1. Introduction
Tea is one of the top three non-alcoholic beverages globally and enjoys significant popularity [
1]. Oolong tea is classified as semi-fermented, with a degree of fermentation that lies between that of green tea and black tea. The production process involving sun-greening and shaking greening imbues oolong tea with its unique aroma and flavor [
2]. Dancong tea, as a prominent representative, offers notable qualities including a high aroma, resistance to brewing, and various health benefits, with studies indicating its efficacy in anti-inflammatory responses and weight management [
3].
The primary processing of oolong tea involves plucking, sun-withering, leaf-tossing, fixation, rolling, and drying, followed by secondary processing steps including sorting, re-roasting, packaging, and storage [
4]. As a representative oolong variety, Dancong tea is typically aged for weeks to months prior to market release [
5]. Recent studies have highlighted dynamic changes in bioactive components, quality attributes, and antimicrobial efficacy during Dancong tea storage [
6,
7], while traditional knowledge attributes gastrointestinal symptom relief (e.g., diarrhea mitigation) to aged oolong consumption. However, the potential of stored Dancong tea to alleviate acute gastric injury remains unexplored.
Recent advancements in food bioactives and gastrointestinal health have highlighted the intricate interactions between phytochemical composition, structural attributes, and biological function. For instance, structural modifications of tea constituents during processing or storage can profoundly influence their bioactivity. Liang et al. (2023) demonstrated how resveratrol’s structural modulation under thermal stress alters its bio-efficacy, reinforcing the notion that thermal or temporal changes in bioactives can enhance or suppress physiological outcomes [
8]. In a related context, Li et al. (2025) explored the nanoscale characteristics of black tea infusions, revealing how compositional features modulate organoleptic and functional properties via structural aggregation [
9]. These insights suggest that the post-processing evolution of tea, such as that occurring during Dancong tea storage, may lead to the formation of novel bioactive complexes with distinct biological effects. Moreover, the gastrointestinal system remains a critical target for dietary interventions, as multiple studies emphasize the relevance of inflammation and oxidative stress pathways in mucosal damage. Compounds such as celastrol and graveoline have been shown to attenuate gastrointestinal and hepatic injury by modulating inflammatory signaling axes, including FOXA1/CLDN4 and JAK1/STAT3, respectively [
10,
11]. These findings underscore the importance of identifying naturally occurring phytochemicals or processed products—like stored Dancong teas—that might achieve similar protective effects via the Nrf-2/HO-1 or NF-κB pathways. Additionally, polysaccharide-rich extracts, like those derived from
Rubus chingii fruits, have demonstrated efficacy in regulating intestinal absorption and barrier function, indicating the broader relevance of plant-derived components in digestive health [
12]. Together, these studies support the hypothesis that storage-induced transformations in Dancong tea may not only modulate chemical profiles but also influence its ability to mitigate gastric injury through anti-inflammatory and antioxidant mechanisms. Critically, the timeline for these transformations remains underexplored; thus, we selected a 6-month storage period based on preliminary data indicating significant stabilization of key bioactive compounds while aligning with traditional aging practices. As new tea undergoes aging, polyphenols, alcohols, and aldehydes gradually diminish due to air oxidation. This process leads to the formation and accumulation of tea pigments [
13]. This alteration may render Dancong tea less irritating to the gastrointestinal mucosa; however, this claim has not been substantiated by research. Furthermore, due to the dual nature of tea components regarding dosage effects on the gastrointestinal tract, it is crucial to examine how varied dosages of stored Dancong tea impact gastric health to guide its scientific utilization.
The gastrointestinal (GI) system, primarily responsible for food digestion and nutrient absorption, encompasses the stomach, small intestine, and large intestine [
14]. Gastrointestinal injuries often result in the loss of epithelial mucus, mucosal damage, and inflammation [
15]. To rigorously evaluate the gastroprotective potential of stored Dancong tea, we employed the hydrochloric acid–ethanol model—a well-established method for inducing acute gastric injury that reliably recapitulates oxidative and inflammatory stress pathways. The hydrochloric acid–ethanol model is a widely utilized method for inducing gastric injury, noted for its simplicity and brevity of the modeling cycle [
16]. Given that inflammation and oxidative stress (mediated by pathways such as NF-κB and Nrf-2/HO-1) are pivotal contributors to gastric mucosal injury, alleviating these factors can be instrumental in mitigating gastric damage [
17].
Here, we hypothesize that aging Dancong tea for six months will reduce its gastrointestinal irritancy. Using a hydrochloric acid/ethanol-induced gastric injury model in mice, we test whether stored Dancong tea (OldT) protects gastric mucosa better than fresh tea (NewT), and elucidate the underlying antioxidant and anti-inflammatory mechanisms. We focus on Lingtou Dancong tea, a widely cultivated oolong tea variety in Guangdong, China [
2], to provide insights into proper storage and consumption practices for gastrointestinal health benefits.
2. Materials and Methods
2.1. Tea Sample Preparation
All Dancong tea leaf samples were harvested in the fall of 2023 from “China Lingtou Dancong Tea Township” in Raoping County, Chaozhou City, Guangdong Province. The tea leaves were standardized to consist of pairs of young leaves and were then processed into tea. Processing involved blending, killing, withering, kneading, and roasting to produce Dancong tea. The processed tea leaves were divided into two batches: one batch was immediately frozen at −80 °C for six months to preserve the characteristics of freshly processed tea (a method adapted from preservation protocols in tea metabolite studies [
18]), while the other batch was stored under ambient conditions (25 °C, 60% relative humidity) for six months to simulate conventional storage.
For phytochemical extraction, 50 g of ground tea leaves from each batch were mixed with pure water at a 1:20 ratio (w/v) and subjected to hot water extraction at 95 °C for 45 min for analytical extract preparation (not simulating a typical cup of tea). The resulting extracts were centrifuged at 8000× g for 15 min to remove insoluble residues. The supernatants were vacuum-concentrated and lyophilized to obtain freeze-dried tea extract powders. These powders, derived from freshly processed tea leaves and aged tea leaves, were defined as NewT and OldT, respectively. Among them, the extraction rate of new tea was 35.72%, and that of old tea was 35.23%. The NewT and OldT samples were subsequently used for animal treatment experiments to evaluate their effects on gastric injury.
2.2. Tea Sample Characterization
The biochemical components were analyzed according to previously established methods [
19]. Briefly, the content of polyphenols was measured using the Folin phenol method (GB/T 8313-2018 [
20]), free amino acids were quantified by the ninhydrin method (GB/T 8314-2013 [
21]), theaflavins, thearubigins, and theabrownins were measured with the People’s Republic of China Agricultural Industry Standard (NY/T 3675-2020 [
22]), and total soluble sugar content was measured by the anthrone–sulfuric acid colorimetric assay. The contents of caffeine and catechins were determined by high-performance liquid chromatography (HPLC). The NewT and OldT samples were extracted with 70% (
v/
v) methanol in a water bath at 70 °C for 10 min. An Agilent column (5 μm, 250 × 4.6 mm) was used for chromatographic separation. Mobile phase A was double-distilled water, phase B was methanol, phase C was 0.05% (
v/
v) phosphoric acid, and phase D was acetonitrile. Gradient elution was performed at the flow rate of 1 mL/min, the sample volume was 10 μL, and the UV detection wavelength was 278 nm. The percentage composition of bioactive compounds (e.g., polyphenols, flavonoids, theaflavins) was calculated as the basis for percentages (dry weight normalization). The biochemical components of NewT and OldT samples are summarized in
Table 1 and
Table 2.
