Bacterial Antimicrobial Resistance in Meat Products—Current Concepts
Abstract
1. Introduction
2. Bacterial AMR in Food-Producing Animals (FPA)
3. Foodborne Infections and AMR in Meat and Meat Products
3.1. AMR Pathogens Transmitted by Meat and Meat Products
3.1.1. Salmonella
3.1.2. Campylobacter spp.
3.1.3. Diarrheagenic E. coli
3.1.4. Shiga-Toxin-Producing E. coli (STEC) Infections
3.1.5. Listeria monocytogenes
3.1.6. Staphylococcus aureus (SA)
4. Transmission of AMR to the Human Population
5. Strategies to Prevent the Occurrence of AMR Microorganism Infections
6. AMR Surveillance in Meat Production and Processing
| Program | Country | Type of Surveillance | Reference | ||
|---|---|---|---|---|---|
| Humans | Food | Animals | |||
| Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) | Denmark | × | × | × | DANMAP [119]. https://www.danmap.org/reports/2023 (accessed on 29 June 2025) |
| French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin (RESAPATH) | France | × | / | × | Anses [120]. https://www.anses.fr/en/content/anses-request-based-opinions-and-reports?titre=RESAPATH (accessed on 29 June 2025) |
| Monitoring of Antimicrobial Resistance and Antibiotic Usage in the Netherlands (MARAN) and NethMap/MARAN report for 2024. | Netherlands | × | × | × | NethMap [121]. https://www.rivm.nl/bibliotheek/rapporten/2024-0117.pdf (accessed on 29 June 2025) |
| Swedish Antibiotic Sales and Resistance in Human Medicine (SWEDRES) and Swedish Veterinary Antibiotic Resistance Monitoring (SVARM) | Sweden | × | / | × | Swedres-Svarm [122]. https://www.sva.se/en/what-we-do/antibiotics/svarm-resistance-monitoring/swedres-svarm-reports/ (accessed on 29 June 2025) |
| A report from five countries in the EU and EEA on AMR monitoring and surveillance in the meat chain | Denmark, France, Netherlands, Sweden, Norway | / | × | / | Mc Nulty et al. [118]. https://doi.org/10.1016/j.tifs.2016.09.010 (accessed on 29 June 2025) |
| ECDC/EFSA/EMA—integrated analysis of the consumption of AM agents and occurrence of AMR in bacteria from humans and FPAs | Europe | × | / | × | ECDC/EFSA/EMEA [112]. https://www.ecdc.europa.eu/en/publications-data/ecdcefsaema-first-joint-report-integrated-analysis-consumption-antimicrobial (accessed on 29 June 2025) |
| Norwegian Surveillance System for Antimicrobial Drug Resistance (NORM/NORM-VET) | Norway | × | × | × | NORM/NORM-VET [123]. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report/_/attachment/inline/78155e88-2b2e-42a1-ac0c-b06071eb0479:21bb52d0f6c051c93f0d4a0605eb001e25e7fe99/NORM%20NORM-VET%202023%20(2).pdf (accessed on 29 June 2025) |
| The surveillance program for methicillin-resistant Staphylococcus aureus in pigs in Norway 2024 | Norway | / | / | × | Urdahl et al. [124]. https://www.vetinst.no/en/surveillance-programmes/mrsa-in-pigs (accessed on 29 June 2025) |
| Global antimicrobial resistance and use surveillance system (GLASS) report (2022). | Global (127 countries, territories, and areas) | × | / | / | WHO [125]. https://www.who.int/publications/i/item/9789240062702 (accessed on 29 June 2025) |
| GBD 2021 AMR Collaborators 1990–2021, with forecasts to 2050 | Global | × | / | / | Naghavi, M. et al. [126]. https://doi.org/10.1016/S0140-6736(24)01867-1 (accessed on 29 June 2025) |
| The European AMR Surveillance Network (EARS-Net) | Europe | × | / | / | European Centre for Disease Prevention and Control [127]. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-annual-epidemiological-report-EARS-Net-2023.pdf (accessed on 29 June 2025) |
| European Centre for Disease Prevention and Control (ECDC) point-prevalence surveys | Europe | × | / | / | European Centre for Disease Prevention and Control [128]. https://data.europa.eu/doi/10.2900/5735023 (accessed on 29 June 2025) |
