Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets, Fish, and Feeding Procedure
2.2. Experimental Sample Collection and Growth Performance Evaluation
- WGR (%) = (final body weight − initial body weight)/initial body weight × 100;
- FCR = feed intake (dry matter)/fish wet weight gain;
- CF = body wet weight (g)/body length (cm)3 × 100.
2.3. Biochemical Analysis
2.4. Analysis of Proximate Composition
2.5. Analysis of FA Composition
- Column: DB-WAX (15 m × 0.25 mm × 0.25 μm, Agilent, Santa Clara, CA, USA);
- Oven program: 120 °C (hold 2 min) → 4 °C/min → 250 °C (hold 5 min);
- Injector: 250 °C, split ratio 10:1;
- Detector (FID): 250 °C;
- Gas flows (mL/min): Carrier (N2): 1.4|Makeup (N2): 25|H2: 40|Air: 400.
- FLQ = (22:6 n-3 + 20:5 n-3)/Σ FA;
- IA = [12:0 + (4 × 14:0) + 16:0]/UFA;
- IT = (14:0 + 16:0 + 18:0)/[(0.5 × MUFA) + (0.5 × n-6 PUFA) + (3 × n-3 PUFA) + (n-3/n-6)];
- HH = (18:1 + PUFA)/(12:0 + 14:0 + 16:0);
- HPI = UFA/[12:0 + (4 × 14:0) + 16:0].
2.6. Analysis of AA Composition
- AAS = AA content of the sample to be tested (mg/g)/AA content of the same AA in the FAO/WHO scoring model (mg/g) × 100;
- CS = AA content of the sample to be tested (mg/g)/reference protein AA content of FAO (1970) [37] whole egg amino acid profile (mg/g) × 100;
- EAAI = (S1 × S2 × ... × Sn)1/n;
- NI = EAAI × protein mass fraction;
- P-BV= 1.09 × EAAI − 11.7.
2.7. Analysis of Muscle Texture Characterization and Physicochemical Indices
- DLP (%) = 100 × (W2 − W1)/W1.
- CMP (%) = 100 × W4/W3.
2.8. Identification and Analysis of VOCs
2.9. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance and Serum Biochemical Indices
3.2. The Proximate Composition, Physicochemical, and Textural Characteristics
3.3. Muscle FA Composition and Nutritional Quality Assessment
3.4. Amino Acid Profile and Nutrient Index of Muscle
3.5. VOC Profiles of Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO Expert Consultation. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. FAO Food Nutr. Pap. 2011, 92, 26369006. [Google Scholar]
- Xie, D.; Chen, C.; Dong, Y.; You, C.; Li, Y. Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Prog. Lipid Res. 2021, 82, 101095. [Google Scholar] [CrossRef]
- Le, V.T.; Knight, S.; Watrous, J.D.; Najhawan, M.; Dao, K.; McCubrey, R.O.; Anderson, J.L. Higher docosahexaenoic acid levels lower the protective impact of eicosapentaenoic acid on long-term major cardiovascular events. Front. Cardiovasc. Med. 2023, 10, 1229130. [Google Scholar] [CrossRef]
- Gronert, K. The protective role of omega-3 in eye disease: New insights. Expert Rev. Ophthalmol. 2011, 6, 493–496. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Gao, J.; Zhang, X.; Xing, J. Eicosapentaenoic acid alleviates inflammatory response and insulin resistance in pregnant mice with gestational diabetes mellitus. Physiol. Res. 2024, 73, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Fish consumption and multiple health outcomes: Umbrella review. Trends Food Sci. Technol. 2020, 99, 273–283. [Google Scholar] [CrossRef]
- Fisheries and Aquaculture Department (FAO). The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018; Available online: https://openknowledge.fao.org/handle/20.500.14283/i9540en (accessed on 30 July 2025).