2.3. Establishment of Mouse Model of Gastric Mucosal Injury
Male ICR mice (to avoid estrous cycle variability), aged six weeks, were procured from Dienen Genetic Technology Co., Ltd. (Guangzhou, Guangdong, China). They were maintained in a 12 h light/dark cycle at 60–70% humidity and at room temperature (22 ± 2 °C), with ad libitum access to food and water. After one week of acclimatization, mice were randomly allocated into groups (n = 10 per group). All mice received daily oral gavage for three consecutive days: (1) normal control (CON), (2) model (MOD), (3) low-dose (200 mg/kg BW) new Dancong tea (L-NewT), (4) high-dose (600 mg/kg BW) new Dancong tea (H-NewT), (5) low-dose (200 mg/kg BW) after-storage Dancong tea (L-OldT), and (6) high-dose (600 mg/kg BW) after-storage Dancong tea (H-OldT). CON/MOD groups received an equal volume of saline. The experimental doses were determined using body surface area normalization, corresponding to human-equivalent daily tea consumption of 3–5 g/day (typical intake) and 10–15 g/day (potential high intake), respectively. This dose range reflects both routine tea-drinking scenarios and exploratory therapeutic applications.
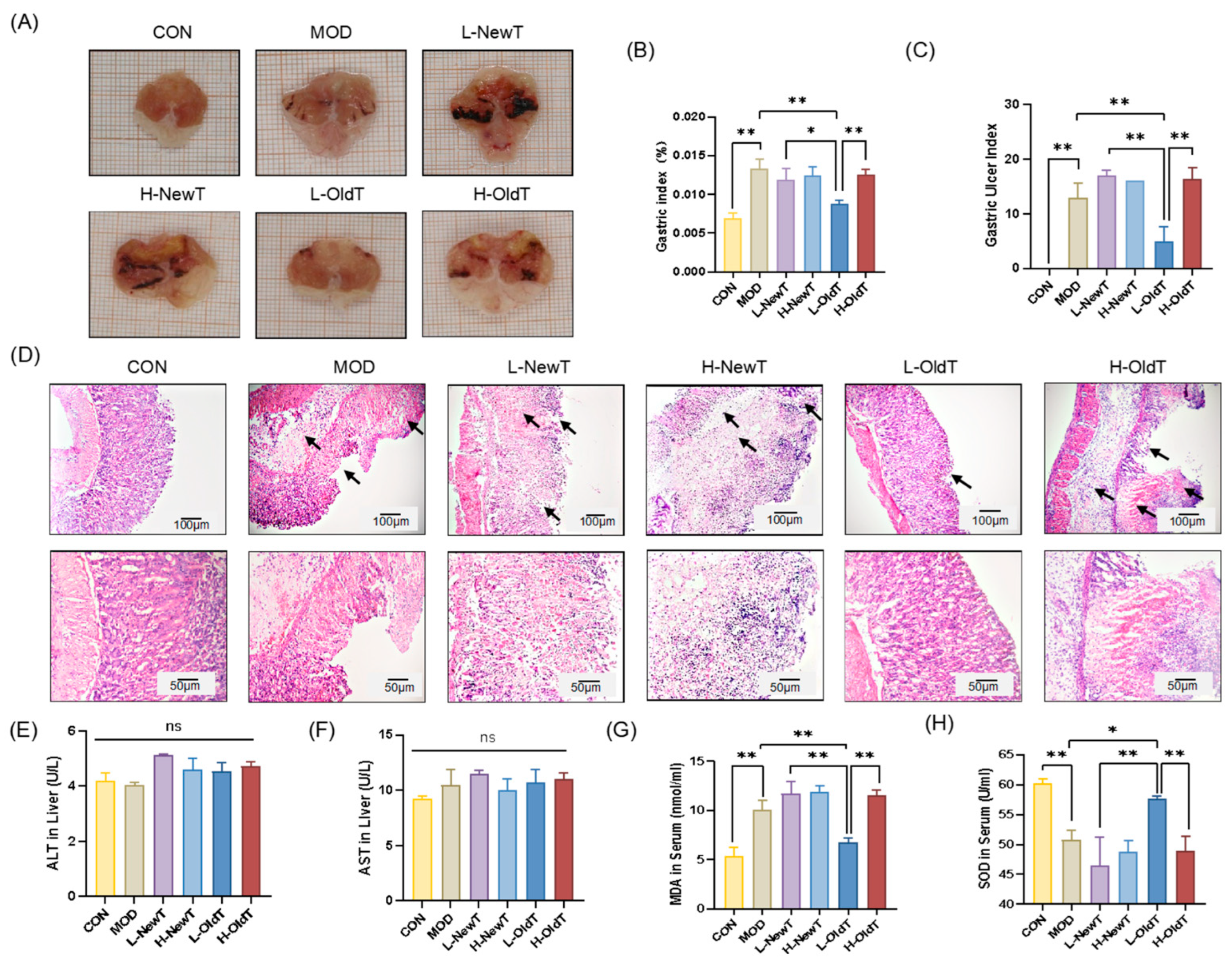
Gastric mucosal injury was induced by a single gavage of 0.4 M HCl and 60% ethanol in all animals except the control group. After modeling, the drug/extract was administered for three consecutive days in the treatment group, while the control and model groups received equal amounts of saline. Two hours post-final treatment, the mice were sacrificed, and blood, stomach, and liver tissues were harvested for further analysis. The stomachs were immediately excised, cut along the greater curvature, photographed, and weighed. One portion was fixed in 4% paraformaldehyde, while the other was rapidly frozen and stored at −80 °C. All experiments adhered to the Guide for the Care and Use of Laboratory Animals, with approval granted by the Animal Care and Welfare Committee of the Tea Research Institute of the Guangdong Academy of Agricultural Sciences (Serial No. 2024016).
2.4. Macroscopic Assessment of Gastric Mucosal Damage
The gastric mucosal ulcer index (UI) was calculated based on the count and diameter of erosions using the formula: UI = Σ[(A × 1) + (B × 2) + (C × 3)], where A, B, and C correspond to the number of lesions measuring ≤1 mm, 1–3 mm, and >3 mm, respectively. Mean scores for each group were calculated to gauge the extent of mucosal injury.
2.5. Histologic Staining
Fixed gastric or intestinal tissues were sectioned into 5 mm blocks and embedded in paraffin. Sections of 5 μm were prepared using a paraffin slicer (Leica, Zurich, Switzerland) and subjected to deparaffinization with xylene and a series of ethanol solutions (100%, 95%, 80%, and 70%). Hematoxylin and eosin (H&E) staining was conducted as per standard protocols. Following staining, sections were dehydrated with 95% and 100% ethanol, permeabilized in xylene, and mounted with neutral resin. Stained sections were observed under a light microscope (Olympus, Japan).
2.6. Biochemical Assay
Gastric tissue samples were homogenized with 9 volumes of saline using an OMNI Bead Ruptor 24 homogenizer. Each sample analyzed in triplicate technical replicates. The supernatant was collected via centrifugation at 2500 rpm for 10 min at 4 °C. Protein content was quantified using a Pierce BCA Protein Assay Kit (BCA, Thermo, Waltham, MA, USA). Levels of aspartate aminotransferase (AST, C010-2-1, Nanjing JianCheng, Nanjing, China), alanine aminotransferase (ALT, C009-1-2, Nanjing JianCheng, Nanjing, China), malondialdehyde (MDA, A003-1-2, Nanjing JianCheng, Nanjing, China), glutathione (GSH, A006-2-1, Nanjing JianCheng, Nanjing, China), catalase (CAT, A007-1-1, Nanjing JianCheng, Nanjing, China), and superoxide dismutase (SOD, A001-3-2, Nanjing JianCheng, Nanjing, China) were assessed using specific assay kits, following manufacturer’s guidelines. Additionally, nitric oxide (NO, MM-0658M1, Meimian, Wuhan, China), ROS (MM-43700M1, Meimian, Wuhan, China), and PGE2 (MM-46884M1, Meimian, Wuhan, China) levels were quantified with appropriate ELISA kits.
