Abstract
Hyperuricemia (HUA), a metabolic disorder characterized by elevated serum uric acid resulting from imbalanced production and excretion, is associated with gout and other serious health issues. This study aimed to screen out the potential probiotics with HUA-alleviating properties among 20 Lactobacillus strains. The results showed that L. reuteri Urob-7 exhibited the highest degradation rates for inosine and guanosine (82.10% and 88.78%, respectively) and strong xanthine oxidase (XOD) inhibitory activity (62.86%). In a HUA mouse model induced by inosine, guanosine, and potassium oxonate, L. reuteri Urob-7 intervention significantly reduced serum uric acid levels by 46.54%, restoring them to levels similar to control groups, and improved kidney function indicators. Moreover, Urob-7 reduced hepatic XOD activity by 37.6% and downregulated XOD expression in the intestines, decreasing excessive uric acid synthesis. It also significantly inhibited the NF-κB/NLRP3 inflammatory pathway, reducing the expression levels of NF-κB and NLRP3 in the kidneys by 39.3% and 47.6%, respectively. Furthermore, L. reuteri Urob-7 increased the abundance of short-chain fatty acid-producing bacteria (e.g., Ruminococcus and Intestinimonas) while reducing the proportion of pathogenic bacteria (e.g., Bacteroides and Anaerovorax), thus ameliorating gut microbiota dysbiosis and intestinal barrier dysfunction. In summary, L. reuteri Urob-7 effectively relieved HUA by modulating uric acid metabolism, suppressing inflammation, and improving gut microbiota balance. These results highlighted its potential as a promising candidate for HUA.
1. Introduction
Hyperuricemia (HUA) is a metabolic disorder characterized by elevated serum uric acid (SUA) concentrations, coupled with imbalanced uric acid synthesis and excretion [1]. HUA is defined as above 7.0 mg/dL (420.0 μmol/L) in males or above 6.0 mg/dL (360.0 μmol/L) in females [2]. HUA is a globally prevalent metabolic disease, with notably high rates in coastal and oceanic regions, such as the United States (21%), Japan (20–25%), and China (15.1%) [3,4,5]. The incidence of HUA is influenced by a variety of factors, including genetics, gender, lifestyle, and diet [6]. Long-term HUA can lead to the deposition of urate crystals in the joints, thereby inducing gout, which is characterized by severe joint pain, inflammation, and swelling [7]. Additionally, HUA is associated with an increased risk of cardiovascular diseases, hypertension, diabetes, and chronic kidney disease [8].
In the human body, urate is the final product of purine metabolism, which involves a multi-step degradation process. Purine nucleosides such as inosine and guanosine are dephosphorylated to form nucleobases, which are then immediately oxidized to produce xanthine, and finally further oxidized to yield urate [9]. Xanthine oxidoreductase (XOD), mainly expressed in the liver and intestines in humans, is a rate-limiting enzyme involved in in vivo uric acid production that catalyzes oxidation from xanthine to uric acid [10,11].The increased activity of XOD can lead to the excessive production of uric acid. In the excretion of uric acid, about 70% of urate is eliminated through the renal system, with the remaining 30% being excreted via the intestinal pathway [12,13]. Urate transporters in intestinal epithelial cells, such as ABCG2, are responsible for transporting uric acid from the blood to the intestinal lumen, where it is directly excreted or decomposed by the intestinal microbiota and then expelled through feces [14].
Current therapeutic approaches for HUA primarily rely on pharmacological interventions, including XOD inhibitors (allopurinol and febuxostat) and uric acid excretion promoters (benzbromarone and rasburicase) [2]. However, these drugs often have significant side effects and poor patient tolerability [15]. In contrast, probiotics offer a safe, cost-effective alternative which can alleviate HUA by reducing uric acid production or increasing uric acid excretion. The metabolites of probiotics, particularly short-chain fatty acids (SCFAs), can reduce lipopolysaccharide (LPS) levels, which in turn decreases inflammatory factors such as IL-1β and alleviates inflammatory responses [16,17]. Specific strains, including Lactobacillus rhamnosus CCFM1130, Lactobacillus rhamnosus CCFM1131, and Lactobacillus reuteri CCFM1132 could promote the production of intestinal SCFAs (especially acetic acid and valeric acid), thereby inhibiting XOD activity. Moreover, probiotic-derived nucleoside hydrolases that can hydrolyze nucleosides, thereby reducing the absorption and metabolism of purines and decreasing uric acid production [18,19]. Additionally, probiotics can restore the balance of the gut microbiota and enhance intestinal barrier disrupted by HUA. Furthermore, probiotics can regulate the expression of uric acid transporters such as URAT1 and ABCG2, thereby modulating the reabsorption and excretion of uric acid [20].
This study aimed to screen out probiotics with nucleoside degradation (inosine and guanosine) and XOD inhibition capabilities in vitro and verify its uric-lowering effects in a high-purine-diet induced HUA mouse model. Specifically, our study delved into the mechanisms of the L. reuteri Urob-7 strain, demonstrating its capability to reduce serum uric acid levels through multiple pathways: the degradation of nucleotides, inhibition of xanthine oxidase (XOD) activity, modulation of uric acid transport, and regulation of the gut microbiota. Importantly, we also highlighted its unique potential in ameliorating inflammation. The study provided a foundation for the development of probiotics as a preventive strategy against HUA.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
In this study, 20 strains of Lactobacillus (Limosilactobacillus fermentum, Lactiplantibacillus plantarum, Limosilactobacillus reuteri, Lacticaseibacillus rhamnosus) isolated from different traditional fermented food stored at −80 °C were selected as candidate strains (Table 1). The strains were inoculated into 2% (v/v) de Man, Rogosa, and Sharp (MRS) medium (Beijing Aoboxing Biotech Co., Ltd., Beijing, China) and subcultured three times at 37 °C for 24 h.

Table 1.
Strains used in this work.
2.2. Determination of Nucleoside Degradation Ability In Vitro
The nucleoside degradation ability of the strains in vitro was determined according to the method described by Xiao Y et al. [21]. After three generations of cultivation, the culture solution was centrifuged at 8000 rpm, 4 °C for 5 min to collect the bacterial pellet. Then, the sediments were washed by sterile phosphate-buffered saline (PBS, pH = 7.2) for three times and re-suspended in 1 mL of a 0.5 g/L inosine-guanosine standard solution and incubated at 37 °C for 60 min. Then, the solution was centrifuged and added with 0.1 mol/L HClO4 (9:1, v/v) to terminate further degradation. A 10 µL of the mixture was filtered through a 0.22-μm filters and injected into the high-performance liquid chromatography (HPLC) system (LC-20A, Shimadzu Corporation, Kyoto, Japan) to analyze the remaining contents of inosine and guanosine. PBS was used as the negative control to minimize background interference and to assess the experimental system’s stability.
HPLC conditions: chromatographic column, INERTSUSTAIN AX-C18, 5 μM, 4.6 × 250 mm; mobile phase, an isocratic gradient of 10 mM KH2PO4 solution to methanol (95:5, v/v); flow rate, 1 mL/min; column temperature, 30 °C; UV detection wave length, 254 nm; total retention time, 20 min. The external standard method was employed for the quantitative determination of inosine and guanosine contents using an HPLC system.
2.3. Determination of In Vitro XOD Inhibitory Activity
A 300 μL of the bacterial fermentation supernatant was combined with equal volume of XOD solution (0.05 U/mL) or 1 × PBS and incubated at 25 °C for 30 min. Then, a 300 μL of the above sample solution was added with 450 μL xanthine (0.48 mM) solution and incubated at 25 °C for 1 h. After centrifugation (8000 rpm, 25 °C, 10 min), the optical density (OD) value at 290 nm was determined using a Microplate reader. XOD inhibitory activity is calculated according to the following formula [22].
where A1 represents the absorbance of the test samples with enzyme; A2 represents the absorbance of the test solution without enzyme; A3 represents the absorbance of the blank group with 1 × PBS with enzyme; A4 represents the absorbance of 1 × PBS without enzyme added.
