Effects of Solid-State Fermentation with Eurotium cristatum on the Physicochemical, Sensory, and Volatile Profiles of Summer–Autumn Green Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Sampling
2.2. Starter Culture and Fermented SAGT Preparation
2.3. Measurement of Key Chemical Components in Tea Samples
High-Performance Liquid Chromatography (HPLC) Evaluation of Catechins
2.4. Sensory Assessment of Tea Samples
2.5. Measurements of the Overall Volatile Profiles and VOCs
2.5.1. Measurement of E-Nose
2.5.2. Measurement of VOCs Determined by GC-MS
2.5.3. Measurement of VOCs Determined by GC-IMS
2.6. Statistical Analysis
3. Results and Discussion
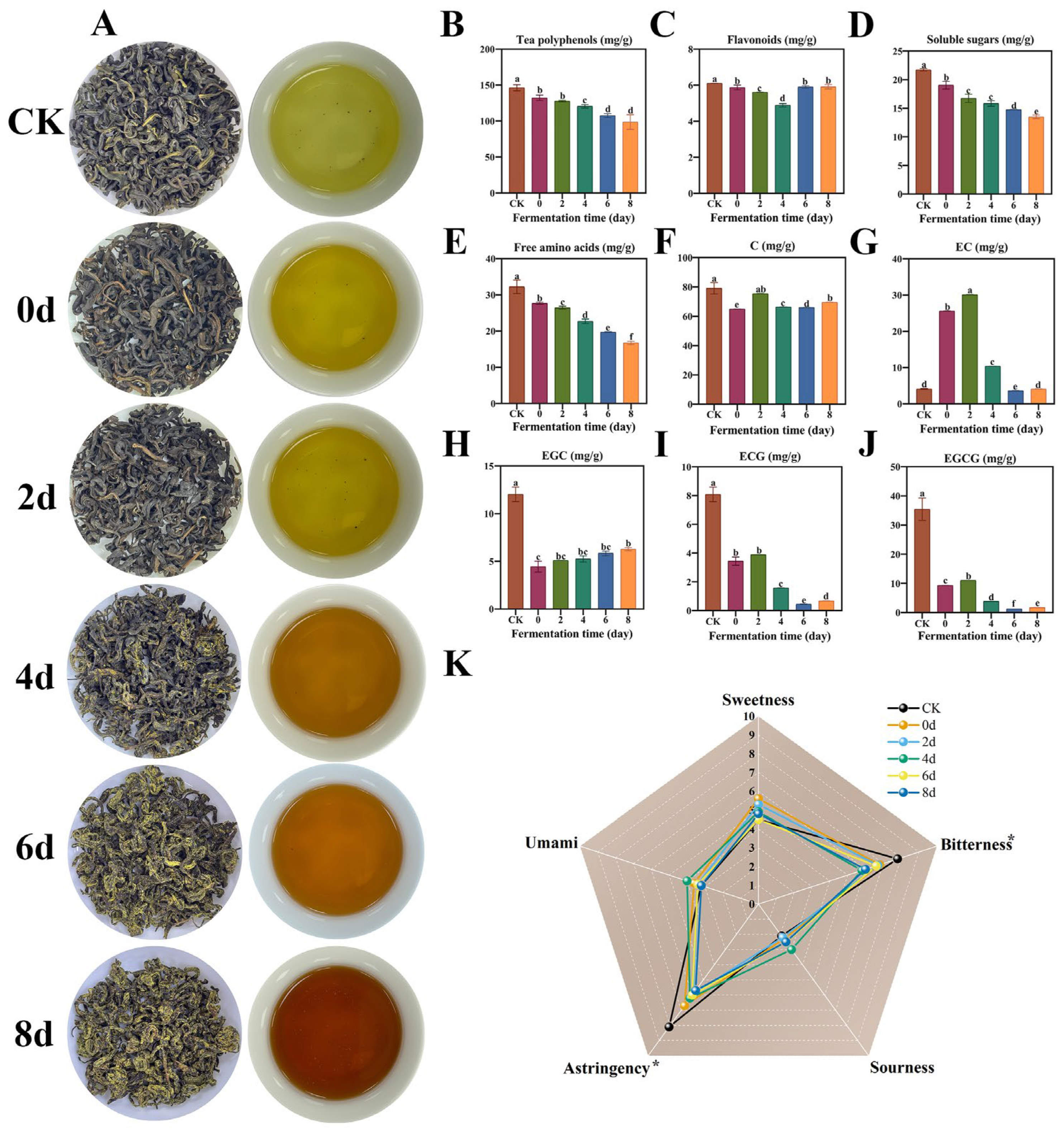
3.1. Variations in Physicochemical and Taste Properties of SAGT in the Progress of Fermentation
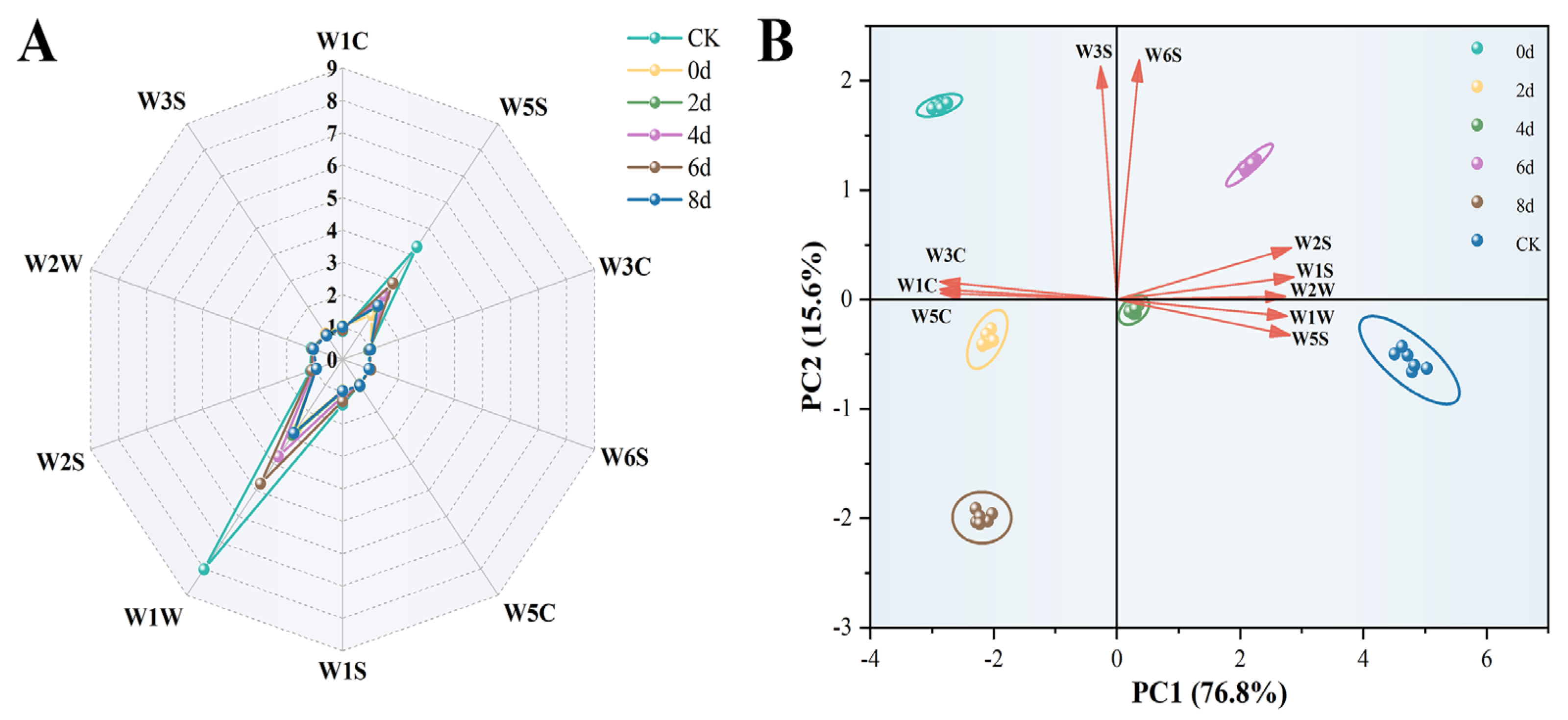
3.2. E-Nose Analysis
3.3. Assessment of VOCs Determined by GC-MS
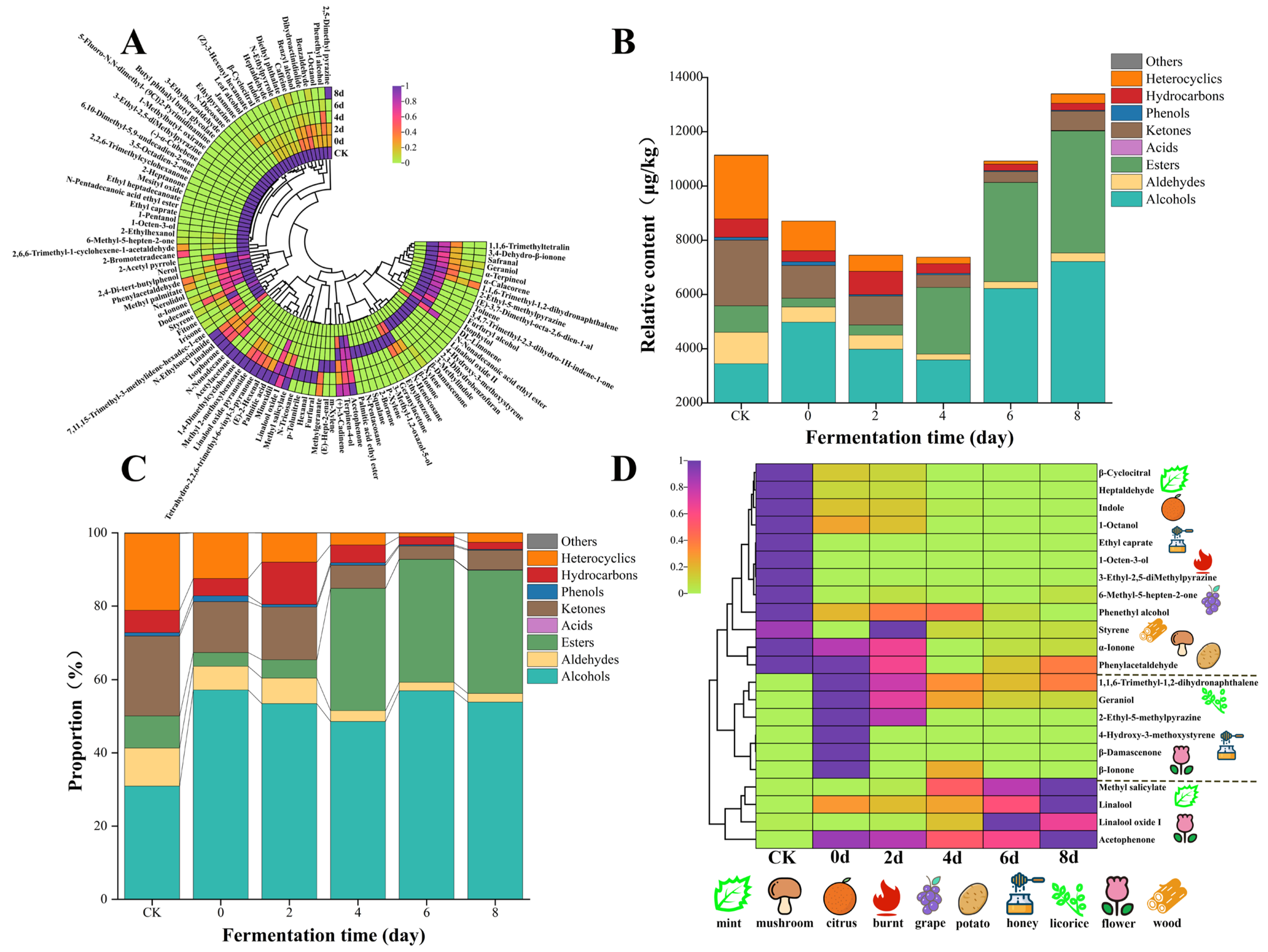
3.3.1. Changes in VOCs During the Progress of Fermentation
3.3.2. ROAV Analysis
3.4. Assessment of VOCs Determined by GC-IMS
3.5. Multivariate Statistical Analysis
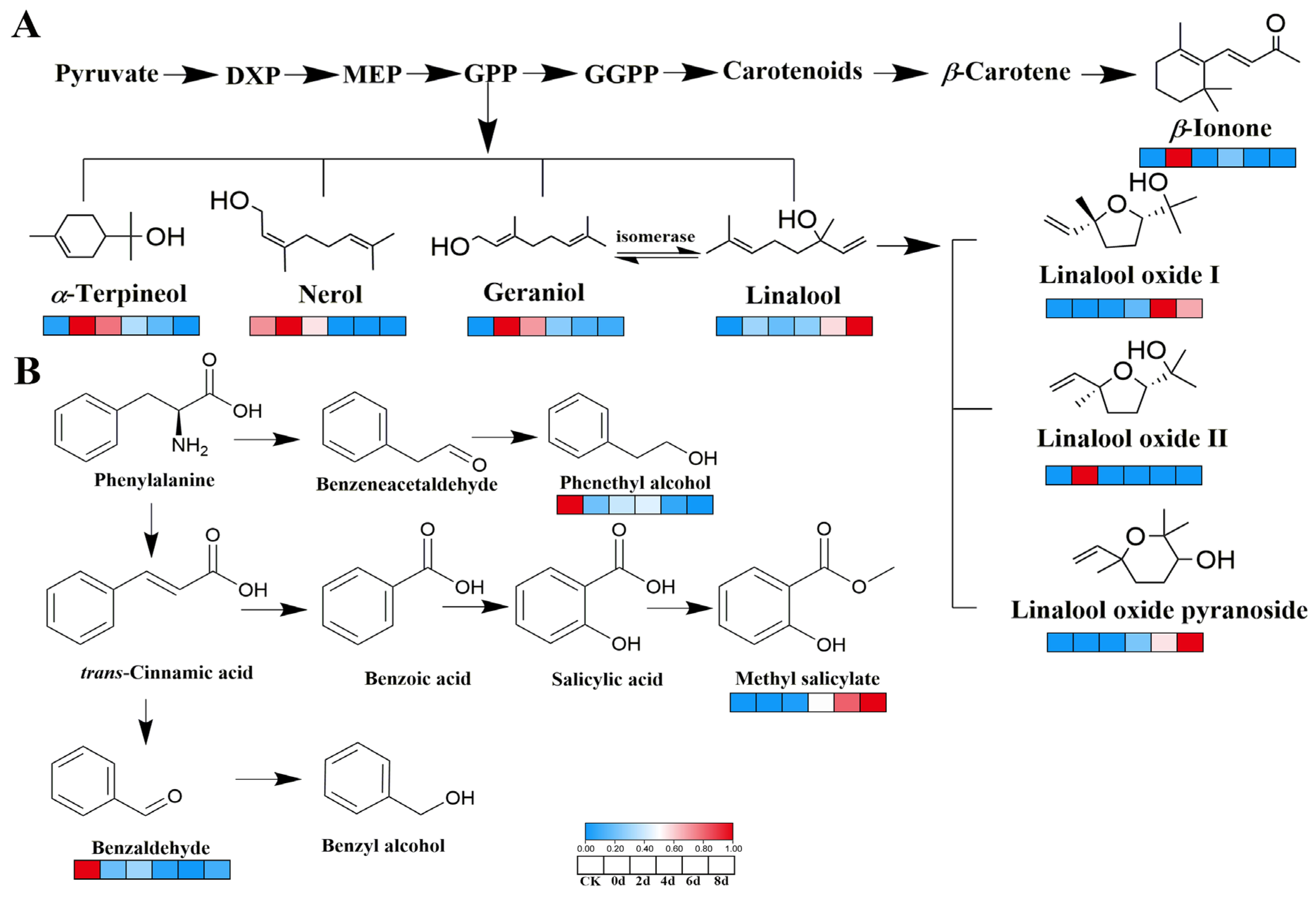