2.7. Immunohistochemistry (IHC)
Paraffin sections were treated with 3% H2O2 to quench endogenous peroxidase activity and subjected to microwave heating in ethylenediaminetetraacetic acid (EDTA) to activate tissue antigens. After blocking with 5% goat serum (SL038, Solarbio, Beijing, China) for 30 min, sections were incubated with primary antibodies, including nuclear factor-erythroid 2-related factor 2 (Nrf-2, Bioss, bs-1074R, Beijing, China, Rabbit, 1:500), heme oxygenase 1 (HO-1, Bioss, bs2075R, Beijing, China, Rabbit, 1:500), interleukin-6 (IL-6, Bioss, bs-0782R, Beijing, China, Rabbit, 1:500), tumor necrosis factor-α (TNF-α, Proteintech, 26405-1-AP, Wuhan, China, Rabbit, 1:500), inducible nitric oxide synthase (iNOS, Proteintech, 22226-1-AP, Wuhan, China, Rabbit, 1:500), cyclooxygenase 2 (COX-2, Proteintech, 12375-1-AP, Wuhan, China, Rabbit, 1:500), and phospho-nuclear factor kappa-B (p-NF-κB, Bioss, bs-0982R, Wuhan, China, Rabbit, 1:500). The following day, sections were probed with corresponding secondary antibodies for one hour, detected using SABC reagent (P0603, Beyotime Biotechnology, Shanghai, China) for 30 min, and developed with DAB (P0203, Beyotime Biotechnology, Shanghai, China) for 2–5 min. Sections were re-stained with hematoxylin (C0107, Beyotime Biotechnology, Shanghai, China) for 90 s, dehydrated through an ethanol gradient, cleared in xylene, and mounted with neutral resin. Quantification was performed by analyzing three random fields per tissue section, with three sections evaluated per mouse, using ImageJ software 1.52a software to calculate mean optical density.
2.8. Western Blot
Gastric tissues were homogenized with tenfold volumes of RIPA lysis buffer (RIPA, Beyotime, Shanghai, China), placed on ice for one hour, and centrifuged at 4 °C for 20 min. Protein concentrations were determined via the Pierce BCA Protein Assay Kit (BCA, Thermo, Waltham, MA, USA). Equal protein amounts were denatured in SDS-PAGE loading buffer (containing 4× DTT) for five minutes before being subjected to SDS-PAGE gel electrophoresis. Proteins were transferred to polyvinylidene fluoride membranes, blocked with 5% skim milk for one hour, and subsequently incubated overnight at 4 °C with primary antibodies including Nrf-2 (Bioss, bs-1074R, Beijing, China, Rabbit, 1:1000), HO-1 (Bioss, bs2075R, Beijing, China, Rabbit, 1:1000), IL-6 (CST, #12912S, Danvers,MA, USA, Mouse, 1:1000), TNF-α (Abcam, ab6671, Cambridge, UK, Rabbit, 1:1000), iNOS (Abcam, ab15323, Cambridge, UK, Rabbit, 1:1000), COX-2 (CST, #12282S, Danvers, MA, USA, Rabbit, 1:1000), inhibitor of nuclear factor kappa-B-α (IκB-α, CST, #9242S, Danvers, MA, USA, Rabbit, 1:1000), phospho-inhibitor of nuclear factor kappa-B-α (p-IκB-α, CST, #9246S, Danvers, MA, USA, Rabbit, 1:1000), NF-κB (CST, #8242S, Danvers, MA, USA, Rabbit, 1:1000), p-NF-κB (CST, #3033S, Danvers, MA, USA, Rabbit, 1:1000), B-cell lymphoma-2-associated X protein (Bax, Abcam, AB32503, Cambridge, UK, Rabbit, 1:1000), B-cell lymphoma-2 (Bcl-2, CST, #2870S, Danvers, MA, USA, Rabbit, 1:1000) and β-actin (CST, #3700S, Danvers, MA, USA, Mouse, 1:1000). Membranes were washed with Tris Buffered Saline with Tween-20 six times, followed by incubation with secondary antibodies for 50 min. Bands were visually developed in the dark using the Tanon system, and gray-scale intensity was normalized to β-actin and quantified using ImageJ.
2.9. Statistical Analysis
Data were analyzed using GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA). To compare the two groups, Student’s t-test was used. For comparisons involving more than two groups, one-way ANOVA with Tukey’s post hoc test was used. Data are presented as the mean ± SD of 10 mice/group (in vivo endpoints), with triplicate technical replicates per sample for biochemical/ELISA assays. * p < 0.05 and ** p < 0.01 indicated significant differences.
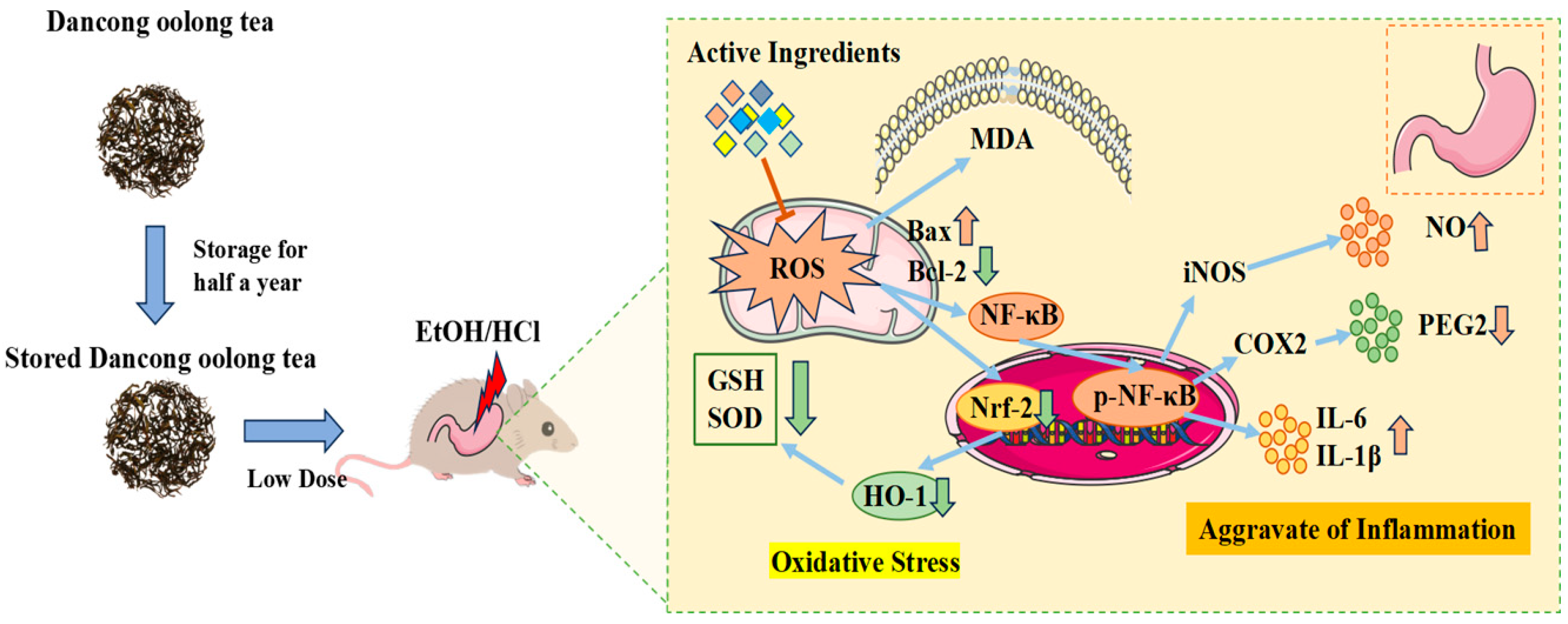
4. Discussion
Dancong tea is one of the representative teas of oolong tea and has health benefits such as anti-inflammatory properties and weight loss [
3]. However, fresh Dancong tea exhibits gastrointestinal irritancy, prompting traditional storage practices to reduce this effect [
2]. The mechanisms and chemical basis underlying this storage-mediated reduction in irritancy remain incompletely understood. This study employed a murine HCl/EtOH-induced gastric injury model to investigate the gastroprotective effects of Dancong tea across different storage durations (fresh vs. 6-month stored) and doses (200 mg/kg vs. 600 mg/kg BW/d, approximating human intakes of 3–5 g/d and 10–12 g/d, respectively). Our central finding is that L-OldT significantly alleviated gastric mucosal damage, whereas H-OldT and both doses of NewT failed to confer protection and often exacerbated injury (
Figure 1). Critically, this establishes a clear interdependence between storage duration and dosage: only the combination of aging (6 months) and low-dose intake (200 mg/kg) conferred gastroprotection.
