, U.; Akpanova, S.; Rysbekov, K.; Kozhakhmetov, S.; Khassenbekova, Z.; Essex, M.; Nurgozhina, A.; Nurgaziyev, M.; Babenko, D. Dynamic changes in microbiome composition following mare’s milk intake for prevention of collateral antibiotic effect. Front. Cell. Infect. Microbiol. 2021, 11, 622735. [Google Scholar] [CrossRef]

| Component | Mare * | Cow * | Goat * | Donkey * | Human * | Functional Note | References |
|---|---|---|---|---|---|---|---|
| Protein (%) | 2.1–2.7 | 3.2–3.5 | 3.0–3.5 | 1.6–1.8 | 1.0–1.2 | Lower in mare/donkey → closer to human milk, easier digestibility | [13,50] |
| Fat (%) | 1.0–1.5 | 3.5–4.0 | 3.8–4.5 | 0.3–1.8 | 3.5–4.5 | Mare’s milk is low-fat, hypoenergetic; donkey similar | [13] |
| Lactose (%) | 6.0–7.0 | 4.6–4.8 | 4.5–4.7 | 6.2–7.0 | 6.5–7.0 | High lactose enhances digestibility and prebiotic effect | [13,50] |
| Casein/whey ratio | ~1:1 | 80:20 | 75:25 | ~50:50 | ~40:60 | Mare/donkey closer to human → digestibility, peptide precursors | [3,44] |
| β-casein | High; multiple phosphorylation sites | High | High | Moderate | High | Precursors for ACE-I peptides | [16] |
| αs1-casein | Low | High | High | Low | Low | Low αs1-CN reduces allergenicity | [16] |
| β-lactoglobulin (g/L) | Very low | 3–4 | 3–4 | Trtableace | Absent | β-LG is main bovine allergen; low in mare’s milk reduces CMA risk | [3,20,51] |
| α-lactalbumin (g/L) | ~1.0 | 1.5 | 1.3 | 1.0–1.2 | 1.2 | Lactose synthesis; antioxidant, antimicrobial peptides | [50,52] |
| Lysozyme (g/L) | 0.25–0.50 | Trace | Trace | 1.0–1.5 | 0.1–0.3 | Strong antimicrobial, gut microbiota modulation | [3,13] |
| Lactoferrin (g/L) | 0.08–0.20 | 0.02–0.1 | 0.02–0.1 | 0.1–0.3 | 1–3 | Antimicrobial, antiviral, immune modulation | [3,13] |
| Immunoglobulins (g/L) | 0.7–1.2 early lactation | 0.6–0.8 | 0.6–0.9 | 0.5–1.0 | 0.5–1.0 | Passive immunity, mucosal defense | [19] |
| Fatty acids (% PUFA) | 18–31% total FA; α-linolenic acid enriched | 3–5% | 5–7% | 10–15% | 15–20% | PUFA content supports cardiovascular health | [3,13,50] |
| Fat globule size (µm) | 2–3 | ~4 | ~4 | 2–3 | 3–4 | Smaller globules aid digestion | [13,53] |
| Oligosaccharides | 6–7% lactose; sialylated OS (3′SL, 6′SL, LSTa–c) | 4.6% lactose | 4.5% | 6–7% + HMO-like OS | Rich in HMOs | Prebiotic and immunomodulatory potential | [3,13] |
| Ca:P ratio | 1.6–1.8:1 | 1.3:1 | 1.3:1 | 1.5:1 | 1.6–2.0:1 | Favorable for bone development | [13,50] |
| Region | Dominant LAB | Dominant Yeasts | Other Taxa Reported | Functional Traits | References |
|---|---|---|---|---|---|
| Inner Mongolia/Xinjiang (China) | Lactobacillus helveticus, Lactococcus lactis, Streptococcus thermophilus | Kluyveromyces marxianus, Saccharomyces cerevisiae | Leuconostoc mesenteroides | Acidification, pathogen suppression, ACE-I peptide generation | [116,117] |
| Kazakhstan | Lactobacillus kefiranofaciens, L. helveticus | Saccharomyces cerevisiae | Pediococcus acidilactici | Exopolysaccharide production, antimicrobial peptides | [118,119] |
| Kyrgyzstan (high pastures) | Lactobacillus delbrueckii ssp. bulgaricus, Lactococcus lactis | Kluyveromyces marxianus | Acinetobacter, Staphylococcus (suppressed post-fermentation) | Proteolysis, pathogen inhibition | [25,120] |
| Russia (Buryatia, Altai) | Lactobacillus helveticus, Lactococcus lactis | Candida kefyr, Kluyveromyces marxianus | Enterococcus faecium | Aroma, antimicrobial activity | [21,121] |
| Europe (experimental koumiss) | Lactobacillus plantarum, L. casei | Saccharomyces cerevisiae | Bifidobacterium breve (starter adjunct) | Probiotic enrichment, DPP-IV inhibition | [122,123] |
| Protein | Concentration in Mare’s Milk * | Comparative Levels | Primary Functions | Peptide Potential | Evidence Strength (Refs) |
|---|---|---|---|---|---|