Inhibition rate (%) = [1 − (A1 − A2)/(A3 − A4)] * 100%
2.4. Animal Experiments
Three-week-old SPF Kunming male mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) weighing 20 ± 2 g were obtained and acclimated for seven days under conditions including a room temperature of 21 ± 2 °C, humidity maintained at 50 ± 10%, and a 12 h light/dark cycle. They were randomly divided into three groups (n = 6): the control group (CK), the HUA model group (MOD), and the Urob-7 intervention group (Urob-7). All groups were fed a basic maintenance diet for mice (Sipeifu Biotechnology Co., Ltd., Beijing, China) and provided with purified drinking water. The HUA model was established by intragastric administration of 250 mg/kg potassium oxonate (OP), 400 mg/kg inosine (INO), and 400 mg/kg guanosine (GUO), administered as a dispersion in 0.5% (w/v) carboxymethylcellulose sodium (CMC-Na). To assess its preventive potential, Limosilactobacillus reuteri Urob-7 was administered intragastrically to the Urob-7 group at a dose of 109 CFU/d starting from the modeling initiation. The CK group received an equal volume of 1 × PBS and 0.5% (w/v) CMC-Na daily. On the 7th, 14th, and 21st days, blood samples were collected from the mice’s orbital sinus, and serum uric acid levels were quantified using a uric acid detection kit (Nanjing Jiancheng, Nanjing, China). On the 21st day following the blood sampling, the mice were anesthetized with isoflurane and euthanized by cervical dislocation. Feces, liver, kidney, spleen, and intestinal tissues were collected and stored at −80 °C until analysis. All experiments were approved by the Animal Care and Use Committee of China Agricultural University and conformed to the Guide for the Care and Use of Laboratory Animals (Approval number: AW42604202-5-1).
2.5. Histopathological Analysis
The intestinal tissues (ileum and colon), liver and kidney tissues samples were fixed in 4% paraformaldehyde for 24 h, then embedded in paraffin. Sections of 5 μm were cut, dewaxed, rehydrated and hematoxylin and eosin (H&E)-stained. Histopathology and inflammation were assessed via optical microscopy. Villus height and crypt depth were measured using ImageJ 1.51 (NIH, Bethesda, MD, USA).
2.6. Liver XOD Activity Measurement
The liver tissue was homogenized at low temperature and centrifuged to obtain the supernatant, and UA synthesis in the liver was evaluated by the activity of xanthine oxidase (Solarbio, BC 1095, Beijing, China) according to the manufacturer’s instructions.
2.7. Serum Biochemical Indicators Examination
Blood samples were left for 2 h after collection and centrifuged (3500 rpm, 4 °C, 15 min) to obtain serum samples. Serum uric acid (UA), creatinine (Cr) and urea nitrogen (BUN) were detected with a commercial assay kit (Jiancheng, Nanjing, China).
2.8. Immunofluorescence Staining
Sections were deparaffinized in xylene, rehydrated with graded ethanol, and underwent antigen retrieval in citrate buffer (pH 6.0) via microwave heating for 20 min. After permeabilization with 1% Triton X-100 and blocking with 5% BSA, sections were incubated with primary antibodies, followed by Alexa Fluor 488-labeled secondary antibodies (1:1000). Nuclei were stained with DAPI, and sections were sealed with an antifluorescence quenching agent. Fluorescence microscopy was used for observation, and ImageJ software quantified ABCG2 expression and localization in the tissue sections.
2.9. Quantitative Reverse Transcription PCR (qRT-PCR)
The tissue samples (50 mg) were homogenized and mixed with 1 mL AG RNAex Pro reagent (Accurate Biotechnology Hunan Co., Ltd., Changsha, China) for total RNA extraction. The RNA was dissolved in RNase-free water and stored at −80 °C. Then, 1 µg of total RNA was reverse-transcribed into cDNA using the Evo M-MLV RT Mix Kit with gDNA Clean. RT-qPCR was performed with the SYBR Green Premix Pro Taq Hs qPCR kit on a QuantStudio™5 system (Thermo Fisher, Wilmington, MA, USA). Primers are listed in Table 2. Target gene expression was quantified relative to β-actin using the 2−∆∆CT method.

Table 2.
qRT-PCR primers used in this work.
2.10. Gut Microbiota 16S rRNA Gene Analysis
Microbial genomic DNA was extracted from fecal samples using the FastPure Stool DNA Isolation Kit (MJYH, Shanghai, China). DNA quality and concentration were assessed via 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer. The V3-V4 region of the 16 S rRNA gene was amplified using primers 338F and 806R with PCR conditions: 95 °C for 3 min, 27 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. PCR products were purified, quantified, and pooled equimolarly for paired-end sequencing on the Illumina NextSeq 2000 platform by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China) Bioinformatics analysis was performed using the Majorbio Cloud platform, including rarefaction curves, alpha diversity indices, PCoA analysis, and identification of differentially abundant taxa.
2.11. Statistical Analysis
GraphPad Prism 9.5 was used for statistical analysis. The results were expressed as mean ± SEM. One-way ANOVA with Tukey’s multiple comparisons test was used to identify the statistically significant differences. A p-value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Screening of Lactobacillus Strains with Uric Acid-Lowering Activity In Vitro
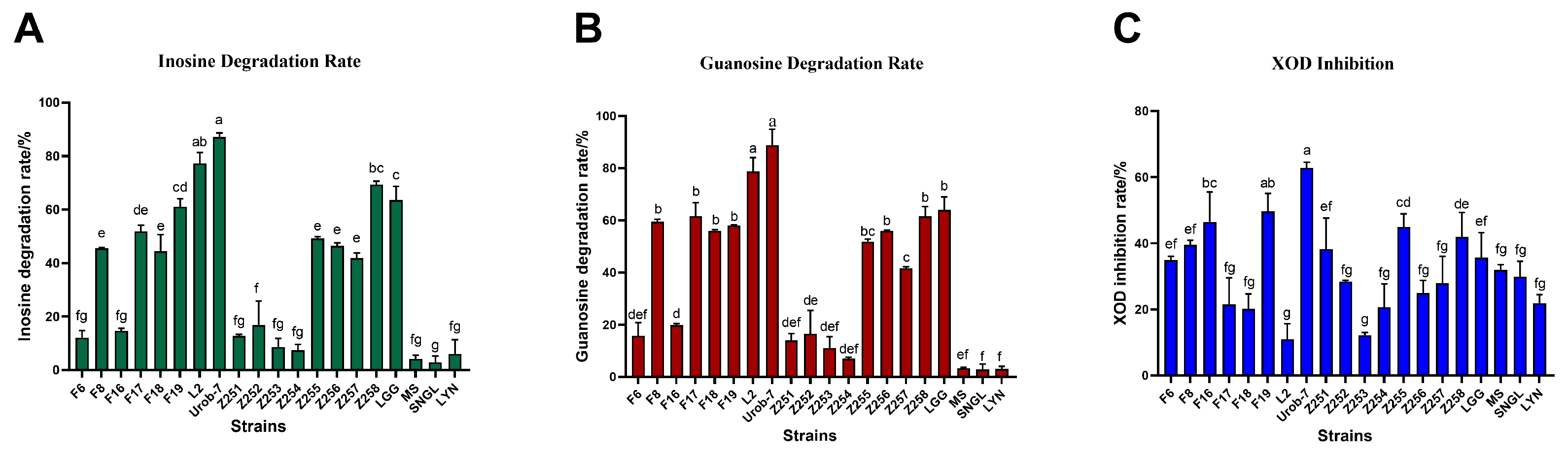
The degradation abilities of inosine and guanosine by 20 Lactobacillus strains were analyzed using HPLC. The results showed that L. reuteri Urob-7 exhibited the highest inosine and guanosine degradation rates with 82.10% and 88.78%, followed by L. reuteri L2 with rates of 75.23% and 78.67% (Figure 1A,B). However, L. reuteri L2 showed relatively low inhibitory activity against XOD at only 10.96%, while Urob-7 the highest inhibition rate among the tested strains at 62.86% (Figure 1C). Therefore, L. reuteri Urob-7 was selected to explore its uric acid-lowering ability in vivo.
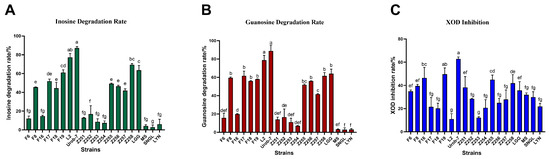
Figure 1.
Nucleoside degradation and XOD inhibition ability of 20 Lactobacillus strains in vitro. (A) Degradation rate of inosine. (B) Degradation rate of guanosine. (C) Inhibition rate of XOD activity. n = 3. Data were presented as mean ± SEM. In each subfigure, different letters above the bars indicate significant differences between groups (p < 0.05). Bars with the same letter are not significantly different.