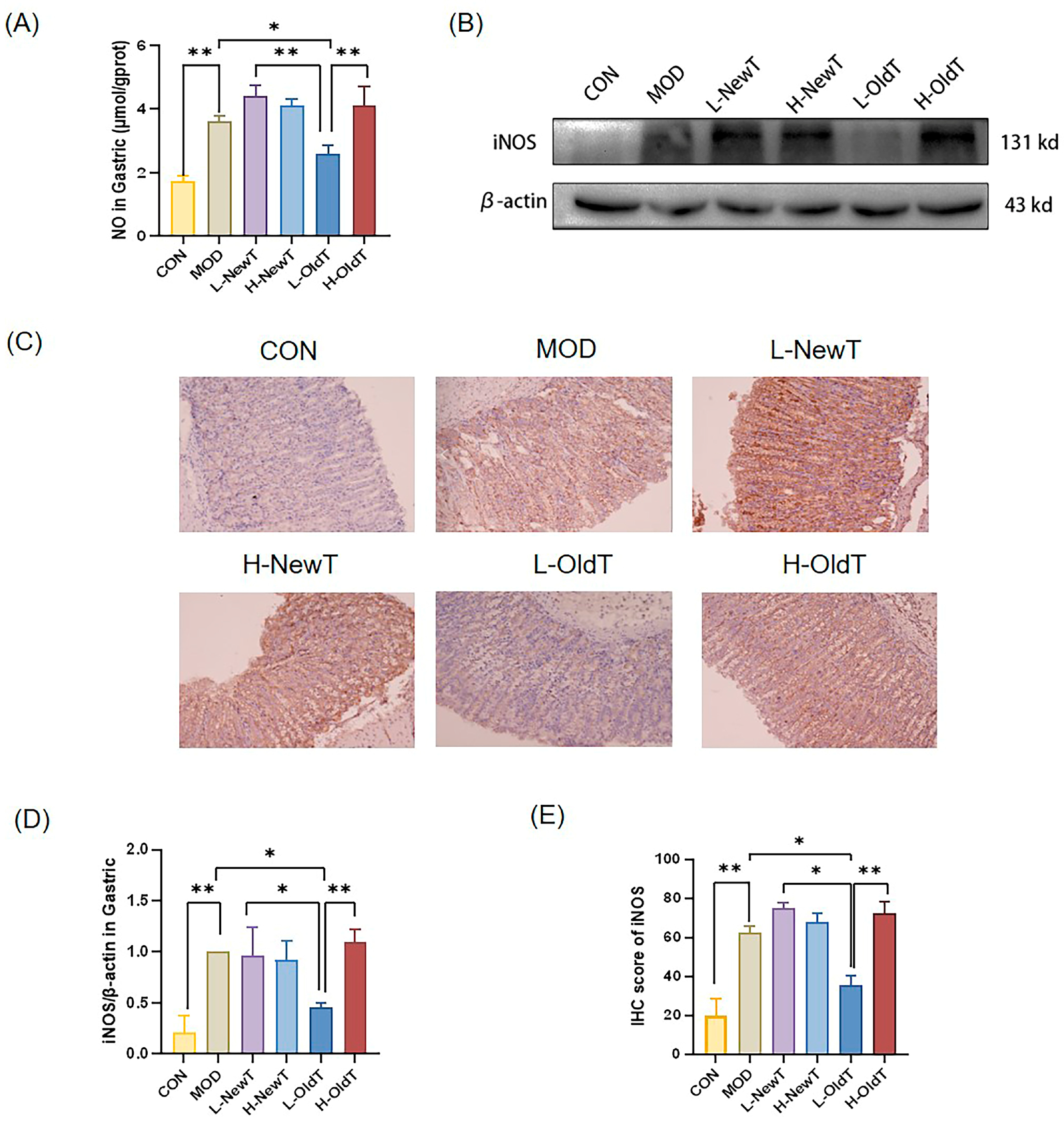
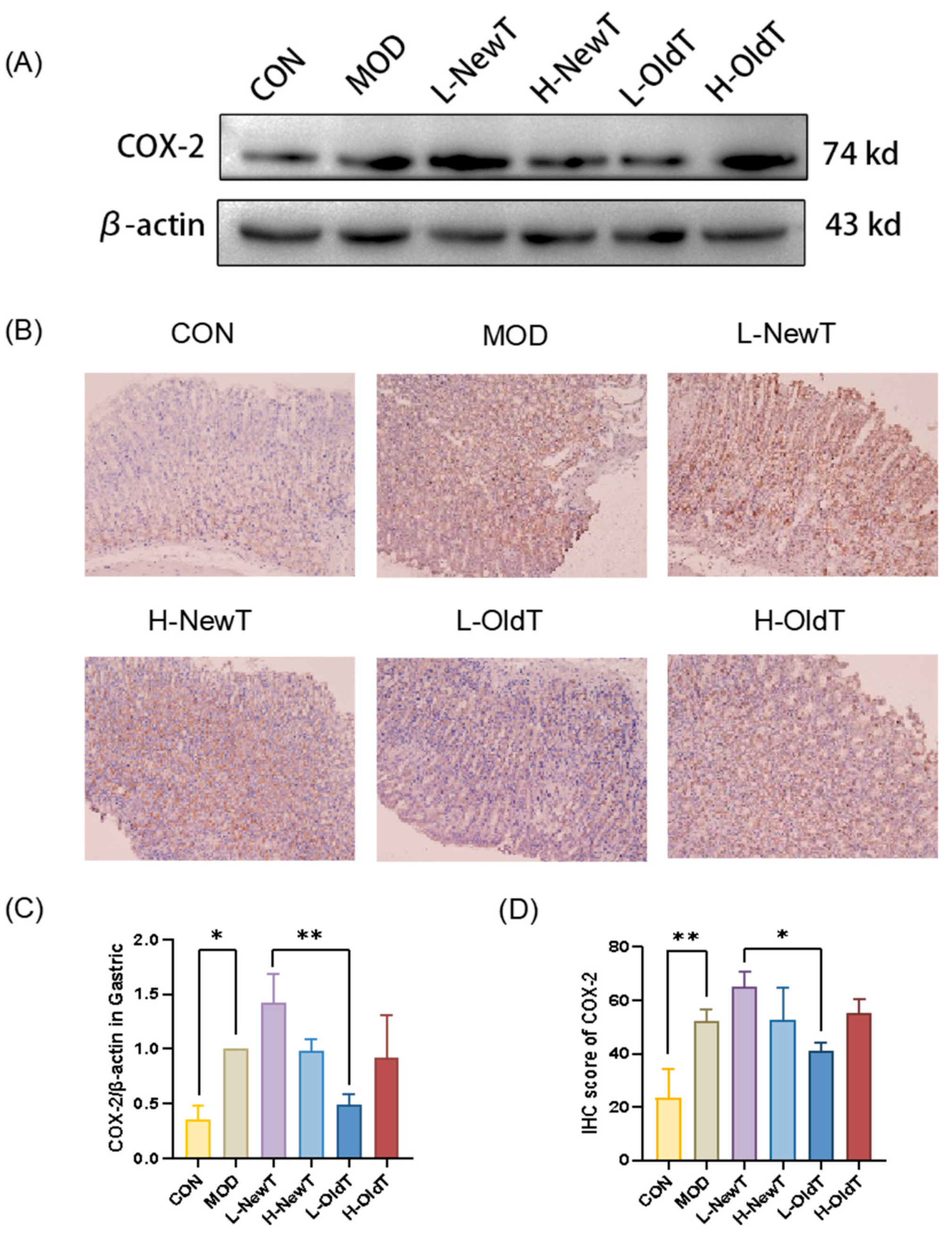
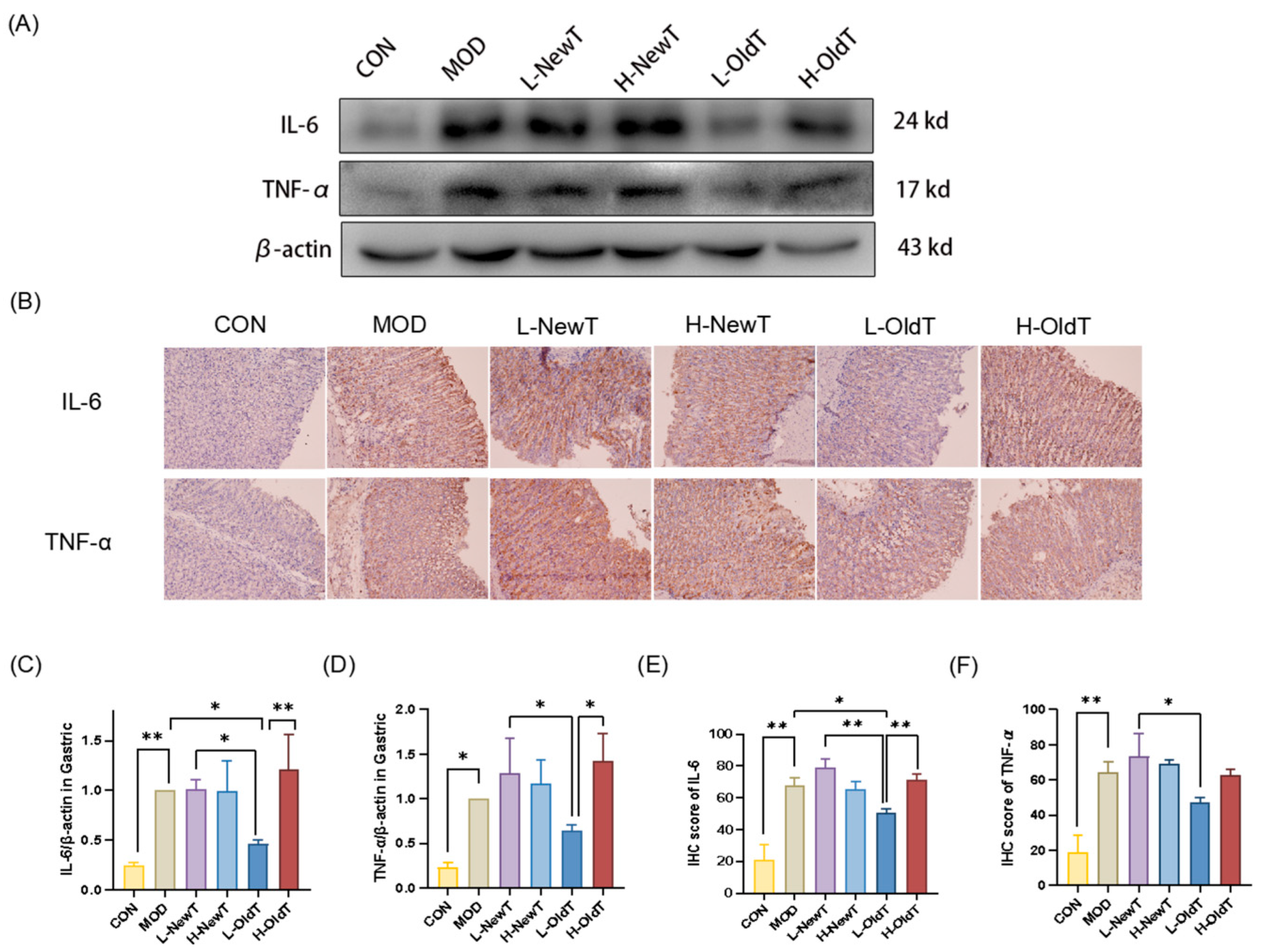
Mechanistically, low-dose properly stored Dancong tea inhibited lipid peroxidation, ROS and NO production, and the expression of inflammatory factors IL-6 and TNF-α, along with inflammatory mediators iNOS and COX-2 through the modulation of the Nrf-2/HO-1 and NF-κB pathways, significantly reducing the deterioration of gastric mucosal damage. In contrast, high doses of Dancong tea and both low and high doses of NewT did not show such effects (
Figure 9). Therefore, moderate consumption of stored Dancong tea (≤200 mg/kg BW/d) can help alleviate gastrointestinal damage in individuals with gastrointestinal discomfort, while high-dose tea (≥600 mg/kg BW/d) and new tea are not advisable.
Traditional wisdom suggests that the stimulant properties of NewT are mainly due to its high content of tea polyphenols, aldehydes, and caffeine. However, after a period of storage, these irritants are reduced and more stable bioactive components are formed [
13]. Compared to fresh Dancong tea, after-storage Dancong tea has significantly lower contents of tea polyphenols, flavonoids, and catechin monomers such as ECG, EC, and GA, along with significantly higher contents of theaflavins, thearubigins, and theafucoxanthins (
Table 1 and
Table 2), which aligns with a related study [
