The Apoptosis Induction and Immunomodulating Activities of Nga-Kee-Mon (Perilla frutescens) Seed Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Cell Lines and Culturing
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
- 0–5 min: 100% B (isocratic);
- 5–10 min: linear gradient from 0 to 20% A;
- 10–20 min: 20% A (isocratic);
- 20–60 min: linear gradient from 20% to 40% A.
2.5. Determination of Phenolic Compound
2.6. Determination of Flavonoid Compounds
2.7. Determination of Antioxidant Activity Using the DPPH Radical Scavenging Assay
2.8. Anti-Proliferation Assay
2.9. Morphological Changes, Acridine Orange/Ethidium Bromide Staining (AO/EB)
2.10. DNA Fragmentation Assay
2.11. Inflammatory Cytokine Production
2.12. Statistical Analysis
3. Results
3.1. Extraction Yield, Total Phenolic and Flavonoid Contents, Antioxidant Capacity, and Phytochemical Profile
3.2. HPLC Analysis of Phenolic Compounds
3.3. Antiproliferative Effect of PSE on HT-29 Cells
3.4. Morphological Changes and Fluorescence Staining Cell
3.5. DNA Fragmentation in HT-29 Cells
3.6. Effect on Pro-Inflammatory Cytokine Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS•+ | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical |
| AO | Acridine orange |
| AO/EB | Acridine orange/Ethidium bromide staining |
| ANOVA | One-way analysis of variance |
| DCFH-DA | 2′,7-dichlorofluorescin diacetate |
| DAD | Diode array detector |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EB | Ethidium bromide |
| FBS | Fetal bovine serum |
| FRAP | Ferric reducing antioxidant power |
| GA | Gallic acid |
| GI | Geographical indication |
| HO-1 | Heme oxygenase-1 |
| HT-29 | Human colon adenocarcinoma cells |
| IC50 | Half-maximal inhibitory concentration |
| IκBα | Inhibitor of nuclear factor kappa B alpha |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| MAPK | Mitogen-activated protein kinase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NF-κB | Nuclear factor kappa-B cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ORAC | Oxygen radical absorbance capacity |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PF | Perilla frutescens |
| PSE | Perilla frutescens seed extract |
| Q | Quercetin |
| RAW 264.7 | Murine macrophage cells |
