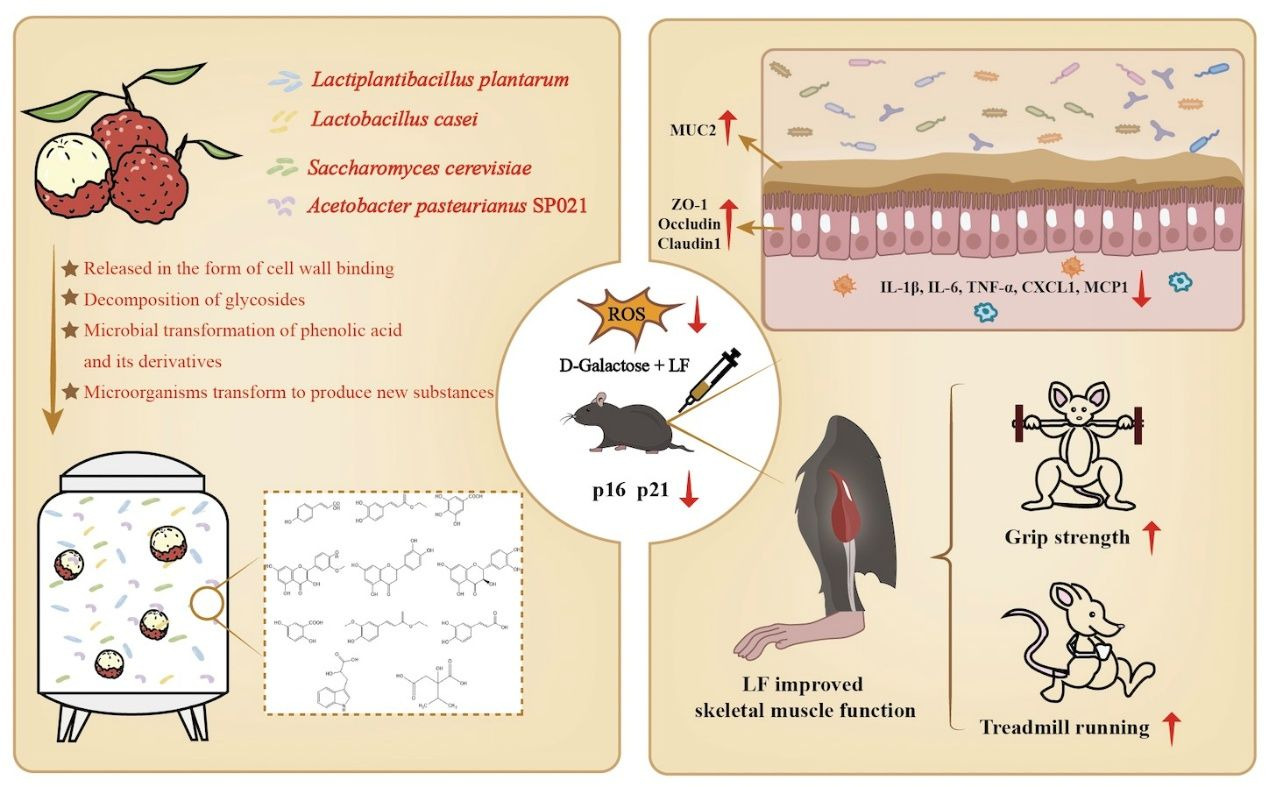
Lychee Fermented by Mixed Probiotic Strains Alleviates D-Galactose-Induced Skeletal Muscle and Intestinal Aging in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Main Reagents
2.2. Lychee Fermentate Preparation
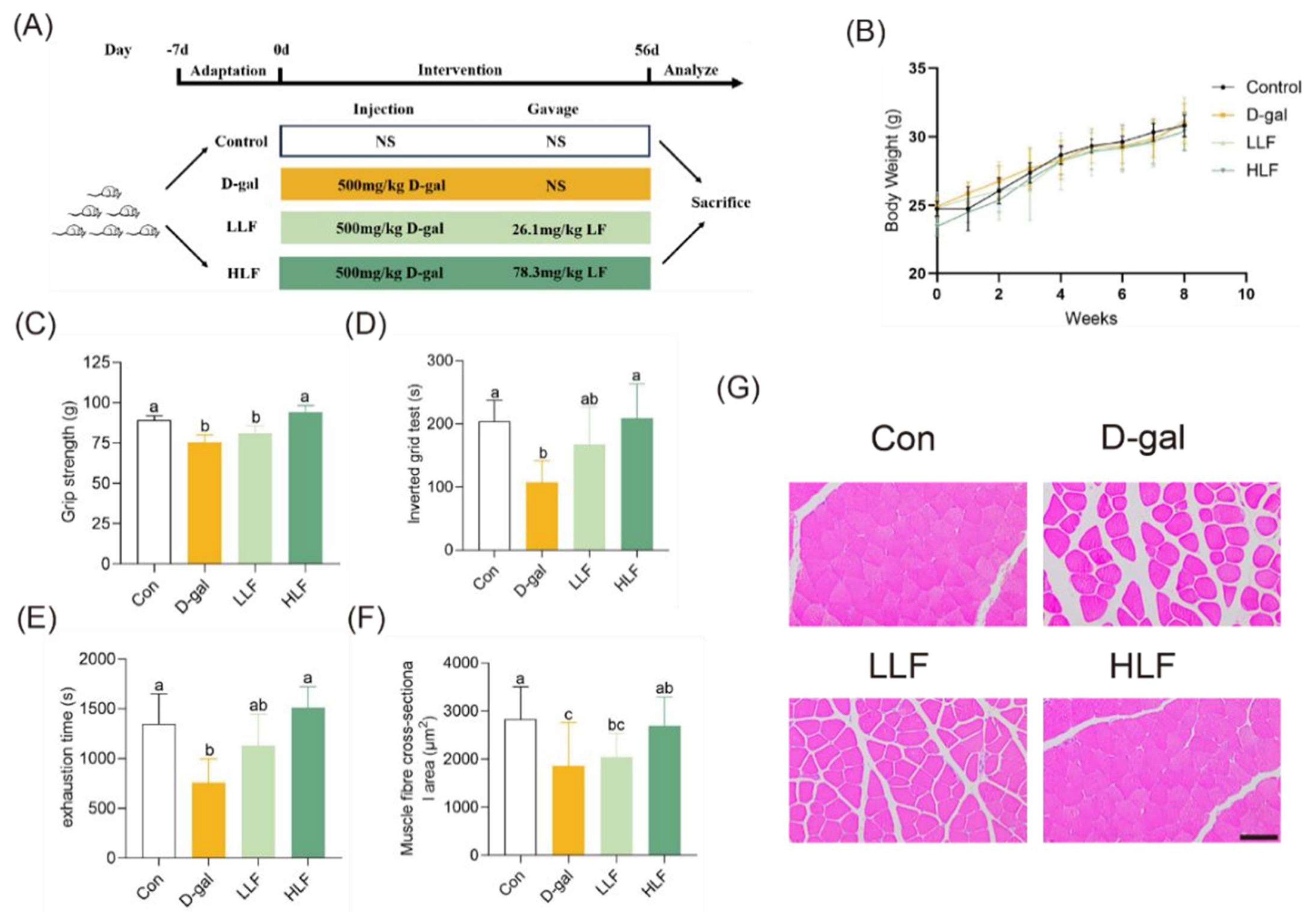
2.3. Animal and Experimental Design
2.4. Muscle Strength and Endurance Analysis
2.5. Histopathological Analysis
2.6. Transcriptional Analysis by Real-Time qPCR
2.7. Immunofluorescence Analysis
2.8. Determination of D-Lactate, MDA and Antioxidant Enzymes
2.9. Microbial Community Analysis
2.10. Untargeted Metabolomics
2.11. Statistical Analysis
3. Results
3.1. The Effects of LF on Skeletal Muscle Function
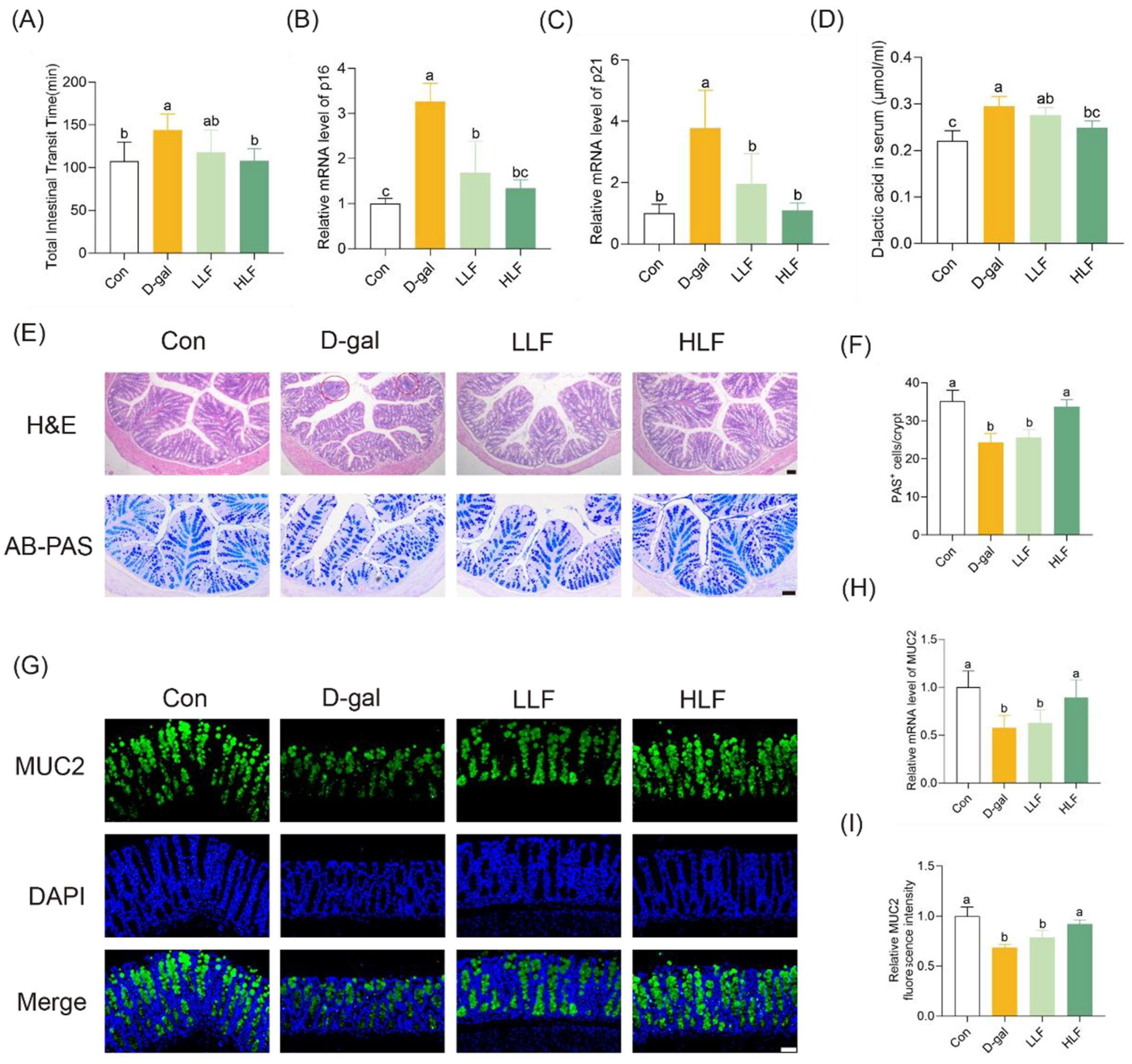
3.2. LF Attenuated Intestinal Aging and Restored Intestinal Barrier Function
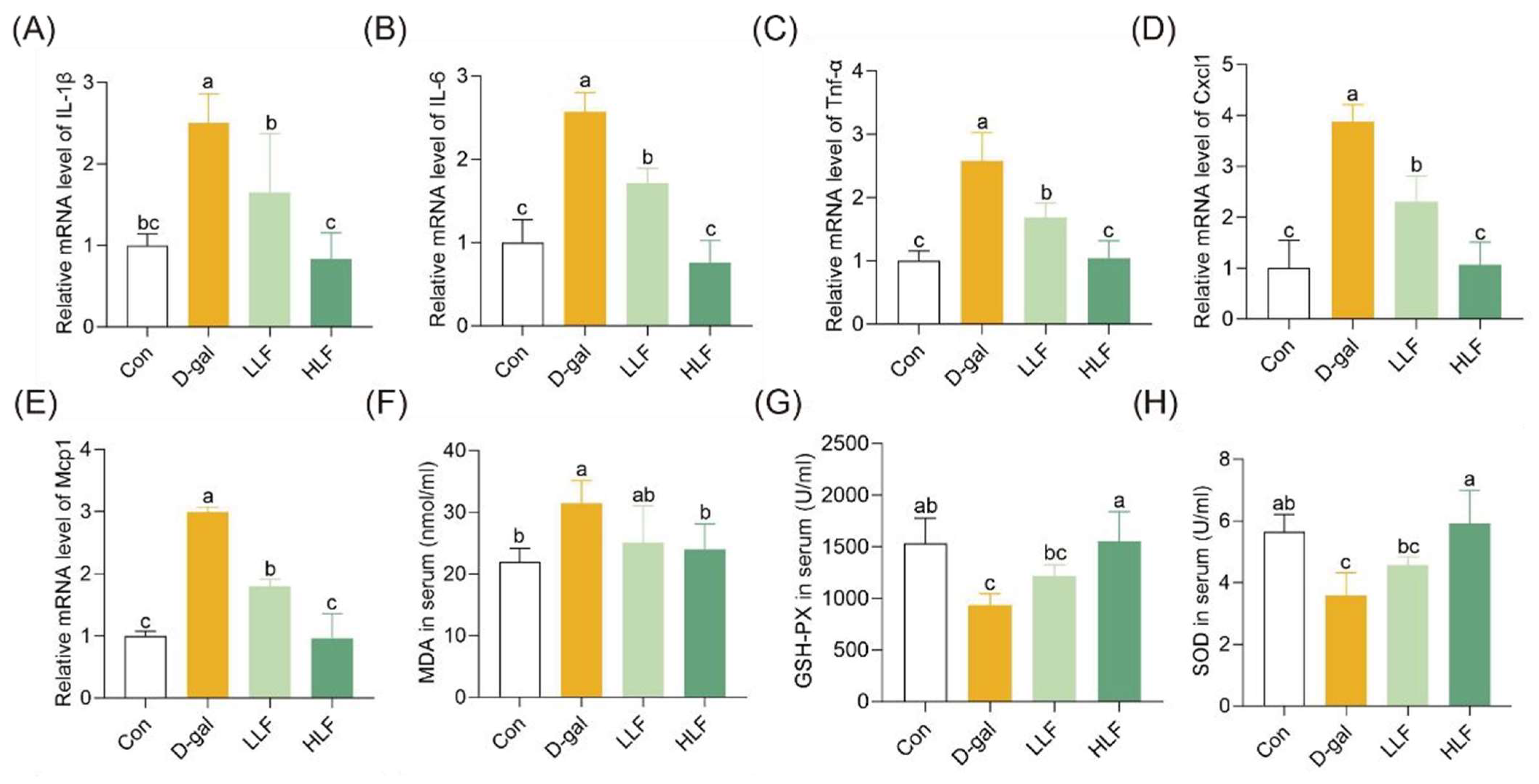
3.3. LF Attenuated Oxidative Stress and Inflammation
3.4. The Modulatory Effects of LF on Gut Microbiota
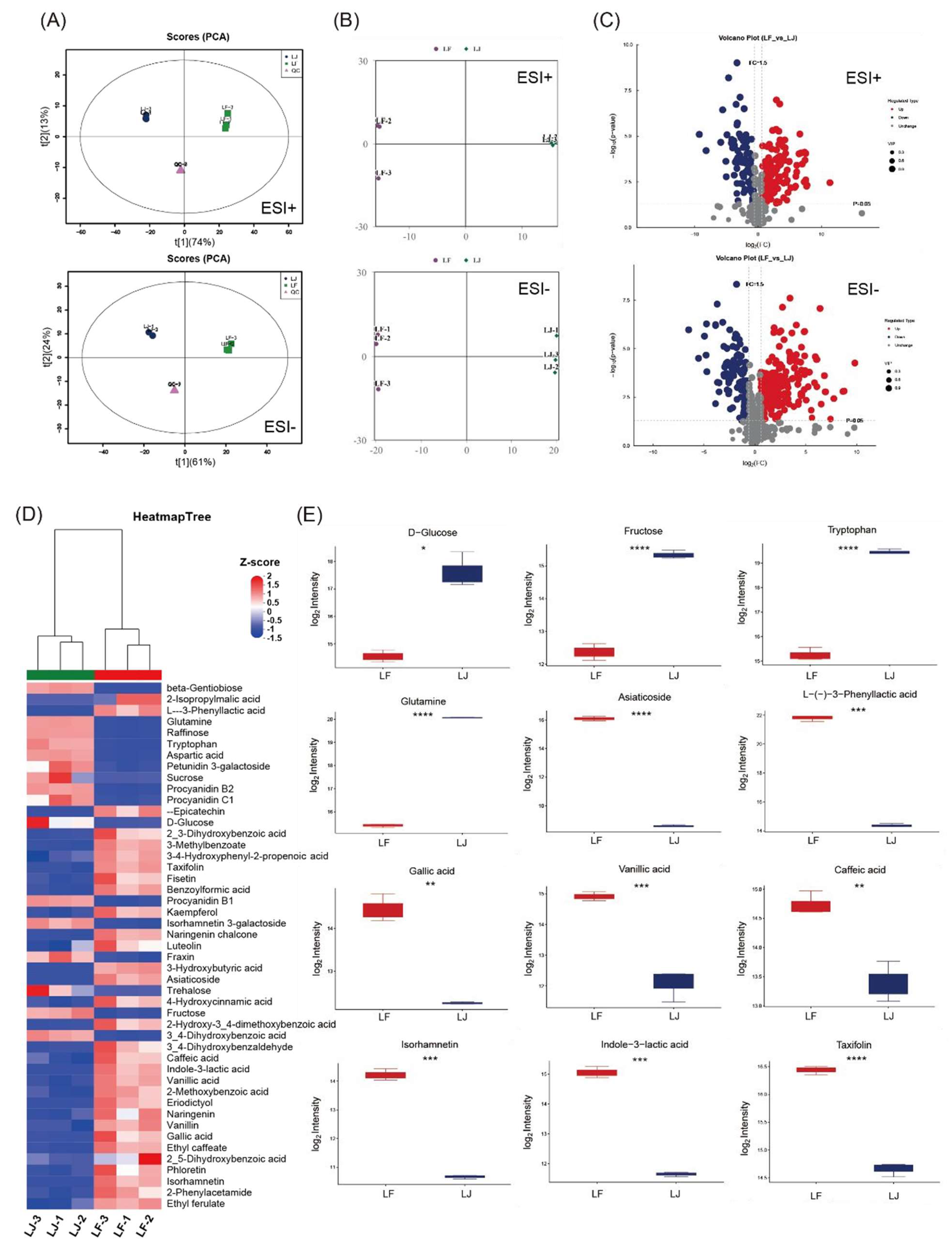
3.5. Phenolic Acids and Flavonoids Were Enriched by Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, D.E.; Canning, D.; Lubet, A. Global population aging: Facts, challenges, solutions & perspectives. Daedalus 2015, 144, 80–92. [Google Scholar] [CrossRef]