13]. In a gastric injury mouse experiment, the model group exhibited severe gastric mucosal injury, including gastric erosion, mucosal necrosis, and epithelial cell loss after ingesting HCl and ethanol for 2 days [
16]. This study revealed that only low-dose post-storage Dancong tea (200 mg/kg) alleviated ethanol/HCl-induced gastric mucosal injury, reducing necrosis, edema, and inflammation. Conversely, high-dose regular Dancong tea (600 mg/kg) and freshly prepared oolong tea (at both doses) exacerbated mucosal damage (
Figure 1). The European Food Safety Authority (EFSA) [
24] conducted extensive studies demonstrating that daily intake of Epigallocatechin gallate(EGCG) exceeding or equaling 800 mg may induce hepatotoxicity. The recommended single-dose limit for EGCG is ≤300 mg. In our study, the low- and high-dose tea aqueous extracts corresponded to human EGCG exposures of 211.8 mg and 635.5 mg/day, respectively, based on body surface area normalization. While serum ALT and AST levels indicated no hepatotoxicity in mice under experimental conditions (
Figure 1E,F), the high-dose group approached risk thresholds. Critically, real-world tea consumption involves multiple infusions of the same leaves, coupled with EGCG’s low bioavailability and instability during digestion [
25]. These factors substantially reduce systemic EGCG exposure, rendering moderate tea consumption—particularly avoiding concentrated brews—both safe and beneficial. This aligns with our findings that low-dose aged Dancong tea alleviates gastric injury, whereas excessive doses exacerbate mucosal damage.