- Peng, L.; Zhang, L.; Xiong, S.; You, J.; Liu, R.; Xu, D.; Huang, Q.; Ma, H.; Yin, T. A comprehensive review of the mechanisms on fish stress affecting muscle qualities: Nutrition, physical properties, and flavor. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13336. [Google Scholar] [CrossRef]
- Lefèvre, F.; Bugeon, J.; Goardon, L.; Kernéis, T.; Labbé, L.; Panserat, S.; Médale, F.; Quillet, E. Seven generations of selection for muscle fat content greatly affect rainbow trout flesh quality and muscle fiber size. Aquac. Rep. 2024, 38, 102343. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Pu, D.; Li, P.; Wei, X.; Li, D.; Gao, L.; Zhai, X.; Zhao, C.; Du, Y. Comparative evaluation of nutritional quality and flavor characteristics for Micropterus salmoides muscle in different aquaculture systems. Food Chem. X 2024, 24, 101787. [Google Scholar] [CrossRef]
- Zhong, Z.; Fan, J.; Su, H.; Zhu, H.; Ma, D. Proximate compositions evaluation, histology and transcriptome analysis revealed the effects of formulated diets on muscle quality in Micropterus salmoides. Reprod. Breed. 2023, 3, 50–58. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Y. Effect of body weight on the nutrient composition of the muscle and skin tissues of Oncorhynchus mykiss. J. Food Compos. Anal. 2023, 124, 105684. [Google Scholar] [CrossRef]
- Duan, Z.; Zhou, Y.; Liu, W.; Shi, C.C.; Li, L.; Dong, Y.; Dong, S. Variations in flavor according to fish size in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 526, 735398. [Google Scholar] [CrossRef]
- De, D.; Mukherjee, S.; Anand, P.S.; Kumar, P.; Suresh, V.R.; Vijayan, K.K. Nutritional profiling of hilsa (Tenualosa ilisha) of different size groups and sensory evaluation of their adults from different riverine systems. Sci. Rep. 2019, 9, 19306. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Wang, L.; Jin, M.; Zhou, Q.; Nie, G. Fillet texture, physicochemical indexes, muscle cellularity and molecular expression in muscle of common carp (Cyprinus carpio haematopterus) in response to dietary hydroxyproline supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- Song, R.; Yao, X.; Jing, F.; Yang, W.; Wu, J.; Zhang, H.; Zhang, P.; Xie, Y.; Pan, X.; Zhao, L.; et al. Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides). Animals 2024, 14, 781. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Huang, Y.; Wang, X.; Li, X.; Zhang, D.; Jiang, G. Dietary DHA affects muscle fiber development by activating AMPK/Sirt1 pathway in blunt snout bream (Megalobrama amblycephala). Aquaculture 2020, 518, 734835. [Google Scholar] [CrossRef]
- Yun, Y.; Song, D.; He, Z.; Mi, J.; Wang, L.; Nie, G. Effects of methionine supplementation in plant protein based diet on growth performance and fillet quality of juveniles common carp (Cyprinus carpio haematopterus). Aquaculture 2021, 549, 737810. [Google Scholar] [CrossRef]
- Xie, D.; Guan, J.; Huang, X.; Xu, C.; Pan, Q.; Li, Y. Tilapia can be a beneficial n-3 LC-PUFA source due to its high biosynthetic capacity in the liver and intestine. J. Agric. Food Chem. 2022, 70, 2701–2711. [Google Scholar] [CrossRef]
- Ji, S.; Xue, R.; Zhou, L.; Sun, J.; Ji, H. The individual and combined effect of DHA and high-fat diet on flesh quality, antioxidant capacity and myofiber characteristics of grass carp (Ctenopharyngodon idellus). Aquaculture 2025, 595, 741487. [Google Scholar] [CrossRef]
- Vinnícius, U.A.; Massamitu, F.W.; Mariana, M.; Paulovski, P.P.A.; Pereira, D.C.T.; Barriviera, F.L.; Tolentino, M.M.; Sampaio, G.G.; Barriviera, F.V.R. Synergistic effects of dietary methionine and taurine on growth performance, blood parameters, expression in hepatic sulfur-metabolism genes, and flesh quality of large Nile tilapia. Anim. Feed. Sci. Tech. 2022, 288, 115291. [Google Scholar] [CrossRef]
- Hua, K.; Suwendi, E.; Bureau, P. Effect of body weight on lysine utilization efficiency in Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 505, 47–53. [Google Scholar] [CrossRef]
- Morais, C.A.R.S.; Santana, T.P.; Santos, C.A.; Passetti, R.A.C.; Melo, J.F.B.; Macedo, F.A.F.; Vesco, A.P.D. Effect of slaughter weight on the quality of Nile tilapia fillets. Aquaculture 2020, 520, 734941. [Google Scholar] [CrossRef]
- Kayan, A.; Boontan, I.; Jaturssitha, S.; Wicke, M.; Kreuzer, M. Effect of Slaughter Weight on Meat Quality of Nile Tilapia (Oreochromisniloticus). Agric. Agric. Sci. Procedia 2015, 5, 159–163. [Google Scholar] [CrossRef][Green Version]
- Nakajima, T.; Hudson, M.J.; Uchiyama, J.; Makibayashi, K.; Zhang, J. Common carp aquaculture in Neolithic China dates back 8000 years. Nat. Ecol. Evol. 2019, 3, 1415–1418. [Google Scholar] [CrossRef]
- Pei, L.; Li, H.; Fei, H.; Yi, B.; Li, F.; Ren, H. Effects of dietary Humulus scandens extract on growth performance, antioxidant responses and intestinal microflora of common carp (Cyprinus carpio). Aquacult. Rep. 2023, 30, 101566. [Google Scholar] [CrossRef]
- Gao, S.; Sun, P.; Ren, H.; Chen, J.; Shen, Y.; Wang, Z.; Huang, Y.; Chen, W. Effects of dietary phosphorus deficiency on the growth performance, hepatic lipid metabolism, and antioxidant capacity of common carp Cyprinus carpio haematopterus. J. Aquat. Anim. Health 2023, 35, 41–49. [Google Scholar] [CrossRef]
- Wang, L.; Jia, S.; Zhang, L.; Ma, F.; Zhang, M.; Yu, M.; Jiang, H.; Qiao, Z.; Li, X. Comparative study on nutritional quality and volatile flavor compounds of muscle in Cyprinus carpio haematopterus under wild, traditional pond and in-pond raceway system culture. Aquacult. Rep. 2022, 25, 101194. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Chen, Y.; Chen, J.; Zhang, M.; Yu, M.; Jiang, H.; Qiao, Z.; Li, X. Mapping Growth-Related Quantitative Trait Locus (QTL) in Commercial Common carp (Cyprinus carpio haematopterus) during Overwintering. Fishes 2022, 7, 7040166. [Google Scholar] [CrossRef]
- He, Z.; Xu, C.; Chen, F.; Lou, Y.; Nie, G.; Xie, D. Dietary DHA Enhanced the Textural Firmness of Common Carp (Cyprinus carpio L.) Fed Plant-Derived Diets through Restraining FoxO1 Pathways. Foods 2022, 11, 3600. [Google Scholar] [CrossRef]
- Official methods of analysis of AOAC international. In 19th Association of Official Analytical Chemists; AOAC: Rockville, MD, USA, 2010.
