Technological Assessment of Bread with the Addition of Cyperus esculentus L. Accessions Flour Grown in the Kuban–Azov Plain
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Plant Material
2.1.2. Bread Material
2.2. Biochemical Analysis
2.2.1. Preparation of Plant Material for Study
2.2.2. Protein Content Analysis
2.2.3. Oil Content Analysis
2.2.4. Starch Content Analysis
2.2.5. Fiber Content Analysis
2.2.6. Dry Matter Content (Moisture) Analysis
2.2.7. Total Phenolic Compounds Analysis
2.2.8. Antioxidant Activity Analysis
2.3. Technological Assessment
2.3.1. Rheological Assessment
Farinographic Evaluation
- Sample Preparation
- Farinographic Indicators Evaluation
Viscoamylographic Assessment
- Sample Preparation
- Viscoamylographic Indicators Evaluation
2.3.2. Sedimentation Analysis (Swelling Capacity of Flour)
Sample Preparation
Sedimentation Indicators Evaluation
2.3.3. Flour Color Evaluation
2.3.4. Baking Evaluation
Bread Sample Preparation
Baking Indicators Evaluation
2.4. Statistical Processing
3. Results and Discussion
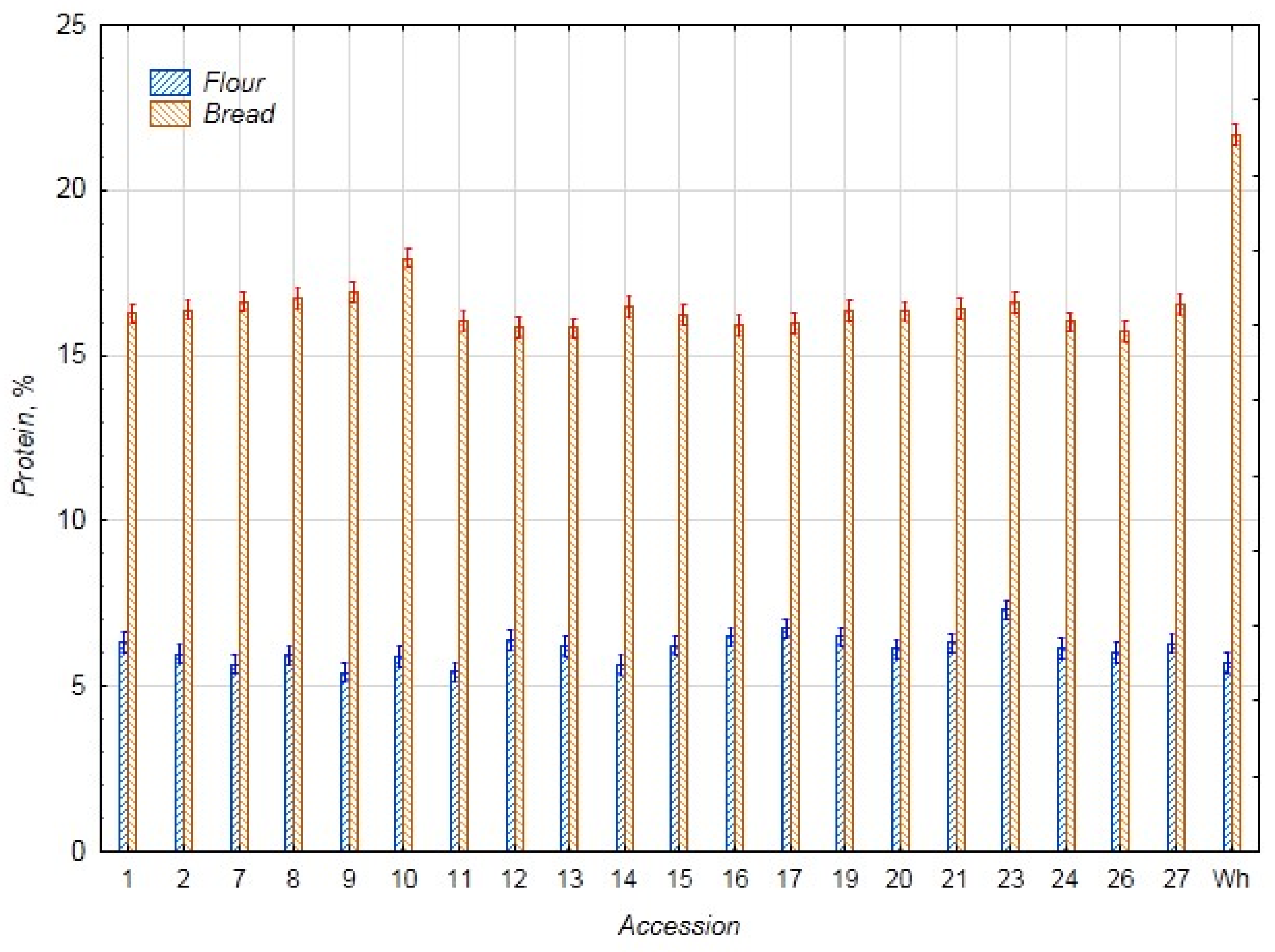
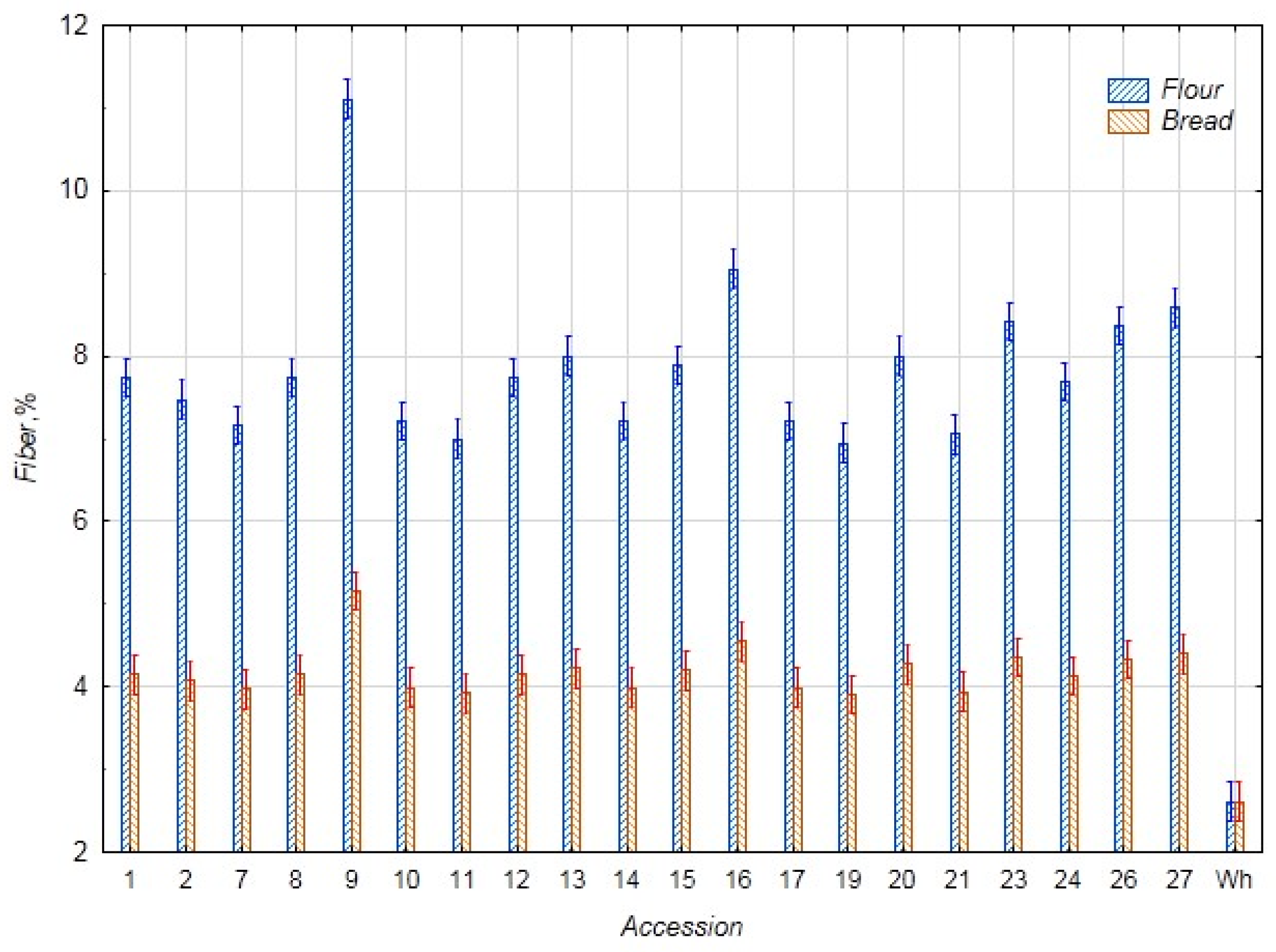
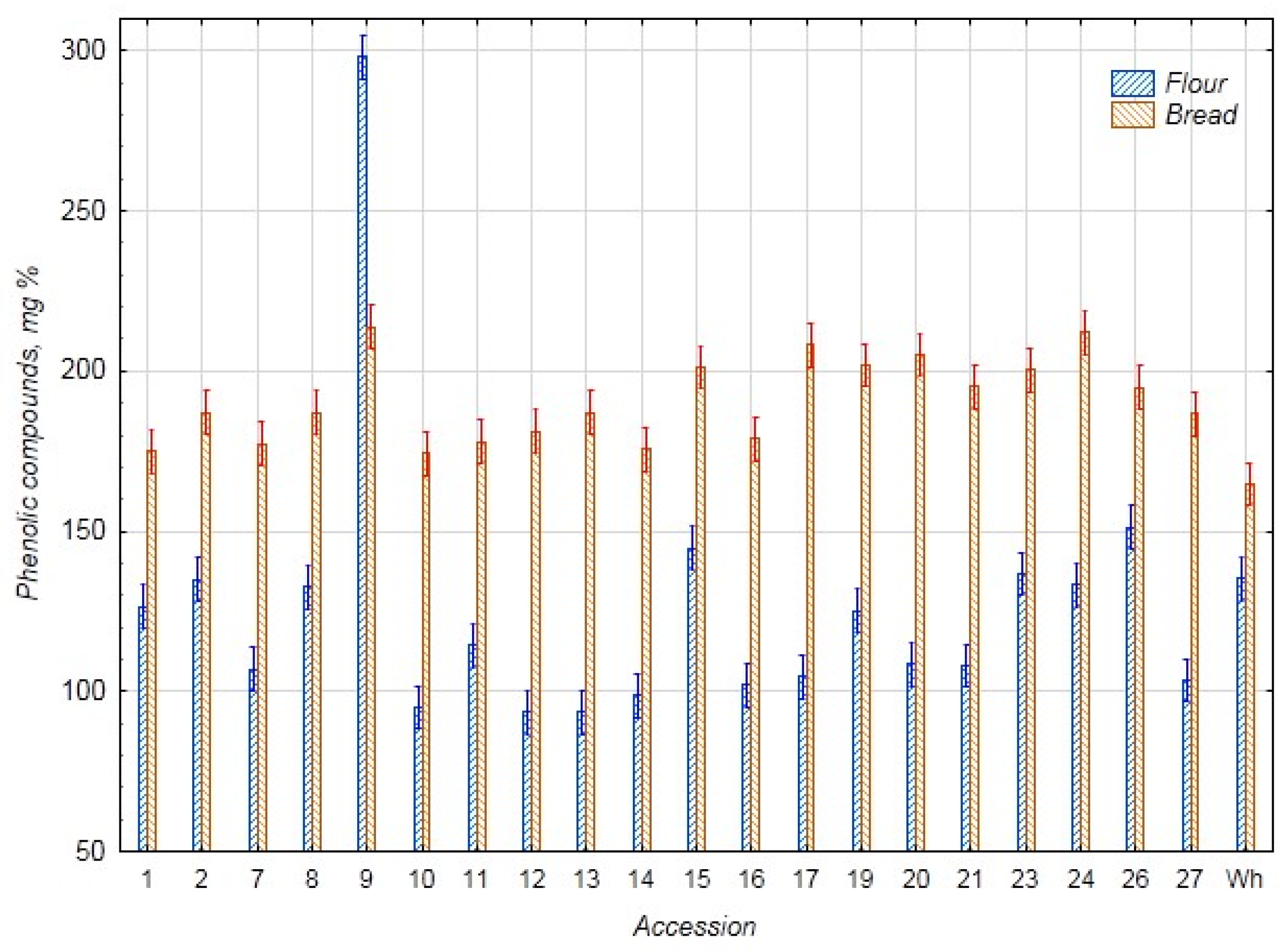
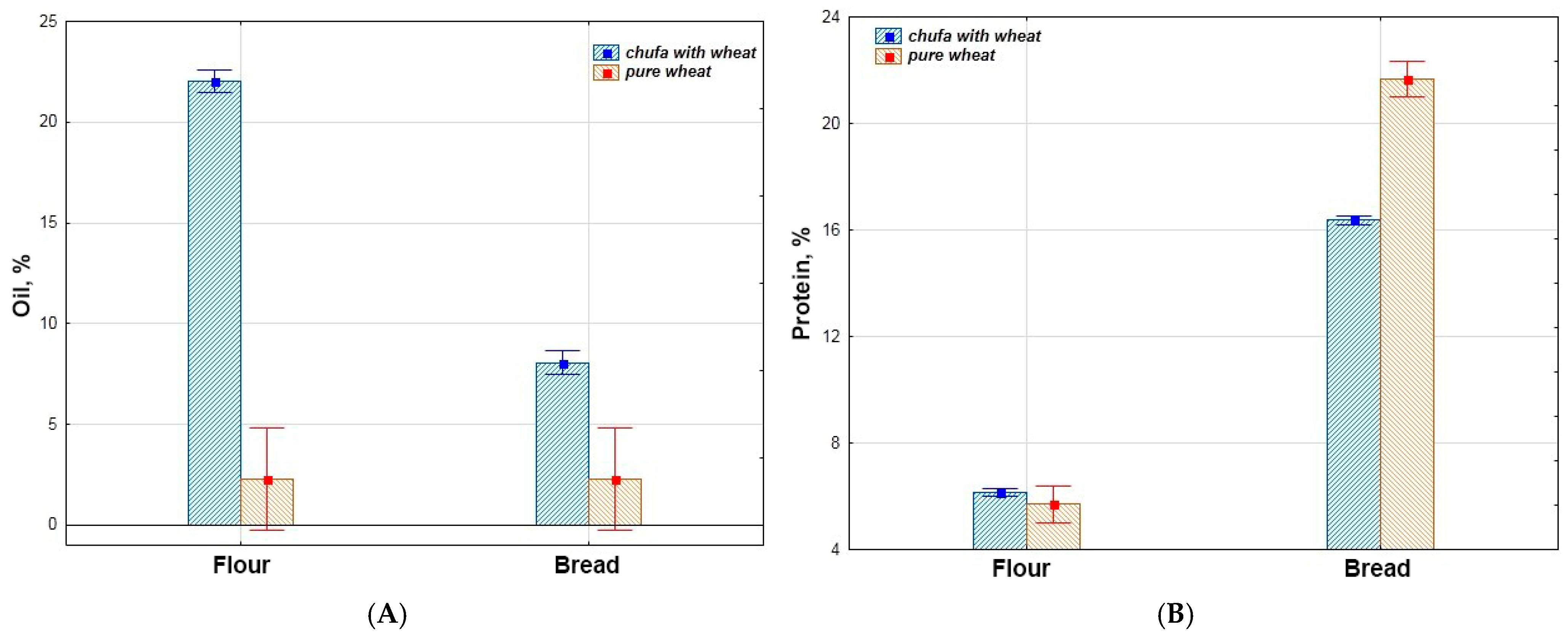
3.1. Biochemical Assessment
3.2. Statistical Evaluation of Biochemical Parameters
3.2.1. Comparative Analysis of the Biochemical Parameters
3.2.2. Correlation Analysis
3.2.3. Analysis of Variance
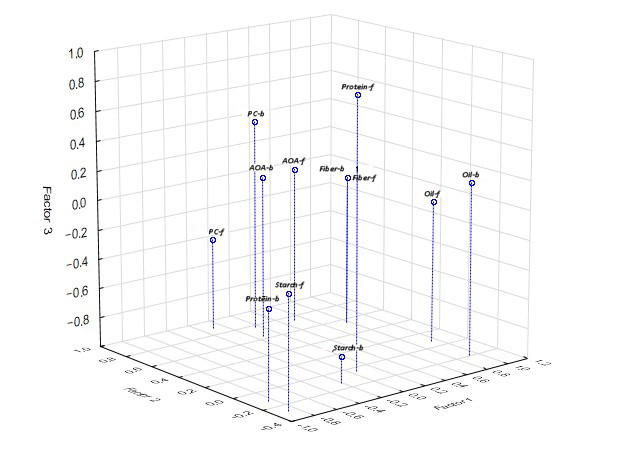
3.2.4. Factorial Analysis
3.3. Technological Assessment Indicators
3.3.1. Sedimentation Analysis
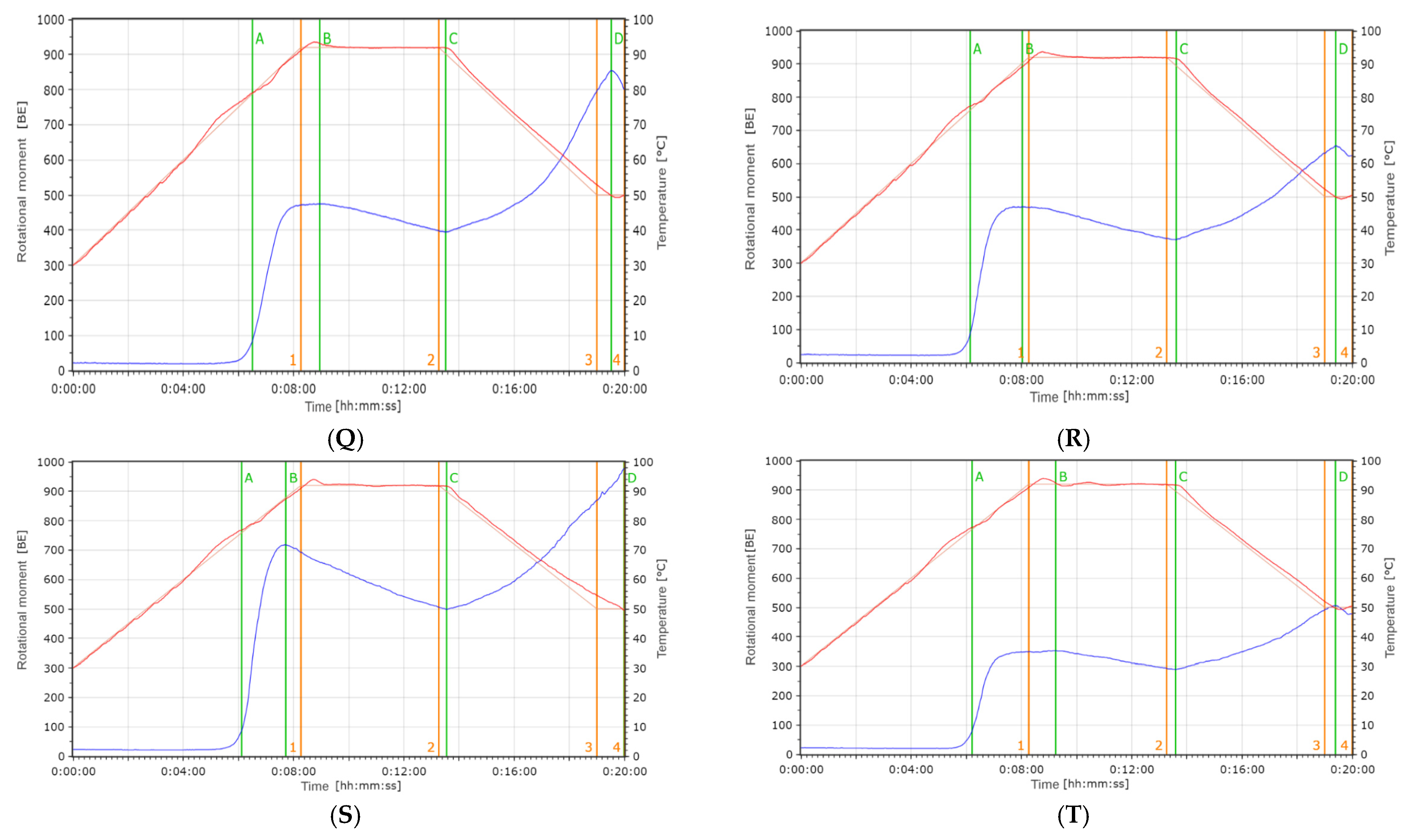
3.3.2. Rheological Assessment Indicators
Viscoamylographic Visco-Mylographic
Farinographic Studies
3.3.3. Evaluation of Flour Color Indicators
3.3.4. Baking Quality
3.4. Results of Statistical Analysis of Technological Assessment
3.4.1. Correlation Analysis of Technological Assessment Parameters
3.4.2. Factor Analysis
3.4.3. Discriminant Analysis by Origin
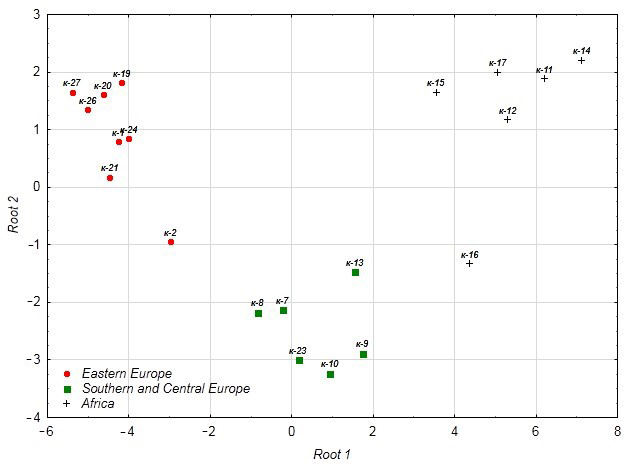
3.4.4. Canonical Analysis
3.4.5. Discriminant Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascual-Seva, N.; Pascual, B. Determination of crop coefficient for chufa crop (Cyperus esculentus L. var. sativus Boeck.) for sustainable irrigation scheduling. Sci. Total Environ. 2021, 768, 144975. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Cheng, Z.; Sun, Y.; Li, L.; Wang, J. Improving Tuber Yield of Tiger Nut (Cyperus esculentus L.) through Nitrogen Fertilization in Sandy Farmland. Plants 2024, 13, 1063. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger nut (Cyperus esculentus L.): Nutrition, processing, function and applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef]
- Kon’kova, N.G.; Safina, G.F. Valuable agronomic traits of chufa (Cyperus esculentus L.) accessions from the VIR collection: Methods of preparing nodules for long-term storage. Proc. Appl. Bot. Genet. Breed. 2021, 182, 34–44. [Google Scholar] [CrossRef]