In this study, the tea polyphenol content of Dancong tea was 18.26%, and the gavage dose of 200 mg/kg BW/d corresponded to an intake of more than 626 mg of tea polyphenols per day in 60 kg gastric-injured individuals, which can exacerbate gastric injury. The polyphenol content of stored Dancong tea was 15.88%, and the low dose was equivalent to less than 545 mg of tea polyphenol per day for 60 kg of people with gastric injury, which is favorable for the repair of gastric injury. Additionally, consuming 10–12 g of Dancong tea at one time also aggravated the gastric injury in mice. Critically, the high-dose model employs fully solubilized extracts to deliver bioactives equivalent to consuming 10–12 g of tea leaves in a single bolus—a scenario divergent from traditional tea preparation where 3–5 g of leaves are steeped multiple times in hot water and consumed gradually over hours. While dosage extrapolation to humans is conceivable, we emphasize that these are only approximations, and human studies are required. However, in daily life, 3–5 g of tea should be brewed at one time and consumed in several portions. Therefore, it is recommended that patients with gastric injury avoid high-dose tea and that regular tea consumption is beneficial for the stomach. Conversely, for other symptoms (e.g., obesity), new tea as well as high concentrations (above 600 mg/kg BW/d) of Dancong tea may provide significant relief [
3].
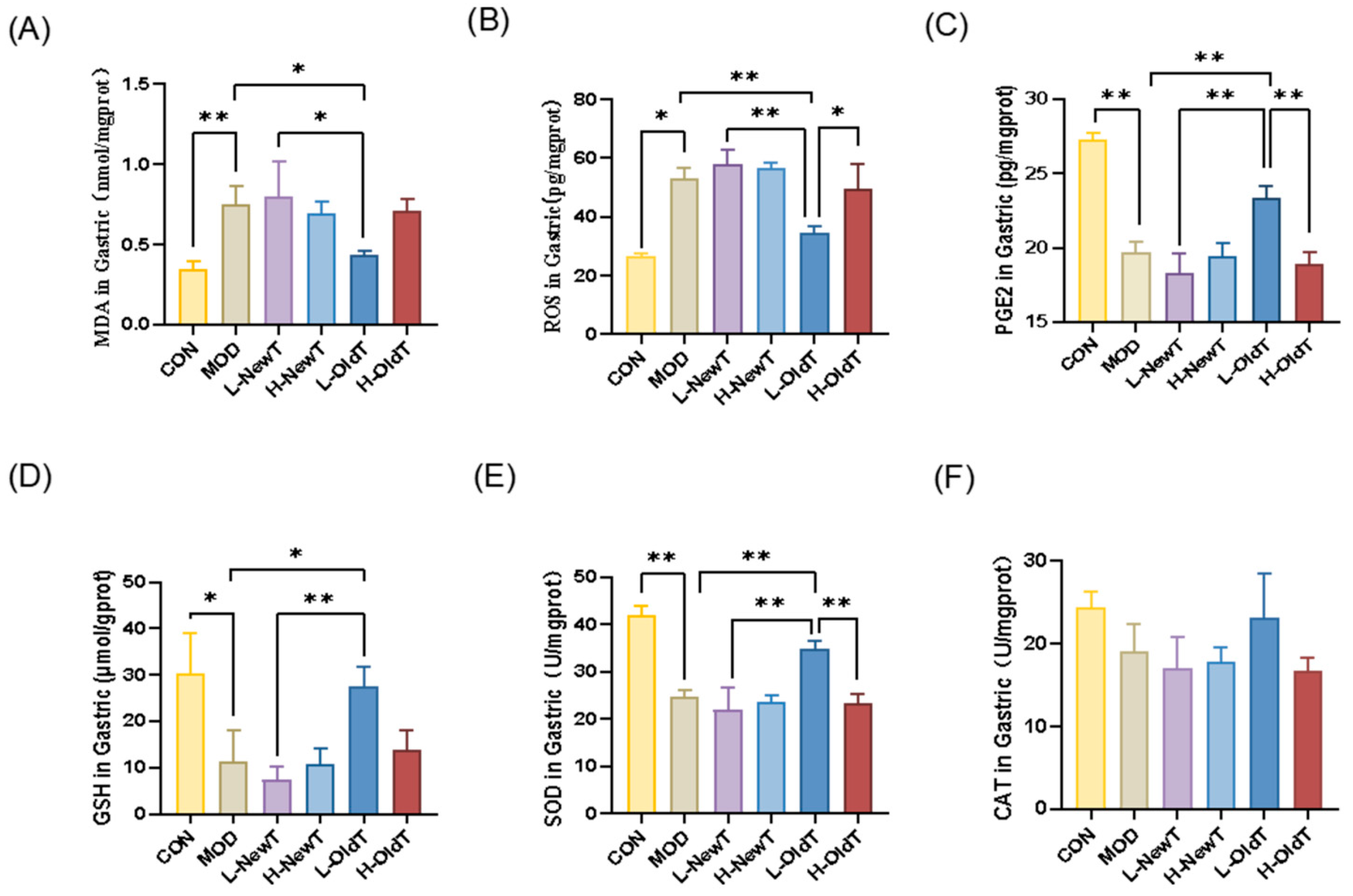
Oxidative stress is one of the major drivers of gastric injury [
23]. Tea, as a natural antioxidant, acts as a scavenger of ROS and is considered to play a significant role in the relief of gastric ulcers [
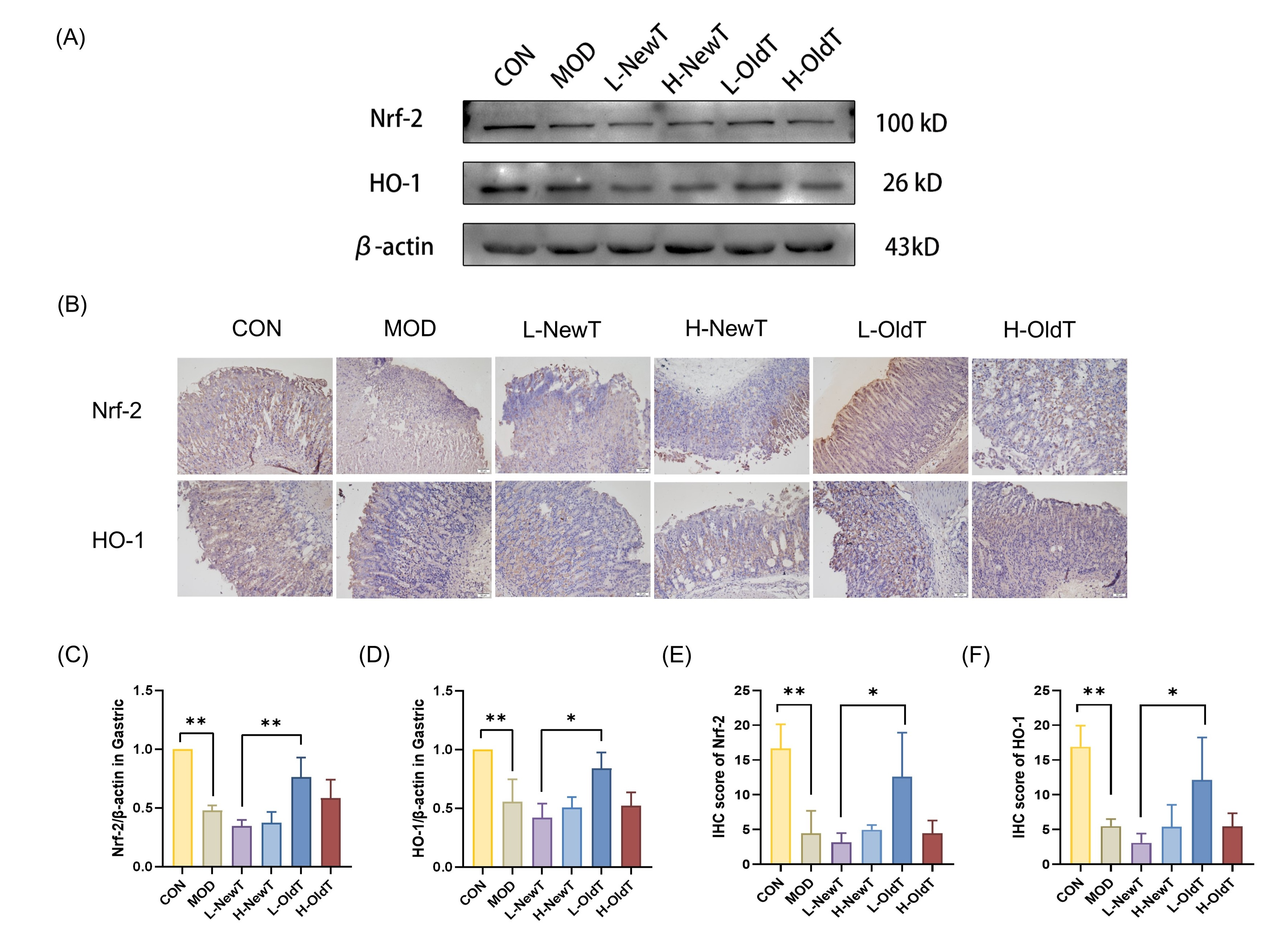