- Zhang, J.; Ren, Y.; Sui, X.; Lu, R.; Cao, X.; Zhang, Y. A modified material-saving method system for detecting and analysing fatty acids in fish using gas chromatography. Aquacu. Res. 2022, 53, 3202–3213. [Google Scholar] [CrossRef]
- Xie, D.; Liu, X.; Wang, S.; You, C.; Li, Y. Effects of dietary LNA/LA ratios on growth performance, fatty acid composition and expression levels of elovl5, Δ 4 fad and Δ 6 /Δ 5 fad in the marine teleost Siganus canaliculatus. Aquaculture 2017, 484, 309–316. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Ijarotimi, O.S.; Keshinro, O.O. Determination of Nutrient Composition and Protein Quality of Potential Complementary Foods Formulated from the Combination of Fermented Popcorn, African Locust and Bambara Groundnut Seed Flour. Pol. J. Food Nutr. Sci. 2013, 63, 155–166. [Google Scholar] [CrossRef]
- Listed, N.A. Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. WHO TRS 1985, 724, 1–206. Available online: https://iris.who.int/handle/10665/39527 (accessed on 30 July 2025).
- FAO/WHO. Amino Acid Content of Foods and Biological Data on Proteins (FAO Nutritional Studies No. 24); Food and Agriculture Organization: Rome, Italy, 1970. [Google Scholar]
- Mi, J.; Liu, D.; Qin, C.; Yan, X.; Pang, P.; Yun, Y.; Wang, L.; Nie, G. Dietary(−)-Epicatechin supplementation regulates myofiber development, fillet quality, and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2023, 572, 739542. [Google Scholar] [CrossRef]
- Li, M.; Sun, M.; Ren, W.; Man, L.; Chai, W.; Liu, G.; Zhu, M.; Wang, C. Characterization of Volatile Compounds in Donkey Meat by Gas Chromatography-Ion Mobility Spectrometry (GC-IMS) Combined with Chemometrics. Food Sci. Anim. Resour. 2024, 44, 165–177. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquacu. Res. 2008, 41, 717–732. [Google Scholar] [CrossRef]
- Nakamura, Y.-N.; Ando, M.; Seoka, M.; Kawasaki, K.-i.; Tsukamasa, Y. Changes of proximate and fatty acid compositions of the dorsal and ventral ordinary muscles of the full-cycle cultured Pacific bluefin tuna Thunnus orientalis with the growth. Food Chem. 2006, 103, 234–241. [Google Scholar] [CrossRef]
- Naeem, M.; Ishtiaq, A. Proximate composition of Mystus bleekeri in relation to body size and condition factor from Nala Daik Sialkot Pakistan. Afr. J. Biotechnol. 2011, 10, 10765–10773. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Liu, C.; Feng, D.; Huang, J.; Jin, Z.; Ma, F.; Xu, J.; Xu, Y.; Zhang, M.; et al. Effects of water flow treatment on muscle quality, nutrient composition and volatile compounds in common carp (Cyprinus carpio). Food Chem X 2025, 26, 102257. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Li, S.; Mi, H.; Tang, L.; et al. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 109. [Google Scholar] [CrossRef]