3.2. L. reuteri Urob-7 Alleviated Hyperuricemia by Decreasing Serum UA Levels
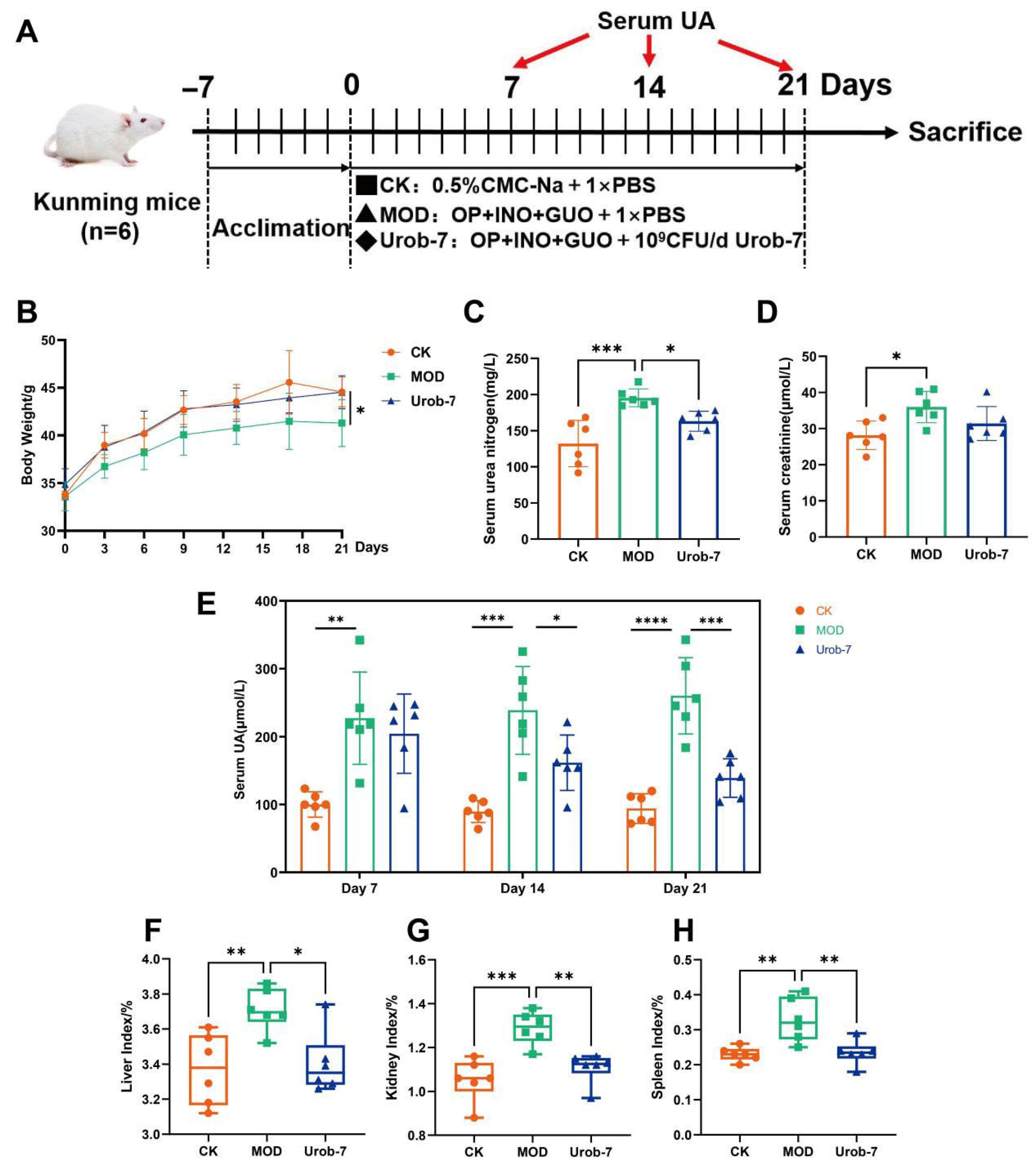
To investigate the effects of Urob-7 on alleviating HUA, a mouse model combined with nucleosides and potassium oxonate was established (Figure 2A). After 21 days’ administration, the body weight of the MOD group decreased by 7.19% compared to the CK group, while there was no significant difference between the Urob-7 group and CK group (Figure 2B). Serum BUN and CR levels were significantly elevated in the MOD group compared to the CK group. Urob-7 intervention restored the BUN levels to that of the CK group, though CR levels remained unchanged relative to the MOD group (Figure 2C,D). Notably, the serum uric acid level in the MOD group remained approximately 2-3-fold higher than that in the CK group. In contrast, the serum uric acid level in the Urob-7 group showed a downward trend from day 7 to day 14 and decreased to nearly the similar level as the CK group at day 21. Compared with CK group, the liver, kidney, and spleen indexes of the MOD group were significantly higher than those of the CK group, increasing by 10.15%, 22.32%, and 42.74%, respectively (Figure 2F–H). The intervention with L. reuteri Urob-7 effectively reduced the organ coefficients to levels comparable to those of the CK group. These results indicated that L. reuteri Urob-7 had therapeutic potential for HUA by reducing serum uric acid, mitigating organ swelling, and modulating kidney function.
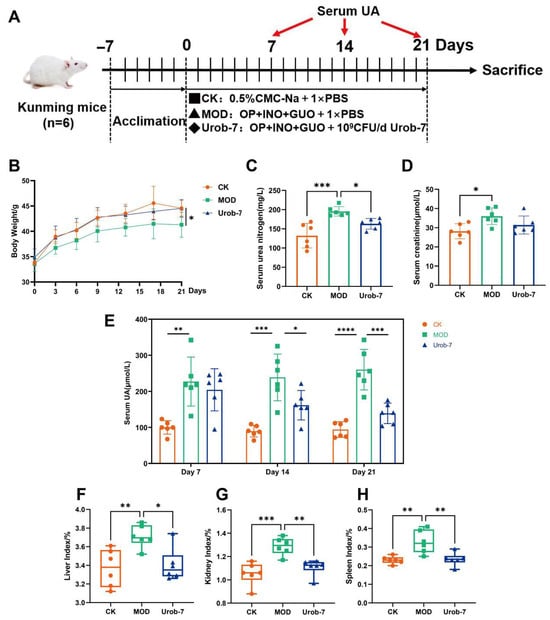
Figure 2.
Effects of L. reuteri Urob-7 on symptoms of HUA. (A) Schematic diagram of the animal experiment. (B) Changes in body weight. (C) Serum BUN levels. (D) Serum Cr levels. (E) Serum uric acid levels during a 21-day period. (F–H) Organ coefficients of livers, kidneys and spleens. n = 6 mice per group. Data were presented as mean ± SEM. p values were determined by one-way ANOVA followed by Tukey’s test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.3. L. reuteri Urob-7 Ameliorated the Liver, Kidney, and Intestinal Structural Damage
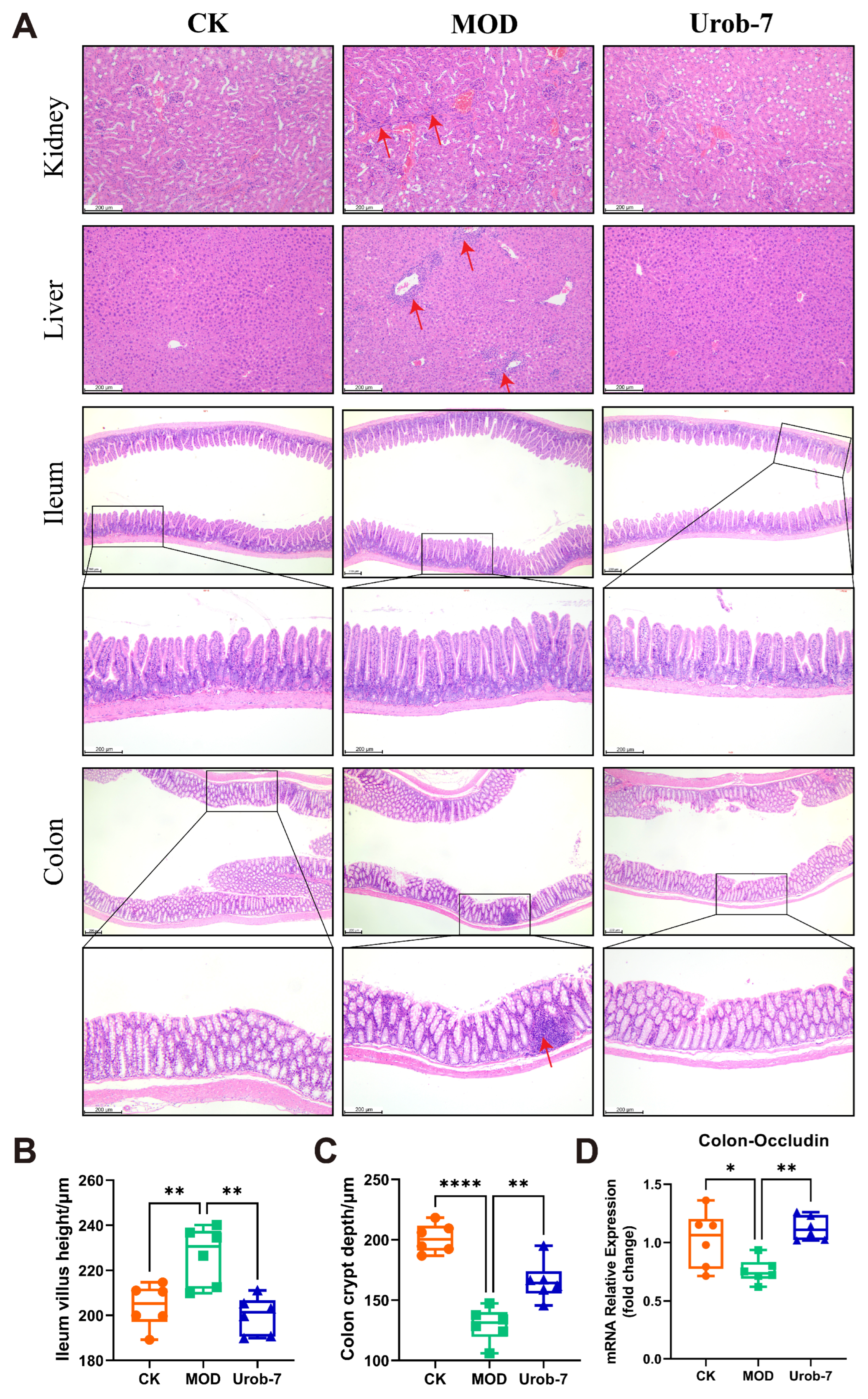
Histopathological analysis (Figure 3A) showed that MOD group mice exhibited marked renal damage, characterized by incomplete glomerular morphology, capillary loop expansion, Bowman’s capsule swelling, and mild inflammatory infiltration. Hepatic sections from MOD mice showed turbid cytoplasm, red blood cell stasis in sinusoids, significant inflammatory infiltration, and variable-sized fat vacuoles. As for the intestines, the colon structure in MOD group mice was distorted with shortened crypts, and marked inflammatory infiltration. Compared with the CK group, the MOD group exhibited