3.6. Metabolic Pathways Analysis of Key VOCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.H.; Zhang, Y.H.; Chen, G.S.; Yin, J.F.; Chen, J.X.; Wang, F.; Xu, Y.Q. Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. npj Sci. Food 2022, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.; Yang, Y.S.; Sun, J.; Dai, H.W.; Luo, Z.F.; Tong, H.R. Characterization of potential aroma compounds in five aroma types of green tea using the sensomics approach. LWT-Food Sci. Technol. 2025, 215, 117177. [Google Scholar] [CrossRef]
- Deng, S.J.; Zhang, G.; Aluko, O.O.; Mo, Z.J.; Mao, J.J.; Zhang, H.B.; Liu, X.H.; Ma, M.; Wang, Q.; Liu, H.B. Bitter and astringent substances in green tea: Composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Res. Int. 2022, 157, 111262. [Google Scholar] [CrossRef]
- Kaczyński, P.; Iwaniuk, P.; Jankowska, M.; Orywal, K.; Socha, K.; Perkowski, M.; Farhan, J.A.; Łozowicka, B. Pesticide residues in common and herbal teas combined with risk assessment and transfer to the infusion. Chemosphere 2024, 367, 143550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Liu, Y.; Xu, S.; Zhong, K.; Wu, Y.; Gao, H. The effect of Eurotium cristatum (MF800948) fermentation on the quality of autumn green tea. Food Chem. 2021, 358, 129848. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Zhu, X.Z.; Ouyang, W.; Chen, M.; Jiang, Y.W.; Wang, J.J.; Hua, J.J.; Yuan, H.B. Effects of electromagnetic roller-hot-air-steam triple-coupled fixation on reducing the bitterness and astringency and improving the flavor quality of green tea. Food Chem.-X 2023, 19, 100844. [Google Scholar] [CrossRef]
- Zheng, X.D.; Xu, S.S.; Yang, Z.C.; Sun, L.; Wu, X.F.; Mu, D.D.; Chen, X.S.; Li, X.J. Mechanisms of single and mixed microbial fermentation to improve summer-autumn green tea. Food Biosci. 2024, 61, 104830. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef]
- Pu, B.; Xu, Y.; Du, C.; Qin, B.; Cao, H.; You, Y. Analyze and Compared the Tea Polyphenol Contents and Caffeine in Different Varieties of Tea. Food Ind. 2017, 38, 301–303. [Google Scholar]
- Cheng, H.; He, W.; Zhao, L.; Hu, X.; Wu, J. Correlation between sensory attributes and chemical components of black and green tea. Trans. Chin. Soc. Agric. Eng. 2012, 28, 375–380. [Google Scholar]
- Liu, P.; Deng, Y.; Yin, J.; Zhang, Y.; Chen, G.; Wang, F.; Chen, J.; Yu, H.; Xu, Y. Quantitative Analysis of the Taste and Its Correlation Research of Chemical Constitutes of Green Tea. J. Chin. Inst. Food Sci. Technol. 