- Rebelo-Marques, A.; De Sousa Lages, A.; Andrade, R.; Ribeiro, C.F.; Mota-Pinto, A.; Carrilho, F.; Espregueira-Mendes, J. Aging hallmarks: The benefits of physical exercise. Front. Endocrinol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Zhai, Z.; Wang, H.; Lin, Y.; Chang, F.; Ge, S.; Sun, X.; Wei, W.; Wang, D.; et al. Bifidobacterium animalis subsp. lactis A6 ameliorates bone and muscle loss via modulating gut microbiota composition and enhancing butyrate production. Bone Res. 2025, 13, 28. [Google Scholar]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 527–539. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Z.; Fang, B.; Wang, R.; Liu, Y.; Li, J.; Lan, H.; Zhao, W.; Hung, W.L.; Zhang, M. Exploring the anti-inflammatory potential of Lacticaseibacillus paracasei postbiotics: Mechanistic insights and functional components. Food Biosci. 2025, 65, 106105. [Google Scholar] [CrossRef]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy apple juice fermented by Lactobacillus prevents obesity via modulating gut microbiota and protecting intestinal tract health. Nutrients 2021, 13, 971. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Xu, R.; Niu, H.; Huang, Q.; Zhou, Y. Lactiplantibacillus plantarum fermentation enhanced the protective effect of kiwifruit on intestinal injury in rats: Based on mitochondrial morphology and function. Food Chem. X 2023, 20, 101025. [Google Scholar] [CrossRef]
