Volatile Organic Compounds in Teas: Identification, Extraction, Analysis, and Application of Tea Aroma
Abstract
1. Introduction
2. Different Tea Types Have Different Flavors
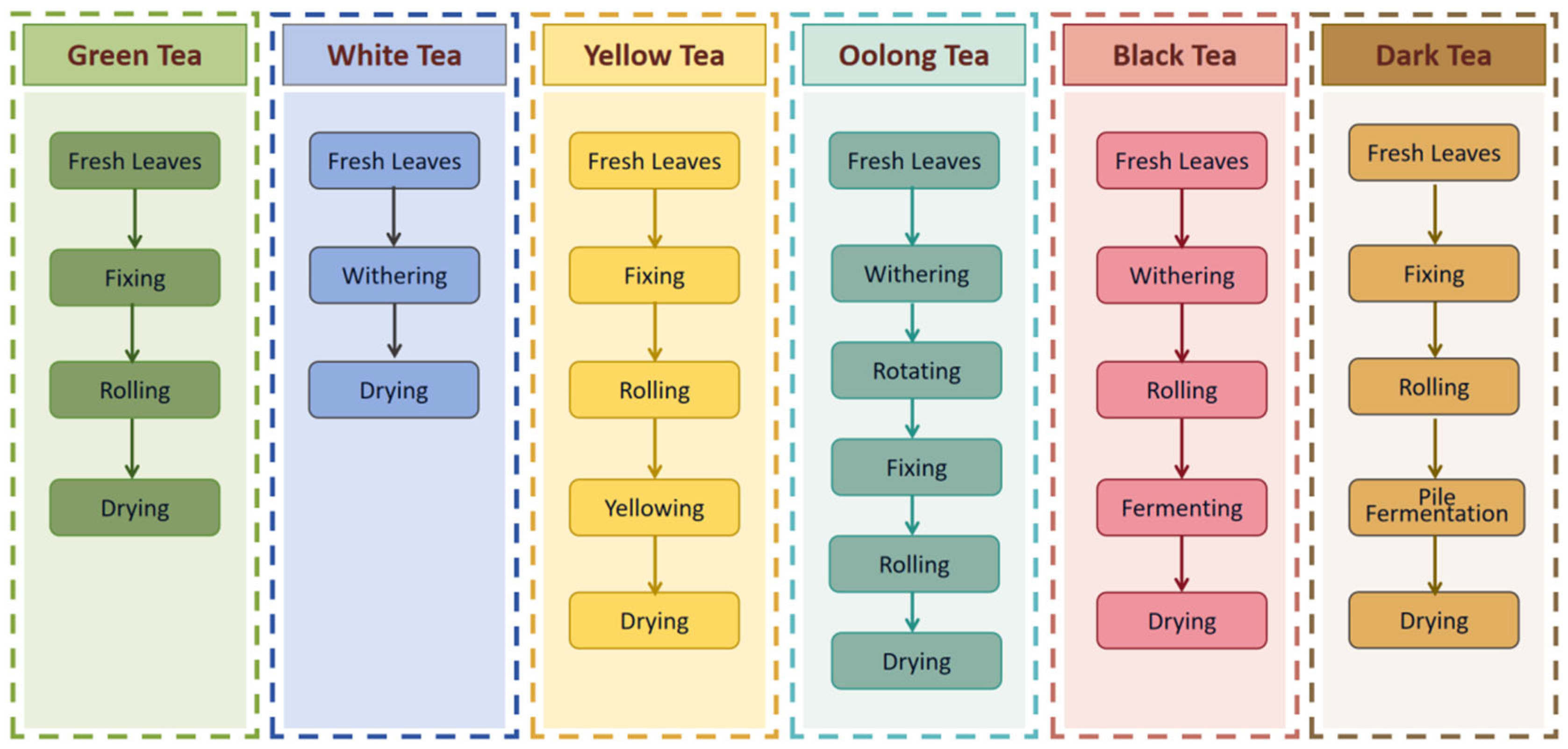
2.1. Tea Types Classification
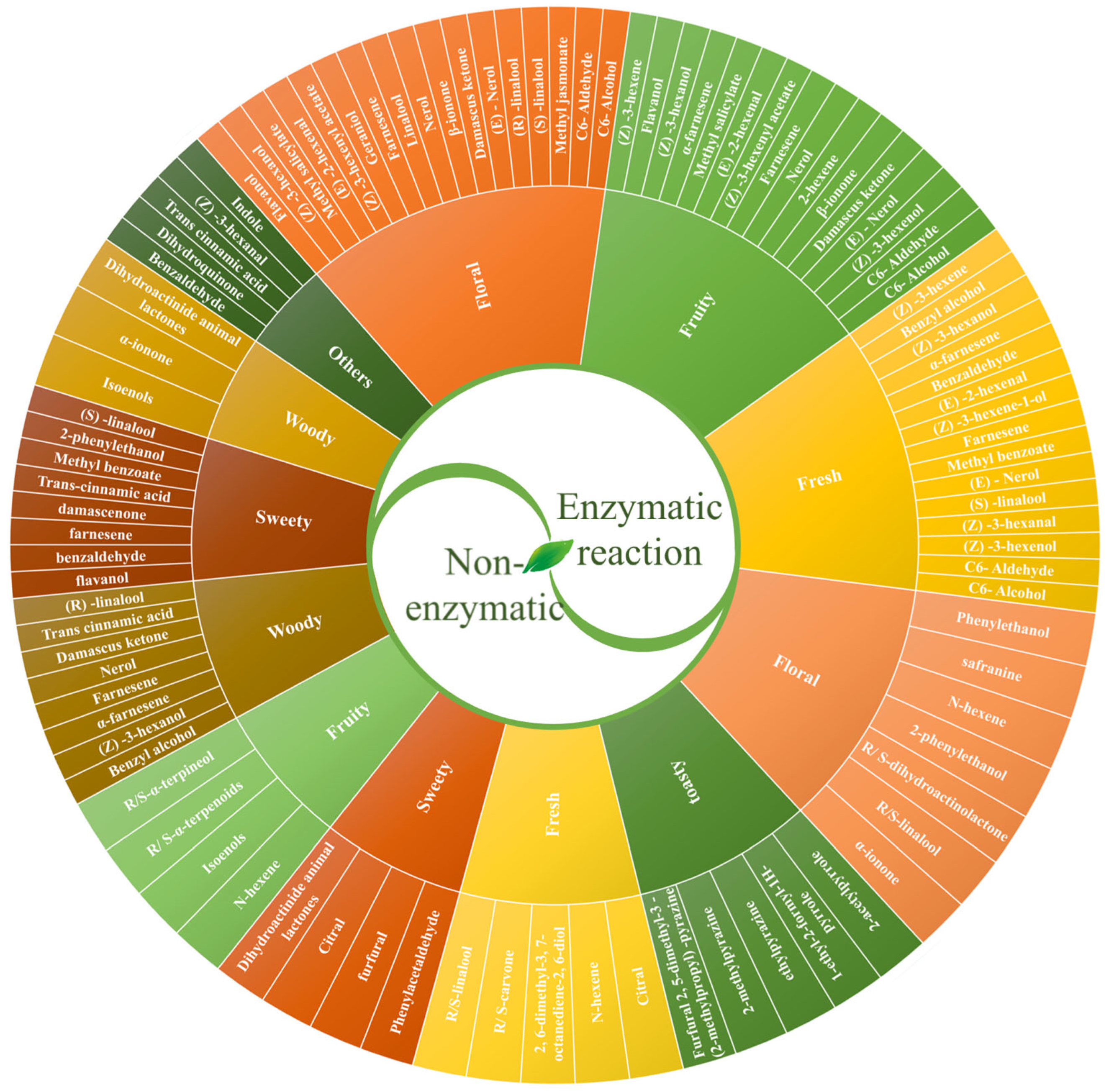
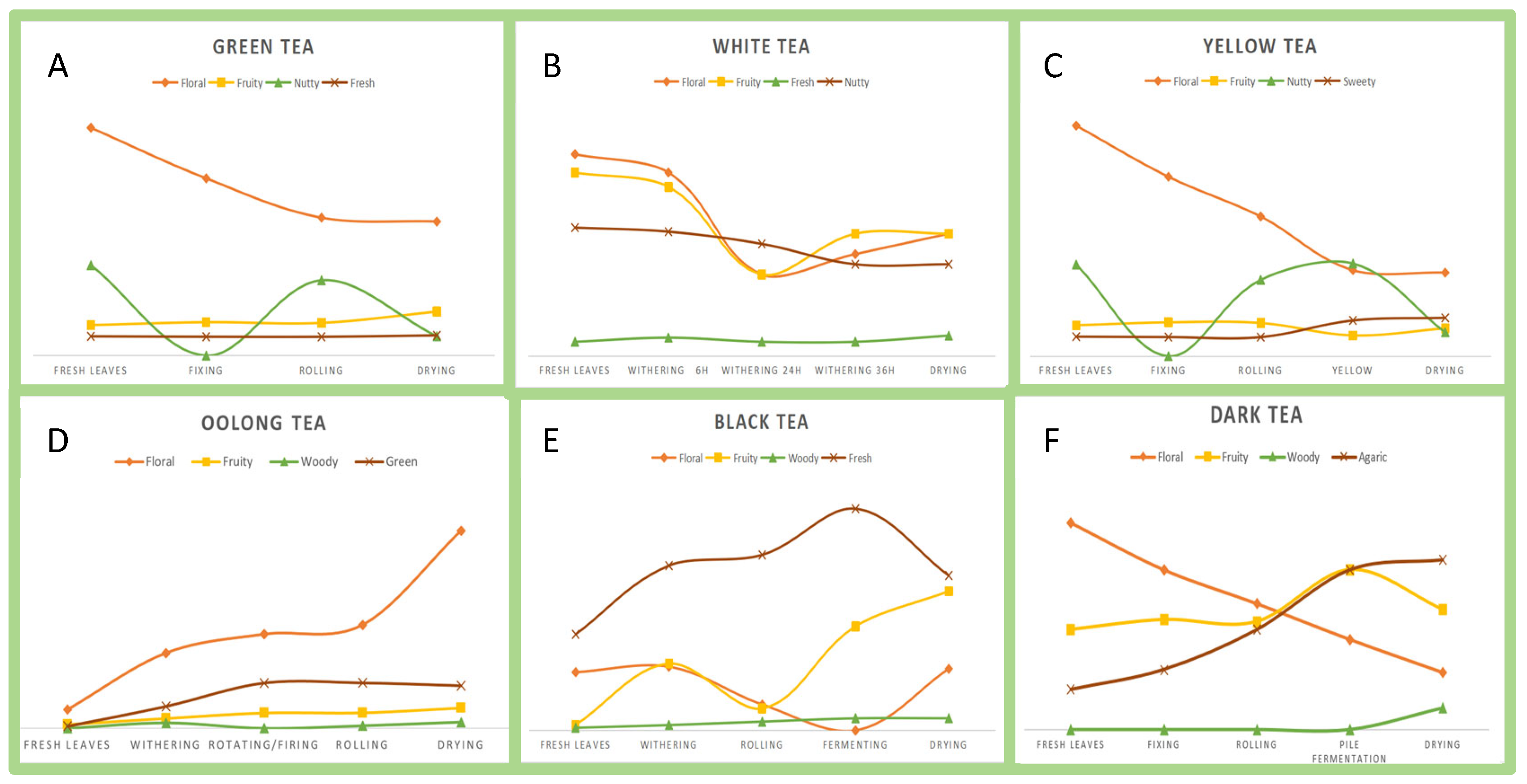
2.2. Tea Aroma Characteristics
| No. | Compound | Odor Descriptor a | Class | CAS | OT (mg/L) b | Key Aroma Compounds (OAV > 1) c | REF | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | WT | YT | OT | BT | DT | |||||||
| 1 | Acetic acid, 4-methylphenyl ester | narcissus, phenol, animalic (⁑) | Ester | 140-39-6 | 0.025 | + | [16] | |||||
| 2 | Acetic acid, hexyl ester | fruity, green, apple, banana, sweet (⁑) | Ester | 142-92-7 | 0.115 | + | [16] | |||||
| 3 | Acetophenone | must, flower, almond (#) | Ketone | 98-86-2 | 0.065 | + | [16] | |||||
| 4 | Anethole | sweet, exotic, flowery, stewed (⁑) | Aromatics | 104-46-1 | 0.015 | + | + | [10,16] | ||||
| 5 | Benzaldehyde | bitter almond-like (‡) | Aldehydes | 100-52-7 | 0.35 | + | + | + | + | [3,9,13,16,17] | ||
| 6 | Benzene, (isothiocyanatomethyl)- | mild, watercress, dusty, medicinal, horseradish, oily (⁑) | Sulfur compounds | 622-78-6 | 0.0007 | + | [16] | |||||
| 7 | Benzene, 1,3-bis(1,1-dimethylethyl)- | – | Aromatics | 1014-60-4 | 1.1 | + | [16] | |||||
| 8 | Benzene, 1-ethyl-3-methyl- | – | Aromatics | 620-14-4 | 0.088 | + | [16] | |||||
| 9 | Benzeneacetaldehyde | hyacinth, honey, clover, hawthorne, cocoa, grapefruit, peanut, floral (†) | Aldehydes | 122-78-1 | 3.33 | + | + | + | + | + | [3,6,10,14,18] | |
| 10 | Benzyl alcohol | bitter almond-like (‡) | Alcohols | 100-51-6 | 0.62 | + | + | [13] | ||||
| 11 | Beta-Ocimene | citrus, herbal, sweet, woody, terpene, herb (†) | Monoterpene | 3779-61-1 | 0.0187 | + | [14] | |||||
| 12 | Biphenyl | pungent, rose, green, geranium (⁑) | Aromatics | 92-52-4 | 0.0033 | + | + | [9,16] | ||||
| 13 | Butanoic acid, 2-methyl-, 2-methylpropyl ester | sweet, fruity (⁑) | Ester | 2445-67-2 | 0.043 | + | [16] | |||||
| 14 | Butanoic acid, 3-methyl-, propyl ester | bitter, sweet, apple, fruity (⁑) | Ester | 557-00-6 | 0.0087 | + | [16] | |||||
| 15 | Butanoic acid, butyl ester | fruity, banana, pineapple, green, cherry, tropical fruit, ripe fruit, juicy fruity (⁑) | Ester | 109-21-7 | 0.028 | + | [16] | |||||
| 16 | Camphor | camphor (⁑) | Terpenoids | 76-22-2 | 0.016 | + | [16] | |||||
| 17 | Carotol | pleasent, mild (⁑) | Terpenoids | 465-28-1 | 0.008 | + | [16] | |||||
| 18 | Carvone | minty, licorice (⁑) | Terpenoids | 99-49-0 | 0.067 | + | [16,17] | |||||
| 19 | Cedrol | wintergreen, woody (‡) | Alcohols | 77-53-2 | 0.064 | + | + | [3,17] | ||||
| 20 | Cis-Linalool Oxide | flower, woody (#) | Heterocyclics | 5989-33-3 | 0.006 | + | [18] | |||||
| 21 | Citronellol | floral, rose, lime (⁑) | Terpenoids | 106-22-9 | 0.04 | + | [16] | |||||
| 22 | Coumarin | sweet, bitter almond-like (‡) | Heterocyclics | 91-64-5 | 4.6 | + | [9] | |||||
| 23 | Decanal | citrus, soap, orange peel, tallow, waxy, floral, sweet, aldehydic (†) | Aldehydes | 112-31-2 | 0.0001 | + | + | + | + | [3,16] | ||
| 24 | Dihydroactinidiolide | fruity (‡) | Esters | 17,092-92-1 | 0.0021 | + | [1,9,17] | |||||
| 25 | Dimethyl sulfide | corn-like (‡) | Sulfur compounds | 75-18-3 | 0.0003 | + | + | [1,2,15] | ||||
| 26 | Dodecane | alkane (†) | Hydrocarbons | 112-40-3 | 0.04 | + | + | [5,16] | ||||
| 27 | Ethanone, 1-(2-aminophenyl)- | grape, sweet (⁑) | Ketone | 551-93-9 | 0.00027 | + | [16] | |||||
| 28 | Ethylbenzene | flower (#) | Aromatics | 100-41-4 | 0.029 | + | [3] | |||||
| 29 | Eucalyptol | eucalyptus, herbal, camphor, medicinal (⁑) | Terpenoids | 470-82-6 | 0.015 | + | [16] | |||||
| 30 | Eugenol | clove-like (‡) | Phenols | 97-53-0 | 0.0025 | + | + | [13,16] | ||||
| 31 | Furan, 2-pentyl- | fruity, green, earthy, beany, vegetable, metallic (⁑) | Heterocyclics | 3777-69-3 | 0.006 | + | [16] | |||||
| 32 | Geraniol | rose-like, citrus-like (‡) | Alcohols | 106-24-1 | 0.797 | + | + | + | + | + | + | [1,2,6,8,9,10,11,12,15,17] |
| 33 | Geranyl formate | fresh, rose, neroli, tea, rose, green (⁑) | Ester | 105-86-2 | 0.2 | + | [16] | |||||
| 34 | Geranyl isobutyrate | sweet, floral, fruity, green, peach, apricot, rose (⁑) | Ester | 2345-26-8 | 0.013 | + | [16] | |||||
| 35 | Geranylacetone | flowery, rose-like (‡) | Alcohols | 3796-70-1 | 8.72 | + | [17] | |||||
| 36 | Heptanal | heavy, planty green odor, apricot-like, nutty flavor (†) | Ketone | 111-71-7 | 0.003 | + | + | + | [2,15] | |||
| 37 | Heptanoic acid, ethyl ester | fruit (#) | Esters | 106-30-9 | 0.00116 | + | [4] | |||||
| 38 | Hexanal | grass, sweaty, tallow, fresh, fatty, fruity, aldehydic (#, †) | Aldehydes | 66-25-1 | 0.005 | + | + | + | + | [1,2,3,4,6,13,15,16] | ||
| 39 | Hexanoic acid | sweaty (‡) | Alkenes | 142-62-1 | 0.673 | + | [14] | |||||
| 40 | Indole | mothball-like, flowery (‡) | Heterocyclics | 120-72-9 | 0.04 | + | + | + | + | + | [1,9,10,11,13,16] | |
| 41 | Isoborneol | balsamic, camphor, herbal, woody (⁑) | Terpenoids | 124-76-5 | 0.0085 | + | [16] | |||||
| 42 | Isobutyl isovalerate | sweet, fruity, apple, raspberry, green, banana (⁑) | Ester | 589-59-3 | 0.034 | + | [16] | |||||
| 43 | Isopentyl hexanoate | fruity, banana, apple, pineapple, green (⁑) | Ester | 2198-61-0 | 0.32 | + | [16] | |||||
| 44 | Jasmine lactone | coconut, sweet (‡) | Esters | 25,524-95-2 | 1.65 | + | [10] | |||||
| 45 | Limonene | fresh orange, lemon-like (†) | Monoterpene | 138-86-3 | 0.01 | + | [5] | |||||
| 46 | Linalool | citrus-like, flowery (‡) | Alcohols | 78-70-6 | 0.0006 | + | + | + | + | + | + | [2,3,5,6,7,8,9,10,11,12,13,14,15,18] |