2014, 14, 173–181. [Google Scholar]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, Y.; Wang, M.; Zhang, H. Detection of bitterness and astringency of green tea with different taste by electronic nose and tongue. PLoS ONE 2018, 13, e0206517. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y.; Ni, D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT-Food Sci. Technol. 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Miyashita, T.; Etoh, H. Improvement of the Bitterness and Astringency of Green Tea by Sub-Critical Water Extraction. Food Sci. Technol. Res. 2013, 19, 471–478. [Google Scholar] [CrossRef][Green Version]
- Xu, X.Y.; Meng, J.M.; Mao, Q.Q.; Shang, A.; Li, B.Y.; Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; et al. Effects of Tannase and Ultrasound Treatment on the Bioactive Compounds and Antioxidant Activity of Green Tea Extract. Antioxidants 2019, 8, 362. [Google Scholar] [CrossRef]
- Song, L.Y.; Ma, F.W.; Chen, H.J.; Fei, Q.; Tao, G.C.; Wu, S.Y.; Shi, D.J.; Deng, J.Y.; Zhao, D.G.; Dong, X.; et al. Dynamic changes in flavor characteristics of black tea during solid-state fermentation with Eurotium cristatum. Food Chem. 2025, 465, 142028. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Q.; Zou, C.; Zhang, Y.H.; Du, Q.Z.; Yin, J.F.; Shi, J.; Xue, S.; Xu, Y.Q. Improving the taste of autumn green tea with tannase. Food Chem. 2019, 277, 432–437. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Chen, Y.; Ho, C.; Wang, Y.; Cai, T.; Li, S.; Ma, J.; Guo, T.; Zhang, L.; et al. Effect of inoculation with different Eurotium cristatum strains on the microbial communities and volatile organic compounds of Fu brick tea. Food Res. Int. 2024, 197, 115219. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Zhang, J.Q.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT-Food Sci. Technol. 2020, 117, 108629. [Google Scholar]
- Liu, T.T.; Liu, X.T.; Huang, G.L.; Liu, L.; Chen, Q.X.; Wang, Q. Theophylline Extracted from Fu Brick Tea Affects the Metabolism of Preadipocytes and Body Fat in Mice as a Pancreatic Lipase Inhibitor. Int. J. Mol. Sci. 2022, 23, 2525. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Xiao, L.; Zhang, X.; Yang, C.; Li, Z.; Zhu, M.; Liu, Z.; Wang, Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS–SPME/GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar]