- Kon’kova, N.G.; Khoreva, V.I.; Popov, V.S.; Yakusheva, T.V.; Malyshev, L.L.; Solovyeva, A.E.; Shelenga, T.V. Variability of the Main Economically Valuable Characteristics of Cyperus esculentus L. in Various Ecological and Geographical Conditions. Plants 2024, 13, 308. [Google Scholar] [CrossRef]
- Zommara, M.A.; Swelam, S.; Raya-Álvarez, E.; Imaizumi, K.; Elmahdy, A.; Alkhudhayri, D.A.; Aljehani, A.A.; Agil, A.; Elmahallawy, E.K. Nutritional and potential health benefits of chufa oil, olive oil, and anhydrous milk fat against gallstone disease in a C57BL/6N mouse model. Front. Nutr. 2024, 11, 1445484. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S. Tiger nut (Cyperus esculentus L.) oil: A review of bioactive compounds, extraction technologies, potential hazards and applications. Food Chem. 2023, 19, 100868. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.S.; Olajide, J.E.; Omale, J.A.; Abbah, O.C.; Ejembi, D.O. Proximate composition, mineral and some vitamin contents of tiger nut (Cyperus esculentus). Clin. Investig. 2018, 8, 161–165. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Olivas, E.; Asensio-Grau, A.; Calvo-Lerma, J.; García-Hern’andez, J.; Heredia, A.; Andrés, A. Content and bioaccessibility of bioactive compounds with potential benefits for macular health in tiger nut products. Food Biosci. 2022, 49, 101879. [Google Scholar] [CrossRef]
- Sanchez-Zapata, E.; Fernandez-Lopez, J.; Sendra, E.; Perez-Alvarez, J.A. Tigernut (Cyperus esculentus) commercialization: Health aspects, composition, properties, and food applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Ani, I.F.; Adeoya, B.K.; Ngori, E.O.; Kehinde, Z.A. Nutritional Composition and Quality Acceptability of Soft Candy (Toffee) Made from Tiger nut. Int. J. Res. Innov. Appl. Sci. 2019, 4, 1–4. [Google Scholar]
- Onwuzuruike, U.A.; Ukegbu, P.; Obasi, N.E.; Ogah, M.; Okereke, I.; Uche, P.C.; Echendu, C. Physical Properties, Dietary Fibre Profile and Peroxide Value of Biscuits Produced from Wheat-Tigernut Flours with Avocado Paste as A Fat Substitute. Indones. Food Sci. Technol. J. 2023, 7, 67–74. [Google Scholar] [CrossRef]
- Kwaghsende, G.S.; Ikala, G.U.; Ochelle, P.O. Quality evaluation of biscuit from wheat-tigernut composite flour. Int. J. Agric. Nutr. 2019, 1, 24–29. [Google Scholar] [CrossRef]
- Akajiaku, L.O.; Kabuo, N.O.; Alagbaoso, S.O.; Orji, I.G.; Nwogu, A.S. Proximate, mineral and sensory properties of cookies made from Tiger-Nut Flour. J. Nutr. Diet. Pract. 2018, 2, 1–5. [Google Scholar]
- Chinma, C.E.; Avu, J.O.; Abubakar, Y.A. Effect of tigernut (Cyperus esculentus) flour addition on the quality of wheat-based cake. Int. J. Food Sci. Technol. 2010, 45, 1746–1752. [Google Scholar] [CrossRef]