| Surveillance for AMR and healthcare-associated infections in Europe—SUSPIRE protocol: registered in the International Prospective Register of Systematic Reviews (PROSPERO) | 32 European countries, (28 EU member states and the four EFTA countries (Iceland, Liechtenstein, Norway, and Switzerland) | × | / | / | Núñez-Núñez et al. [129]. https://doi.org/10.1016/j.cmi.2017.07.014 (accessed on 29 June 2025) |
| European Sales and Use of Antimicrobials for Veterinary Medicine (ESUAvet) annual surveillance reports | Europe | / | / | × | European Sales and Use of Antimicrobials for veterinary medicine (ESUAvet) [130]. https://www.ema.europa.eu/en/documents/report/european-sales-use-antimicrobials-veterinary-medicine-annual-surveillance-report-2023_en.pdf (accessed on 29 June 2025) |
| The Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR) network—a joint initiative of the WHO Regional Office for Europe | 30 countries of the EU and EEA | × | / | / | European Centre for Disease Prevention and Control and WHO Regional Office for Europe [127]. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-ECDC-WHO-executive-summary-2023-data.pdf (accessed on 29 June 2025) |
| EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). (2024). | 27 EU Member States (MSs), the United Kingdom (Northern Ireland), and four non-MSs. | × | × | × | EFSA and ECDC [131,132]. https://doi.org/10.2903/j.efsa.2024.8583 (accessed on 29 June 2025) |
| The National Antimicrobial Resistance Monitoring System (NARMS) | USA | × | × | × | FDA Requests Public Comments to Inform Development of National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan for 2026–2030 [133]. https://www.fda.gov/animal-veterinary/antimicrobial-resistance/national-antimicrobial-resistance-monitoring-system (accessed on 29 June 2025) |
| Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) | Canada | × | × | × | CIPARS [134]. https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars.html (accessed on 29 June 2025) |
| Food and Agriculture Organization Antimicrobial Resistance Action Plan (FAO AMR) | 150 countries worldwide | / | × | × | FAO [135]. https://openknowledge.fao.org/server/api/core/bitstreams/dd6c0ba1-fd85-4a3e-b398-53b610c35318/content (accessed on 29 June 2025) |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Atta, S.A.; Nasr, S.M.; Mouneir, S.M. Current perspective on veterinary drug and chemical residues in food of animal origin. Environ. Sci. Pollut. Res. 2022, 29, 15282–15302. [Google Scholar] [CrossRef] [PubMed]
- Lobanovska, M.; Pilla, G. Focus: Drug development: Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.E.A.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, A.; Ravi, K. Antibiotics Misuse and Antimicrobial Resistance Development in Agriculture: A Global Challenge. Environ. Health 2024, 2, 618–622. [Google Scholar] [CrossRef]
- Karabasil, N.; Mirković, M.; Vićić, I.; Perić, I.; Zlatković, N.; Luković, B.; Gajić, I. Antimicrobial Resistance in Diverse Ecological Niches—One Health Perspective and Food Safety. Antibiotics 2025, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; Herman, L. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Händel, N.; Schuurmans, J.M.; Brul, S.; ter Kuile, B.H. Compensation of the metabolic costs of antibiotic resistance by physiological adaptation in Escherichia coli. Antimicrob. Agents Chemother. 2013, 57, 3752–3762. [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarthg, S.; Hindlerh, J.F.; Kahlmeteri, G.; Olsson-Liljequistj, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Pruden, A.; Alvarez, P.J.J.; Aga, D.; Bürgmann, H.; Li, X.; Manaia, C.M.; Nambi, I.; Wigginton, K.; Zhang, T.; et al. Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit: A critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Viale, P.; Giannella, M. MDR/XDR/PDR or DTR? Which definition best fits the resistance profile of Pseudomonas aeruginosa? Curr. Opin. Infect. Dis. 2023, 1, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Almakrami, M.; Salmen, M.; Aldashel, Y.A.; Alyami, M.H.; Alquraishah, N.; AlZureea, M.; Almakrami, J. Prevalence of multidrug-, extensively drug-, and pandrug-resistant bacteria in clinical isolates from King Khaled Hospital, Najran, Saudi Arabia. Discov. Med. 2024, 1, 108. [Google Scholar] [CrossRef]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Trajković, L.; Cvetković, M.; Mitrović, M.; Pravdić, Z.; Pantić, N.; Sabljić, N.; Jaković, L.; Vidović, A.; Suvajdžić-Vuković, N.; Virijević, M. Antibiotic resistance patterns of multidrug resistant bacteria in acute myeloid leukemia patients during induction treatment. J. Infect. Dev. Ctries. 2025, 19, 362–369. [Google Scholar] [CrossRef]
- Shoaib, M.; He, Z.; Geng, X.; Tang, M.; Hao, R.; Wang, S.; Shang, R.; Wang, X.; Zhang, H.; Pu, W. The emergence of multi-drug resistant and virulence gene carrying Escherichia coli strains in the dairy environment: A rising threat to the environment, animal, and public health. Front. Microbiol. 2023, 14, 1197579. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.; Ali, M.M.; Ijaz, M.; Sattar, H.; Ghaffar, A.; Sajid Hasni, M.; Bhutta, Z.A.; Ashfaq, K.; Kulyar, M.F.E.A.; et al. Tracking Infection and Genetic Divergence of Methicillin-Resistant Staphylococcus aureus at Pets, Pet Owners, and Environment Interface. Front. Vet. Sci. 2022, 9, 900480. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Abalkhail, A.; Edrees, H.M.; Ellethy, A.T.; Almuzaini, A.M.; Ibrahem, M.; Almujaidel, A.; Alzaben, F.; Alqrni, A.; et al. Microbial Food Safety and Antimicrobial Resistance in Foods: A Dual Threat to Public Health. Microorganisms 2025, 13, 1592. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Mercanoglu, T.B. A State-of-Art Review on Multi-Drug Resistant Pathogens in Foods of Animal Origin: Risk Factors and Mitigation Strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef]
- Pineda-Cortel, M.R.; del Rosario, E.H.; Villaflores, O.B. Use of veterinary medicinal products in the Philippines: Regulations, impact, challenges, and recommendations. J. Vet. Sci. 2024, 25, e33. [Google Scholar] [CrossRef] [PubMed]
- Borzi, M.M.; Cardozo, M.V.; Oliveira, E.S.; Pollo, A.S.; Guastalli, E.A.L.; Santos, L.F.D.; Santos, L.F.D.; Ávila, F.A.D. Characterization of avian pathogenic Escherichia coli isolated from free-range helmeted guineafowl. Braz. J. Microbiol. 2018, 49 (Suppl. 1), 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Riley, L.W. A population-based surveillance study of shared genotypes of Escherichia coli isolates from retail meat and suspected cases of urinary tract infections. mSphere 2018, 3, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Jimenez, D.; Garcia-Menino, I.; Fernandez, J.; Garcia, V.; Mora, A. Chicken and turkey meat: Consumer exposure to multidrug-resistant Enterobacteriaceae including mcr-carriers, uropathogenic E. coli and high-risk lineages such as ST131. Int. J. Food Microbiol. 2020, 331, 108750. [Google Scholar] [CrossRef]
- Interagency Coordination Group on Antimicrobial Resistance. No Time to Wait: Infections from Drug-Resistant Securing the Future from Drug-Resistant Infections; Technical Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lechner, I.; Freivogel, C.; Stärk, K.D.; Visschers, V.H. Exposure pathways to antimicrobial resistance at the human-animal interface—A qualitative comparison of Swiss expert and consumer opinions. Front. Public Health 2020, 8, 345. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). About Antimicrobial Resistance. 2015. Available online: https://www.cdc.gov/antimicrobial-resistance/about/index.html (accessed on 24 May 2025).