- Duan, W.; Zhou, L.; Ren, Y.; Liu, F.; Xue, Y.; Wang, F.Z.; Lu, R.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; et al. Lactic acid fermentation of goji berries (Lycium barbarum) prevents acute alcohol liver injury and modulates gut microbiota and metabolites in mice. Food Funct. 2024, 15, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, Y.; Deng, Z.; Yang, J.; Gan, D. Boosting the Antioxidant Potential of Polymeric Proanthocyanidins in Litchi (Litchi chinensis Sonn.) Pericarp via Biotransformation of Utilizing Lactobacillus plantarum. Foods 2023, 12, 2384. [Google Scholar] [CrossRef]
- Ibrahim, S.R. and Mohamed, G.A. Litchi chinensis: Medicinal uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2015, 174, 492–513. [Google Scholar] [CrossRef]
- Lin, S.; Yang, B.; Chen, F.; Jiang, G.; Li, Q.; Duan, X.; Jiang, Y. Enhanced DPPH radical scavenging activity and DNA protection effect of litchi pericarp extract by Aspergillus awamori bioconversion. Chem. Cent. J. 2012, 6, 108. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Zhao, L.; Wang, K.; Wu, D.; Hu, Z.; Liu, X. GABA and fermented litchi juice enriched with GABA promote the beneficial effects in ameliorating obesity by regulating the gut microbiota in HFD-induced mice. Food Funct. 2023, 14, 8170–8185. [Google Scholar] [CrossRef]
- Wen, J.; Ma, L.; Xu, Y.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef]