| Lysozyme | ~99 mg/L (0.099 g/L) | ~5× higher than human milk (~21 mg/L); trace in bovine | Antimicrobial (especially Gram-positive), immune modulation | Stable across fermentation; fragments likely antimicrobial | Strong—compositional and functional data [44] |
| Lactoferrin | ~80–218 mg/L (0.08–0.22 g/L) | Higher than bovine; lower than human milk (~1–3 g/L) | Antimicrobial, antiviral, immunomodulatory, iron sequestration | Precursors to lactoferricin/lactoferrampin peptides | Moderate—quantified values from mare milk [95] |
| Immunoglobulins (IgG, IgA, IgM) | ~0.88 g/L IgG at 0–12 h postpartum; plus IgA (~36.5 g/L), IgM (~14.1 g/L) [149] | Higher than typical cow milk (not quantified), lower than colostrum | Passive and mucosal immunity, cytokine modulation | Fermentation-derived peptides possible but uncharacterized | Moderate—based on temporal Ig quantifications [149] |
| α-Lactalbumin (α-LA) | Qualitatively abundant; similar to human milk and higher than bovine, exact g/L not provided | Higher than cow, similar to human milk [3,50] | Involved in lactose synthesis, antioxidant, immunomodulatory | Digestion releases antimicrobial and bioactive peptides | Moderate—qualitative abundance supported [3,50] |
| β-Lactoglobulin (β-LG) | Very low/reduced levels (specific values not provided) | High in bovine (~3–4 g/L), absent in human milk | Major bovine allergen; low levels reduce allergenicity | Low abundance limits peptide generation during fermentation | Moderate—clinical tolerance literature [53] |
| Study Type | Model/Population | Intervention | Endpoints | Key Findings | Limitations | Reference |
|---|---|---|---|---|---|---|
| Animal—immunity | Immunosuppressed rats (cyclophosphamide) | Koumiss | Spleen/thymus index; leukocytes, lymphocytes, CD4+/CD8+ ratio; Peyer’s patches | Improved immune organs and lymphocyte recovery | Limited sample sizes; composition undefined | [110] |
| Animal—cardiovascular/metabolic | Hyperlipidemic models | Koumiss or fermented mare’s milk | Lipid profiles; ACE-inhibitory activity | Lipid metabolism and ACE-I activity—supported by peptidomic data | No clinical comparator; short duration | [210] |
| Animal—anti-inflammatory/gut health | Mouse ulcerative colitis model | Fermented mare’s milk | Colitis scores, inflammation markers | Reduced colitis inflammation, modulated flora | Preclinical; specific peptides not profiled | [108] |
| Human—allergy/tolerability | (Some studies exist, but koumiss-specific small) | Equid milk intake | Tolerance, allergic reactions | Mare/donkey milk generally tolerated in CMA patients | Small numbers; not koumiss-specific in all; more data needed | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokrollahi, B.; Choi, J.-Y.; Won, M.; Kim, E.-T.; Lee, S.-E.; Ham, J.-S. Koumiss (Fermented Mare’s Milk) as a Functional Food: Bioactive Proteins, Peptides, and Future Perspectives. Foods 2025, 14, 3954. https://doi.org/10.3390/foods14223954
Shokrollahi B, Choi J-Y, Won M, Kim E-T, Lee S-E, Ham J-S. Koumiss (Fermented Mare’s Milk) as a Functional Food: Bioactive Proteins, Peptides, and Future Perspectives. Foods. 2025; 14(22):3954. https://doi.org/10.3390/foods14223954
Chicago/Turabian StyleShokrollahi, Borhan, Jae-Young Choi, Miyoung Won, Eun-Tae Kim, Seung-Eun Lee, and Jun-Sang Ham. 2025. "Koumiss (Fermented Mare’s Milk) as a Functional Food: Bioactive Proteins, Peptides, and Future Perspectives" Foods 14, no. 22: 3954. https://doi.org/10.3390/foods14223954
APA StyleShokrollahi, B., Choi, J.-Y., Won, M., Kim, E.-T., Lee, S.-E., & Ham, J.-S. (2025). Koumiss (Fermented Mare’s Milk) as a Functional Food: Bioactive Proteins, Peptides, and Future Perspectives. Foods, 14(22), 3954. https://doi.org/10.3390/foods14223954