| SD | Standard deviation |
| SH-SY5Y | Human neuroblastoma cell line |
| TC | Tocopherol |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TNF-α | Tumor necrosis factor-alpha |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
References
- Jun, H.I.; Kim, B.T.; Song, G.S.; Kim, Y.S. Structural characterization of phenolic antioxidants from purple Perilla frutescens (var. acuta) leaves. Food Chem. 2014, 148, 367–372. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2008, 14, 133–140. [Google Scholar] [CrossRef]
- Huang, B.P.; Lin, C.H.; Chen, Y.C.; Kao, S.H. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264.7 cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Kuo, C.L.; Wang, J.P.; Cheng, J.S.; Huang, Z.W.; Chen, C.F. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef]
- He, D.-L.; Jin, R.-Y.; Li, H.-Z.; Liu, Q.-Y.; Zhang, Z.-J. Identification of a novel anticancer oligopeptide from Perilla frutescens (L.) Britt. and its enhanced anticancer effect by targeted nanoparticles in vitro. Int. J. Polym. Sci. 2018, 2018, 1782734. [Google Scholar] [CrossRef]
- Dissook, S.; Umsumarng, S.; Mapoung, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Dejkriengkraikul, P. Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID. Front. Med. 2022, 9, 1072056. [Google Scholar]
- Khanaree, C.; Pintha, K.; Tantipaiboonwong, P.; Suttajit, M.; Chewonarin, T. The effect of Perilla frutescens leaf on 1,2-dimethylhydrazine-induced initiation of colon carcinogenesis in rats. J. Food Biochem. 2018, 42, e12493. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens Britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef]
- Sirichatnach, P.; Pintha, K.; Tantipaiboonwong, P.; Thephinlap, C.; Suttajit, M.; Kaowinn, S.; Kangwan, N.; Suwannaloet, W.; Pangjit, K. Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed. Biomedicines 2025, 13, 851. [Google Scholar] [CrossRef]
- Deethai, P.; Siriwathanakul, C.; Pitchakarn, P.; Imsumran, A.; Wongnoppavich, A.; Dissook, S.; Chewonarin, T. Perilla frutescens Seed Residue Extract Restores Gut Microbial Balance and Enhances Insulin Function in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2025, 26, 8176. [Google Scholar] [CrossRef]