- Huang, R.; Yao, H.; Ji, S.; Wu, J.; Lin, Q.; Gupta, T.B.; Gan, D.; Wu, X. A lychee fermentate with enriched acetate but lowered GABA attenuates DSS-induced colitis by reinforcing gut barrier function and modulating intestinal microbiota. Food Biosci. 2024, 59, 104089. [Google Scholar] [CrossRef]
- Tang, S.; Luo, N.; Zeng, Q.; Dong, L.; Zhang, R.; He, S.; Nag, A.; Huang, F.; Su, D. Lychee pulp phenolics fermented by mixed lactic acid bacteria strains promote the metabolism of human gut microbiota fermentation in vitro. Food Funct. 2023, 14, 7672–7681. [Google Scholar] [CrossRef]
- Sheng, J.; Shan, C.; Liu, Y.; Zhang, P.; Li, J.; Cai, W.; Tang, F. Comparative evaluation of the quality of red globe grape juice fermented by Lactobacillus acidophilus and Lactobacillus plantarum. Int. J. Food Sci. Technol. 2022, 57, 2235–2248. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent advances of fermented fruits: A review on strains, fermentation strategies, and functional activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory bowel diseases and sarcopenia: The role of inflammation and gut microbiota in the development of muscle failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, M.; Zhang, S.; Wang, J.; Zhang, R.; Dong, L.; Huang, F.; Su, D.; Deng, M. Unveiling biotransformation of free flavonoids into phenolic acids and chromones alongside dynamic migration of bound phenolics in Lactobacillus-fermented lychee pulp. Food Chem. 2024, 457, 140115. [Google Scholar] [CrossRef]