| 47 | Methyl salicylate | minty (‡) | Esters | 119-36-8 | 0.977 | + | + | + | + | + | + | [1,3,4,5,9,10,12,13,14,15,17] |
| 48 | Naphthalene | strong mothball odor, dry, pungent, tarry (#, †, *) | Heterocyclics | 91-20-3 | 0.05 | + | + | + | [3,9,16] | |||
| 49 | Naphthalene, 1,2-dihydro-1,1,6-trimethyl- | licorice (⁑) | Aromatics | 30,364-38-6 | 0.0025 | + | [16] | |||||
| 50 | Naphthalene, 1-methyl- | naphthyl, chemical, medicinal, camphor (⁑) | Aromatics | 90-12-0 | 0.008 | + | [16] | |||||
| 51 | Naphthalene, 2,6-dimethyl- | grassy (⁑) | Aromatics | 581-42-0 | 0.01 | + | [16] | |||||
| 52 | Naphthalene, 2-methyl- | sweet, floral, woody (⁑) | Aromatics | 91-57-6 | 0.004 | + | [16] | |||||
| 53 | Nonanal | citrus-like, soapy (‡) | Aldehydes | 124-19-6 | 0.001 | + | + | + | + | [1,2,3,4,6,7,17] | ||
| 54 | Octanal | citrus-like, green (‡) | Aldehydes | 124-13-0 | 0.0007 | + | + | [1,3,4] | ||||
| 55 | Octanoic acid, 3-methylbutyl ester | sweet, oily, fruity, green, soapy, pineapple, coconut (⁑) | Ester | 2035-99-6 | 0.07 | + | [16] | |||||
| 56 | Octanoic acid, ethyl ester | fruit, fat (#) | Ester | 106-32-1 | 0.01287 | + | [4] | |||||
| 57 | Pentanoic acid, 4-methyl-, ethyl ester | fruity (⁑) | Ester | 25,415-67-2 | 0.006 | + | [16] | |||||
| 58 | Phenol, 4-propyl- | medicinal, phenol (⁑) | Phenol | 645-56-7 | 0.107 | + | [16] | |||||
| 59 | Phenylethyl Alcohol | honey, rose, lilac (#) | Alcohols | 60-12-8 | 0.399 | + | + | + | + | + | [2,4,9,13,15] | |
| 60 | Styrene | balsamic, gasoline (#) | Alkenes | 100-42-5 | 0.000035 | + | [9] | |||||
| 61 | Trans-anethole | sweet, anisic, licorice, mimosa (⁑) | Aromatics | 4180-23-8 | 0.057 | + | [16] | |||||
| 62 | Trans-Rose oxide | floral (⁑) | Terpenoids | 876-18-6 | 0.0005 | + | [16] | |||||
| 63 | Trans-β-Ionone | violet-like, flower, raspberry-like aroma (†) | Ketone | 79-77-6 | <0.000001 | + | + | + | [3,6,9] | |||
| 64 | Tridecane | alkane (†) | Hydrocarbons | 629-50-5 | 0.042 | + | [16] | |||||
| 65 | Vanillin | vanilla-like, sweet (‡) | Aldehydes | 121-33-5 | 0.053 | + | [16] | |||||
| 66 | α-Farnesene | fruity (‡) | Sulfur compounds | 502-61-4 | - | + | [17] | |||||
| 67 | α-ionone | floral, sweet, woody, fruity, orris, raspberry, violet (†) | Ketone | 127-41-3 | 0.0004 | + | + | + | + | + | [4,9,11,13] | |
| 68 | β-cyclocitral | mint, saffron, damascone, sweet, fruity, rose oxide (†) | Others | 432-25-7 | 0.427 | + | + | [10,11,17] | ||||
| 69 | β-ionone | violet, orange, jam, seaweed, orris, raspberry, cedar wood odor (†, *) | Ketone | 79-77-6 | 0.574 | + | + | + | + | [1,8,10,11,15,17,18] | ||
| 70 | β-Myrcene | balsamic, must, spice (#) | Alkenes | 123-35-3 | 0.013 | + | + | [7] | ||||
| 71 | γ-Nonalactone | coconut-like (‡) | Esters | 104-61-0 | 0.03 | + | [11] | |||||
| 72 | (+)-Alpha-Pinene | – | Monoterpene | 7785-70-8 | 0.0053 | + | [14] | |||||
| 73 | (2E,4Z)-2,4-Decadienal | fried, fatty, geranium, green, waxy (⁑) | Aldehyde | 25,152-83-4 | 0.00007 | + | [16] | |||||
| 74 | (E)-2-Decenal | fatty, green (‡) | Aldehydes | 3913-81-3 | 0.572 | + | + | [10] | ||||
| 75 | (E)-2-Hexenal | green apple-like (‡) | Aldehydes | 6728-26-3 | 0.11 | + | [13] | |||||
| 76 | (E)-2-Nonenal | green, tallow-like (#) | Aldehyde | 40,435-58-1 | 0.00008 | + | + | [3] | ||||
| 77 | (E)-2-Octenal | fatty, nutty (‡) | Aldehydes | 2548-87-0 | 0.025 | + | + | + | [3,4] | |||
| 78 | (E)-2-Pentenal | fatty, fruity (‡) | Aldehydes | 1576-87-0 | 0.005 | + | [10] | |||||
| 79 | (E)-3-Penten-2-one | Fruity (#) | Ketone | 4549-33-3 | 0.0015 | + | [3] | |||||
| 80 | (E)-Nerolidol | flowery, woody (‡) | Alcohols | 40,716-66-3 | 0.006 | + | + | [3,10,11] | ||||
| 81 | (E)-β-Ionone | flowery, violet-like (‡) | Alcohols | 79-77-6 | 0.000036 | + | + | + | [2,7,15] | |||
| 82 | (E, E)-2,4-Heptadienal | fatty, flowery (‡) | Aldehydes | 4313-03-5 | 0.000032 | + | + | + | [1,8,17] | |||
| 83 | (E, E)-3,5-Octadien-2-One | creamy, fruity (#) | Ketone | 1569-08-4 | 0.0005 | + | [3] | |||||
| 84 | (Z)-3-Hexen-1-ol | green, grassy (‡) | Alcohols | 928-96-1 | 0.0039 | + | + | + | + | [1,5,9,13] | ||
| 85 | (Z)-Hex-3-En-1-Yl Hexanoate | tender, fresh, clean aroma (⁑) | Esters | 74,298-89-8 | 0.016 | + | [3] | |||||
| 86 | (Z)-Jasmone | woody, flowery (‡) | Alcohols | 488-10-8 | 0.00002 | + | + | [9,13] | ||||
| 87 | α-Ionone | sweet, woody, floral, violet, orris, tropical, fruity (⁑) | Terpenoids | 127-41-3 | 0.00378 | + | [16] | |||||
| 88 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | spicy, warm, balsamic, woody (⁑) | Aromatics | 607-91-0 | 0.088 | + | [16] | |||||
| 89 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | floral, green, waxy, citrus, woody (⁑) | Terpenoids | 7212-44-4 | 0.01 | + | [16] | |||||
| 90 | 1-Decanol | fatty, waxy, floral, orange, sweet, watery (⁑) | Alcohol | 112-30-1 | 0.023 | + | [16] | |||||
| 91 | 1-hexanol | oil, alcoholic, ethereal, resin, sweet, fruity, flower, green (†) | Alcohols | 111-27-3 | 0.0056 | + | + | [4,13] | ||||
| 92 | 1-Methylnaphthalene | pungent, rancid (#) | Aromatics | 90-12-0 | 0.00002 | + | [3] | |||||
| 93 | 1-Nonanol | fresh, clean, fatty, floral, rose, orange, dusty, wet, oily (⁑) | Alcohol | 143-08-8 | 0.0053 | + | [16] | |||||
| 94 | 1-Octanol | fresh orange rose odor penetrating aromatic odor, chemical, metal, burnt (#, †) | Alcohol | 111-87-5 | 0.022 | + | + | + | [4,12,16] | |||
| 95 | 1-Octen-3-Ol | mushroom, lavender, rose, hay (†) | Alcohols | 3391-86-4 | 0.001 | + | + | + | + | [5,6,9,15,17] | ||
| 96 | 2(3H)-Furanone, 5-ethyldihydro- | sweet, caramel (⁑) | Ester | 695-06-7 | 0.26 | + | [16] | |||||