- Chantana, W.; Hu, R.; Buddhasiri, S.; Thiennimitr, P.; Tantipaiboonwong, P.; Chewonarin, T. The extract of Perilla frutescens seed residue attenuated the progression of aberrant crypt foci in rat colon by reducing inflammatory processes and altered gut microbiota. Foods 2023, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, A.; Chopra, R.; Garg, M. A review on nutritional value, functional properties and pharmacological application of Perilla frutescens. Biomed. Pharmac. J. 2019, 12, 649–660. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Pintha, K.; Yodkeeree, S.; Pitchakarn, P.; Limtrakul, P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac. J. Cancer Prev. 2014, 15, 4601–4607. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Elin Novia, S.; Berna, E.; Rani, S. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Sundaram, S.G.; Milner, J.A. Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett. 1993, 74, 85–90. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef]
- Ha, J.Y.; Yi, G.; Bae, H.H.; Go, Y.S.; Kim, Y.J.; Lee, K.M.; Hong, C.O.; Kim, K.K. Isolation, identification, and apoptosis activity of the photosensitizer methyl pheophorbide A from Perilla frutescens leaves. Appl. Biol. Chem. 2022, 65, 52. [Google Scholar]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A rich source of pharmacological active compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-A.; Do, T.-H.; Pham, T.-T.-H.; Huynh, N.-T. In vitro anti-melanogenic effect of Perilla frutescens leaf extracts. J. Herbmed Pharmacol. 2022, 11, 48–54. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A traditional medicine and food homologous plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y. Antioxidant compounds and activities of Perilla frutescens var. crispa and its processed products. Food Sci. Biotechnol. 2024, 33, 1123–1133. [Google Scholar] [CrossRef]
- Kongkeaw, S.; Riebroy, S.; Chaijan, M. Comparative studies on chemical composition, phenolic compounds and antioxidant activities of brown and white Perilla frutescens seeds. Chiang Mai J. Sci. 2015, 42, 896–906. [Google Scholar]