- Chrzanowski, G. Saccharomyces cerevisiae—An interesting producer of bioactive plant polyphenolic metabolites. Int. J. Mol. Sci. 2020, 21, 7343. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (poly) phenolic compounds in food fermentations by lactic acid bacteria: Novel insights into metabolic pathways and functional metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Cui, S.; Chen, W. Anti-aging effects and mechanisms of anthocyanins and their intestinal microflora metabolites. Crit. Rev. Food Sci. Nutr. 2024, 64, 2358–2374. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Cîrîc, A.I.; Begea, M. Anti-aging effects of flavonoids from plant extracts. Foods 2024, 13, 2441. [Google Scholar] [CrossRef]
- Luo, Q.; Gong, P.; Shi, R.; Chen, W.; Wang, C.; Zhang, C.; Wu, Z. Syringic acid alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota. J. Agric. Food Chem. 2023, 71, 8458–8470. [Google Scholar] [CrossRef] [PubMed]
- Riemschneider, S.; Hoffmann, M.; Slanina, U.; Weber, K.; Hauschildt, S.; Lehmann, J. Indol-3-carbinol and quercetin ameliorate chronic DSS-induced colitis in C57BL/6 mice by AhR-mediated anti-inflammatory mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 2262. [Google Scholar] [CrossRef]