| 97 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- | musky, coumarin (⁑) | Heterocyclic compound | 17,092-92-1 | 0.0021 | + | [16] | |||||
| 98 | 2,4-Decadienal, (E, E)- | dusty, waxy, oily, soapy (⁑) | Aldehyde | 25,152-84-5 | 0.00007 | + | [16] | |||||
| 99 | 2,4-Di-tert-butylphenol | Phenol (⁑) | Phenol | 96-76-4 | 0.5 | + | [16] | |||||
| 100 | 2,6-Nonadienal, (E, E)- | fresh, citrus, green, cucumber, melon (⁑) | Aldehyde | 17,587-33-6 | 0.0005 | + | [16] | |||||
| 101 | 2,6-Nonadienal, (E, Z)- | cucumber, green (⁑) | Aldehyde | 557-48-2 | 0.00001 | + | [16] | |||||
| 102 | 2-Acetylthiazole | nutty, popcorn, roasted, peanut, hazelnut (⁑) | Heterocyclic compound | 24,295-03-2 | 0.004 | + | [16] | |||||
| 103 | 2-Cyclopenten-1-one, 2-hydroxy-3,4-dimethyl- | strong, caramel (⁑) | Ketone | 21,835-00-7 | 0.02 | + | [16] | |||||
| 104 | 2-Decenal, (Z)- | Tallow (⁑) | Aldehyde | 2497-25-8 | 0.05 | + | [16] | |||||
| 105 | 2-Dodecenal, (E)- | citrus, metallic, mandarin, orange, waxy, aldehydic (⁑) | Aldehyde | 20,407-84-5 | 0.0073 | + | [16] | |||||
| 106 | 2-hexenal | apple, cheesy, vegetable, banana, rancid, fatty, plum, almond (#, †, *) | Aldehydes | 6728-26-3 | 0.00527 | + | + | [14,17] | ||||
| 107 | 2-Methylbutanal | Malty (‡) | Aldehydes | 96-17-3 | 0.0015 | + | [1] | |||||
| 108 | 2-methylisoborneol | must, earth (†) | Alcohols | 2371-42-8 | 0.000036 | + | [14] | |||||
| 109 | 2-Methylpropanal | malty, pungent (‡) | Aldehydes | 78-84-2 | 0.00049 | + | [1] | |||||
| 110 | 2-Nonenal, (Z)- | orris, fatty, waxy, cucumber (⁑) | Aldehyde | 60,784-31-8 | 0.0045 | + | [16] | |||||
| 111 | 2-Octen-1-ol, (E)- | green, citrus, vegetable, fatty (⁑) | Alcohol | 18,409-17-1 | 0.02 | + | [16] | |||||
| 112 | 2-Pentylfuran | bean-like, fruity, green, earthy and vegetable-like smell (†) | Furan | 3777-69-3 | 0.006 | + | + | [3] | ||||
| 113 | 2-Propenal, 3-phenyl- | sweet, spicy, aldehydic, aromatic, balsamic, cinnamyl, resinous, honey, powdery (⁑) | Aldehyde | 104-55-2 | 0.024 | + | [14,16] | |||||
| 114 | 3-Mercapto-3-methylbutyl formate (ester) | sulfury, catty, caramel, onion, roasted coffee, roasted meat, tropical (⁑) | Ester | 50,746-10-6 | 0.000002 | + | [16] | |||||
| 115 | 3-Mercaptohexanol | sulfury, fruity, tropical (⁑) | Alcohol | 51,755-83-0 | 0.00006 | + | [16] | |||||
| 116 | 3-Methylbutanal | malty (‡) | Aldehydes | 590-86-3 | 0.0005 | + | [1,3] | |||||
| 117 | 3-Octanol | earthy, mushroom, herbal, melon, citrus, woody, spicy, minty (⁑) | Alcohol | 589-98-0 | 0.078 | + | [16] | |||||
| 118 | 3-Octanone | fresh, herbal, lavender, sweet, mushroom (⁑) | Ketone | 106-68-3 | 0.0013 | + | [16] | |||||
| 119 | 4-Decenoic acid, methyl ester, Z- | fruity, pear, mango, fishy, peach skin, green (⁑) | Ester | 7367-83-1 | 0.003 | + | [16] | |||||
| 120 | 4-Ethyl-1,2-Dimethoxybenzene | Mature (†) | Ethers | 5888-51-7 | 0.0034 | + | [18] | |||||
| 121 | 4-Phenyl-2-butanol | floral, peony, foliage, sweet, mimosa, heliotrope (⁑) | Alcohol | 2344-70-9 | 0.0043 | + | [16] | |||||
| 122 | 5-Methyl-2-thiophenecarboxaldehyde | sweet, almond, cherry, furfural, woody, acetophenone (⁑) | Heterocyclic compound | 13,679-70-4 | 0.001 | + | [16] | |||||
| 123 | 6-Methyl-5-hepten-2-one | fruity, nutty (‡) | Alcohols | 110-93-0 | 0.656 | + | + | + | [1,10,15] | |||
| 124 | 6-Nonenal, (E)- | – | Aldehyde | 2277-20-5 | 0.000022 | + | [16] | |||||
| 125 | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | citronella oil, rose, leafy, oily, petal (⁑) | Terpenoids | 1117-61-9 | 0.04 | + | [16] | |||||
3. VOCs Extraction in Teas
3.1. Simultaneous Distillation–Extraction
3.2. Solvent-Assisted Flavor Evaporation
3.3. Headspace Analysis
3.4. Supercritical Fluid Extraction
3.5. Solid-Phase Microextraction
4. VOCs Analysis in Teas
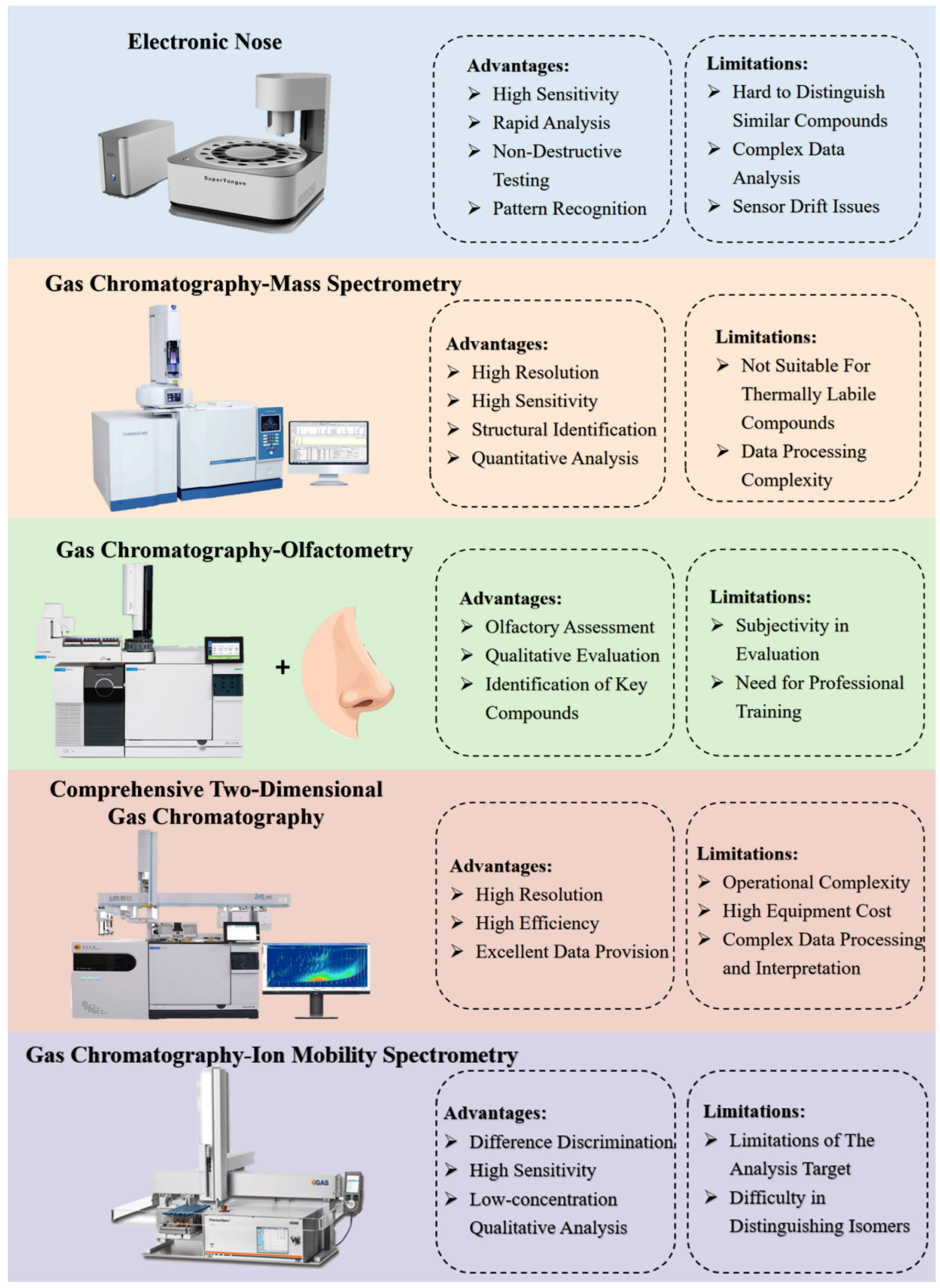
4.1. E-Nose in VOCs Analysis
4.2. GC-MS in VOCs Analysis
4.3. GC-O in VOCs Analysis
4.4. GC × GC in VOCs Analysis
4.5. GC-IMS in VOCs Analysis
4.6. Advanced Analysis in Aroma Identification
5. Application of Tea Aroma
5.1. Essential Oil Application
5.2. Food Additive Application
5.3. Daily Necessities Application
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOC | Volatile Organic Compounds |
| OAV | Odor Activity Value |
| SDE | Simultaneous Distillation-Extraction |
| SAFE | Solvent-Assisted Flavor Evaporation |
| HSA | Headspace Analysis |
| SFE | Supercritical Fluid Extraction |
| SPME | Solid-Phase Microextraction |
| E-nose | Electronic Nose |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| GC-O | Gas Chromatography-Olfactometry |
| GC × GC | Comprehensive Two-Dimensional Gas Chromatography |
| GC-IMS | Gas Chromatography-Ion Mobility Spectrometry |
| GC × GC-TOFMS | Comprehensive Two-Dimensional Gas Chromatography Time-Of-Flight Mass Spectrometry |
| ATD-GC-MS | Automated Thermal Desorption-Gas Chromatography-Mass Spectrometry |
| GC-QqQ MS | Gas Chromatography- quadrupole Mass Spectrometry |
| GC-TOF MS | Gas Chromatography-Time-of-Flight Mass Spectrometry |
| MALDI | Matrix-Assisted Laser Desorption/Ionization |
| ESI | Electrospray Ionization |
| BC | Breast Carcinoma |
| RSM | Response Surface Methodology |
| AI | Artificial Intelligence |
| PCA-SVM | Principal Component Analysis-Support Vector Machine |
| PCA-WNN | Principal Component Analysis-Wavelet Neural Network |
References