- Adam, G.; Robu, S.; Flutur, M.M.; Cioanca, O.; Vasilache, I.A.; Adam, A.M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens extracts in clinical practice. Antioxidants 2023, 12, 600. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108, 375–391. [Google Scholar] [CrossRef]
- Asif, M. Phytochemical study of polyphenols in Perilla frutescens as an antioxidant. Avicenna J. Phytomed. 2012, 2, 169–178. [Google Scholar]
- Hu, Z.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.; Liu, F. Mechanism of antifungal activity of Perilla frutescens essential oil against Aspergillus flavus by transcriptomic analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Kang, R.; Helms, R.; Stout, M.; Jaber, H.; Chen, Z.; Nakatsu, T. Antimicrobial activity of the volatile constituents of Perilla frutescens and its synergistic effects with polygodial. J. Agric. Food Chem. 1992, 40, 232–236. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yeo, E.J.; Moon, S.C.; Kim, Y.W.; Ahn, H.J.; Park, S.C. Perilla leaf, Perilla frutescens, induces apoptosis and G1 phase arrest in human leukemia HL-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J. Med. Food 2009, 12, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, N.; Guo, G.; Lu, S.; Li, Z.; Mu, Y.; Xia, X.; Xu, Z.; Hu, Y.; Xiang, X. Perilla seed oil: A review of health effects, encapsulation strategies and applications in food. Foods 2024, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, W.; Xiang, J.; Chen, H.; Quek, S.Y. Fabrication, characterisation and oxidative stability of perilla seed oil emulsions and microcapsules stabilised by protein and polysaccharides. J. Food Process. Preserv. 2022, 46, e16023. [Google Scholar] [CrossRef]
- Yi, D.; Wang, Z.; Peng, M. Comprehensive review of Perilla frutescens: Chemical composition, pharmacological mechanisms, and industrial applications in food and health products. Foods 2025, 14, 700. [Google Scholar] [CrossRef]
- Assefa, A.D.; Jeong, Y.J.; Kim, D.J.; Jeon, Y.A.; Ok, H.C.; Baek, H.J.; Sung, J.S. Characterization, identification, and quantification of phenolic compounds using UPLC-Q-TOF-MS and evaluation of antioxidant activity of 73 Perilla frutescens accessions. Food Res. Int. 2018, 111, 153–167. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens leaf extract and fractions: Polyphenol composition, antioxidant, enzymes (α-glucosidase, acetylcholinesterase, and tyrosinase) inhibitory, anticancer, and antidiabetic activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Pintha, K.; Chaiwangyen, W.; Suttajit, M.; Khanaree, C.; Khantamat, O. Bioefficacy of Nga-Mon (Perilla frutescens) fresh and dry leaf: Assessment of antioxidant, antimutagenicity, and anti-inflammatory potential. Plants 2023, 12, 2210. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Parr, A.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Wu, H.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Chang, Y.L. New analytical method for investigating the antioxidant power of food extracts on the basis of their electron-donating ability: Comparison to the ferric reducing/antioxidant power (FRAP) assay. J. Agric. Food Chem. 2010, 58, 8477–8480. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- McHugh, P.; Turina, M. Apoptosis and necrosis: A review for surgeons. Surg. Infect. 2006, 7, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Shen, Y.; Zhu, L.; Yao, L.; Wang, X.; Zhang, A.; Li, J.; Wu, J.; Qin, L. Rosmarinic acid, the active component of Rubi Fructus, induces apoptosis of SGC-7901 and HepG2 cells through mitochondrial pathway and exerts anti-tumor effect. Naunyn Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3743–3755. [Google Scholar] [CrossRef]
- Synakiewicz, A.; Stanisławska-Sachadyn, A.; Owczarzak, A.; Skuza, M.; Stachowicz-Stencel, T. Cytokine IL-6, but not IL-1β, TNF-α and NF-κB, is increased in paediatric cancer patients. Acta Biochim. Pol. 2023, 70, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tetsuka, T.; Yoshida, S.; Watanabe, N.; Kobayashi, M.; Matsui, N.; Okamoto, T. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from TNF-α- or IL-1β-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000, 465, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Craig, R.; Larkin, A.; Mingo, A.M.; Thuerauf, D.J.; Andrews, C.; McDonough, P.M.; Glembotski, C.C. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release: Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000, 275, 23814–23824. [Google Scholar] [CrossRef]
- Mahdiani, S.; Omidkhoda, N.; Heidari, S.; Hayes, A.W.; Karimi, G. Protective effect of luteolin against chemical and natural toxicants by targeting NF-κB pathway. Biofactors 2022, 48, 744–762. [Google Scholar] [CrossRef]