- Zhu, W.; Zhou, S.; Guo, H.W.; Hu, J.L.; Cao, Y.Y.; Xu, Y.X.; Lin, X.C.; Tian, B.M.; Fan, F.Y.; Gong, S.Y.; et al. Golden-flower fungus (Eurotiwm cristatum) presents fungal flower aroma as well as accelerates the aging of white tea (Shoumei). Food Chem. 2024, 451, 139452. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Zhu, M.; He, C.; Li, Z.; Wang, Y.; Liu, Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT-Food Sci. Technol. 2022, 169, 113925. [Google Scholar]
- Qadir, M.; Muhammad, T.; Bakri, M.; Gao, F. Determination of total polyphenols in tea by a flow injection-fiber optic spectrophotometric system. Instrum. Sci. Technol. 2018, 46, 185–193. [Google Scholar] [CrossRef]
- GB/T 8314-2013; Methodology for Sensory Evaluation of Tea. National Standard of the People’s Republic of China: Beijing China, 2013.
- Yu, J.; Liu, Y.; Zhang, S.; Luo, L.; Zeng, L. Effect of brewing conditions on phytochemicals and sensory profiles of black tea infusions: A primary study on the effects of geraniol and β-ionone on taste perception of black tea infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef]
- GB/T 8313-2018; Methodology for Sensory Evaluation of Tea. National Standard of the People’s Republic of China: Beijing China, 2018.
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. National Standard of the People’s Republic of China: Beijing China, 2018.