- Ru, Y.; Luo, Y.; Liu, D.; Huang, Q.; Zhou, X.; Linghu, M.; Luo, X.; Lv, Z.; Wu, Y.; Zhang, H.; et al. Isorhamnetin alleviates ferroptosis-mediated colitis by activating the NRF2/HO-1 pathway and chelating iron. Int. Immunopharmacol. 2024, 135, 112318. [Google Scholar] [CrossRef]
- Wang, R.; Shen, L.; Li, H.; Peng, H. Eriodictyol attenuates dextran sodium sulphate-induced colitis in mice by regulating the sonic hedgehog signalling pathway. Pharm. Biol. 2021, 59, 972–983. [Google Scholar] [CrossRef]
- Kim, J.G.; Sharma, A.R.; Lee, Y.H.; Chatterjee, S.; Choi, Y.J.; Rajvansh, R.; Chakraborty, C.; Lee, S.S. Therapeutic potential of quercetin as an antioxidant for bone-muscle-tendon regeneration and aging. Aging Dis. 2024, 16, 1414. [Google Scholar] [CrossRef]
- Mukai, R.; Matsui, N.; Fujikura, Y.; Matsumoto, N.; Hou, D.X.; Kanzaki, N.; Shibata, H.; Horikawa, M.; Iwasa, K.; Hirasaka, K.; et al. Preventive effect of dietary quercetin on disuse muscle atrophy by targeting mitochondria in denervated mice. J. Nutr. Biochem. 2016, 31, 67–76. [Google Scholar] [CrossRef]
- Wang, F.; Qi, Y.; Gao, Y.; Wang, Z.; Shen, X.; Wu, H. Syringic acid suppresses ferroptosis of skeletal muscle cells to alleviate lower limb ischemia/reperfusion injury in mice via the HMGB1 pathway. Chem. Biol. Drug Des. 2023, 102, 1387–1398. [Google Scholar] [CrossRef]
- Chen, T.L.; Zhu, G.L.; Wang, J.A.; Zhang, G.D.; Liu, H.F.; Chen, J.R.; Wang, Y.; He, X.L. Protective effects of isorhamnetin on apoptosis and inflammation in TNF-α-induced HUVECs injury. Int. J. Clin. Exp. Pathol. 2015, 8, 2311. [Google Scholar]