- Graham, J.P.; Boland, J.J.; Silbergeld, E. Growth promoting antibiotics in food animal production: An economic analysis. Public Health Rep. 2007, 122, 79–87. [Google Scholar] [CrossRef]
- Giubilini, A.; Birkl, P.; Douglas, T.; Savulescu, J.; Maslen, H. Taxing Meat: Taking Responsibility for One’s Contribution to Antibiotic Resistance. J. Agric. Environ. Ethics 2017, 30, 179–198. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- Szoke, Z.; Fauszt, P.; Mikolas, M.; David, P.; Szilagyi-Tolnai, E.; Pesti-Asboth, G.; Homoki, J.R.; Kovacs-Forgacs, I.; Gal, F.; Stundl, F.; et al. Comprehensive analysis of antimicrobial resistance dynamics among broiler and duck intensive production systems. Sci. Rep. 2025, 15, 4673. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Buonavoglia, A.; Farooq, M.; Festino, A.R.; Ruffini, F.; Paludi, D.; Di Francesco, C.E.; Vergara, A.; Smoglica, C. Antibiotic resistance in Italian poultry meat production chain: A one-health perspective comparing antibiotic free and conventional systems from the farming to the slaughterhouse. Front. Food Sci. Technol. 2023, 3, 1168896. [Google Scholar] [CrossRef]
- Musuka, G.; Machakwa, J.; Mano, O.; Iradukunda, P.G.; Gashema, P.; Moyo, E.; Nsengimana, A.; Manhokwe, S.; Dhliwayo, T.; Dzinamarira, T. Antimicrobial Resistance and Its Impact on Food Safety Determinants Along the Beef Value Chain in Sub-Saharan Africa—A Scoping Review. Trop. Med. Infect. Dis. 2025, 10, 82. [Google Scholar] [CrossRef]
- Cameron, A.; McAllister, T.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016, 7, 68. [Google Scholar] [CrossRef]
- Morel, C. Transmission of antimicrobial resistance from livestock agriculture to humans and from humans to animals. In OECD Food, Agriculture and Fisheries Papers, No. 133; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- McMahon, M.A.S.; Xu, J.; Moore, J.E.; Blair, I.S.; McDowell, D.A. Environmental stress and antibiotic resistance in food-related pathogens. Appl. Environ. Microbiol. 2007, 73, 211–217. [Google Scholar] [CrossRef]
- Buncic, S.; Nychas, G.-J.; Lee, M.R.F.; Koutsoumanis, K.; Hébraud, M.; Desvaux, M.; Chorianopoulos, N.; Bolton, D.; Blagojevic, B.; Antic, D. Microbial pathogen control in the beef chain: Recent research advances. Meat Sci. 2014, 97, 288–297. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- EMA Regulation (EC) No. 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying Down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No. 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No. 726/2004 of the European Parliament and of the CouncilText with EEA Relevance. Available online: http://data.europa.eu/eli/reg/2009/470/oj (accessed on 21 June 2024).
- Almashhadany, D.A. Detecting Antibiotic Residues Among Sheep Milk Using YCT, DDA, and Acidification Method in Erbil City, Kurdistan Region, Iraq. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2020, 77, 29–35. [Google Scholar] [CrossRef]
- Almashhadany, D.A. Monitoring of Antibiotic Residues among Sheep Meat in Erbil City and Thermal Processing Effect on Their Remnants. Iraqi J. Vet. Sci. 2020, 34, 217–222. [Google Scholar] [CrossRef]
- Horn, N.; Bhunia, A.K. Food-associated stress primes foodborne pathogens for the gastrointestinal phase of infection. Front. Microbiol. 2018, 9, 1962. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Trymers, M.; Chajęcka-Wierzchowska, W.; Tkacz, K.; Zadernowska, A.; Modzelewska-Kapituła, M. Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective. Pathogens 2024, 13, 1123. [Google Scholar] [CrossRef]