- Yang, X.; Liu, Y.; Mu, L.; Wang, W.; Zhan, Q.; Luo, M.; Tian, H.; Lv, C.; Li, J. Discriminant research for identifying aromas of non-fermented Pu-erh tea from different storage years using an electronic nose. J. Food Process. Preserv. 2018, 42, e13721. [Google Scholar]
- Li, H.H.; Luo, L.Y.; Wang, J.; Fu, D.H.; Zeng, L. Lexicon development and quantitative descriptive analysis of Hunan fuzhuan brick tea infusion. Food Res. Int. 2019, 120, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Gao, H. Insight into effects of isolated Eurotium cristatum from Pingwu Fuzhuan brick tea on the fermentation process and quality characteristics of Fuzhuan brick tea. J. Sci. Food Agric. 2020, 100, 3598–3607. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zu, Z.Q.; Shen, S.S.; An, T.T.; Zhang, H.W.; Lu, H.Q.; Fu, M.Y.; Wen, Y.; Chen, Q.; Gao, X.L. Dynamic changes in the metabolite profile and taste characteristics of loose-leaf dark tea during solid-state fermentation by Eurotium cristatum. LWT-Food Sci. Technol. 2023, 176, 114528. [Google Scholar] [CrossRef]
- Zheng, W.J.; Wan, X.C.; Bao, G.H. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Ye, J.H.; Ye, Y.; Yin, J.F.; Jin, J.; Liang, Y.R.; Liu, R.-Y.; Tang, P.; Xu, Y.Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Xiao, Y.; He, C.; Chen, Y.L.; Ho, C.T.; Wu, X.; Huang, Y.X.; Gao, Y.; Hou, A.X.; Li, Z.J.; Wang, Y.L.; et al. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef]
- Ma, Y.; Ling, T.J.; Su, X.Q.; Jiang, B.; Nian, B.; Chen, L.J.; Liu, M.L.; Zhang, Z.Y.; Wang, D.P.; Mu, Y.Y.; et al. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2021, 334, 127560. [Google Scholar] [CrossRef]
- Zhou, H.C.; Liu, Y.Q.; Wu, Q.; Zhang, X.L.; Wang, H.; Lei, P.D. The manufacturing process provides green teas with differentiated nonvolatile profiles and influences the deterioration of flavor during storage at room temperature. Food Chem.-X 2024, 22, 101371. [Google Scholar] [CrossRef]
- Jiang, G.X.; Xue, R.; Xiang, J.; Wang, Y.F.; Liu, B.; Yuan, Y.; Pu, Q.; Fang, X.; Hu, X.M.; Liu, X.Y.; et al. Dynamic changes in the aroma profiles and volatiles of Enshi Yulu tea throughout its industrial processing. Food Chem. 2024, 458, 140145. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.L.; Jiang, R.G.; Xu, Y.Q.; Zhang, Y.Y.; Luo, Y.; Huang, J.N.; Wang, K.B.; Liu, Z.H. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Ma, W.J.; Zhu, Y.; Shi, J.; Wang, J.T.; Wang, M.Q.; Shao, C.Y.; Yan, H.; Lin, Z.; Lv, H.P. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chem. 2021, 346, 128906. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mo, H.; Yan, M.; Zhu, Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. J. Sci. Food Agric. 2007, 87, 1502–1504. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.D.; Luo, Y.; Xiao, L.Z.; Wang, K.B.; Huang, J.N.; Liu, Z.H. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. LWT-Food Sci. Technol. 2020, 127, 109355. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Gok, R.; Selhorst, P.; Kiene, M.; Noske, T.; Ziegler, M.; Fischer, U.; Winterhalter, P. Target-Guided Isolation of Progenitors of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) from Riesling Wine by High-Performance Countercurrent Chromatography. Molecules 2022, 27, 5378. [Google Scholar] [CrossRef]
- Liu, N.F.; Shen, S.S.; Huang, L.F.; Deng, G.J.; Wei, Y.M.; Ning, J.M.; Wang, Y.J. Revelation of volatile contributions in green teas with different aroma types by GC-MS and GC-IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Yang, X.B.; Chen, Q.H.; Liu, S.C.; Hong, P.Z.; Zhou, C.X.; Zhong, S.Y. Characterization of the effect of different cooking methods on volatile compounds in fish cakes using a combination of GC-MS and GC-IMS. Food Chem.-X 2024, 22, 101291. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Li, L.H.; Wang, Y.Q.; Wu, Y.Y.; Li, C.S.; Chen, S.J.; Zhao, Y.Q.; Wang, D.; Xiang, H.; Wei, Y. Discrimination and characterization of volatile organic compound fingerprints during sea bass fermentation by combining GC-IMS and GC-MS. Food Biosci. 