- Hao, M.; Yi, L.; Cheng, W.; Zhu, J.; Zhao, S. Lipidomics analysis reveals new insights into crisp grass carp associated with meat texture. Heliyon 2024, 10, e32179. [Google Scholar] [CrossRef] [PubMed]
- Jakob, G.; Rafea, N.; Margaret, B. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, F.; Zhang, W.; Parisi, G.; Du, Z.; Zhang, M. The flesh texture of teleost fish: Characteristics and interventional strategies. Rev. Aquacult. 2023, 16, 508–535. [Google Scholar] [CrossRef]
- Keenan, S.R.; Currie, P.D. The Developmental Phases of Zebrafish Myogenesis. J. Dev. Biol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Zhang, Z.; Limbu, S.M.; Zhao, S.; Chen, L.; Luo, Y.; Zhang, M.; Qiao, F.; Du, Z. Dietary l-carnitine supplementation recovers the increased pH and hardness in fillets caused by high-fat diet in Nile tilapia (Oreochromis niloticus). Food Chem. 2022, 382, 132367. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Rudchenko, A.E.; Gladyshev, M.I. Effect of season and trophic level on fatty acid composition and content of four commercial fish species from Krasnoyarsk Reservoir (Siberia, Russia). Fish. Res. 2017, 187, 178–187. [Google Scholar] [CrossRef][Green Version]
- El-Sayed, A.F.M.; Mansour, C.R.; Ezzat, A.A. Effects of dietary lipid source on spawning performance of Nile tilapia (Oreochromis niloticus) broodstock reared at different salinities. Aquaculture 2005, 248, 187–196. [Google Scholar] [CrossRef]
- Köprücü, K.; Yonar, M.E.; Özcan, S. Effect of dietary n-3 polyunsaturated fatty acids on antioxidant defense and sperm quality in rainbow trout (Oncorhynchus mykiss) under regular stripping conditions. Anim. Reprod. Sci. 2015, 163, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Jaya-Ram, A.; Kuah, M.K.; Lim, P.S.; Kolkovski, S.; Shu-Chien, A.C. Influence of dietary HUFA levels on reproductive performance, tissue fatty acid profile and desaturase and elongase mRNAs expression in female zebrafish Danio rerio. Aquaculture 2008, 277, 275–281. [Google Scholar] [CrossRef]