- Tang, H.; Zhang, M.; Liu, J.; Cai, J. Metabolomic and Transcriptomic Analyses Reveal the Characteristics of Tea Flavonoids and Caffeine Accumulation and Regulation between Chinese Varieties (Camellia sinensis Var. Sinensis) and Assam Varieties (C. sinensis Var. Assamica). Genes 2022, 13, 1994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Jian, G.; Zeng, L.; Gu, D.; Su, X.; Yang, Z.; Zhou, X.; Yang, J.; Jian, G.; et al. Role of Histone Deacetylase CsHDA8 in Regulating the Accumulation of Indole during the Oolong Tea Manufacturing Process. Beverage Plant Res. 2022, 2, 17. [Google Scholar] [CrossRef]
- Zou, D.; Yin, X.L.; Gu, H.W.; Peng, Z.X.; Ding, B.; Li, Z.S.; Hu, X.C.; Long, W.J.; Fu, H.Y.; She, Y.B. Insight into the Effect of Cultivar and Altitude on the Identification of EnshiYulu Tea Grade in Untargeted Metabolomics Analysis. Food Chem. 2024, 436, 137768. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.; Wan, X. Flavor of Tea (Camellia sinensis): A Review on Odorants and Analytical Techniques. Comp. Rev. Food Sci. Food Safe 2022, 21, 3867–3909. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, C.; Guo, Y.; Xu, Y.; Gong, S.; Chu, Q.; Chen, P. Unraveling the Contributing Factors of Stale Odor in Longjing Tea through a Sensomics Approach. Food Chem. 2024, 441, 138301. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Wang, J.Q.; Wu, Z.Q.; Huang, J.; Li, Z.B.; Xu, J.Y.; Long, D.; Ye, T.; Wang, G.N.; Yin, J.F.; et al. The Role of Glutathione in Stabilizing Aromatic Volatile Organic Compounds in Rougui Oolong Tea: A Comprehensive Study from Content to Mechanisms. Food Chem. 2024, 437, 137802. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Guo, W.; Liang, J.; Liu, Z. Unraveling the Key Cooked Off-Flavor Compounds in Thermally Sterilized Green Tea Beverages, and Masking Effect of Tea Raw Material Baking. Food Chem. 2025, 464, 141671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Yin, H.X.; Yuan, H.B.; Jiang, Y.W.; Dong, C.W.; Deng, Y.L. Characterization of the Volatile Components in Green Tea by IRAE-HS-SPME/GC-MS Combined with Multivariate Analysis. PLoS ONE 2018, 13, e0193393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, Z.; Yin, P.; Ma, B.; Ma, C.; Xu, C.; Wang, J.; Wang, Z.; Yin, D.; Xia, T. Impact of Prolonged Withering on Phenolic Compounds and Antioxidant Capability in White Tea Using LC-MS-Based Metabolomics and HPLC Analysis: Comparison with Green Tea. Food Chem. 2022, 368, 130855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Liu, Y.; Liu, Y.; Dong, C.; Lin, Z.; Teng, J. Black Tea Aroma Formation during the Fermentation Period. Food Chem. 2022, 374, 131640. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Schieberle, P. Characterization of the Key Aroma Compounds in the Beverage Prepared from Darjeeling Black Tea: Quantitative Differences between Tea Leaves and Infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Lv, J.; Qiu, G.; Tian, H. Description of Key Aroma Components of Green Tea and the Influence of Processing. J. Food Compos. Anal. 2025, 141, 107367. [Google Scholar] [CrossRef]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the Biosyntheses and Stress Response Mechanisms of Aroma Compounds in Tea (Camellia sinensis) to Safely and Effectively Improve Tea Aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Yu, Y.; Zhu, Y.; Ma, J.; Liu, Z.; Ni, L.; Lin, C.-C.; Wang, K.; Liu, Y. Exploration of the Flavor Diversity of Oolong Teas: A Comprehensive Analysis Using Metabolomics, Quantification Techniques, and Sensory Evaluation. Food Res. Int. 2024, 195, 114868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Kun, J.R.; Dai, H.; Tong, H.R. Changes in Aroma Compounds and Selected Precursors of Sun-Dried Green Tea during Processing. FOOD Sci. 2024, 45, 133. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of Key Odorants Responsible for Chestnut-like Aroma Quality of Green Teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma Formation and Dynamic Changes during White Tea Processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yin, X.; Wu, H.; Zhao, M.; Huang, J.; Zhang, J.; Li, T.; Ning, J. Improving the Flavor of Summer Green Tea (Camellia sinensis L.) Using the Yellowing Process. Food Chem. 2022, 388, 132982. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, Y.; Zhang, Q.; Wang, Y.; Chen, M.; Li, M.; Huang, Y.; Wu, Z.; Ye, J.; Wang, H. Effects of Processing Procedures on the Formation of Aroma Intensity and Odor Characteristic of Benshan Tea (Oolong Tea, Camellia sentences). Heliyon 2023, 9, e14855. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hua, J.; Deng, Y.; Jiang, Y.; Qian, M.C.; Wang, J.; Li, J.; Zhang, M.; Dong, C.; Yuan, H. Aroma Dynamic Characteristics during the Process of Variable-Temperature Final Firing of Congou Black Tea by Electronic Nose and Comprehensive Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. Food Res. Int. 2020, 137, 109656. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhong, Q.; Wang, L.; Lin, Z. Changing Law of Aroma Components during Processing of Pu’er Tea. Tea Sci. 2009, 29, 95–101. [Google Scholar]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of Volatile Contributions in Green Teas with Different Aroma Types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Li, C.; Lv, S.; Gao, J.; Xia, Y.; Geng, Y. Study on the Effects of Processes on Aroma Compounds in Rizhao Green Tea Based on the Gas Chromatography-Ion Mobility Spectrometry. J. Food Process. Preserv. 2023, 2023, 7447. [Google Scholar] [CrossRef]