- Martín-Esparza, E.; González-Martínez, C. Horchata de chufa: A traditional Spanish beverage with exceptional organoleptic, nutritive, and functional attributes. Funct. Prop. Tradit. Foods 2016, 12, 371–375. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M.; Cantavella-Ferrero, Y. Enhancing Bioactive Antioxidants’ Extraction from “Horchata de Chufa” By-Products. Foods 2018, 7, 161. [Google Scholar] [CrossRef]
- Rios, M.; Tinitana, F.; Jarrín, Р.; Donoso, N.; Romero-Benavides, J.С. “Horchata” drink in Southern Ecuador: Medicinal plants people’s well-being. J. Ethnobiol. Ethnomed. 2017, 13, 18. [Google Scholar] [CrossRef]
- Orhevba, B.A.; Bankole, O.S. Effect of process treatments on the proximate composition of tigernut-soy milk blends. Afr. J. Food Sci. 2019, 13, 261–280. [Google Scholar] [CrossRef]
- Adebayo-Oyetoro, A.O.; Ogundipe, O.O.; Adeyeye, S.A.O.; Akande, E.A.; Akinyele, A.B. Production and evaluation of tiger nut (Cyperus esculentus) milk flavoured with moringa oleifera leaf extract. Curr. Res. Nutr. Food Sci. 2019, 7, 265–271. [Google Scholar] [CrossRef]
- Sobhy, H.; El-Nawawy, M.; Hegazi, N.; El-Dieb, S.; Abd El-Razik, F.; Elkashef, H. Tiger nut tuber milk: Using dairy byproducts and probiotic bacteria. Acta Sci. Pol. Technol. Aliment. 2022, 21, 271–280. [Google Scholar] [CrossRef]
- Madsen, M.; Thulesen, E.; Mohammadifar, M.; Bang-Berthelsen, C. Chufa drink: Potential in developing a new plant-based fermented dessert. Foods 2021, 10, 3010. [Google Scholar] [CrossRef]
- Özcan, M.M. Quality Evaluation of Bread Prepared from Wheat–Chufa Tuber Composite Flour. Foods 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, N.; Albanell, E.; Minarro, B.; Guamis, B.; Marta Capellas, M. Effect of tiger nut-derived products in gluten-free batter and bread. Food Sci. Technol. Int. 2014, 1, 323–331. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, nu-merical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, E.E.; Anisimova, I.N.; Ryazanova, M.K.; Kibkalo, I.A.; Alpatieva, N.V. Newly Developed Restorer Lines of Sorghum [Sorghum bicolor (L.) Moench] Resistant to Green-bug. Plants 2024, 13, 425. [Google Scholar] [CrossRef]
- Pozdnyakov, V.V.; Vasilenko, A.A. Use of test systems for evaluation of total antioxidant activity of seeds. Plant Breed. Seed Prod. 2017, 112, 153–163. [Google Scholar] [CrossRef]
- Shaban, H.H.; El-Hadidy, G.S.; Hamouda, A.M. Evaluation of Breadsticks Prepared from Chufa Tubers as Partial Substitute of Wheat Flour. Asian J. Food Res. Nutr. 2023, 2, 73–84. [Google Scholar] [CrossRef]
- Kibkalo, I. Effectiveness of and perspectives for the sedimentation analysis method in grain quality evaluation in various cere-al crops for breeding purposes. Plants 2022, 11, 1640. [Google Scholar] [CrossRef]
- Kibkalo, I.A.; Loskutov, I.G.; Voitsutskaya, N.P.; Solovyova, M.V.; Obukhova, N.S.; Blinova, E.V. Development of methodo-logical approaches to assessing the technological properties of oat grain. Plant Biotechnol. Breed. 2024, 7, 6–15. (In Russian) [Google Scholar] [CrossRef]