- Olędzka, A.J.; Czerwińska, M.E. Role of plant-derived compounds in the molecular pathways related to inflammation. Int. J. Mol. Sci. 2023, 24, 4666. [Google Scholar] [CrossRef]
- Hahn, D.; Shin, S.H.; Bae, J.S. Natural antioxidant and anti-inflammatory compounds in foodstuff or medicinal herbs inducing heme oxygenase-1 expression. Antioxidants. 2020, 9, 1191. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.H.; Park, S.Y. Synergistic apoptotic effects of rosmarinic acid and luteolin via oxidative stress modulation in human colorectal cancer cells. Food Chem. Toxicol. 2022, 165, 113087. [Google Scholar]
- Bansal, M.P. ROS/Redox regulation with dietary phytochemicals and role in anticancer activity. In Redox Regulation and Therapeutic Approaches in Cancer; Springer Nature: Singapore, 2023; pp. 91–131. [Google Scholar]
- Li, X.; Chen, L.; Zhao, Y. Combined polyphenolic treatment with luteolin and rosmarinic acid suppresses NF-κB activation and pro-inflammatory cytokine release in LPS-stimulated macrophages. Phytomedicine 2021, 90, 153633. [Google Scholar]
- Park, H.J.; Kim, D.W.; Lee, S.Y. Neuroprotective synergy of rosmarinic acid and luteolin through Nrf2/HO-1 activation in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Sci. 2020, 21, 4393. [Google Scholar]
- Veeramani, M.; Sakthi Abirami, M.; Kanimozhi, R. A review of phytochemical and pharmacological activity of Perilla frutescens (L.) Britton. Int. J. Pharm. Pharm. Res. 2024, 30, 1000–1015. [Google Scholar]
- Sharma, A.R.; Sharma, G.; Nath, S.; Lee, S.-S. Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application. Green Process. Synth. 2024, 13, 200–210. [Google Scholar] [CrossRef]
- Sa, K.J.; Jang, S.J.; Lee, S.; Park, H.; Cho, J.; Sung, J.; Lee, J.K. Characterization of volatile compounds of Perilla frutescens L. in South Korea. Appl. Biol. Chem. 2023, 66, 41. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Zheng, J.; Xu, J.; Wang, H.; Du, J.; Zhou, D.; Sun, Y.; Shen, B. Toxic effects of Perilla frutescens (L.) Britt. essential oil and its main component on Culex pipiens pallens (Diptera: Culicidae). Plants 2023, 12, 1600. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Dönmez, A.A. Composition of the essential oil of Perilla frutescens (L.) Britton from Turkey. Flavour Fragr. J. 2003, 18, 122–123. [Google Scholar] [CrossRef]