- Dixit, O.V.A.; Behruznia, M.; Preuss, A.L.; O’Brien, C.L. Diversity of antimicrobial resistant bacteria isolated from Australian chicken and pork meat. Front. Microbiol. 2024, 15, 1347597. [Google Scholar] [CrossRef] [PubMed]
- Gousia, P.; Economou, V.; Sakkas, H.; Leveidiotou, S.; Papadopoulou, C. Antimicrobial Resistance of Major Foodborne Pathogens from Major Meat Products. Foodborne Pathog. Dis. 2011, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tagar, S.; Qambrani, N.A. Bacteriological quality assessment of poultry chicken meat and meat contact surfaces for the presence of targeted bacteria and determination of antibiotic resistance of Salmonella spp. in Pakistan. Food Control 2023, 151, 109786. [Google Scholar] [CrossRef]
- Zelalem, A.; Sisay, A.; Vipham, J.L.; Abegaz, K.; Kebede, A.; Terefe, Y. The prevalence and antimicrobial resistance profiles of bacterial isolates from meat and meat products in Ethiopia: A systematic review and meta-analysis. Int. J. Food Contam. 2019, 6, 1. [Google Scholar] [CrossRef]
- Ojha, A.K.; Shah, N.P.; Mishra, V.; Emanuel, N.; Taneja, N.K. Prevalence of antibiotic resistance in lactic acid bacteria isolated from traditional fermented Indian food products. Food Sci. Biotechnol. 2023, 32, 2131–2143. [Google Scholar] [CrossRef] [PubMed]
- Vinayamohan, P.G.; Viju, L.S.; Joseph, D.; Venkitanarayanan, K. Fermented Foods as a Potential Vehicle of Antimicrobial-Resistant Bacteria and Genes. Fermentation 2023, 9, 688. [Google Scholar] [CrossRef]
- Wolfe, B.E. Are fermented foods an overlooked reservoir of antimicrobial resistance? Curr. Opin. Food Sci. 2023, 51, 101018. [Google Scholar] [CrossRef]
- Fraqueza, M.J. Antibiotic resistance of lactic acid bacteria isolated from dry-fermented sausages. Int. J. Food Microbiol. 2015, 212, 76–88. [Google Scholar] [CrossRef]
- Leroy, S.; Christieans, S.; Talon, R. Tetracycline Gene Transfer in Staphylococcus xylosus in situ During Sausage Fermentation. Front. Microbiol. 2019, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Wang, P.; Zhao, X.; Liang, L.; Ji, Q.; Ge, Y.; Chen, Y. Metagenomic profiles of the antimicrobial resistance in traditional Chinese fermented meat products: Core resistome and co-occurrence patterns. Int. J. Food Microbiol. 2024, 418, 110740. [Google Scholar] [CrossRef] [PubMed]
- Shahraz, F.; Dadkhah, H.; Khaksar, R.; Mahmoudzadeh, M.; Hosseini, H.; Kamran, M.; Bourke, P. Analysis of antibiotic resistance patterns and detection of mecA gene in Staphylococcus aureus isolated from packaged hamburger. Meat Sci. 2012, 90, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Galán-Relaño, Á.; Díaz, A.V.; Lorenzo, B.H.; Gómez-Gascón, L.; Mena, M.Á.; Carrasco, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and salmonellosis: An update on public health implications and control strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- Pavelquesi, S.L.S.; de Oliveira Ferreira, A.C.A.; Rodrigues, L.F.S.; Silva, C.M.S.; Silva, I.C.R.; Orsi, D.C. Prevalence and antimicrobial resistance of Salmonella spp. isolated from chilled chicken meat commercialized at retail in Federal District. J. Food Prot. 2023, 86, 100130. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf (accessed on 3 October 2024).
- NARMS. National Antimicrobial Resistance Monitoring System for Enteric Bacteria: Antimicrobial Resistance Facts. Antimicrobial Resistance Facts|NARMS|CDC; 2024. Available online: https://www.cdc.gov/narms/resistance/index.html (accessed on 1 October 2024).