- Chernykh, V.Y.; Kostyuchenko, M.N.; Baluyan, K.A.; Smetanin, D.O.; Kandrokov, R.K. Reducing the staling rate of bakery products made from wheat flour. Polzunovsky Bull. 2024, 2, 96–106. (In Russian) [Google Scholar] [CrossRef]
- Anisimova, L.V.; Serebrennikova, E.S.; Bondarenko, V.E.; Basov, V.Y. Rheological properties of dough from a mixture of wheat and lupin flour. Polzunovsky Bull. 2018, 4, 40–44. (In Russian) [Google Scholar] [CrossRef]
- Chikpah, S.K.; Korese, J.K.; Hensel, O.; Sturm, B.; Pawelzik, E. Rheological properties of dough and bread quality characteristics as influenced by the proportion of wheat flour substitution with orange-fleshed sweet potato flour and baking conditions. LWT 2021, 147, 111515. [Google Scholar] [CrossRef]
- Anisimova, L.V.; Soltan, O. Rheological properties of dough from a mixture of wheat and whole oat flour. Polzunovsky Bull. 2017, 3, 9–13. (In Russian) [Google Scholar]
- Diosi, G.; More, M.; Sipos, P. Role of the farinograph test in the wheat our quality determination. Acta Univ. Sapientiae Aliment. 2015, 8, 104–110. [Google Scholar] [CrossRef]
- Gómez, A.; Ferrero, C.; Calvelo, A.; Añón, M.C.; Puppo, M.C. Effect of Mixing Time on Structural and Rheological Properties of Wheat Flour Dough for Bread making. Int. J. Food Prop. 2011, 14, 583–598. [Google Scholar] [CrossRef]
- Shteinberg, T.S.; Shvedova, O.G.; Kandrokov, R.K.; Bolotov, V.I. Selection of color characteristics for the development of a method for identifying the admixture of soft wheat flour in durum wheat flour. Bull. AGAU 2017, 12, 175–181. (In Russian) [Google Scholar]
- Mcguire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Sammalisto, S.; Laitinen, M.; Sontag-Strohm, T. Baking quality assessment of twenty whole grain oat cultivar samples. Foods 2021, 10, 2461. [Google Scholar] [CrossRef]
- Efremova, E.N. Quality evaluation of bakery products. Agrar. Bull. North Cauc. 2016, 3, 20–24. (In Russian) [Google Scholar]
- Nashat, S.; Abdullah, M.Z. In Computer Vision Technology for Food Quality Evaluation, Sun., D.-W., Ed.; Quality Evaluation of Bakery Products, 2nd ed.; Academic Press: Penang, Malaysia, 2016; pp. 525–589. [Google Scholar] [CrossRef]









| Сatalog Number | Origin |
|---|---|
| 1 | Russia |
| 2 | Russia |
| 7 | Poland |
| 8 | Bulgaria |
| 9 | Bulgaria |
| 10 | Bulgaria |
| 11 | Mali |
| 12 | Benin |
| 13 | Germany |
| 14 | Ivory Coast |
| 15 | Ivory Coast |
| 16 | Ivory Coast |
| 17 | Ivory Coast |
| 19 | Russia |
| 20 | Ukraine |
| 21 | Belarus |
| 23 | France |
| 24 | Ukraine |
| 26 | Russia |
| 27 | Russia |
| Level | Flour | Bread | ||||||
|---|---|---|---|---|---|---|---|---|
| Chufa | Wheat | Chufa 30%/Wheat 70% | Wheat 100% | |||||
| X | Sx | X | Sx | X | Sx | X | Sx | |
| Oil, % | 22.0 | 0.39 | 2.3 | 0.02 | 8.1 | 0.12 | 2.3 | 0.00 |