- Furuita, H.; Tanaka, H.; Yamamoto, T.; Suzuki, N.; Takeuchi, T. Effects of high levels of n-3 HUFA in broodstock diet on egg quality and egg fatty acid composition of Japanese flounder, Paralichthys olivaceus. Aquaculture 2002, 210, 323–333. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Xie, J. Progress on odor deterioration of aquatic products: Characteristic volatile compounds, analysis methods, and formation mechanisms. Food Biosci. 2023, 53, 102666. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Tadele, W.W.; Yi, J.; Wibowo, S.; Kebede, B.T.; Loey, A.M.V.; Grauwet, T.; Hendrickx, M.E. Insight into the evolution of flavor compounds during cooking of common beans utilizing a headspace untargeted fingerprinting approach. Food Chem. 2018, 275, 224–238. [Google Scholar] [CrossRef]
- An, Y.; Wen, L.; Li, W.; Zhang, X.; Hu, Y.; Xiong, S. Insight into the evolution of aroma compounds during thermal processing of surimi gel from silver carp (Hypophthalmichthys molitrix). Food Chem. 2021, 374, 131762. [Google Scholar] [CrossRef]
- Chen, F.; He, Y.; Li, X.; Zhu, H.; Li, Y.; Xie, D. Improvement in muscle fatty acid bioavailability and volatile flavor in tilapia by dietary α-linolenic acid nutrition strategy. Foods 2024, 13, 1005. [Google Scholar] [CrossRef]
- Sprague, M.; Xu, G.; Betancor, M.B.; Olsen, R.E.; Torrissen, O.; Glencross, B.D.; Tocher, D.R. Endogenous production of n-3 long-chain PUFA from first feeding and the influence of dietary linoleic acid and the α-linolenic: Linoleic ratio in Atlantic salmon (Salmo salar). Br. J. Nutr. 2019, 122, 1091–1102. [Google Scholar] [CrossRef]
- Yadav, A.K.; Rossi, W.; Habte-Tsion, H.-M.; Kumar, V. Impacts of dietary eicosapentaenoic acid EPA and docosahexaenoic acid DHA level and ratio on the growth, fatty acids composition and hepatic-antioxidant status of largemouth bass (Micropterus salmoides). Aquaculture 2020, 529, 735683. [Google Scholar] [CrossRef]
- Chen, F.; Lou, Y.; Guan, J.; Lan, X.; Su, Z.; Xu, C.; Li, Y.; Xie, D. Rapeseed and palm oils can improve the growth, muscle texture, fatty acids and volatiles of marine teleost golden pompano fed low fish oil diets. Foods 2025, 14, 788. [Google Scholar] [CrossRef]
- Turchini, G.M.; Moretti, V.M.; Mentasti, T.; Orban, E.; Valfre, F. Effects of dietary lipid source on fillet chemical composition, flavour volatile compounds and sensory characteristics in the freshwater fish tench (Tinca tinca L.). Food Chem. 2007, 102, 1144–1155. [Google Scholar] [CrossRef]

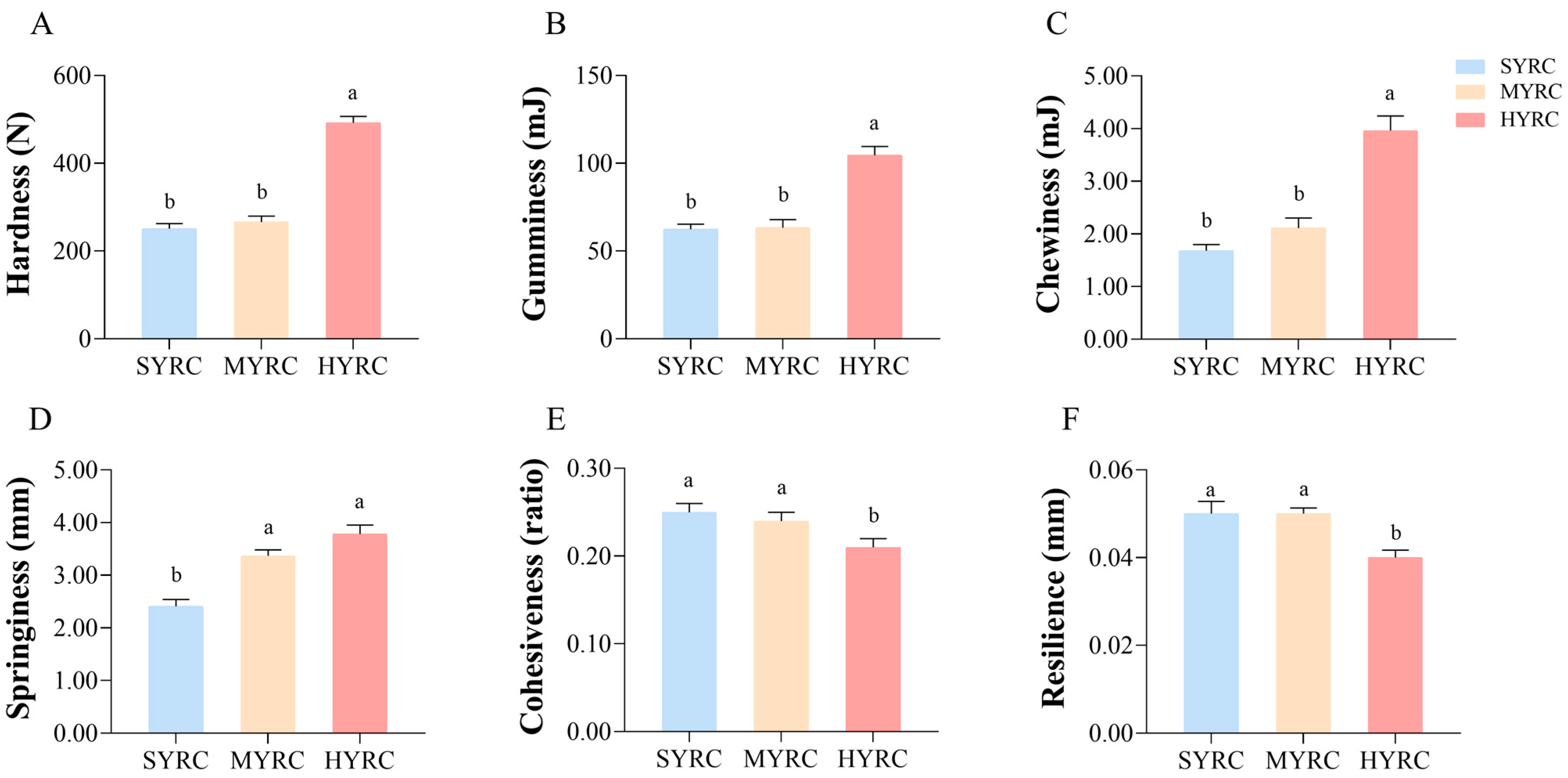


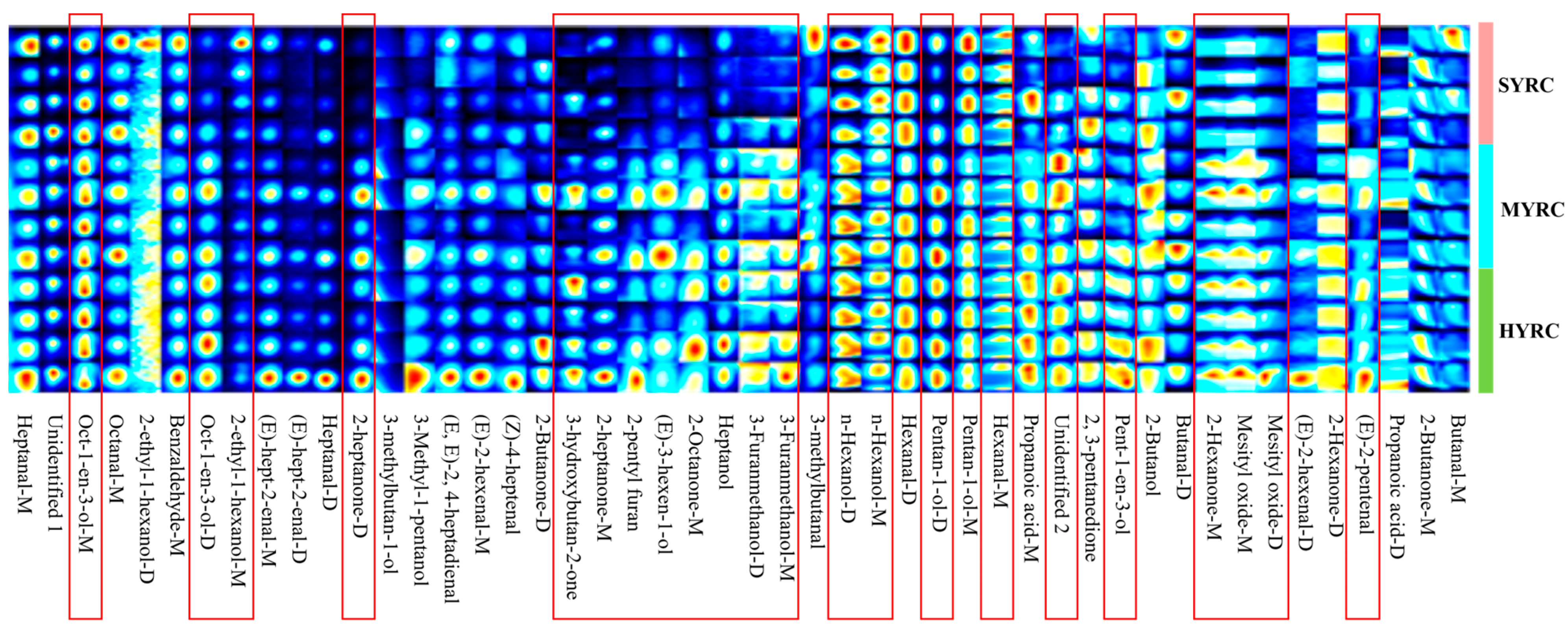

| Index | Groups | ||
|---|---|---|---|
| SYRC | MYRC | HYRC | |
| Growth | |||
| IBW (g) | 11.62 ± 0.53 | 66.84 ± 0.93 | 266.13 ± 1.86 |
| FBW (g) | 82.18 ± 2.67 c | 263.50 ± 1.10 b | 637.07 ± 3.33 a |
| WGR (%) | 707.07 ± 22.97 a | 294.22 ± 1.66 b | 139.39 ± 1.19 c |
| FCR | 1.36 ± 0.06 b | 1.54 ± 0.01 a | 1.41 ± 0.01 b |
| CF (g/cm3) | 2.39 ± 0.02 b | 2.55 ± 0.05 a | 2.39 ± 0.02 b |