- Chai, S.J.; Cole, D.; Nisler, A.; Mahon, B.E. Poultry: The most common food in outbreaks with known pathogens, United States, 1998–2012. Epidemiol. Infect. 2017, 145, 316–325. [Google Scholar] [CrossRef]
- Al-Qadiri, H.M.; Al-Holy, M.A.; Al-Abdallat, A.M.; Olaimat, A.N.; Saleh, M.; Bakri, F.G.; Abutarbush, S.M.; Rasco, B.A. Antimicrobial resistance of Salmonella spp. recovered from raw chicken meat at abattoirs and retail markets in Jordan. Ital. J. Food Sci. 2025, 37, 216–227. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. Available online: https://www.cdc.gov/mmwr/volumes/67/ss/ss6710a1.htm (accessed on 29 June 2025). [CrossRef] [PubMed]
- Smadi, H.; Sargeant, J.M. Quantitative risk assessment of human salmonellosis in Canadian broiler chicken breast from retail to consumption. Risk Anal. 2013, 33, 232–248. [Google Scholar] [CrossRef]
- Borges, K.A.; Furian, T.Q.; Souza, S.N.; Salle, C.T.P.; Moraes, H.L.S.; Nascimento, V.P. Antimicrobial resistance and molecular characterization of Salmonella enterica serotypes isolated from poultry sources in Brazil. Braz. J. Poult. Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Perin, A.P.; Martins, B.; Barreiros, M.; Yamatogi, R.S.; Nero, L.A.; Dos Santos Bersot, L. Occurrence, quantification, pulse types, and antimicrobial susceptibility of Salmonella spp. Isolated from chicken meat in the state of Paraná, Brazil. Braz. J. Microbiol. 2020, 51, 335–345. [Google Scholar] [CrossRef]
- Cunha-Neto, A.D.; Carvalho, L.A.; Carvalho, R.C.T.; dos Prazeres Rodrigues, D.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Salmonella isolated from chicken carcasses from a slaughterhouse in the state of Mato Grosso, Brazil: Antibiotic resistance profile, serotyping, and characterization by repetitive sequence-based PCR system. Poult. Sci. 2018, 97, 1373–1381. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibioticresistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, S.; Hansen, M.; Miranda, R.; Newsom-Stewart, K.; Rogers, J.E. Prevalence and antibiotic resistance of Salmonella and Campylobacter isolates from raw chicken breasts in retail markets in the United States and comparison to data from the plant level. Life 2023, 13, 642. [Google Scholar] [CrossRef]
- Zaiko, E.V.; Bataeva, D.S.; Yushina, Y.K.; Grudistova, M.A.; Velebit, B. Prevalence, serovar, and antimicrobial resistance of Salmonella isolated from meat and minced meat used for production smoked sausage. In Proceedings of the 61st International Meat Industry Conference, Zlatibor, Serbia, 26–29 September 2021; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 854, p. 012108. [Google Scholar] [CrossRef]
- Tack, B.; Vanaenrode, J.; Verbakel, J.Y.; Toelen, J.; Jacobs, J. Invasive non-typhoidal Salmonella infections in sub-Saharan Africa: A systematic review on antimicrobial resistance and treatment. BMC Med. 2020, 18, 212. [Google Scholar] [CrossRef]
- Terentjeva, M.; Avsejenko, J.; Streikiša, M.; Utināne, A.; Kovaļenko, K.; Bērziņš, A. Prevalence and antimicrobial resistance of Salmonella in meat and meat products in Latvia. Ann. Agric. Environ. Med. 2017, 24, 317–321. [Google Scholar] [CrossRef] [PubMed]
- ISO 6579:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2002.