- Xie, J.; Wang, L.; Deng, Y.; Yuan, H.; Zhu, J.; Jiang, Y.; Yang, Y. Characterization of the Key Odorants in Floral Aroma Green Tea Based on GC-E-Nose, GC-IMS, GC-MS and Aroma Recombination and Investigation of the Dynamic Changes and Aroma Formation during Processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, L.; Zhang, Y.; Liu, Y.; Yang, R.; Dai, X.; Wang, T.; Lv, C.; Zuo, L.; Liu, Y.; et al. Effect of Drying Methods on Aroma Profiling of Large-Leaf Green Tea (Camellia sinensis Var. Assamica) Determined by HS-SPME-GC-MS. Foods 2025, 14, 1275. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, Y.; Lin, J.; Lv, H.; Zhou, W.; Zhou, X.; Qian, Y.; Zeng, X.; He, W.; Ye, Y. Comparison of Volatile and Nonvolatile Metabolites in Green Tea under Hot-Air Drying and Four Heat-Conduction Drying Patterns Using Widely Targeted Metabolomics. Food Chem. X 2023, 19, 100767. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Si, X.; Li, P.; Li, J.; Chen, Z.; Li, P.; Chen, C.; Liu, Z.; Zhao, J. Dynamic Change of Tea (Camellia sinensis) Leaf Cuticular Wax in White Tea Processing for Contribution to Tea Flavor Formation. Food Res. Int. 2023, 163, 112182. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Zheng, X.; Li, S.M. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.-X.; Zhu, Y.-L.; Lin, Z.-Y.; Chen, D.; Zhang, Y.; Lv, H.-P.; Dai, W.-D.; Ni, D.-J.; Lin, Z.; et al. Comprehensive Comparison of Aroma Profiles and Chiral Free and Glycosidically Bound Volatiles in Fujian and Yunnan White Teas. Food Chem. 2024, 448, 139067. [Google Scholar] [CrossRef] [PubMed]
- Gemert, L.V. Compilations of Odour Threshold Values in Air, Water and Other Media, Second Enlarged and Revised ed.; Boelens Aroma Chemical Information Service: Huizen, The Netherlands, 2003. [Google Scholar]
- Sheng, C.; Lu, M.; Liu, Q.; Zhou, H.; Xiong, Z.; Li, T.; Wei, Y.; Zhang, J.; Ke, H.; Wu, Y.; et al. Differences in the Aroma Quality of Large-Leaf Yellow Tea Subjected to Different Roasting Methods. LWT 2024, 204, 116475. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Q.; Qin, M.; Xu, W.; Wang, X.; Zhou, J.; He, C.; Chen, Y.; Yu, Z.; Ni, D. Study on Color, Aroma, and Taste Formation Mechanism of Large-Leaf Yellow Tea during an Innovative Manufacturing Process. Food Chem. 2024, 438, 138062. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, C.; Jiang, R.; Hu, T.; Zheng, X.; Huang, J.; Liu, Z.; Li, Q. Characterization of the Key Aroma Compounds in Different Aroma Types of Chinese Yellow Tea. Foods 2023, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Han-yu, Y. Research Progress of Factors Effecting Yellow Tea Quality and Processing Technology. Tea Commun. 2013, 40, 4. [Google Scholar]
- Wang, Q.; Zhao, X.; Qian, Y.; Wang, R. In Vitro Antioxidative Activity of Yellow Tea and Its in Vivo Preventive Effect on Gastric Injury. Exp. Ther. Med. 2013, 6, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Schwab, W.; Ho, C.T.; Song, C.K.; Wan, X.C. Characterization of the Aroma Profiles of Oolong Tea Made from Three Tea Cultivars by Both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Peng, Z.; Zhu, Q.; Chen, C.; Liu, J.; Fu, S.; Zhang, J. Analysis of Tieguanyin Aged Aroma Compounds and Their Correlation with Microbial Communities. LWT 2023, 185, 115205. [Google Scholar] [CrossRef]
- He, C.; Zhou, J.; Li, Y.; Zhang, D.; Ntezimana, B.; Zhu, J.; Wang, X.; Xu, W.; Wen, X.; Chen, Y.; et al. The Aroma Characteristics of Oolong Tea Are Jointly Determined by Processing Mode and Tea Cultivars. Food Chem. X 2023, 18, 100730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Zhao, Y.; Zhu, M.; Li, J.; Wang, K. A Meta-Analysis of Dynamic Changes of Key Aroma Compounds during Black Tea Processing. Food Biosci. 2024, 58, 103784. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and Identification of Minor and Aroma-Active Constituents in Kangra Orthodox Black Tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea Aroma Formation from Six Model Manufacturing Processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, Q.; Li, Q.; Ou, X.; Li, S.; Liu, Z.; Huang, J. Multiplex PCR Identification of Aspergillus Cristatus and Aspergillus Chevalieri in Liupao Tea Based on Orphan Genes. Foods 2022, 11, 2217. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Wang, M.Q.; Liu, C.M.; Ma, W.J.; Zhu, Y.; Lin, Z.; Lü, H.P. Analysis of Volatile Composition and Key Aroma Compounds of Liupao Tea. Food Sci. 2020, 41, 191. [Google Scholar] [CrossRef]
- Li, Q.; Hong, X.; Zheng, X.; Xu, Y.; Lai, X.; Teng, C.; Wu, W.; Huang, J.; Liu, Z. Characterization of Key Aroma Compounds and Core Functional Microorganisms in Different Aroma Types of Liupao Tea. Food Res. Int. 2022, 152, 110925. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Guo, X.; Liu, G.; Song, Y.; Ho, C.; Hou, R.; Zhang, L.; Wan, X. A Comparative Analysis for the Volatile Compounds of Various Chinese Dark Teas Using Combinatory Metabolomics and Fungal Solid-State Fermentation. J. Food Drug Anal. 2018, 26, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, Y.; Wang, K.; Niu, Y.; Xiao, Z. Characterization of Key Aroma Compounds and Enantiomer Distribution in Longjing Tea. Food Chem. 2021, 361, 130096. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhou, J.; Luo, Q.; Zhu, J.; Yu, Z.; Zhang, D.; Ni, D.; Chen, Y. The Key Aroma Components of Steamed Green Tea Decoded by Sensomics and Their Changes under Different Withering Degree. Food Chem. 2024, 439, 138176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, T.; Xie, H.; Ou, X.; Huang, J.; Wang, C.; Liu, Z.; Li, Q. Characterization of the Key Odor-Active Compounds in Different Aroma Types of Fu Brick Tea Using HS-SPME/GC-MSO Combined with Sensory-Directed Flavor Analysis. Food Chem. 2023, 426, 136527. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Feng, J.; Chen, Q.; Lin, H.; Zhou, X.; Zhuang, J.; Wang, J.; Tan, Y.; Sun, Z.; Wang, Y.; et al. Comparative Volatiles Profiling in Milk-Flavored White Tea and Traditional White Tea Shoumei via HS-SPME-GC-TOFMS and OAV Analyses. Food Chem. X 2023, 18, 100710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.C.; Wang, M.Q.; Ni, D.J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Chen, Z.; Xiong, Z.; Feng, W.; Wei, Y.; Li, T.; Ning, J. Sensory-Directed Flavor Analysis of Jinggu White Tea: Exploring the Formation Mechanisms of Sweet and Fruity Aromas. Food Chem. X 2024, 24, 102026. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Hu, Y.; Pei, Z.; Yu, J.; Li, M.; Zhang, L.; Ho, C.-T.; Zhang, Y.; Wan, X. Insights into the Key Odorants in Large-Leaf Yellow Tea (Camellia sinensis) by Application of the Sensomics Approach. J. Agric. Food Chem. 2023, 71, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, M.; Dong, Z.; Zhu, Y.; Shi, J.; Ma, W.; Lin, Z.; Lv, H. Volatile Components and Key Odorants of Chinese Yellow Tea (Camellia sinensis). LWT 2021, 146, 111512. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Z.; Zhang, L.; Dai, H.; Wu, W.; Zheng, Z.; Lin, F.; Xu, J.; Huang, Y.; Sun, W. Characterization of Volatile Compounds and Identification of Key Aroma Compounds in Different Aroma Types of Rougui Wuyi Rock Tea. Food Chem. 2024, 455, 139931. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Lin, S.; Hong, L.; Kang, J.; Chen, Y.; Li, M.; Jia, Y.; Jia, X.; Wu, Z.; et al. Effect of Processing on Aroma Intensity and Odor Characteristic of Shuixian (Camellia sinensis) Tea. Food Chem. X 2023, 17, 100616. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Wang, Y.J.; Li, J.Y.; Zhang, J.X.; Wei, Y.M.; Yan, Y.X.; Wang, H.P.; Pan, Y.; Xiong, Z.C.; Wang, R.J.; et al. Aroma Formation Mechanism by the Drying Step during Congou Black Tea Processing: Analyses by HP-SPME and SAFE with GC-MS. LWT 2024, 198, 116019. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Wen, S.; Chen, R.; Sun, S.; Lai, X.; Li, Q.; Zhang, Z.; Lai, Z.; Li, Z.; et al. Analysis of Aroma Quality Changes of Large-Leaf Black Tea in Different Storage Years Based on HS-SPME and GC–MS. Food Chem. X 2023, 20, 100991. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Sheng, C.; Ke, H.; Li, T.; Liu, Q.; Zhang, J.; Li, L.; Wang, Y.; Ning, J. Revealing the Differences in Aroma of Black Tea under Different Drying Methods Based on GC–MS, GC-O. Food Chem. X 2024, 23, 101782. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, H.; Lu, M.; Li, Y. HS-SPME-GC-MS Untargeted Metabolomics Reveals Key Volatile Compound Changes during Liupao Tea Fermentation. Food Chem. X 2024, 23, 101764. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, L.; Kun, J.; He, S.; Tong, H.; Chen, Y. The Unique Aroma of Ripened Pu-Erh Tea, Liupao Tea and Tietban Tea: Associated Post-Fermentation Condition and Dominant Microorganism with Key Aroma-Active Compound. Food Chem. 2025, 464, 141788. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tian, Y.; Zhao, F.; Wang, R.; Zhou, H.; Zhang, N.; Wang, Y.; Shan, Z.; Zhang, C. Analysis of the Key Aroma Components of Pu’er Tea by Synergistic Fermentation with Three Beneficial Microorganisms. Food Chem. X 2024, 21, 101048. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, G.B.; Likens, S.T. Gas Chromatography Evidence for the Occurrence of Hop Oil Components in Beer. J. Chromatogr. A 1966, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Shigematsu, H.; Shiratsuchi, H.; Osajima, Y. Comparison of the Odor Concentrates by SDE and Adsorptive Column Method from Green Tea Infusion. J. Agric. Food Chem. 1995, 43, 1616–1620. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation—A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, X.; Zou, C.; Yin, J. Tea Aroma Analysis Based on Solvent-Assisted Flavor Evaporation Enrichment. JoVE 2023, 195. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.C.; García-Sarrió, M.J.; Sanz, M.L. Volatile Sampling by Headspace Techniques. TrAC Trends Anal. Chem. 2015, 71, 85–99. [Google Scholar] [CrossRef]
- Wang, Y.; McCaffrey, J.; Norwood, D.L. Recent Advances in Headspace Gas Chromatography. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1823–1851. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.; Xu, H.; Wang, X. A Novel Quality Evaluation Index and Strategies to Identify Scenting Quality of Jasmine Tea Based on Headspace Volatiles Analysis. Food Sci. Biotechnol. 2013, 22, 331–340. [Google Scholar] [CrossRef]
- Zheng, X.T.; Zeng, X.Y.; Lin, X.L.; Chen, D.S.; Li, Y.; Huang, J.J.; Yu, Z.C.; Zhu, H. Exploring Aromatic Components Differences and Composition Regularity of 5 Kinds of These 4 Aroma Types Phoenix Dancong Tea Based on GC–MS. Sci. Rep. 2024, 14, 2727. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Benado, A.; Zollweg, J.; Daniels, J. Supercritical Fluid Extraction—Fundamental Principles and Modeling Methods. Food Technol. 1986, 40, 55–65. [Google Scholar]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and Possibilities of Supercritical CO2 Extraction in Food Processing Industry: An Overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Gruosso, T.; Mieulet, V.; Cardon, M.; Bourachot, B.; Kieffer, Y.; Devun, F.; Dubois, T.; Dutreix, M.; Vincent-Salomon, A.; Miller, K.M.; et al. Chronic Oxidative Stress Promotes H2 AX Protein Degradation and Enhances Chemosensitivity in Breast Cancer Patients. EMBO Mol. Med. 2016, 8, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Catherine, L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Baptista, J.; Tavares, J.; Carvalho, R. Comparison of Catechins and Aromas among Different Green Teas Using HPLC/SPME-GC. Food Res. Int. 1998, 31, 729–736. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wu, X.; Zhang, Y.; He, Z.; Zhang, Y.; Zhang, X.; Li, Q.; Huang, J.; Liu, Z. Pu-Erh Tea Unique Aroma: Volatile Components, Evaluation Methods and Metabolic Mechanism of Key Odor-Active Compounds. Trends Food Sci. Technol. 2022, 124, 25–37. [Google Scholar] [CrossRef]