| Protein, % | 6.2 | 0.07 | 5.7 | 0.00 | 16.4 | 0.08 | 21.7 | 0.08 |
| Starch, % | 24.7 | 0.25 | 54.4 | 0.57 | 42.7 | 0.70 | 52.7 | 0.00 |
| Fiber, % | 7.9 | 0.15 | 2.6 | 0.00 | 4.2 | 0.05 | 2.6 | 0.00 |
| Phenolic substances, mg% | 125.6 | 6.96 | 135.2 | 0.35 | 191.0 | 2.07 | 164.8 | 2.95 |
| Antioxidant activity, mg% | 19.6 | 0.78 | 1.9 | 0.09 | 22.3 | 0.39 | 20.6 | 0.35 |
| Oil | Protein | Starch | Fiber | PC | АОА | B/Oil | B/Pr | B/St | B/F | B/PC | B/АОА | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil | 1.000 | 0.068 | −0.826 | 0.728 | −0.001 | 0.453 | 0.800 | −0.811 | −0.136 | 0.728 | 0.213 | 0.189 |
| Protein | 0.771 | 1.000 | −0.134 | 0.124 | −0.313 | 0.036 | 0.201 | −0.295 | −0.459 | 0.125 | 0.312 | −0.004 |
| Starch | 0.000 | 0.563 | 1.000 | −0.784 | −0.069 | −0.698 | −0.766 | 0.887 | 0.490 | −0.783 | −0.469 | −0.285 |
| Fiber | 0.000 | 0.593 | 0.000 | 1.000 | 0.443 | 0.775 | 0.568 | −0.708 | −0.252 | 1.000 | 0.524 | 0.315 |
| PC | 0.995 | 0.167 | 0.767 | 0.044 | 1.000 | 0.564 | −0.379 | 0.112 | 0.172 | 0.439 | 0.468 | 0.429 |
| АОА | 0.039 | 0.878 | 0.000 | 0.000 | 0.008 | 1.000 | 0.384 | −0.509 | −0.249 | 0.774 | 0.722 | 0.389 |
| B/Oil | 0.000 | 0.383 | 0.000 | 0.007 | 0.090 | 0.086 | 1.000 | −0.784 | −0.277 | 0.568 | 0.087 | 0.014 |
| B/Pr | 0.000 | 0.194 | 0.000 | 0.000 | 0.628 | 0.019 | 0.000 | 1.000 | 0.534 | −0.708 | −0.433 | −0.224 |
| B/St | 0.557 | 0.037 | 0.024 | 0.269 | 0.456 | 0.277 | 0.225 | 0.013 | 1.000 | −0.253 | −0.404 | −0.177 |
| B/F | 0.000 | 0.589 | 0.000 | 0.000 | 0.047 | 0.000 | 0.007 | 0.000 | 0.269 | 1.000 | 0.527 | 0.318 |
| B/PC | 0.353 | 0.169 | 0.032 | 0.015 | 0.032 | 0.000 | 0.708 | 0.050 | 0.069 | 0.014 | 1.000 | 0.435 |
| B/АОА | 0.412 | 0.987 | 0.210 | 0.165 | 0.052 | 0.081 | 0.952 | 0.329 | 0.442 | 0.160 | 0.049 | 1.000 |
| Sign | Reliability (p) | (η2) The Proportion of The Source’s Influence | |||||
|---|---|---|---|---|---|---|---|
| Variant | Culture | Variant*Culture | Variant | Culture | Variant*Culture | У | |
| Oil, % | 0.000 | 0.000 | 0.000 | 14.8 | 49.6 | 14.8 | 20.8 |
| Protein, % | 0.000 | 0.000 | 0.000 | 90.0 | 3.1 | 4.4 | 2.6 |
| Starch, % | 0.000 | 0.000 | 0.000 | 8.5 | 50.4 | 12.5 | 28.7 |
| Fiber, % | 0.000 | 0.000 | 0.000 | 11.8 | 40.9 | 11.8 | 35.5 |
| Phenolic substances, mg% | 0.005 | 0.613 | 0.279 | 9.3 | 0.3 | 1.3 | 89.1 |
| Antioxidant activity, mg% | 0.000 | 0.000 | 0.000 | 19.5 | 16.2 | 11.0 | 53.4 |
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| Oil | 0.937 | 0.068 | −0.034 |
| Protein | 0.051 | −0.044 | 0.828 |
| Starch | −0.878 | −0.286 | −0.237 |
| Fiber | 0.768 | 0.555 | 0.022 |
| PC | −0.098 | 0.885 | −0.375 |
| АОА | 0.510 | 0.746 | 0.061 |
| B/Oil | 0.913 | −0.206 | 0.176 |
| B/Pr | −0.856 | −0.131 | −0.387 |
| B/St | −0.215 | −0.140 | −0.815 |
| B/F | 0.768 | 0.555 | 0.024 |
| B/PC | 0.169 | 0.774 | 0.432 |
| B/АОА | 0.070 | 0.640 | 0.101 |