a significant increase in ileum villus height. In contrast, Urob-7 group mice showed less severe kidney damage, with neatly arranged proximal tubular epithelial cells and clearer cytoplasm. Urob-7 intervention also reduced hepatic fat deposition and inflammation, leading to more regular and uniform hepatocyte morphology. Compared with the MOD group, ImageJ analysis further confirmed that mice in the Urob-7 group exhibited significantly reduced ileum villus height and increased colon crypt depth (Figure 3B,C). Additionally, the expression level of Occludin was higher in the Urob-7 group than in the MOD group (Figure 3D), indicating that Urob-7 could ameliorate intestinal pathological changes induced by HUA. Overall, these findings suggested that L. reuteri Urob-7 intervention effectively alleviated HUA-induced pathological changes and inflammation in the liver, kidneys, and intestines.
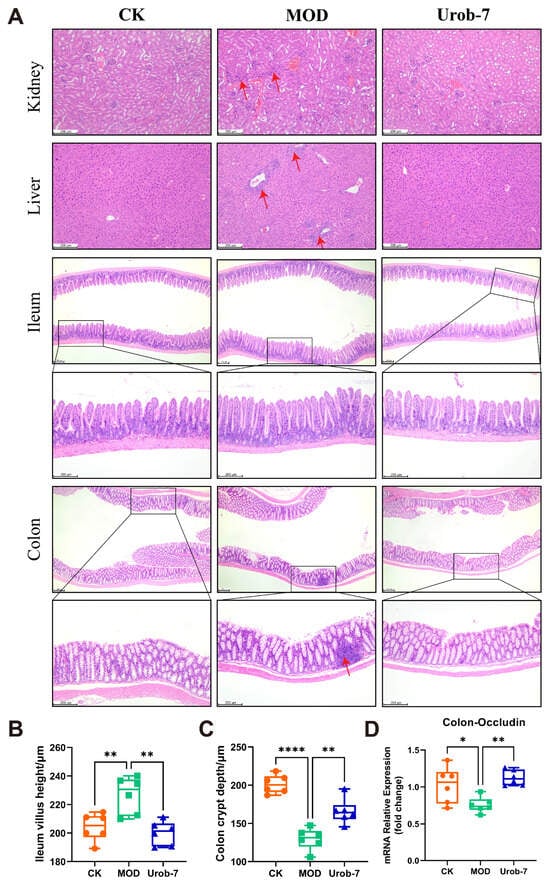
Figure 3.
Effects of L. reuteri Urob-7 on histopathological damage to the kidney, liver, and intestines in HUA mice. (A) Representative images of H&E staining in the kidney, liver, ileum and colon. The red arrows represent inflammatory cell infiltration. (B) The villi height of ileum. (C) The crypt depth of colon. (D) Expression levels of Occludin mRNA in the colon. n = 6 mice per group. Data were presented as mean ± SEM. p values were determined by one-way ANOVA followed by Tukey’s test. * p < 0.05, ** p < 0.01, and **** p < 0.0001.
3.4. L. reuteri Urob-7 Regulated Uric Acid Re-Absorption and Excretion Transporter
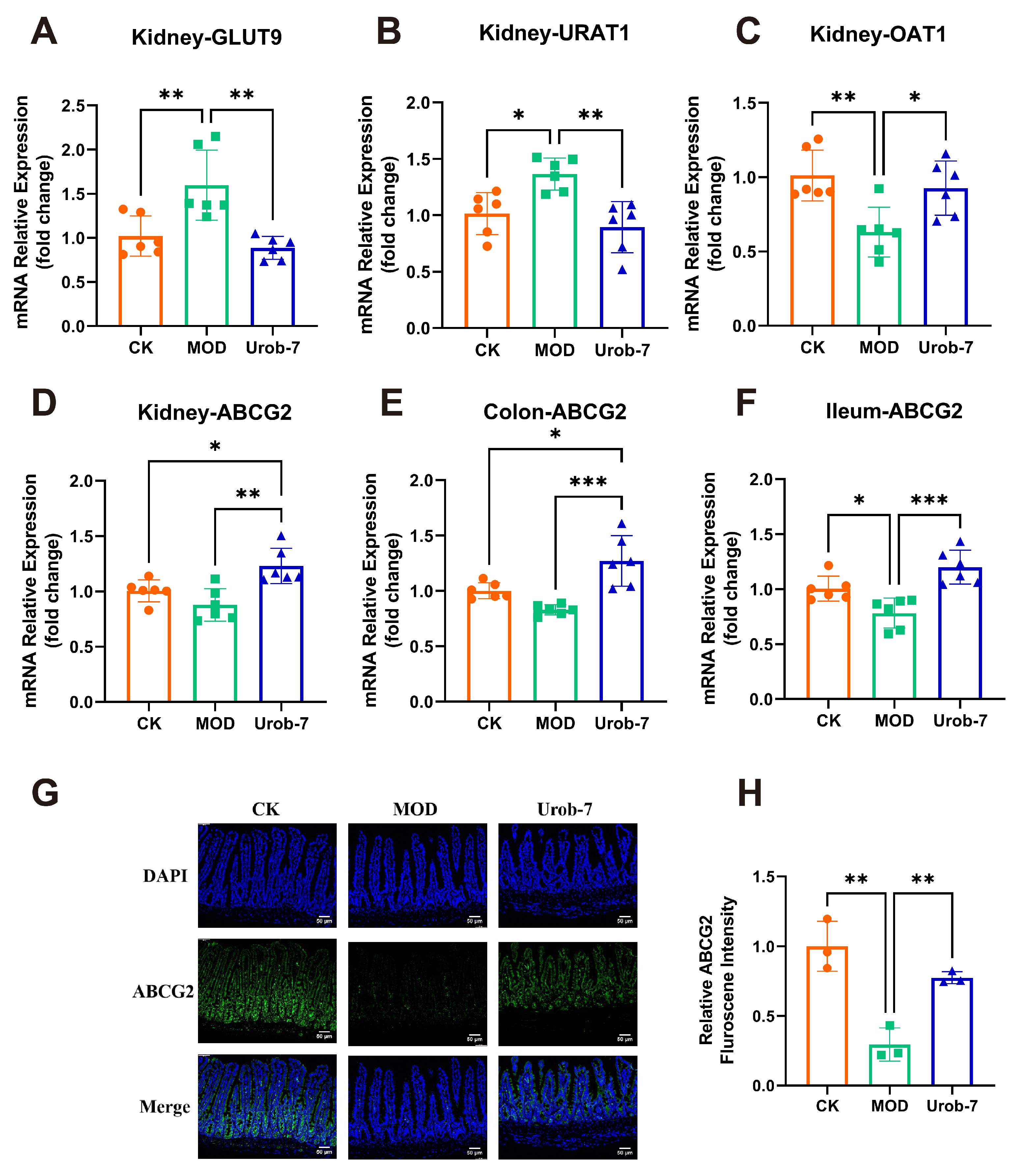
To investigate the effects of L. reuteri Urob-7 on uric acid metabolism, re-absorption transporters (GLUT9 and URAT1) and excretion transporters (ABCG2 and OAT1) were analyzed. The results showed that mRNA levels of GLUT9 and URAT1 significantly increased by 56.57% and 34.45%, while mRNA levels of OAT1 and ABCG2 decreased by 37.62% and 12.61% in the MOD group (Figure 4A–D). Moreover, ABCG2 expression in the ileum and colon of MOD group also significantly decreased (Figure 4E,F). L. reuteri Urob-7 intervention reversed these changes by significantly down-regulating the mRNA expression of GLUT9 and URAT1 and upregulating OAT1 and ABCG2 in the kidney. Furthermore, ABCG2 expression in the intestinal tissues was significantly upregulated. Immunofluorescence analysis confirmed that ABCG2 protein expression in the ileum of the Urob-7 group was significantly higher than in the MOD group (Figure 4G,H). These findings suggested that L. reuteri Urob-7 effectively promoted uric acid excretion and reduced uric acid reabsorption by regulating the expression of these key transporters, thereby alleviating HUA.