2022, 50, 102048. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Huang, Y.; Liu, C.; Yue, T.; Cao, W. Identification and biotransformation analysis of volatile markers during the early stage of Salmonella contamination in chicken. Food Chem. 2024, 431, 137130. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Wang, Y.D.; Zeng, H.; Qiu, S.Z.; Han, H.Y.; Wang, B. Identification of key aroma compounds and core functional microorganisms associated with aroma formation for Monascus-fermented cheese. Food Chem. 2024, 434, 137401. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Feng, X.Y.; Yang, G.; Peng, X.W.; Du, M.Y.; Song, J.; Kan, J.Q. Impact of aroma-enhancing microorganisms on aroma attributes of industrial Douchi: An integrated analysis using E-nose, GC-IMS, GC-MS, and descriptive sensory evaluation. Food Res. Int. 2024, 182, 114181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.X.; Wang, L.Q.; Yao, H.B.; Wang, J.; An, H.M.; Li, Q.; Wang, C.; Huang, J.A.; Liu, Z.H. Unraveling the unique profile of Fu brick tea: Volatile components, analytical approaches and metabolic mechanisms of key odor-active compounds. Trends Food Sci. Technol. 2025, 156, 104879. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Q.; Jiang, R.; Liu, Y.; Xie, H.; Ou, X.; Li, Q.; Liu, Z.; Huang, J. Characteristic volatiles of Fu brick tea formed primarily by extracellular enzymes during Aspergillus cristatus fermentation. Food Res. Int. 2024, 177, 113854. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, J.; Zhang, Z.; Tian, S.; Liu, Z.; Shen, C. Biochemical components and fungal community dynamics during the flowering process of Moringa-Fu brick tea, a novel microbially fermented blended tea. LWT-Food Sci. Technol. 2021, 140, 110822. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.Y.; Chen, M.X.; Li, M.R.; Zhang, H.W.; Song, P.P.; An, T.T.; Yue, P.X.; Gao, X.L. Influence of Eurotium cristatum and Aspergillus niger individual and collaborative inoculation on volatile profile in liquid-state fermentation of instant dark teas. Food Chem. 2021, 350, 129234. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, R.Y.; Chen, Y.L.; Ho, C.T.; Hou, A.X.; Zhang, X.L.; Zhu, M.Z.; Zhang, C.Y.; Wang, Y.L.; Liu, Z.H.; et al. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Biosci. 2023, 55, 585–599. [Google Scholar] [CrossRef]
- Nie, C.; Zhong, X.; He, L.; Gao, Y.; Zhang, X.; Wang, C.; Du, X. Comparison of different aroma-active compounds of Sichuan Dark brick tea Camellia sinensis and Sichuan Fuzhuan brick tea using gas chromatography-mass spectrometry (GC-MS) and aroma descriptive profile tests. Eur. Food Res. Technol. 2019, 245, 1963–1979. [Google Scholar] [CrossRef]
- Xu, S.; Song, L.Y.; Shi, D.J.; Wu, S.Y.; Ma, F.W.; Chen, H.J.; Meng, Q.L.; Fei, Q.; Meng, L.S.; Wu, W.N.; et al. Dynamic variations in flavor profiles of Guizhou high-mountain white tea produced by Eurotium cristatum using solid-state fermentation. Food Biosci. 2025, 68, 106725. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Song, L.; Zhao, Y.; Zhao, D. Effects of Solid-State Fermentation with Eurotium cristatum on the Physicochemical, Sensory, and Volatile Profiles of Summer–Autumn Green Tea. Foods 2025, 14, 3681. https://doi.org/10.3390/foods14213681
Xu S, Song L, Zhao Y, Zhao D. Effects of Solid-State Fermentation with Eurotium cristatum on the Physicochemical, Sensory, and Volatile Profiles of Summer–Autumn Green Tea. Foods. 2025; 14(21):3681. https://doi.org/10.3390/foods14213681
Chicago/Turabian StyleXu, Su, Linyao Song, Yichen Zhao, and Degang Zhao. 2025. "Effects of Solid-State Fermentation with Eurotium cristatum on the Physicochemical, Sensory, and Volatile Profiles of Summer–Autumn Green Tea" Foods 14, no. 21: 3681. https://doi.org/10.3390/foods14213681
APA StyleXu, S., Song, L., Zhao, Y., & Zhao, D. (2025). Effects of Solid-State Fermentation with Eurotium cristatum on the Physicochemical, Sensory, and Volatile Profiles of Summer–Autumn Green Tea. Foods, 14(21), 3681. https://doi.org/10.3390/foods14213681