| The variance proportion by factor | 39.6 | 26.1 | 16.1 |
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | Factor 8 | |
|---|---|---|---|---|---|---|---|---|
| Oil | 0.648 | −0.121 | −0.263 | −0.407 | −0.357 | −0.130 | −0.252 | −0.007 |
| Protein | −0.403 | 0.261 | 0.523 | −0.349 | −0.290 | 0.259 | −0.098 | 0.125 |
| Starch | −0.101 | −0.088 | −0.199 | −0.621 | −0.071 | 0.212 | −0.048 | 0.028 |
| Fiber | −0.628 | 0.153 | −0.652 | 0.054 | 0.124 | 0.013 | −0.110 | −0.081 |
| PC | −0.454 | 0.215 | −0.140 | 0.509 | −0.028 | −0.003 | 0.549 | −0.177 |
| АОА | −0.275 | 0.215 | 0.113 | 0.257 | 0.080 | 0.258 | 0.025 | −0.718 |
| WAC | 0.191 | 0.569 | −0.359 | −0.216 | −0.150 | −0.135 | 0.593 | −0.132 |
| DDT | 0.017 | 0.307 | 0.210 | −0.051 | 0.169 | 0.889 | 0.005 | 0.009 |
| DS | −0.169 | 0.319 | −0.013 | −0.070 | 0.258 | 0.809 | 0.079 | −0.120 |
| DD | 0.361 | −0.362 | −0.010 | −0.363 | −0.413 | 0.049 | 0.073 | 0.217 |
| VN | −0.105 | 0.375 | 0.116 | −0.037 | 0.213 | 0.871 | −0.000 | −0.020 |
| PSC1 | 0.263 | −0.054 | 0.233 | 0.038 | 0.033 | 0.042 | 0.896 | −0.116 |
| PSC2 | 0.221 | 0.140 | 0.482 | 0.190 | 0.097 | 0.063 | 0.684 | 0.331 |
| P2-1 | 0.041 | 0.323 | 0.496 | 0.255 | 0.124 | 0.054 | −0.059 | 0.696 |
| PT | 0.110 | −0.507 | 0.549 | 0.043 | 0.570 | 0.112 | 0.193 | −0.051 |
| IPV | 0.363 | 0.174 | −0.033 | 0.257 | −0.628 | −0.030 | 0.205 | −0.340 |
| PTS | 0.010 | −0.464 | 0.684 | 0.051 | 0.503 | 0.075 | 0.150 | −0.107 |
| TPV | −0.055 | 0.223 | 0.155 | −0.200 | 0.799 | 0.288 | 0.141 | 0.197 |
| PVHS | 0.050 | −0.764 | 0.011 | 0.107 | −0.532 | −0.208 | −0.157 | 0.101 |
| PVT | −0.098 | 0.208 | −0.178 | 0.131 | 0.886 | 0.135 | 0.027 | 0.031 |
| MHPV | 0.034 | −0.882 | −0.071 | 0.093 | −0.307 | −0.196 | −0.211 | 0.096 |
| CPPV | 0.056 | −0.959 | −0.068 | −0.001 | −0.176 | −0.188 | −0.057 | 0.009 |
| VEM | 0.080 | −0.934 | −0.114 | 0.001 | −0.236 | −0.194 | −0.095 | 0.004 |
| VDPT | 0.073 | −0.328 | 0.084 | 0.083 | −0.833 | −0.101 | 0.010 | 0.169 |
| VIUC | 0.066 | −0.967 | −0.063 | −0.055 | −0.093 | −0.176 | 0.034 | −0.042 |
| VIHC | 0.050 | −0.940 | −0.125 | −0.095 | 0.176 | −0.130 | 0.044 | −0.076 |
| L1 | 0.421 | 0.191 | 0.849 | 0.069 | −0.094 | 0.077 | 0.097 | 0.079 |
| L2 | 0.410 | 0.166 | 0.857 | 0.063 | −0.083 | 0.103 | 0.046 | 0.092 |
| a1 | −0.959 | 0.036 | −0.241 | −0.009 | 0.052 | 0.036 | −0.090 | −0.037 |
| a2 | −0.958 | 0.034 | −0.240 | −0.014 | 0.054 | 0.041 | −0.096 | −0.033 |
| b1 | 0.716 | −0.007 | 0.678 | 0.029 | −0.057 | 0.027 | 0.104 | 0.034 |
| b2 | 0.720 | −0.011 | 0.670 | 0.040 | −0.041 | 0.008 | 0.121 | 0.046 |
| С1 | −0.970 | 0.059 | 0.106 | 0.001 | 0.056 | 0.079 | −0.077 | −0.056 |
| С2 | −0.975 | 0.047 | 0.081 | 0.018 | 0.066 | 0.074 | −0.055 | −0.050 |
| h1 | 0.959 | −0.036 | 0.240 | 0.012 | −0.049 | −0.039 | 0.091 | 0.027 |
| h2 | 0.959 | −0.033 | 0.241 | 0.011 | −0.048 | −0.037 | 0.093 | 0.031 |