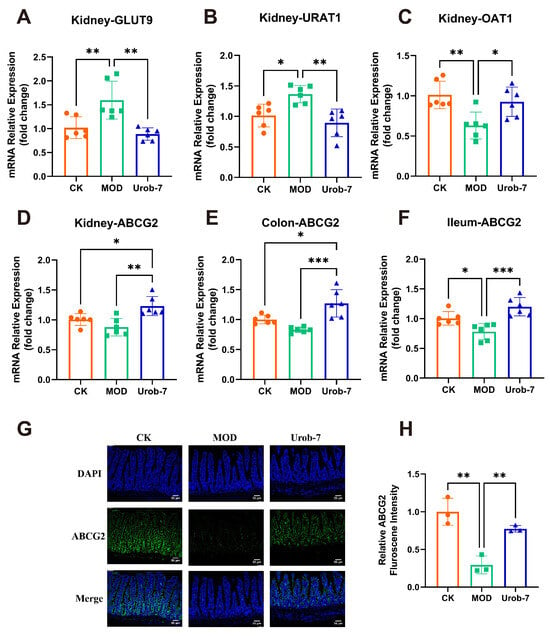
Figure 4.
Effects of L. reuteri Urob-7 on uric acid transporter. The mRNA expression levels of (A) GLUT9, (B) URAT1, (C) OAT1 and (D) ABCG2 in the kidney. The expression levels of ABCG2 in the colon (E) and ileum (F). n = 6 mice per group. (G) Representative immunofluorescence staining images of ABCG2 (green). Nuclei were stained by DAPI (blue). Scale bar means 50 μm. (H) Quantification of ABCG2 fluorescence intensity. n = 3 mice per group. Data were presented as mean ± SEM. p values were determined by one-way ANOVA followed by Tukey’s test. * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.5. L. reuteri Urob-7 Ameliorated NLRP3 Inflammatory Response and Inhibited Uric Acid Synthesis
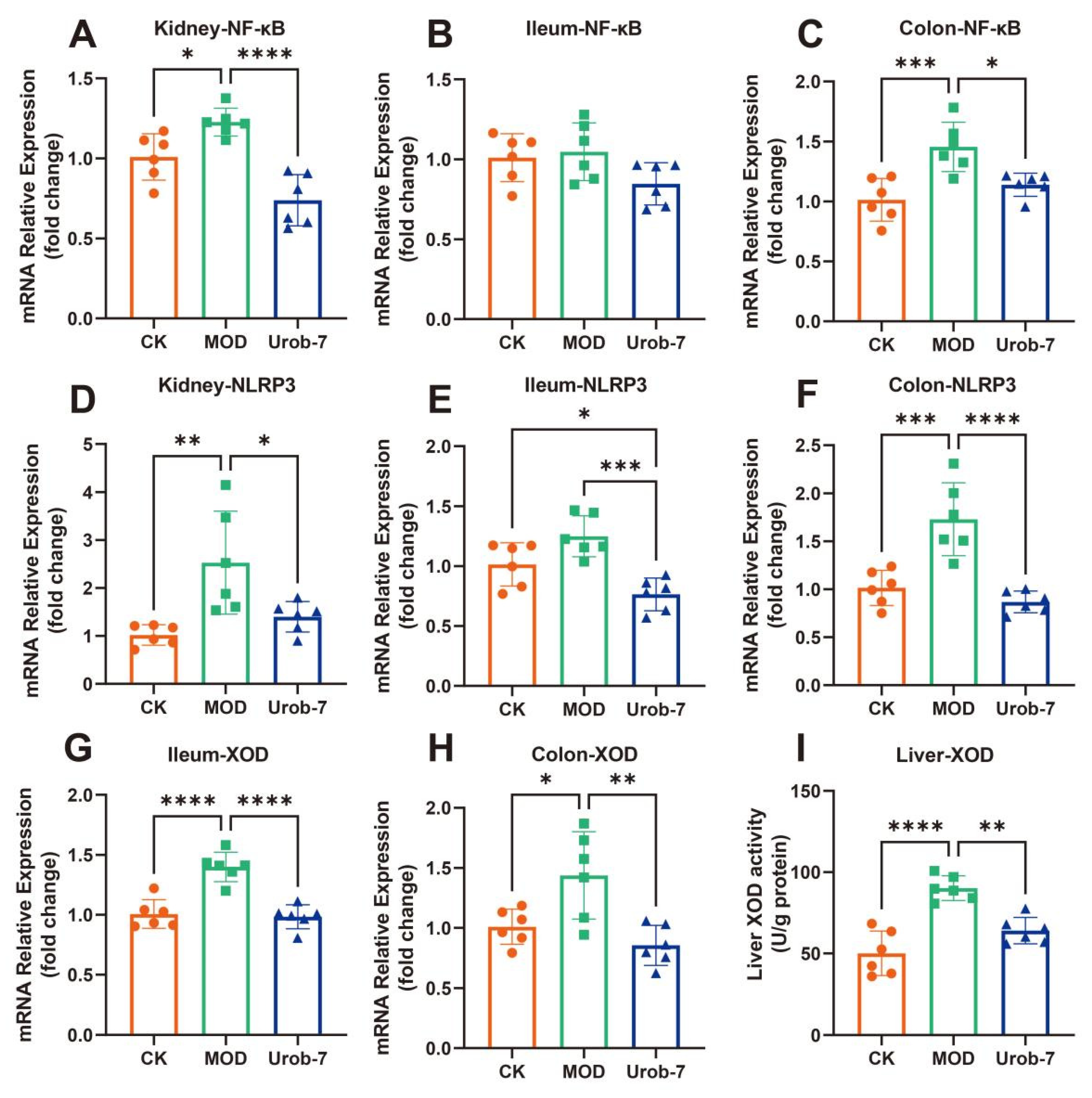
The expressions of inflammatory signaling pathways and key enzymes in uric acid metabolism were evaluated in three groups of mice (Figure 5A–F). The results showed that the gene expression of NF-κB was significantly upregulated in the kidney and colon of the MOD group, increasing by 39.30% and 43.63% respectively compared with the CK group. Similarly, the mRNA expression levels of NLRP3 in the kidney and colon of MOD group mice were also significantly upregulated. However, there were no significant differences in NF-κB expression among the three groups in the ileum, and significant differences in NLRP3 expression were only observed between the Urob-7 group and the other two groups. Notably, L.reuteri Urob-7 intervention effectively down-regulated the gene expression at the mRNA level, restoring the levels of NF-κB and NLRP3 in the kidney and colon to levels similar to those of the CK group.