| Serum biochemical indexes | |||
| TAG (mmol/L) | 2.25 ± 0.18 b | 3.58 ± 0.20 a | 3.34 ± 0.24 a |
| T-CHO (mmol/L) | 3.43 ± 0.14 b | 5.36 ± 0.24 a | 4.88 ± 0.22 a |
| LDL-C (mmol/L) | 1.41 ± 0.11 b | 2.80 ± 0.15 a | 0.76 ± 0.07 c |
| HDL-C (mmol/L) | 1.24 ± 0.08 b | 2.34 ± 0.11 a | 2.17 ± 0.14 a |
| HDL-C/LDL-C | 0.89 ± 0.03 b | 0.85 ± 0.05 b | 2.95 ± 0.15 a |
| Index | Groups | ||
|---|---|---|---|
| SYRC | MYRC | HYRC | |
| FA composition | |||
| 14:0 | 0.13 ± 0.01 b | 0.26 ± 0.02 a | 0.15 ± 0.01 b |
| 16:0 | 6.40 ± 0.41 ab | 7.25 ± 0.43 a | 5.86 ± 0.28 b |
| 18:0 | 2.11 ± 0.12 | 2.23 ± 0.50 | 1.95 ± 0.10 |
| 20:0 | 0.03 ± 0.00 c | 0.07 ± 0.01 a | 0.05 ± 0.00 b |
| SFA | 8.67 ± 0.52 | 9.80 ± 0.91 | 8.00 ± 0.39 |
| 16:1n-7 | 0.11 ± 0.02 c | 0.89 ± 0.12 a | 0.48 ± 0.04 b |
| 18:1n-9 | 6.73 ± 0.79 b | 12.77 ± 0.74 a | 7.75 ± 0.49 b |
| 20:1n-9 | 0.29 ± 0.03 b | 0.48 ± 0.03 a | 0.38 ± 0.02 b |
| MUFA | 7.14 ± 0.82 b | 14.13 ± 0.86 a | 8.61 ± 0.54 b |
| 18:2n-6 | 9.27 ± 0.94 b | 12.04 ± 1.13 a | 8.90 ± 0.56 b |
| 18:3n-6 | 0.26 ± 0.02 | 0.23 ± 0.01 | 0.22 ± 0.01 |
| 20:3n-6 | 1.04 ± 0.04 a | 0.68 ± 0.01 c | 0.91 ± 0.02 b |
| 20:4n-6 | 1.33 ± 0.07 a | 1.02 ± 0.09 b | 1.45 ± 0.05 a |
| n-6 PUFA | 11.90 ± 1.02 | 13.97 ± 1.19 | 11.48 ± 0.58 |
| 18:3n-3 | 0.69 ± 0.08 b | 1.20 ± 0.12 a | 0.82 ± 0.04 b |
| 20:3n-3 | 0.01 ± 0.00 c | 0.04 ± 0.00 a | 0.03 ± 0.00 b |
| 20:5n-3 | 0.14 ± 0.00 a | 0.08 ± 0.01 b | 0.07 ± 0.00 b |
| 22:6n-3 | 1.39 ± 0.10 a | 0.71 ± 0.05 c | 1.10 ± 0.02 b |
| n-3 PUFA | 2.23 ± 0.09 | 2.03 ± 0.15 | 2.03 ± 0.06 |
| LC-PUFA | 3.91 ± 0.10 a | 2.52 ± 0.16 b | 3.56 ± 0.06 a |
| Nutritional quality | |||
| HPI | 3.07 ± 0.10 b | 3.63 ± 0.11 a | 3.42 ± 0.06 a |
| FLQ | 1.53 ± 0.10 a | 0.79 ± 0.06 c | 1.17 ± 0.02 b |
| HH | 3.18 ± 0.11 b | 3.83 ± 0.13 a | 3.54 ± 0.07 a |
| n-3/n-6 | 0.19 ± 0.02 a | 0.15 ± 0.01 b | 0.18 ± 0.01 ab |
| IA | 0.33 ± 0.01 a | 0.28 ± 0.01 b | 0.29 ± 0.01 b |
| IT | 0.53 ± 0.01 | 0.48 ± 0.03 | 0.49 ± 0.01 |
| Class | Compound | CAS# | RI | Rt [sec] | Dt [a.u.] |
|---|---|---|---|---|---|
| Alcohol (16) | oct-1-en-3-ol-M | C3391864 | 986.7 | 555.741 | 1.1534 |
| oct-1-en-3-ol-D | C3391864 | 985 | 551.777 | 1.58779 | |
| n-Hexanol-M | C111273 | 872.8 | 355.129 | 1.32201 | |
| n-Hexanol-D | C111273 | 874.5 | 357.364 | 1.65695 | |
| 2-ethyl-1-hexanol-M | C104767 | 1046.5 | 665.6 | 1.41635 | |
| 2-ethyl-1-hexanol-D | C104767 | 1045.3 | 663.335 | 1.79977 | |
| (E)-3-hexen-1-ol | C928972 | 855.5 | 333.891 | 1.25166 | |
| 3-Methyl-1-pentanol | C589355 | 849.3 | 326.625 | 1.30311 | |
| pentan-1-ol-M | C71410 | 763 | 237.62 | 1.24994 | |