| SS | −0.003 | 0.407 | 0.713 | 0.121 | 0.257 | 0.232 | 0.274 | −0.169 |
| CD | −0.157 | 0.244 | 0.462 | 0.361 | 0.563 | 0.323 | −0.106 | −0.255 |
| CH | −0.002 | −0.294 | 0.130 | 0.523 | −0.229 | 0.546 | 0.308 | −0.216 |
| S24 | 0.053 | 0.266 | 0.104 | −0.703 | 0.406 | −0.133 | −0.058 | −0.095 |
| TQS | 0.428 | 0.284 | 0.219 | 0.190 | 0.127 | 0.242 | 0.552 | 0.064 |
| share of total | 21.8 | 17.9 | 14.3 | 5.9 | 11.8 | 8.1 | 6.8 | 4.1 |
| Trait | Abbreviation | p |
|---|---|---|
| Oil | Oil | 0.975 |
| Protein | Protein | 0.098 |
| Starch | Starch | 0.817 |
| Fiber | Fiber | 0.510 |
| Phenolic compounds | PC | 0.576 |
| Antioxidant activity | АОА | 0.162 |
| Water absorption capacity | WAC | 0.250 |
| Dough development time | DDT | 0.107 |
| Dough stability | DS | 0.101 |
| Dilution of the dough | DD | 0.612 |
| Valorimeter number | VN | 0.098 |
| Flour swelling capacity phase 1 | PSC1 | 0.148 |
| Flour swelling capacity phase 2 | PSC2 | 0.541 |
| Phase 2/Phase 1 | P2-1 | 0.778 |
| Pasting temperature | PT | 0.350 |
| Initial pasting viscosity | IPV | 0.005 |
| Pasting temperature start | PTS | 0.188 |
| Time to peak viscosity | TPV | 0.934 |
| Peak viscosity in the hot state | PVHS | 0.791 |
| Peak viscosity temperature | PVT | 0.380 |
| Minimum hot paste viscosity | MHPV | 0.554 |
| Cold paste peak viscosity | CPPV | 0.433 |
| Viscosity at the end of measurement | VEM | 0.500 |
| Viscosity drop at peak temperature | VDPT | 0.528 |
| Viscosity increase upon cooling | VIUC | 0.388 |
| Viscosity increase from hot to cold | VIHC | 0.269 |
| L1 Flour color brightness | L1 | 0.832 |
| L2 Flour color brightness | L2 | 0.925 |
| a1 The red–green range of the spectrum | a1 | 0.300 |
| a2 The red–green range of the spectrum | a2 | 0.282 |
| b1 Yellow–blue range of the spectrum | b1 | 0.393 |
| b2 Yellow–blue range of the spectrum | b2 | 0.349 |
| С1 Chromaticity index | c1 | 0.416 |
| С2 Chromaticity index | c2 | 0.437 |
| h1 Shade angle | h1 | 0.272 |
| h2 Shade angle | h2 | 0.280 |
| Shape stability | SS | 0.824 |
| Crumb density | CD | 0.807 |
| Crumb hardness | CH | 0.186 |
| Staling in 24 h | S24 | 0.421 |
| Taste quality score | TQS | 0.114 |
| Eastern Europe | South and Central Europe | Africa | |
|---|---|---|---|
| Initial pasting viscosity (IPV) | −166.4 | −172.1 | −174.2 |
| Dough stability (DS) | −445.3 | −452.3 | −458.2 |
| Peak viscosity temperature (PVT) | 260.6 | 259.5 | 263.9 |
| Pasting temperature Start (PTS) | 2718.7 | 2740.6 | 2775.1 |
| Pasting temperature (PT) | −11,199.4 | −11,304.5 | −11,443.1 |
| Valorimeter number (VN) | 450.0 | 457.9 | 463.1 |
| Oil | 1022.1 | 1034.7 | 1047.6 |
| Dough development time (DDT) | −750.5 | −762.2 | −771.1 |
| Constant number | −90,778.1 | −91,787.8 | −94,197.1 |
| Correct Decisions | Eastern Europe | South and Central Europe | Africa | |
|---|---|---|---|---|