- Maheshwari, N.; Khan, A.A.; Mahmood, R. Oral administration of curcumin and gallic acid alleviates pentachlorophenol-induced oxidative damage in rat intestine. Food Chem. Toxicol. 2023, 176, 113745. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Sun, L.; Liu, X.; Yin, Y.; Hao, J.; Zhang, W. p-Hydroxybenzoic acid ameliorates colitis by improving the mucosal barrier in a gut microbiota-dependent manner. Nutrients 2022, 14, 5383. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Fang, J.; Dai, Y.; Chen, J.; Zhang, H.; Li, H.; Chen, W. Lactiplantibacillus plantarum CCFM8610 mitigates oxidative stress-related intestinal aging through its metabolite indole-3-lactic acid. Food Biosci. 2025, 63, 105822. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Qin, G.; Huang, Y.; Cheng, S.; Chen, Z.; Zhang, Y.; Shu, Y.; Zeng, X.; Guo, R. Lonicera japonica polysaccharides alleviate D-galactose-induced oxidative stress and restore gut microbiota in ICR mice. Int. J. Biol. Macromol. 2023, 245, 125517. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Sepulveda, M.; Wang, P.; Rasic, M.; Tullius, S.G.; Perkins, D.; Alegre, M.L. Microbiota-dependent and-independent effects of obesity on transplant rejection and hyperglycemia. Am. J. Transplant. 