26]. The results of the present study showed that low-dose OldT inhibited gastric injury-induced ROS and lipid peroxide accumulation via Nrf-2/HO-1 pathway activation, whereas high-dose or freshly prepared oolong tea lacked antioxidant efficacy and exacerbated oxidative stress (
Figure 1 and
Figure 6). These findings align with related studies on the antioxidant activity of tea [
1,
17], reflecting the importance of suitable storage for the antioxidant regulatory capacity of Dancong tea.
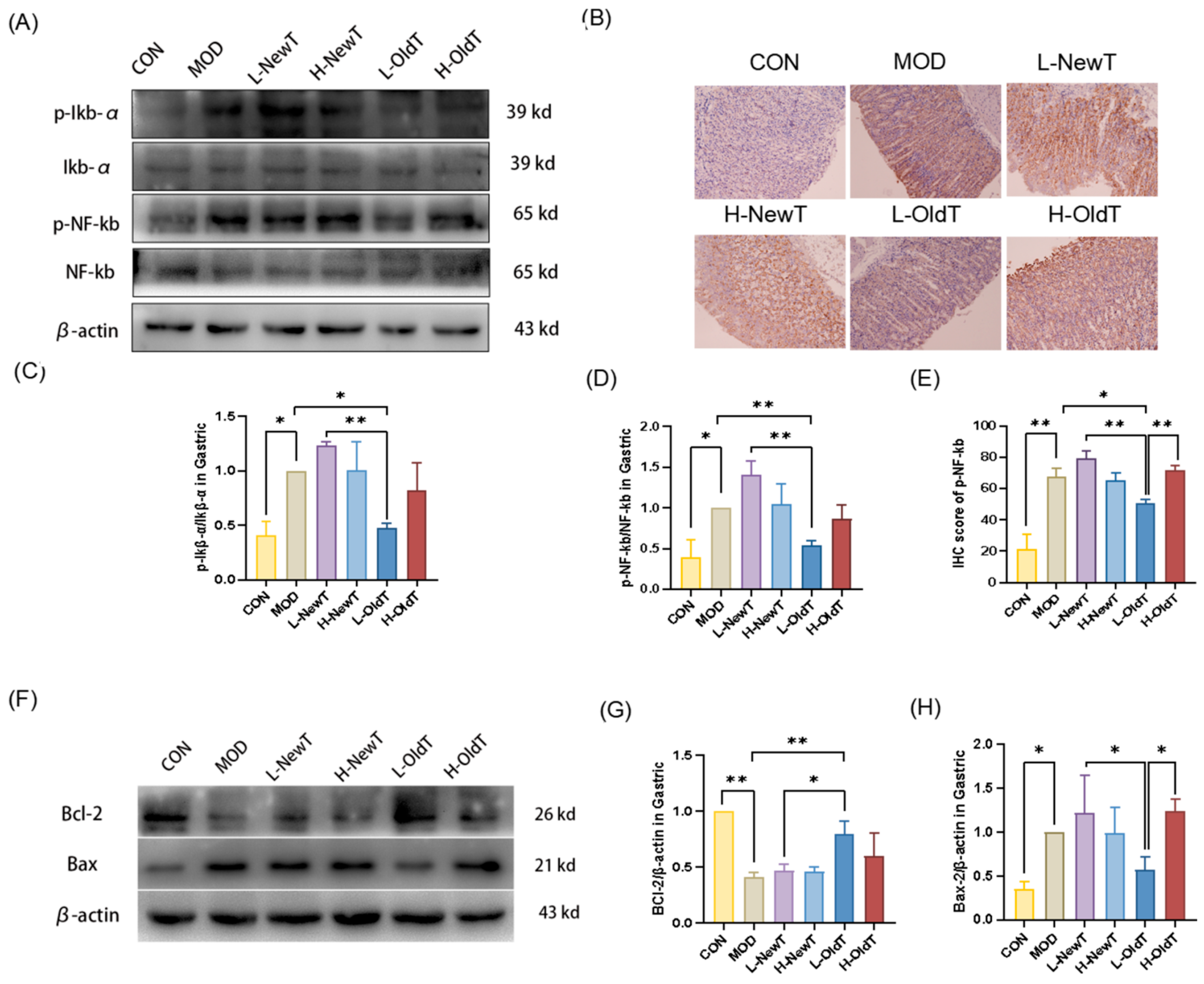
Imbalances in the antioxidant system exacerbate ROS accumulation, triggering NF-κB activation via IκB-α dissociation and NF-κB p65 release, thereby upregulating pro-inflammatory factors (e.g., TNF-α, IL-6) and amplifying inflammation. This study demonstrated that stored Dancong tea, unlike NewT, suppressed NF-κB signaling and downstream cytokines (
Figure 5 and
Figure 7), consistent with reports of NF-κB inhibition alleviating gastric injury. Additionally, L-OldT downregulated iNOS and COX-2 expression (linked to excess NO and PGE2 depletion [
27]), restoring NO/PGE2 balance (
Figure 2,
Figure 3 and
Figure 4), akin to findings with fermented soybeans. Crosstalk between NF-κB and Nrf-2/HO-1 pathways likely underpins this effect, as NF-κB modulation interacts with Nrf-2-mediated antioxidant defenses. Furthermore, L-OldT reduced apoptosis by restoring the Bcl-2/Bax balance disrupted by ROS, paralleling studies on tea-mediated hepatoprotection and gastric apoptosis inhibition. Thus, stored Dancong tea mitigates gastric injury by blocking ROS-driven Nrf-2/NF-κB crosstalk, alleviating oxidative stress, inflammation, and apoptosis. However, the causative role of these pathways in attenuating gastric injury remains to be mechanistically validated through further inhibitor experiments or genetic knockdown animal models.