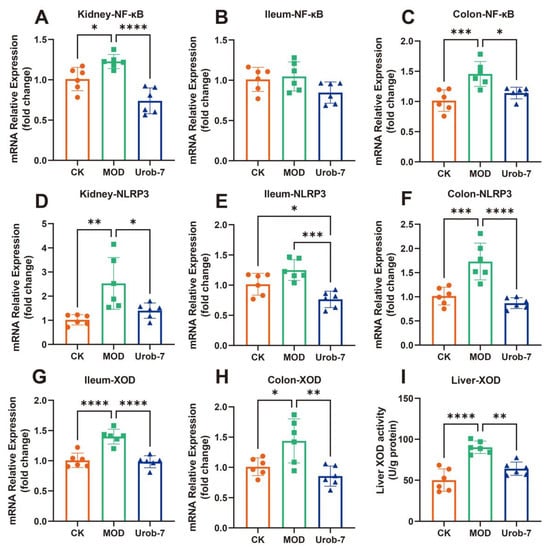
Figure 5.
Effects of L. reuteri Urob-7 on inflammatory response and XOD in vivo. The mRNA expression levels of (A) Kidney NF-κB, (B) Ileum NF-κB, (C) Colon NF-κB, (D) Kidney NLRP3, (E) Ileum NLRP3, (F) Colon NLRP3, (G) Ileum XOD, (H) Colon XOD. (I) Liver XOD activity. n = 6 mice per group. Data were presented as mean ± SEM. p values were determined by one-way ANOVA followed by Tukey’s test. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
XOD gene expression was significantly upregulated in both the colon and ileum in MOD group, but Urob-7 intervention reversed this effect (Figure 5G,H). Meanwhile, XOD activity in the liver was significantly increased in the MOD group, while oral administration of L.reuteri Urob-7 markedly reduced XOD activity in the livers of HUA mice (Figure 5I). These findings suggested that Urob-7 alleviated HUA by suppressing NLRP3-mediated inflammation and modulating XOD expression and activity, thereby reducing uric acid production and its associated inflammatory responses.
3.6. L. reuteri Urob-7 Reshaped Gut Microbiota Composition in Mice with HUA
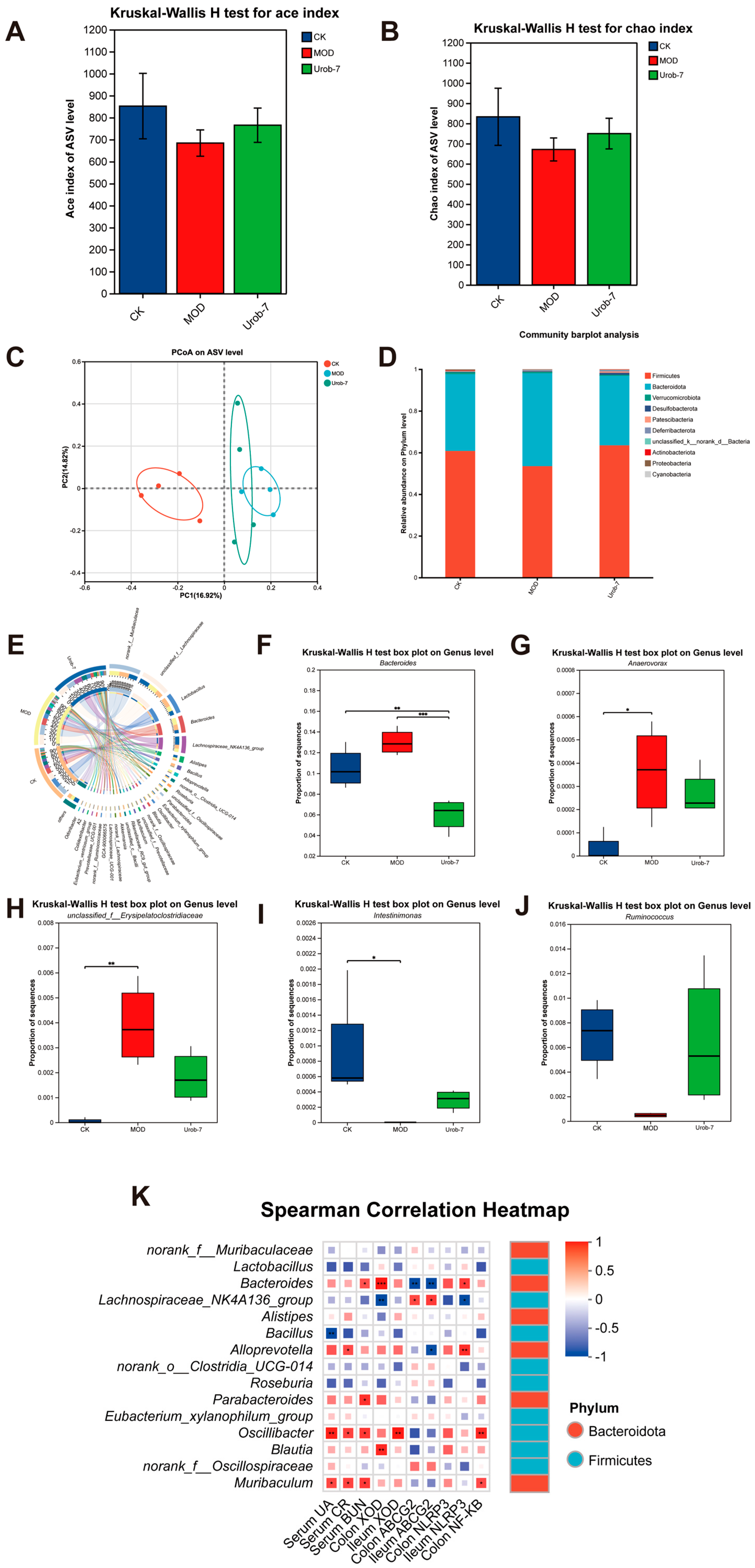
To illustrate the effect of L. reuteri Urob-7 on the gut microbiota composition in HUA mice, the diversity and abundance of gut microbiota were detected in fecal samples via 16S rRNA sequencing. Compared with the CK group, the Ace index and Chao index of mice in the MOD group decreased but did not exhibit a significant difference. The Ace index and Chao index of the Urob-7 group increased compared with those of the MOD group mice, but did not restore to the same level as the CK group (Figure 6A,B). A principal coordinates analysis (PCoA) showed a significant difference between the CK and MOD groups at the ASV level (PCoA 1 = 16.92%, PCoA 2 = 14.82%) (Figure 6C). Moreover, the Urob-7 group exhibited a trend towards the separation and clustering of microbiota structures from the MOD group, indicating L. reuteri Urob-7 treatment may change the gut microbiota composition. In addition, compared to the CK group, the relative abundance of Firmicutes and Actinobacteriota was significantly reduced, while that of Bacteroidetes was significantly increased in the MOD group (Figure 6D). The administration of L. reuteri Urob-7 reversed this imbalance and restored the ratio of Bacteroidetes to Firmicutes (Bac/Firm Ratio) to the level of the CK group. At the genus level, the circus plot analysis showed that norank_f__Muribaculaceae, unclassified_f__Lachnospiraceae, Lactobacillus, Bacteroides, Lachnospiraceae_NK4A136_group, and Alistipes are the shared core genera in the three groups of mice (Figure 6E). Furthermore, compared with the CK group, the relative abundance of Bacteroides, unclassified_f__Erysipelatoclostridiaceae, and Anaerovorax significantly increased (p < 0.05) in the MOD group, while the relative abundance of Ruminococcus and Intestinimonas significantly decreased (p < 0.05) (Figure 6F–J). The Spearman correlation heatmap analysis showed that the relative abundance of Bacteroides was positively correlated with serum biomarker concentrations (UA, CR, and BUN), XOD expression, and inflammation-related genes, while negatively correlated with ABCG2 expression in the ileum and colon (Figure 6K). These results suggested that Urob-7 intervention effectively reversed the HUA-induced imbalance in gut microbiota.
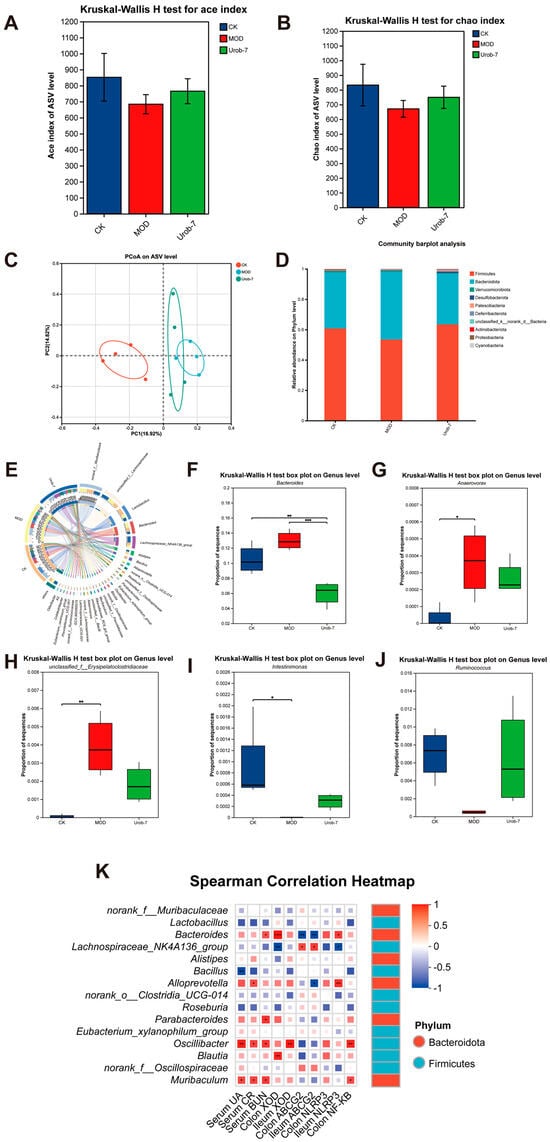
Figure 6.