2023, 23, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Meng, F.; Wang, X.; Han, Y.; Yang, W.; Wang, C.; Shan, Z. Effects of iodine intake on gut microbiota and gut metabolites in Hashimoto thyroiditis-diseased humans and mice. Commun. Biol. 2024, 7, 136. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Tao, J.; Bai, X.; Jing, Y.; Zhai, Z.; Luo, J.; Zhang, W.; Gan, D.; Hao, Y. Lychee Fermented by Mixed Probiotic Strains Alleviates D-Galactose-Induced Skeletal Muscle and Intestinal Aging in Mice. Foods 2025, 14, 3684. https://doi.org/10.3390/foods14213684
Han H, Tao J, Bai X, Jing Y, Zhai Z, Luo J, Zhang W, Gan D, Hao Y. Lychee Fermented by Mixed Probiotic Strains Alleviates D-Galactose-Induced Skeletal Muscle and Intestinal Aging in Mice. Foods. 2025; 14(21):3684. https://doi.org/10.3390/foods14213684
Chicago/Turabian StyleHan, Huixian, Jin Tao, Xiaoyue Bai, Yizhi Jing, Zhengyuan Zhai, Junjie Luo, Wanxiang Zhang, Dan Gan, and Yanling Hao. 2025. "Lychee Fermented by Mixed Probiotic Strains Alleviates D-Galactose-Induced Skeletal Muscle and Intestinal Aging in Mice" Foods 14, no. 21: 3684. https://doi.org/10.3390/foods14213684
APA StyleHan, H., Tao, J., Bai, X., Jing, Y., Zhai, Z., Luo, J., Zhang, W., Gan, D., & Hao, Y. (2025). Lychee Fermented by Mixed Probiotic Strains Alleviates D-Galactose-Induced Skeletal Muscle and Intestinal Aging in Mice. Foods, 14(21), 3684. https://doi.org/10.3390/foods14213684