- Sun, L.; Dong, X.; Ren, Y.; Agarwal, M.; Ren, A.; Ding, Z. Profiling Real-Time Aroma from Green Tea Infusion during Brewing. Foods 2022, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, E.; Duncan, S.E.; Kuhn, D.D.; Dietrich, A.M.; O’Keefe, S.F. SDE and SPME Analysis of Flavor Compounds in Jin Xuan Oolong Tea. J. Food Sci. 2016, 81, C348–C358. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Xu, M.; Wei, K.; Dai, Q.; Wan, X.; Leong, O.; Lin, R.; Cui, C.; Hou, R. The Chemical Basis of Aroma/Taste and Color Formation in Green Tea Infusion during Cold Brewing Revealed by Metabolomics Analysis. Food Chem. 2025, 479, 143788. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R. Development of a Low-Cost e-Nose to Assess Aroma Profiles: An Artificial Intelligence Application to Assess Beer Quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and Recent Advances in Electronic Nose for Quality Inspection of Agricultural and Food Products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile Compounds, Affecting Factors and Evaluation Methods for Rice Aroma: A Review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Wen, M.C.; Zhu, M.T.; Han, Z.S.; Ho, C.T.; Granato, D.; Zhang, L. Comprehensive Applications of Metabolomics on Tea Science and Technology: Opportunities, Hurdles, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4890–4924. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Ren, L.; Shi, X.; Chang, Z. Soil Pesticides Pollution Detection and Specific Recognition Using Electronic Nose. Sens. Actuators B Chem. 2024, 408, 135492. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, F.; Shimizu, K.; Kinoshita, T.; Kanamori, M.; Morita, A.; Watanabe, N. Identification of Coumarin-Enriched Japanese Green Teas and Their Particular Flavor Using Electronic Nose. J. Food Eng. 2009, 92, 312–316. [Google Scholar] [CrossRef]
- Qin, Z.; Pang, X.; Chen, D.; Cheng, H.; Hu, X.; Wu, J. Evaluation of Chinese Tea by the Electronic Nose and Gas Chromatography–Mass Spectrometry: Correlation with Sensory Properties and Classification According to Grade Level. Food Res. Int. 2013, 53, 864–874. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wang, H.; Cheng, C.; Zhang, X.; Xue, J.; Zhou, S.; Li, B.; Li, T.; Zhang, Y.; et al. Comparative Analysis of Asparagus Tea Processing and Flavor Component Analysis. LWT 2024, 194, 115795. [Google Scholar] [CrossRef]
- Cronin, D.A. Principles and Applications of Gas Chromatography in Food Analysis: Edited by Michael H. Gordon, Ellis Horwood, 1990. £59.95 (368 Pages) ISBN 0 7476 0053 8. Trends Food Sci. Technol. 1990, 1, 94. [Google Scholar] [CrossRef]
- Zhang, S.; Long, X.; Xing, Y.; Ee, K.-H.; Goh, R.M.V.; Huang, Y.; Pua, A.; Jublot, L.; Liu, S.Q.; Yu, B. A Systematic Approach to Analyzing Catechins and Catechin Derivatives in Ceylon Black Tea Using Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry. Food Chem. X 2025, 28, 102621. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Li, S.; Wang, H.; Yu, P.; Shao, H.; Wang, S.; Wang, H.; Jin, F. Unveiling Key Aroma-Active Compounds of Two Distinct Aroma-Types Oriental Melon: An Integrated Sensomics Approach Utilizing GC × GC-O-TOF-MS, GC–MS, Aroma Recombination and Omission Experiments. Food Chem. X 2025, 28, 102517. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zou, Y.; Liao, G.; Zheng, Z.; Chen, G.; Zhong, Y.; Wang, G. Identification of Characteristic Flavor Compounds and Small Molecule Metabolites during the Ripening Process of Nuodeng Ham by GC-IMS, GC–MS Combined with Metabolomics. Food Chem. 2024, 440, 138188. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the Changes of Aroma Profiles in Large-Leaf Yellow Tea during Processing Using GC–MS and Electronic Nose Analysis. Food Chem. X 2025, 27, 102507. [Google Scholar] [CrossRef] [PubMed]
- Dellacassa, E.; Minteguiaga, M.A. Gas Chromatography-Olfactometry (GC-O) of Essential Oils and Volatile Extracts. In Essential Oils; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 477–500. ISBN 978-1-119-82961-4. [Google Scholar]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the Key Aroma-Active Compounds in High-Grade Dianhong Tea Using GC-MS and GC-O Combined with Sensory-Directed Flavor Analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Huang, G.; Ren, L.; Li, Y.; Yu, J.; Lu, Q.; Yang, Y.; Deng, X.; Li, Y.; Zhou, H. Effects of Monascus Purpureus on Ripe Pu-Erh Tea in Different Fermentation Methods and Identification of Characteristic Volatile Compounds. Food Chem. 2024, 440, 138249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, N.; Yu, T.; Gao, J.; Fan, Y.; Wang, W.; Wang, J.; Wu, Y.; Zhang, J.; Ning, J. The Enhancement of Flowery-like Aroma in Green Tea under Optimized Processing Conditions by Sensory-Directed Flavor Analysis. Food Chem. X 2024, 22, 101427. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.; Shellie, R.; Cornwell, C. Gas Chromatographic Technologies for the Analysis of Essential Oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, Q.; Guo, T. Variation of Taste and Odor Compounds in Tea Beverage after Microbial Fermentation by HPLC–MS, GC×GC–O–MS, GC–MS, and Sensory Evaluation. J. Food Compos. Anal. 2024, 128, 106075. [Google Scholar] [CrossRef]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.P.; Zhang, Y.; Dai, W.D.; Guo, L.; Tan, J.F.; Peng, Q.H.; et al. Identification and Quantification of Key Odorants in the World’s Four Most Famous Black Teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Lan, X.; Wang, C.; Chen, W.; Zhan, S.; Sun, Y.; Su, W.; Lin, C.-C.; Liu, W.; et al. Unveiling the Aromatic Intricacies of Wuyi Rock Tea: A Comparative Study on Sensory Attributes and Odor-Active Compounds of Rougui and Shuixian Varieties. Food Chem. 2024, 435, 137470. [Google Scholar] [CrossRef] [PubMed]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26, 5457. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, T.; Yang, D.; Xie, J. Application of Gas Chromatography-Ion Mobility Spectrometry in the Analysis of Food Volatile Components. AChrom 2023, 35, 35–45. [Google Scholar] [CrossRef]