Effects of L. reuteri Urob-7 on gut microbiota composition in HUA mice. (A) Ace index of Alpha diversity. (B) Chao index of Alpha diversity. (C) Principal coordinates analysis (PCoA) plot on ASV level. (D) Relative abundance of microbiota at the phylum level. (E) Circos diagram for community diversity analysis at the genus level. (F–J) The relative abundance of Bacteroides, Ruminococcus, Intestinimonas, unclassified_f__Erysipelatoclostridiaceae and Anaerovorax. (K) Spearman correlation analysis between microbiota at the genus level and biochemical parameters. Box colors indicated the level of correlation coefficients. n = 6 mice per group. Data were presented as mean ± SEM. p values were determined by one-way ANOVA followed by Tukey’s test. * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
Purine bases in food are mainly present in the form of nucleoproteins. During digestion, gastric acid initiates the breakdown of nucleoproteins into nucleic acids and proteins. In the small intestine, pancreatic nucleases further degrade nucleic acids into mononucleotides. These mononucleotides are subsequently hydrolyzed by enzymes such as 5’-nucleotidase to produce nucleosides and phosphate [23]. The purine-containing nucleosides are absorbed by intestinal epithelial cells and are eventually metabolized into uric acid in the liver [24]. Thus, reducing the absorption of nucleosides into the bloodstream is crucial for controlling blood uric acid levels. As precursors of uric acid synthesis, inosine and guanosine can be hydrolyzed into purine bases by enterocytes. Probiotics can decrease the host’s nucleoside absorption, thereby lowering uric acid production. Li et al. [25] discovered three genes annotated as ribonucleoside hydrolase (RihA, RihB and RihC) involved in the conversion of inosine and guanosine in the L. plantarum genome. Similarly, L. reuteri has been reported to harbor the ribonucleoside hydrolase gene RihC [26]. The protein encoded by this gene can efficiently degrade inosine, reducing its intestinal circulation and hepatic accumulation. XOD is the rate-limiting enzyme in uric acid synthesis, and inhibiting its activity can also significantly reduce uric acid production. In this study, L. reuteri Urob-7 demonstrated high nucleoside degradation and XOD inhibiting activities. As a probiotic naturally present in the human intestinal tract, L. reuteri exhibits tolerance to gastrointestinal fluids [27]. Notably, we report for the first time that L. reuteri Urob-7 demonstrates robust nucleoside hydrolase activity as well as potent XOD inhibition.
HUA is often accompanied by inflammatory responses [28]. Monosodium urate (MSU) crystals can activate the NF-κB pathway via TLR2/4-MyD88 signaling, inducing NLRP3 and pro-IL-1β expression. Meanwhile, MSU-induced lysosomal disruption or ROS production triggers NLRP3 inflammasome assembly, activating caspase-1 to cleave pro-IL-1β into mature IL-1β. The released IL-1β binds to IL-1 receptors (IL-1R), reactivating the MyD88/NF-κB pathway and forming a positive feedback loop [29,30]. Increased XOD activity leads to excessive uric acid production and MSU crystal formation, further aggravating the inflammatory response [31]. Recently, probiotics have shown potential in alleviating HUA by modulating NLRP3 inflammasome activation and inflammatory factor release. Lactobacillus acidophilus F02 suppressed urate-induced upregulation of pro-IL-1β, IL-1β, NLRP3, ASC, and caspase-1 expression, thereby mitigating HUA-induced hepatorenal injury [32]. In this study, L. reuteri Urob-7 effectively suppresses the NLRP3 inflammasome pathway, thereby mitigating inflammation associated with hyperuricemia. This integrated approach offers a more comprehensive strategy for hyperuricemia management compared to probiotics that act through single mechanistic actions. For example, L. rhamnosus Fmb14 has been shown to alleviate hyperuricemia-induced hepatocyte pyroptosis by inhibiting the NLRP3 inflammasome cascade [33], but it does not exhibit notable nucleoside degradation or XOD inhibitory capabilities. Similarly, L. plantarum has been reported to reduce uric acid levels by enhancing colonic uric acid excretion, yet its effect on XOD activity and nucleoside metabolism remains limited [20]. The multifaceted mechanism of L. reuteri Urob-7 makes it a promising candidate for the development of natural therapeutics against hyperuricemia, offering a broader spectrum of benefits including uric acid metabolism regulation, inflammation suppression, and gut microbiota restoration.
HUA is closely linked to gut microbiota dysbiosis [34]. Compared to healthy individuals, the proportion of Firmicutes decreases, while Bacteroidota and Proteobacteria increase at the phylum level in HUA patients [35]. At the genus level, Blautia, Collinsella, and Turicibacter are positively correlated with HUA, while SCFAs-producing bacteria like Alistipes, Roseburia, Parabacteroides, and Clostridium are negatively correlated [36]. These SCFA-producing bacteria play critical roles in maintaining gut barrier integrity and modulating inflammatory pathways, and their reduced abundance may disrupt intestinal homeostasis, exacerbate metabolic disorders, and promote systemic inflammation [37,38]. In this study, Urob-7 intervention increased the relative abundance of Firmicutes and Actinobacteriota and decreased Bacteroidota at the phylum level. Notably, Actinobacteriota may alleviate HUA-associated inflammation through Treg cell activation or NF-κB pathway suppression [39]. At the genus level, the relative abundance of Bacteroides, unclassified_f__Erysipelatoclostridiaceae, and Anaerovorax decreased significantly in the Urob-7 group, while the relative abundance of Ruminococcus and Intestinimonas increased. Bacteroides has been implicated in the metabolic conversion of purines to uric acid. A reduction in the abundance of Bacteroides may suppress this metabolic pathway, thereby potentially mitigating uric acid accumulation in the host [34]. Its reduced abundance may mitigate urate accumulation and enhance gut barrier integrity, thereby curbing bacterial translocation and inflammatory cytokine release [40]. Previous studies have shown that the bacterial genera Ruminococcus and Intestinimonas are capable of degrading resistant starch into butyrate via specific metabolic pathways [41]. Butyrate, by activating the PPARγ pathway, exerts anti-inflammatory effects, upregulates gut ABCG2 transporter expression, and facilitates uric acid excretion [42,43]. Collectively, these functional shifts in the gut microbiota highlight potential mechanisms through which microbiota influence uric acid metabolism and systemic inflammation.
Studies have demonstrated a significant positive correlation between serum uric acid concentrations and pro-inflammatory cytokines, including IL-6 and IL-1β, in elderly inpatients [44]. This aligns with the growing body of evidence linking uric acid metabolism to inflammatory responses, which is of particular relevance in the context of hyperuricemia and associated co-morbidities. By modulating uric acid levels and mitigating inflammation, L. reuteri Urob-7 may offer a synergistic approach to managing hyperuricemia, with potential implications for improving patient outcomes in clinical settings. This integrated mechanism highlights the probiotic’s promise in addressing the complex interplay between metabolic dysfunction and inflammation, as often observed in conditions like hyperuricemia. Furthermore, it underscores the need for broader exploration of such multi-targeted strategies in the development of novel therapeutic interventions. In conclusion, L. reuteri Urob-7 effectively alleviated HUA by degrading nucleosides, inhibiting XOD activity, reducing inflammation, and restoring gut microbiota homeostasis.