- Kalchayanand, N.; Dass, S.C.; Zhang, Y.; Oliver, E.L.; Wang, B.; Wheeler, T.L. Efficacy of Antimicrobial Interventions Used in Meat Processing Plants against Antimicrobial Tolerant Non–Antibiotic-Resistant and Antibiotic-Resistant Salmonella on Fresh Beef. J. Food Prot. 2022, 85, 1114–1121. [Google Scholar] [CrossRef]
- Jung, D.; Morrison, B.J.; Rubin, J.E. A review of antimicrobial resistance in imported foods. Can. J. Microbiol. 2022, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar]
- Bukari, Z.; Emmanuel, T.; Woodward, J.; Ferguson, R.; Ezughara, M.; Darga, N.; Lopes, B.S. The Global Challenge of Campylobacter: Antimicrobial Resistance and Emerging Intervention Strategies. Trop. Med. Infect. Dis. 2025, 10, 25. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic Resistance in Campylobacter: Emergence, Transmission and Persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef]
- Du, Y.; Wang, C.; Ye, Y.; Liu, Y.; Wang, A.; Li, Y.; Zhou, X.; Pan, H.; Zhang, J.; Xu, X. Molecular Identification of Multidrug-Resistant Campylobacter Species from Diarrheal Patients and Poultry Meat in Shanghai, China. Front. Microbiol. 2018, 9, 1642. [Google Scholar] [CrossRef]
- Signorini, M.L.; Rossler, E.; Díaz David, D.C.; Olivero, C.R.; Romero-Scharpen, A.; Soto, L.P.; Astesana, D.M.; Berisvil, A.P.; Zimmermann, J.A.; Fusari, M.L.; et al. Antimicrobial Resistance of Thermotolerant Campylobacter Species Isolated from Humans, Food-Producing Animals, and Products of Animal Origin: A Worldwide Meta-Analysis. Microb. Drug Resist. 2018, 24, 1174–1190. [Google Scholar] [CrossRef]
- Schiaffino, F.; Colston, J.M.; Paredes-Olortegui, M.; François, R.; Pisanic, N.; Burga, R.; Peñataro-Yori, P.; Kosek, M.N. Antibiotic Resistance of Campylobacter Species in a Pediatric Cohort Study. Antimicrob. Agents Chemother. 2019, 63, e01911-18. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ramamurthy, T.; Bhattacharya, M.K.; Rajendran, K.; Mukhopadhyay, A.K. Campylobacter jejuni in hospitalized patients with diarrhea, Kolkata, India. Emerg. Infect. Dis. 2013, 19, 1155–1156. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Raketić, S.V.; Mašković, J.M.; Mašković, P.Z.; Kurćubić, L.V.; Heinz, V.; Tomasevic, I.B. Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin. Plants 2023, 12, 3236. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Đurović, V.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Mašković, P.Z.M.; Mašković, J.; Mitić, M.; Živković, V.; et al. Multitarget Phytocomplex: Focus on Antibacterial Profiles of Grape Pomace and Sambucus ebulus L. Lyophilisates Against Extensively Drug-Resistant (XDR) Bacteria and In Vitro Antioxidative Power. Antibiotics 2024, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Madhup, S.K.; Shrestha, R.; Panta, R.; Chauguthi, L.; Katu-wal, N.; Shrestha, S. Prevalence of pathogenic bacteria in meat products and their antimicrobial resistance pattern. ACCLM 2021, 4, 13–19. [Google Scholar] [CrossRef]
- Crespi, E.; Ghiglione, B.; Crivelli, X.B.; Cundon, C.; Espinosa, R.F.; Penzotti, P.; Bentancor, A. Analysis of Escherichia coli isolated from minced meat: Implications for public health in Tierra del Fuego, Argentina. Rev. Argent. Microbiol. 2025, in press. [Google Scholar] [CrossRef]
- Gweshe, W.M.; Muteveri, T.; Gufe, C.; Marumure, J.; Hodobo, T.C. Antimicrobial-resistant Shiga-toxin producing Escherichia coli Isolated from Ready-to-eat Meat Products and Fermented Milk Sold in the Formal and Informal Sectors in Harare, Zimbabwe. J. Pure Appl. Microbiol. 2020, 14, 1157–1165. [Google Scholar] [CrossRef]
- Zhang, W.; Jayarao, B.M.; Knabel, S.J. Multi-virulence-locus sequence typing of Listeria monocytogenes. Appl. Environ. Microbiol. 2004, 70, 913–920. [Google Scholar] [CrossRef]
- Moreno, L.Z.; Paixão, R.; Gobbi, D.D.S.; Raimundo, D.C.; Ferreira, T.P.; Moreno, A.M.; Matté, M.H. Characterization of antibiotic resistance in Listeria spp. isolated from slaughterhouse environments, pork and human infections. J. Infect. Dev. Ctries. 2014, 8, 416–423. [Google Scholar] [CrossRef]
- Walsh, D.; Duffy, G.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A. Antibiotic resistance among Listeria, including Listeria monocytogenes, in retail food. J. Appl. Microbiol. 2001, 90, 517–522. [Google Scholar] [CrossRef]