| pentan-1-ol-D | C71410 | 763 | 237.62 | 1.52125 | |
| 3-methylbutan-1-ol | C123513 | 735.2 | 212.453 | 1.23788 | |
| pent-1-en-3-ol | C616251 | 682.5 | 173.319 | 1.37858 | |
| 3-Furanmethanol-M | C4412913 | 978.7 | 538.125 | 1.10407 | |
| 3-Furanmethanol-D | C4412913 | 978.3 | 537.186 | 1.34958 | |
| Heptanol | C53535334 | 976.1 | 532.495 | 1.39346 | |
| 2-Butanol | C78922 | 587.6 | 136.563 | 1.16311 | |
| Aldehyde (16) | Octanal-M | C124130 | 1011.7 | 603.309 | 1.42214 |
| (E)-hept-2-enal-M | C18829555 | 957.1 | 493.577 | 1.25369 | |
| (E)-hept-2-enal-D | C18829555 | 958.6 | 496.525 | 1.6573 | |
| (Z)-4-heptenal | C6728310 | 900.8 | 394.019 | 1.14015 | |
| (E)-2-hexenal-M | C6728263 | 851.2 | 328.861 | 1.17816 | |
| (E)-2-hexenal-D | C6728263 | 848.8 | 326.066 | 1.50786 | |
| (E, E)-2,4-heptadienal | C4313035 | 1021.3 | 619.857 | 1.19829 | |
| (E)-2-pentenal | C1576870 | 748.8 | 224.46 | 1.10294 | |
| Heptanal-M | C111717 | 902.4 | 396.486 | 1.35351 | |
| Heptanal-D | C111717 | 904.2 | 399.281 | 1.68845 | |
| Hexanal-D | C66251 | 792.4 | 266.579 | 1.55595 | |
| Hexanal-M | C66251 | 793.7 | 267.834 | 1.28602 | |
| Benzaldehyde-M | C100527 | 963 | 505.37 | 1.14816 | |
| butanal-M | C123728 | 588.8 | 136.968 | 1.10489 | |
| butanal-D | C123728 | 589.9 | 137.372 | 1.28822 | |
| 3-methylbutanal | C590863 | 639 | 155.383 | 1.18664 | |
| Ketone (11) | 2-Hexanone-M | C591786 | 797.3 | 271.309 | 1.21854 |
| 2-Hexanone-D | C591786 | 793 | 267.161 | 1.49324 | |
| 3-hydroxybutan-2-one | C513860 | 717.7 | 197.979 | 1.32651 | |
| 2,3-pentanedione | C600146 | 690.5 | 177.349 | 1.22624 | |
| 2-Butanone-M | C78933 | 588.6 | 136.904 | 1.0781 | |
| 2-Butanone-D | C78933 | 589.6 | 137.248 | 1.24155 | |
| 2-heptanone-M | C110430 | 891.5 | 379.72 | 1.26111 | |
| 2-heptanone-D | C110430 | 892 | 380.279 | 1.6181 | |
| 2-Octanone-M | C111137 | 996.2 | 577.26 | 1.33063 | |
| Mesityl oxide-M | C141797 | 800.4 | 274.272 | 1.12174 | |
| Mesityl oxide-D | C141797 | 795.5 | 269.532 | 1.42392 | |
| Furan (1) | 2-pentyl furan | C3777693 | 996.2 | 577.26 | 1.23912 |
| Acid (2) | Propanoic acid-M | C79094 | 712.7 | 193.96 | 1.10182 |
| Propanoic acid-D | C79094 | 713.5 | 194.576 | 1.27144 | |
| Unidentified (2) | unidentified 1 | - | 987 | 556.307 | 1.43952 |
| unidentified 2 | - | 715.4 | 196.135 | 1.16306 |
| Compound | Class | Groups | ||
|---|---|---|---|---|
| SYRC | MYRC | HYRC | ||
| 2-heptanone-D | Ketone | 391.70 ± 70.55 b | 1639.99 ± 271.92 a | 1500.31 ± 291.76 a |
| 2-heptanone-M | Ketone | 654.17 ± 82.12 b | 1035.02 ± 63.58 a | 981.47 ± 73.53 a |