| Eastern Europe | 100.0 | 8 | 0 | 0 |
| South and Central Europe | 100.0 | 0 | 6 | 0 |
| Africa | 100.0 | 0 | 0 | 6 |
| Total | 100.0 | 8 | 6 | 6 |
| Catalog | The Observed Classification | Russia | Europe | Africa |
|---|---|---|---|---|
| k-1 | Russia | 1.000 | 0.000 | 0.000 |
| k-2 | Russia | 0.994 | 0.006 | 0.000 |
| k-7 | Europe | 0.000 | 1.000 | 0.000 |
| k-8 | Europe | 0.000 | 1.000 | 0.000 |
| k-9 | Europe | 0.000 | 1.000 | 0.000 |
| k-10 | Europe | 0.000 | 1.000 | 0.000 |
| k-11 | Africa | 0.000 | 0.000 | 1.000 |
| k-12 | Africa | 0.000 | 0.000 | 1.000 |
| k-13 | Europe | 0.000 | 1.000 | 0.000 |
| k-14 | Africa | 0.000 | 0.000 | 1.000 |
| k-15 | Africa | 0.000 | 0.000 | 1.000 |
| k-16 | Africa | 0.000 | 0.015 | 0.985 |
| k-17 | Africa | 0.000 | 0.000 | 1.000 |
| k-19 | Russia | 1.000 | 0.000 | 0.000 |
| k-20 | Russia | 1.000 | 0.000 | 0.000 |
| k-21 | Russia | 1.000 | 0.000 | 0.000 |
| k-23 | Europe | 0.000 | 1.000 | 0.000 |
| k-24 | Russia | 1.000 | 0.000 | 0.000 |
| k-26 | Russia | 1.000 | 0.000 | 0.000 |
| k-27 | Russia | 1.000 | 0.000 | 0.000 |
| Sign | Root 1 | Root 2 |
|---|---|---|
| Initial pasting viscosity (IPV) | −2.115 | 1.221 |
| Dough stability (DS) | −5.110 | 0.494 |
| Peak viscosity temperature (PVT) | 0.635 | 1.600 |
| Pasting temperature start (PTS) | 5.423 | 1.814 |
| Pasting temperature (PT) | −4.474 | −0.961 |
| Valorimeter number (VN) | 10.659 | −2.759 |
| Oil | 2.146 | 0.103 |
| Dough development time (DDT) | −7.375 | 1.127 |
| Variance Explained by the Factor | 85.8 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kon’kova, N.G.; Khoreva, V.I.; Popov, V.S.; Yakusheva, T.V.; Kibkalo, I.A.; Malyshev, L.L.; Solovyеva, A.E.; Shelenga, T.V. Technological Assessment of Bread with the Addition of Cyperus esculentus L. Accessions Flour Grown in the Kuban–Azov Plain. Foods 2025, 14, 3680. https://doi.org/10.3390/foods14213680
Kon’kova NG, Khoreva VI, Popov VS, Yakusheva TV, Kibkalo IA, Malyshev LL, Solovyеva AE, Shelenga TV. Technological Assessment of Bread with the Addition of Cyperus esculentus L. Accessions Flour Grown in the Kuban–Azov Plain. Foods. 2025; 14(21):3680. https://doi.org/10.3390/foods14213680
Chicago/Turabian StyleKon’kova, Nina G., Valentina I. Khoreva, Vitaliy S. Popov, Tamara V. Yakusheva, Ilya A. Kibkalo, Leonid L. Malyshev, Alla E. Solovyеva, and Tatiana V. Shelenga. 2025. "Technological Assessment of Bread with the Addition of Cyperus esculentus L. Accessions Flour Grown in the Kuban–Azov Plain" Foods 14, no. 21: 3680. https://doi.org/10.3390/foods14213680
APA StyleKon’kova, N. G., Khoreva, V. I., Popov, V. S., Yakusheva, T. V., Kibkalo, I. A., Malyshev, L. L., Solovyеva, A. E., & Shelenga, T. V. (2025). Technological Assessment of Bread with the Addition of Cyperus esculentus L. Accessions Flour Grown in the Kuban–Azov Plain. Foods, 14(21), 3680. https://doi.org/10.3390/foods14213680