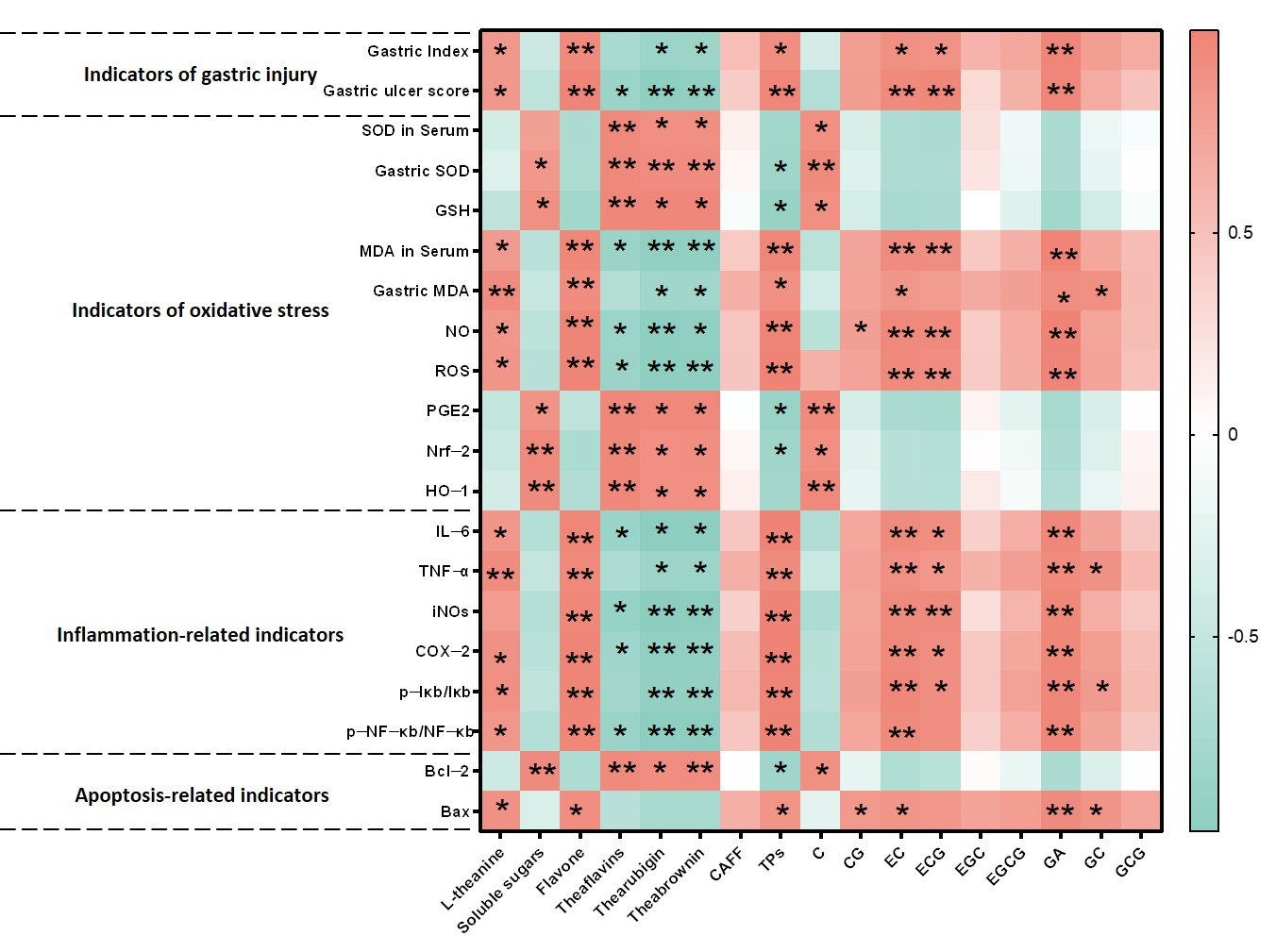
Our correlation analyses reveal a dual role of tea components in gastric mucosal protection, modulated by storage-induced chemical transformations. NewT containing elevated levels of tea polyphenols, L-theanine, EC, ECG, and GA exhibited paradoxical pro-oxidative and pro-inflammatory effects at high concentrations, as evidenced by their positive correlations with gastric injury biomarkers (NF-κB activation, Bax elevation) and negative associations with antioxidant defenses (SOD, GSH) and Nrf-2 expression (
Figure 8). This concentration-dependent duality aligns with previous findings where low-dose polyphenols demonstrated mucosal protection, while excessive levels exacerbated oxidative damage through redox cycling mechanisms [
28,
29,
30]. The observed dose–response relationship provides a plausible explanation for the gastric irritancy of newly processed Dancong tea.
Correlations between NewT and OldT components (e.g., theaflavins) and Nrf-2/NF-κB expression suggest candidate protective agents; however, definitive mechanistic validation requires future studies using pathway inhibitors or genetic models. These findings are mechanistically supported by Alanazi et al.’s demonstration of theaflavins’ dual regulation of Nrf-2 antioxidant and NF-κB inflammatory pathways in renal protection [
31], and Adhikary et al.’s report on theaflavin-mediated gastric ulcer healing through enhanced antioxidant capacity [
32]. The structural evolution from monomeric catechins to polymeric pigments during storage appears critical—oxidative polymerization not only increases molecular stability but reduces phenolic hydroxyl exposure, thereby lowering gastrointestinal astringency while preserving redox-modulating capacity [
33]. Our quantification of this transformation (
Table 1 and
Table 2) provides direct evidence for the traditional practice of tea aging in gastrointestinal health preservation. Three key implications emerge from these findings: First, the catechin-theaflavin conversion appears to shift tea’s biological activity from potential irritancy to mucosal protection. Second, the Nrf-2/HO-1/NF-κB axis serves as a pivotal regulatory node connecting chemical composition with gastric outcomes. Third, the time-dependent nature of these transformations underscores the importance of storage duration optimization, though our single timepoint analysis currently limits precise temporal recommendations.
While this study establishes important correlations between chemical transformations and the gastroprotective effects of stored Dancong tea, several limitations warrant acknowledgment. First, the experimental design focused on a single storage timepoint (6 months) and utilized frozen-stored tea as a proxy for NewT—conditions that may not fully replicate commercial aging processes or fresh tea. Second, the HCl/EtOH-induced mouse model reflects acute gastric injury rather than chronic human pathologies (e.g., H. pylori infections or non-steroidal anti-inflammatory drugs-induced ulcers), and interspecies differences in mucosal homeostasis limit direct clinical extrapolation. Third, the absence of a positive control (e.g., sucralfate) precludes efficacy benchmarking against established therapeutics. Fourth, human-equivalent dosing (200–600 mg/kg) assumes theoretical bioavailability without clinical validation. Finally, our compositional analysis, though comprehensive, cannot fully resolve storage-driven complexity (e.g., microbial bioconversion versus oxidative polymerization). To address these gaps, we are currently employing high-resolution metabolomics (UPLC-QTOF-MS) to systematically map the dynamic chemical landscape of aging Dancong tea across multiple storage durations. This ongoing work aims to (1) identify novel transformation products beyond the characterized theaflavins/thearubigins, and (2) establish temporal thresholds for the catechin–polymer conversion process. The resulting chemical atlas will enable targeted mechanistic studies to decipher how specific transformation pathways (e.g., oxidative polymerization vs. microbial bioconversion) differentially modulate gastric protective mechanisms through Nrf-2/NF-κB crosstalk. Future studies should prioritize dose–response trials in human cohorts and investigate long-term safety and efficacy.