| 2-Hexanone-M | Ketone | 1955.30 ± 72.47 b | 2325.51 ± 76.84 a | 2306.75 ± 38.12 a |
| 3-hydroxybutan-2-one | Ketone | 1536.04 ± 664.20 b | 3406.34 ± 1086.37 ab | 5189.92 ± 652.16 a |
| 2-Octanone-M | Ketone | 190.04 ± 15.09 b | 321.02 ± 48.82 ab | 408.47 ± 53.80 a |
| pentan-1-ol-D | Alcohol | 2470.53 ± 420.17 b | 4711.10 ± 556.58 a | 4846.99 ± 397.81 a |
| 3-Furanmethanol-D | Alcohol | 140.10 ± 29.77 b | 274.11 ± 29.23 a | 304.27 ± 21.12 a |
| 3-Furanmethanol-M | Alcohol | 577.11 ± 94.41 b | 932.40 ± 67.57 a | 1135.35 ± 65.62 a |
| oct-1-en-3-ol-D | Alcohol | 1257.79 ± 272.53 b | 2628.42 ± 290.25 a | 3134.26 ± 217.34 a |
| oct-1-en-3-ol-M | Alcohol | 5386.57 ± 541.89 b | 6961.14 ± 151.95 a | 7336.22 ± 164.75 a |
| n-Hexanol-D | Alcohol | 4632.20 ± 269.15 b | 7455.97 ± 241.84 a | 7439.56 ± 285.79 a |
| Mesityl oxide-D | Alcohol | 2275.51 ± 138.51 b | 2934.47 ± 223.47 a | 2932.77 ± 159.07 a |
| Heptanol | Alcohol | 514.50 ± 39.56 b | 803.34 ± 59.54 a | 867.75 ± 81.78 a |
| (E)-3-hexen-1-ol | Alcohol | 119.68 ± 16.78 b | 241.13 ± 31.24 a | 174.71 ± 16.38 a |
| Mesityl oxide-M | Alcohol | 1539.25 ± 71.83 b | 1611.87 ± 135.17 b | 1915.81 ± 23.83 a |
| pent-1-en-3-ol | Alcohol | 2049.83 ± 285.57 b | 2614.82 ± 430.85 b | 4005.95 ± 456.65 a |
| 2-ethyl-1-hexanol-M | Alcohol | 661.13 ± 98.68 a | 480.14 ± 10.63 ab | 461.1.59 b |
| n-Hexanol-M | Alcohol | 5619.22 ± 201.04 a | 4795.00 ± 275.89 b | 5253.34 ± 148.38 ab |
| (E)-2-pentenal | Aldehyde | 358.07 ± 62.82 b | 399.32 ± 41.56 b | 573.42 ± 54.64 a |
| Hexanal-M | Aldehyde | 2553.37 ± 36.42 a | 1915.24 ± 177.62 b | 2160.42 ± 129.08 ab |
| 2-pentyl furan | Furan | 183.25 ± 38.88 b | 483.40 ± 82.19 a | 513.18 ± 91.91 a |
| unidentified 2 | - | 2433.63 ± 459.98 b | 4227.97 ± 326.38 a | 4514.08 ± 94.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wang, J.; Wei, Y.; Yan, X.; Li, Y.; Xie, D.; Nie, G. Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile. Foods 2025, 14, 2794. https://doi.org/10.3390/foods14162794
He Z, Wang J, Wei Y, Yan X, Li Y, Xie D, Nie G. Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile. Foods. 2025; 14(16):2794. https://doi.org/10.3390/foods14162794
Chicago/Turabian StyleHe, Zijie, Junli Wang, Yun Wei, Xiao Yan, Yuanyou Li, Dizhi Xie, and Guoxing Nie. 2025. "Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile" Foods 14, no. 16: 2794. https://doi.org/10.3390/foods14162794
APA StyleHe, Z., Wang, J., Wei, Y., Yan, X., Li, Y., Xie, D., & Nie, G. (2025). Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile. Foods, 14(16), 2794. https://doi.org/10.3390/foods14162794