- Yan, F.; Chen, X.; Qu, D.; Huang, W.; He, L.; Wan, T.; Zhang, L.; Wang, Q.; Hu, C.Y. Determination of Geographical Origin of Southern Shaanxi Congou Black Teas Using Sensory Analysis Combined with Gas Chromatography–Ion Mobility Spectrometry. Foods 2024, 13, 3904. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Wan, X.C.; Ho, C.T. Application of Gas Chromatography-Ion Mobility Spectrometry in Tea (Camellia sinensis): A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70119. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rong, Y.; Liu, F.; Jiang, Y.; Deng, Y.; Dong, C.; Yuan, H. Rapid Characterization of the Volatile Profiles in Pu-erh Tea by Gas Phase Electronic Nose and Microchamber/Thermal Extractor Combined with TD-GC-MS. J. Food Sci. 2021, 86, 2358–2373. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yan, T.; Yang, J.; Xu, H. Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose. Horticulturae 2023, 9, 885. [Google Scholar] [CrossRef]
- Xu, H.; Jing, T.; Cheng, Y.; Zheng, M.; Li, Y.; Gu, L.; Rao, Y.; Song, C.; Jing, H.; Li, K. Machine Learning-Assisted ZnO-Based Sensor for Multi-Species Recognition of Volatile Aroma Components in Tea Plant. Sens. Actuators B Chem. 2025, 430, 137337. [Google Scholar] [CrossRef]
- An, H.; Ou, X.; Chen, J.; Li, J.; Li, S.; Liu, Y.; Jiang, H.; Li, C.; Fang, L.; Liu, Z.; et al. Preliminary Exploration of Acceptance and Emotional Responses to the Key Floral Volatile Compounds of Pu’er Crude Tea. Food Front. 2024, 5, 1765–1775. [Google Scholar] [CrossRef]
- Youn, B.; Kim, Y.; Yoo, S.; Hur, M. Antimicrobial and Hand Hygiene Effects of Tea Tree Essential Oil Disinfectant: A Randomised Control Trial. Int. J. Clin. Pract. 2021, 75, e14206. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.K.; Kim, B.K.; Kim, A.Y. The Impact of Aromatherapy-Based Oral Care on Oral Conditions, Salivary pH, and Halitosis in Older Adults with Dementia: Pilot Study. Geriatr. Nurs. 2023, 53, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Chang, Y.Y.; Wu, X.F.; Wang, Y.C.; Shen, M.H.; Yeh, C.; Zheng, Z.F.; Wang, J.J. Effectiveness of Aroma-Tea Tree Oil and Eucalyptus Oil in Alleviating COVID-19 Vaccine Discomfort Side Effects. EXPLORE 2023, 19, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Qian, L.; Kayama, K.; Wu, F.; Su, Z.; Liu, X. Cake of Japonica, Indica and Glutinous Rice: Effect of Matcha Powder on the Volatile Profiles, Nutritional Properties and Optimal Production Parameters. Food Chem. X 2023, 18, 100657. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Zhang, Y.; Qiu, R.; Li, L.; Zong, X.Y. Effects of Tea Addition on Antioxidant Capacity, Volatiles, and Sensory Quality of Beer. Food Chem. X 2024, 21, 101193. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Bakry, A.M.; Shi, L.; Zhan, P.; He, W.; Eid, W.A.M.; Ferweez, H.; Hamed, Y.S.; Ismail, H.A.; Tian, H.; et al. Mechanistic Insights into Cross-Modal Aroma-Taste Interactions Mediating Sweetness Perception Enhancement in Fu Brick Tea. Food Chem. 2025, 489, 144933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile Compound Analysis and Aroma Evaluation of Tea-Scented Roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, S.; Chen, Y.; Huang, R.; Cheng, B.; Mao, Q.; Liu, T.; Liu, Y.; Zhao, K.; Pan, H.; et al. Multi-Omics Analyzes of Rosa Gigantea Illuminate Tea Scent Biosynthesis and Release Mechanisms. Nat. Commun. 2024, 15, 8469. [Google Scholar] [CrossRef] [PubMed]
- Joichi, A.; Nakamura, Y.; Haze, S.; Ishikawa, T.; Atoji, H.; Nishida, T.; Sakurai, K. Volatile Constituents of Blue-coloured Hybrid Tea Rose Flowers. Flavour. Fragr. J. 2013, 28, 180–187. [Google Scholar] [CrossRef]
- Shen, J.X.; Rana, M.M.; Liu, G.F.; Ling, T.J.; Gruber, M.Y.; Wei, S. Differential Contribution of Jasmine Floral Volatiles to the Aroma of Scented Green Tea. J. Food Qual. 2017, 2017, 5849501. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Wang, H.; Tuo, J.; Ding, Y.; Cao, H.; Yue, C. Volatile Organic Compounds in Teas: Identification, Extraction, Analysis, and Application of Tea Aroma. Foods 2025, 14, 2574. https://doi.org/10.3390/foods14152574
Zeng Q, Wang H, Tuo J, Ding Y, Cao H, Yue C. Volatile Organic Compounds in Teas: Identification, Extraction, Analysis, and Application of Tea Aroma. Foods. 2025; 14(15):2574. https://doi.org/10.3390/foods14152574
Chicago/Turabian StyleZeng, Qin, Huifeng Wang, Jiaojiao Tuo, Yumeng Ding, Hongli Cao, and Chuan Yue. 2025. "Volatile Organic Compounds in Teas: Identification, Extraction, Analysis, and Application of Tea Aroma" Foods 14, no. 15: 2574. https://doi.org/10.3390/foods14152574
APA StyleZeng, Q., Wang, H., Tuo, J., Ding, Y., Cao, H., & Yue, C. (2025). Volatile Organic Compounds in Teas: Identification, Extraction, Analysis, and Application of Tea Aroma. Foods, 14(15), 2574. https://doi.org/10.3390/foods14152574