Author Contributions
Conceptualization, Y.J. and H.Q.; methodology, X.B.; software, Y.W.; validation, Y.J., X.B. and Y.H. (Yan Hao); formal analysis, Y.J.; investigation, X.B.; resources, Z.Z. (Zhengyuan Zhai); data curation, H.Q.; writing—original draft preparation, H.Q.; writing—review and editing, Y.J. and Y.H. (Yanling Hao); visualization, X.B.; supervision, Y.H. (Yanling Hao); project administration, Z.Z. (Zhu Zhang); funding acquisition, Y.H. (Yanling Hao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Key R&D Program of China, grant number 2022YFF1100100.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of China Agricultural University Institutional Animal Care and Use Committee (protocol code Aw42604202-5-1 and 2024.6.13 of approval).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, L.J.; Zhang, Y.P.; Zeng, C.C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181. [Google Scholar]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Hakoda, M. Epidemiology of hyperuricemia and gout in Japan. Nihon Rinsho 2008, 66, 647–652. [Google Scholar] [PubMed]
- Piao, W.; Zhao, L.Y.; Yang, Y.X.; Fang, H.Y.; Ju, L.H.; Cai, S.Y.; Yu, D.M. The Prevalence of Hyperuricemia and Its Correlates among Adults in China: Results from CNHS 2015–2017. Nutrients 2022, 14, 4095. [Google Scholar] [CrossRef] [PubMed]
- Yokose, C.; McCormick, N.; Choi, H.K. Dietary and Lifestyle-Centered Approach in Gout Care and Prevention. Curr. Rheumatol. Rep. 2021, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Neogi, T.; George, J.; Rekhraj, S.; Struthers, A.D.; Choi, H.; Terkeltaub, R.A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012, 64, 327–338. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabo, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid-Key Ingredient in the Recipe for Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mount, D.B. The Molecular Physiology of Uric Acid Homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef]
- Xu, X.X.; Li, C.H.; Zhou, P.; Jiang, T.L. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Pascart, T.; Richette, P. Investigational drugs for hyperuricemia, an update on recent developments. Expert Opin. Investig. Drugs 2018, 27, 437–444. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Li, X.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activity via a short-chain fatty acid-dependent mechanism. Food Funct. 2021, 12, 7054–7067. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Saito, C.; Murayama-Chiba, Y.; Kano, H.; Asami, Y.; Itoh, H. Lactobacillus gasseri PA-3 utilizes the purines GMP and guanosine and decreases their absorption in rats. Nucleosides Nucleotides Nucleic Acids 2018, 37, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Saito-Iwamoto, C.; Nakamura, M.; Soeda, M.; Chiba, Y.; Kano, H.; Asami, Y. Lactobacillus gasseri PA-3 Uses the Purines IMP, Inosine and Hypoxanthine and Reduces their Absorption in Rats. Microorganisms 2017, 5, 10. [Google Scholar] [CrossRef]
- Lee, Y.; Werlinger, P.; Suh, J.W.; Cheng, J.H. Potential Probiotic Lacticaseibacillus paracasei MJM60396 Prevents Hyperuricemia in a Multiple Way by Absorbing Purine, Suppressing Xanthine Oxidase and Regulating Urate Excretion in Mice. Microorganisms 2022, 10, 851. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, C.; Zeng, X.; Yuan, Z. Microecological treatment of hyperuricemia using Lactobacillus from pickles. BMC Microbiol. 2020, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef]
- Koguchi, T.; Tadokoro, T. Beneficial Effect of Dietary Fiber on Hyperuricemia in Rats and Humans: A Review. Int. J. Vitam. Nutr. Res. 2019, 89, 89–108. [Google Scholar] [CrossRef]
- Sanderson, I.R.; He, Y. Nucleotide uptake and metabolism by intestinal epithelial cells. J. Nutr. 1994, 124 (Suppl. S1), 131s–137s. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; Guo, Z.; Gao, R.; Ni, Z.; Cui, H.; Zong, M.; Van Bockstaele, F.; Lou, W. Lactiplantibacillus plantarum enables blood urate control in mice through degradation of nucleosides in gastrointestinal tract. Microbiome 2023, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, L.A.; Chikurova, N.Y.; Atroshenko, D.L.; Savin, S.S.; Kleymenov, S.Y.; Chernobrovkina, A.V.; Pometun, E.V.; Minyaev, M.E.; Matyuta, I.O.; Hushpulian, D.M.; et al. Structure-Functional Examination of Novel Ribonucleoside Hydrolase C (RihC) from Limosilactobacillus reuteri LR1. Int. J. Mol. Sci. 2023, 25, 538. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Li, D.; Yuan, S.; Deng, Y.; Wang, X.; Wu, S.; Chen, X.; Li, Y.; Ouyang, J.; Lin, D.; Quan, H.; et al. The dysregulation of immune cells induced by uric acid: Mechanisms of inflammation associated with hyperuricemia and its complications. Front. Immunol. 2023, 14, 1282890. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rokad, D.; Malovic, E.; Luo, J.; Harischandra, D.S.; Jin, H.; Anantharam, V.; Huang, X.; Lewis, M.; Kanthasamy, A.; et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 2019, 12, eaat9900. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Sui, X.L.; Xu, Y.P.; Gu, F.J.; Zhang, A.S.; Chen, J.H. Association between Nod-like receptor protein 3 inflammasome and gouty nephropathy. Exp. Ther. Med. 2020, 20, 195–204. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Ren, Z.; Li, Y.; Huang, J.; Lin, Z.; Zhang, B. Overnutrition-induced gout: An immune response to NLRP3 inflammasome dysregulation by XOD activity increased in quail. Front. Immunol. 2022, 13, 1074867. [Google Scholar] [CrossRef]
- Meng, Y.P.; Hu, Y.S.; Wei, M.; Wang, K.M.; Wang, Y.Y.; Wang, S.L.; Hu, Q.; Wei, H.; Zhang, Z.H. Amelioration of hyperuricemia by Lactobacillus acidophilus F02 with uric acid-lowering ability via modulation of NLRP3 inflammasome and gut microbiota homeostasis. J. Funct. Foods 2023, 111, 105903. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Chen, X.Y.; Zhang, L.; Meng, F.Q.; Zhou, L.B.; Lu, Z.X.; Lu, Y.J. Lacticaseibacillus rhamnosus Fmb14 ameliorates hyperuricemia-induced hepatocyte pyroptosis via NLRP3 inflammasome cascade inhibition. Food Sci. Hum. Wellness 2024, 13, 2174–2186. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Z.; Zhang, F.L.; Wang, B.; Wei, Z.J.; Liao, N.Q.; Zhang, M.; Liang, J.; Wang, L.S. Causal effects of gut microbiota on gout and hyperuricemia: Insights from genome-wide Mendelian randomization, RNA-sequencing, 16S rRNA sequencing, and metabolomes. Biosci. Rep. 2024, 44, BSR20240595. [Google Scholar] [CrossRef]
- Singh, A.K.; Durairajan, S.S.K.; Iyaswamy, A.; Williams, L.L. Elucidating the role of gut microbiota dysbiosis in hyperuricemia and gout: Insights and therapeutic strategies. World J. Gastroenterol. 2024, 30, 4404–4410. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Liu, L.D.; Zhu, J.Y.; Zhu, S.S.; Ye, B.Q.; Yang, J.L.; Huang, J.Y.; Huang, Z.H.; You, Y.; Li, W.K.; et al. Alistipes indistinctus-derived hippuric acid promotes intestinal urate excretion to alleviate hyperuricemia. Cell Host Microbe 2024, 32, 366–381.e9. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, D.; Li, D.; Bao, B.; Chen, Q.; Wang, S.; Han, S.; Zhao, M. Lactobacillus paracasei N1115 alleviates hyperuricemia in mice: Regulation of uric acid metabolism as well as its impact on gut microbiota and short-chain fatty acids. Front. Nutr. 2025, 12, 1651214. [Google Scholar] [CrossRef]
- Polesso, F.; Sarker, M.; Anderson, A.; Parker, D.C.; Murray, S.E. Constitutive expression of NF-κB inducing kinase in regulatory T cells impairs suppressive function and promotes instability and pro-inflammatory cytokine production. Sci. Rep. 2017, 7, 14779. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.S.; Xia, D.Y.; Luo, X.D.; Luo, H.T.; Pan, J.; Ma, W.Q.; Li, J.Z.; Mo, Q.Y.; Tu, Q.; et al. Lactobacillus rhamnosus GG ameliorates hyperuricemia in a novel model. npj Biofilms Microbiomes 2024, 10, 25. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. Isme J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Husejko, J.; Kozakiewicz, M.; Gackowski, M.; Mądra-Gackowska, K.; Wojtasik, J.; Strzała, D.; Pesta, M.; Ciesielska, J.; Ratajczak, D.; Kędziora-Kornatowska, K. Higher uric acid associated with elevated IL-6 and IL-1β levels in older inpatients: A cross-sectional study. Rheumatol. Int. 2025, 45, 177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).