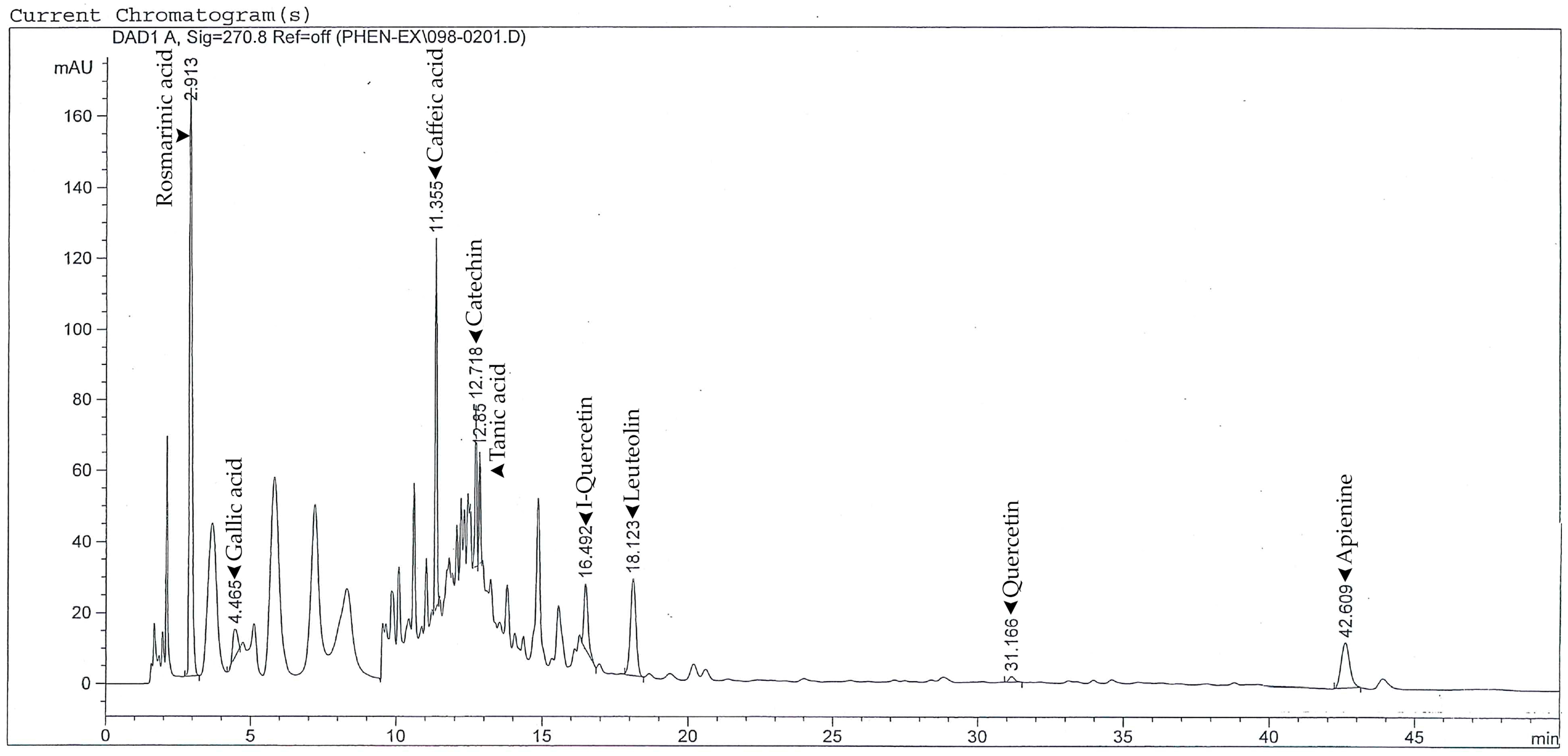


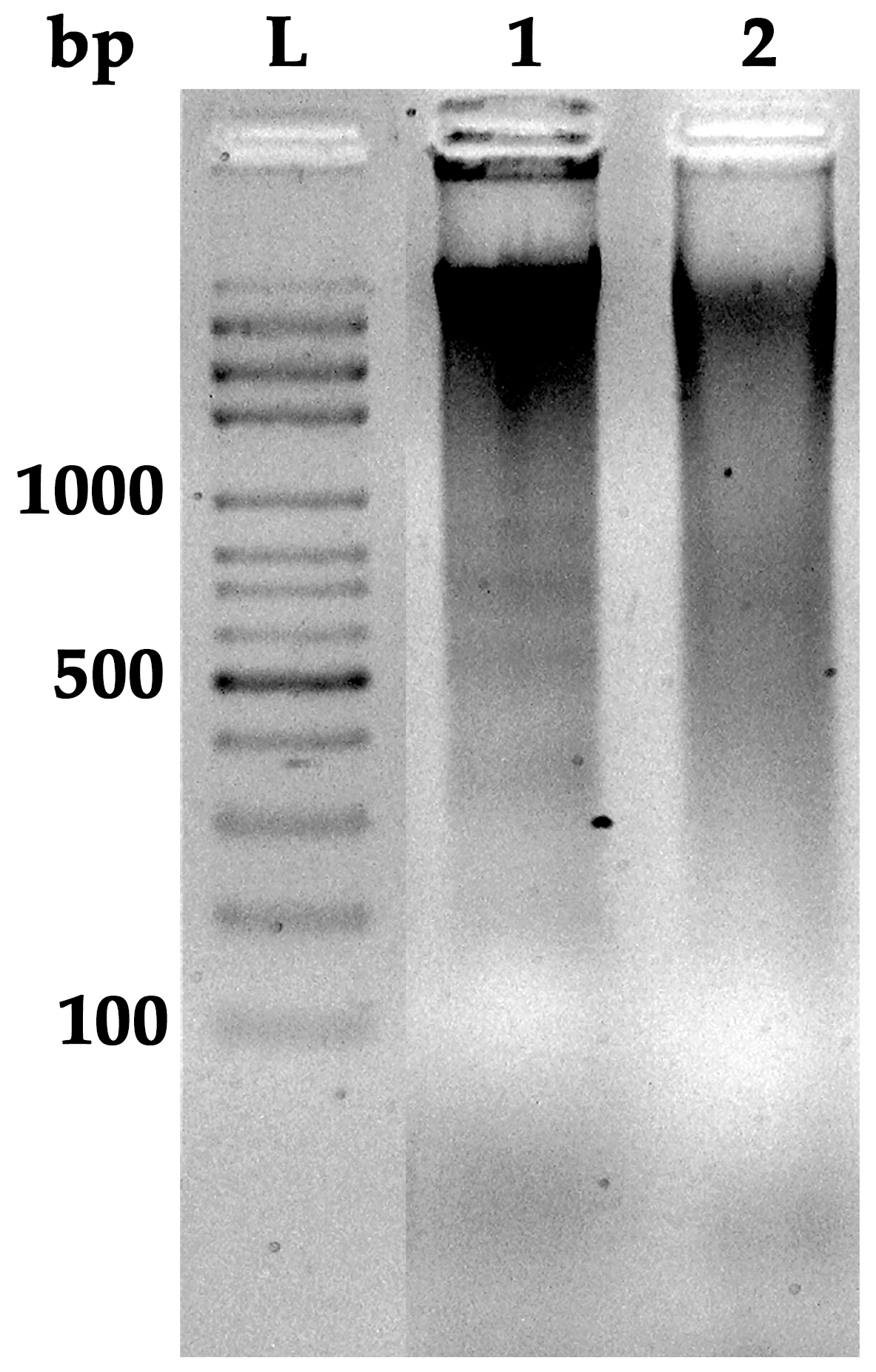
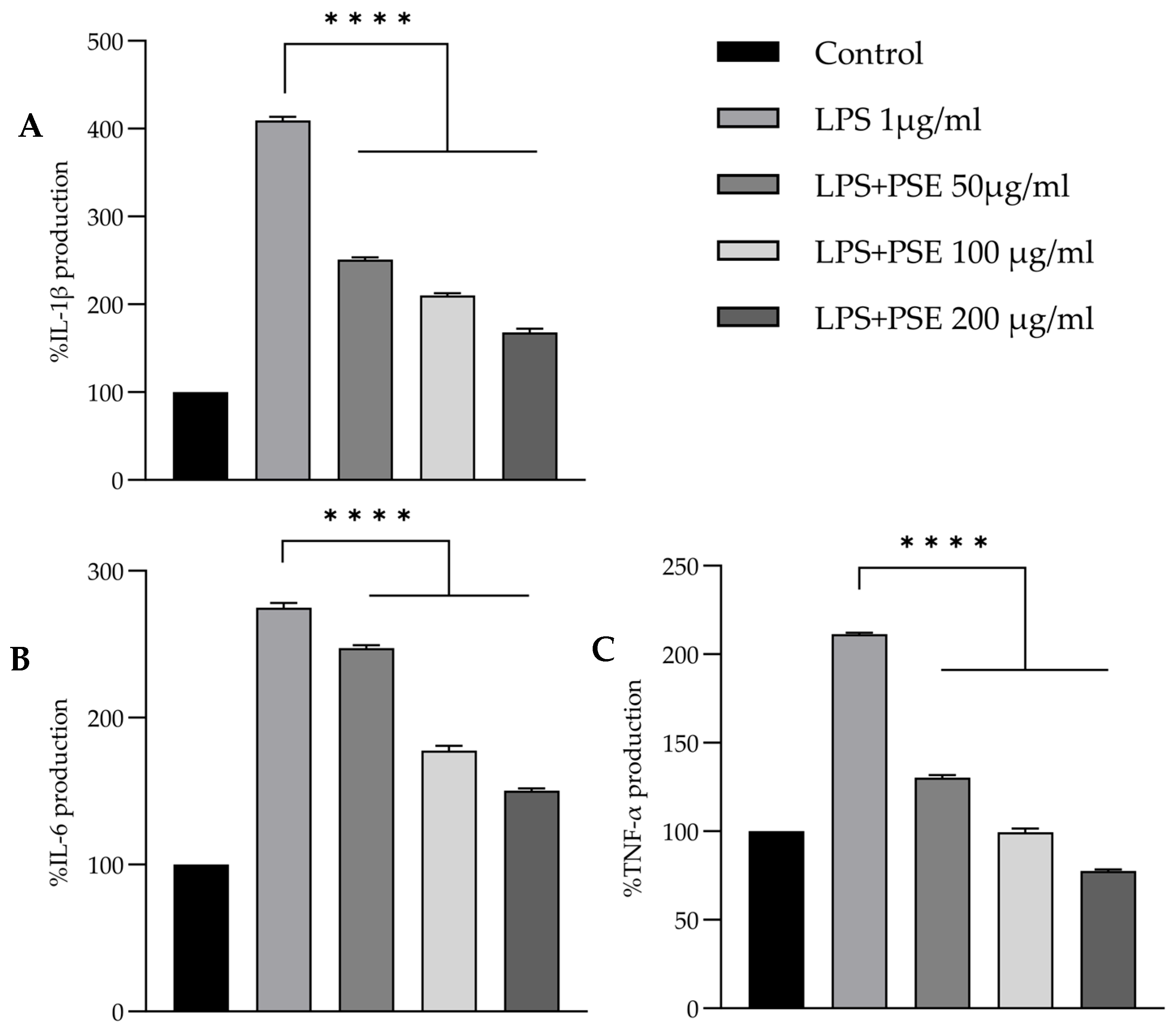
| Parameter | Value (Mean ± SD) | Unit | % w/w |
|---|---|---|---|
| Extraction of PF seed by 70% ethanol | 6.85 ± 0.71 | g from 100 g of dried weight | 6.85 g% |
| Antioxidant capacity of PSE using DPPH assay | 312.87 ± 12.98 | mg eq.Trolox/100 g of dry extract | - |
| Total phenolic contents | 375.04 ± 11.45 | mg GAE/g extract | 37.5 g% |
| Total flavonoid contents | 223.45 ± 16.02 | mg QE/g extract. | 22.4 g% |
| Rosmarinic acid | 1157.36 ± 55.66 | µg/g dry weight | 0.116 g% |
| Luteolin | 103.28 ± 2.71 | µg/g dry weight | 0.010 g% |
| Apigenin | 27.45 ± 1.12 | µg/g dry weight | 0.003 g% |
| Gallic acid | 24.89 ± 1.93 | µg/g dry weight | 0.002 g% |
| Quercetin | 22.22 ± 1.18 | µg/g dry weight | 0.002 g% |
| Caffeic acid | 21.30 ± 1.09 | µg/g dry weight | 0.002 g% |
| Tannic | 16.69 ± 0.92 | µg/g dry weight | 0.0016 g% |
| I-Quecetin | 14.32 ± 0.82 | µg/g dry weight | 0.0014 g% |
| Catechin | 6.06 ± 0.51 | µg/g dry weight | 0.0006 g% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhumtanom, P.; Wongta, A.; Chaiyana, W. The Apoptosis Induction and Immunomodulating Activities of Nga-Kee-Mon (Perilla frutescens) Seed Extract. Foods 2025, 14, 3685. https://doi.org/10.3390/foods14213685
Dhumtanom P, Wongta A, Chaiyana W. The Apoptosis Induction and Immunomodulating Activities of Nga-Kee-Mon (Perilla frutescens) Seed Extract. Foods. 2025; 14(21):3685. https://doi.org/10.3390/foods14213685
Chicago/Turabian StyleDhumtanom, Pongsathorn, Anurak Wongta, and Wantida Chaiyana. 2025. "The Apoptosis Induction and Immunomodulating Activities of Nga-Kee-Mon (Perilla frutescens) Seed Extract" Foods 14, no. 21: 3685. https://doi.org/10.3390/foods14213685
APA StyleDhumtanom, P., Wongta, A., & Chaiyana, W. (2025). The Apoptosis Induction and Immunomodulating Activities of Nga-Kee-Mon (Perilla frutescens) Seed Extract. Foods, 14(21), 3685. https://doi.org/10.3390/foods14213685