- Morobe, I.C.; Obi, C.L.; Nyila, M.A.; Gashe, B.A.; Matsheka, M.I. Prevalence, antimicrobial resistance profiles of Listeria monocytognes from various foods in Gaborone, Botswana. Afr. J. Biotechnol. 2009, 8, 285. [Google Scholar] [CrossRef]
- Wilson, A.; Gray, J.; Chandry, P.S.; Fox, E.M. Phenotypic and genotypic Analysis of antimicrobial resistance among Listeria monocytogenes isolated from Australian food production chains. Genes 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.K.; Lu, P.L.; Chen, J.Y.; Lin, F.M.; Chang, S.C. High-level expression of AmpC β-lactamase due to insertion of nucleotides between -10 and -35 promoter sequences in Escherichia coli clinical isolates: Cases not responsive to extended-spectrum-cephalosporin treatment. Antimicrob. Agents Chemother. 2003, 47, 2138–2144. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Arnold, C.; Threlfall, E.J. Rapid detection of gyrA and parC mutations in quinolone-resistant Salmonella enterica using Pyrosequencing® technology. J. Microbiol. Methods 2007, 68, 163–171. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Haskell, K.J.; Schriever, S.R.; Fonoimoana, K.D.; Haws, B.; Hair, B.B.; Wienclaw, T.M.; Holmstead, J.G.; Barboza, A.B.; Berges, E.T.; Heaton, M.J.; et al. Antibiotic resistance is lower in Staphylococcus aureus isolated from antibiotic-free raw meat as compared to conventional raw meat. PLoS ONE 2018, 13, e0206712. [Google Scholar] [CrossRef] [PubMed]
- Ajose, D.J.; Abolarinwa, T.O.; Oluwarinde, B.O.; Montso, P.K.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens. Biomedicines 2022, 10, 2426. [Google Scholar] [CrossRef]
- Abukhattab, S.; Hosch, S.; Abu-Rmeileh, N.M.E.; Hasan, S.; Vonaesch, P.; Crump, L.; Hattendorf, J.; Daubenberger, C.; Zinsstag, J.; Schindler, T. Whole-genome sequencing for One Health surveillance of antimicrobial resistance in conflict zones: A case study of Salmonella spp. and Campylobacter spp. in the West Bank, Palestine. Appl. Environ. Microbiol. 2023, 89, e00658-23. [Google Scholar] [CrossRef]
- Collignon, P.; Powers, J.H.; Chiller, T.M.; Aidara-Kane, A.; Aarestrup, F.M. World health organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 2016, 49, 132–141. [Google Scholar] [CrossRef]
- Fumagalli, D. Antibiotic Resistance, Meat Consumption and the Harm Principle. Ethics Policy Environ. 2023, 26, 53–68. [Google Scholar] [CrossRef]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Global Action Plan on Antimicrobial Resistance. World Health Organization. 2015. Available online: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf (accessed on 24 May 2025).
- Pan, Y.; Deng, Z.; Shahidi, F. Natural bioactive substances for the control of food-borne viruses and contaminants in food. Food Prod. Process. Nutr. 2020, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Na, F.; Zhang, M.; Yang, W. Microbial Control in the Processing of Low Temperature Meat Products: Non-Thermal Sterilization and Natural Antimicrobials. Foods 2025, 14, 225. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines on Use of Medically Important Antimicrobials in Food–Producing Animals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- O’Neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste. The Review on Antimicrobials Resistance. 2015. Available online: https://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf (accessed on 24 May 2025).
- O’Neill, J. Tackling Drug Resistant Infections Globally: Final Report and Recommendations, The Review on Antimicrobial Resistance. 2016. Available online: http://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 24 May 2025).
- Grace, D. Review of Evidence on Antimicrobial Resistance and Animal Agriculture in Developing Countries; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2015. [Google Scholar] [CrossRef]
- ECDC/EFSA; EMEA; European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2015, 13, 114. [Google Scholar] [CrossRef]
- Anachinaba, I.A.; Adzitey, F.; Teye, G.A.; Brown, C.A.; Boateng, E.F. Knowledge and perception of consumers on microbiological meat safety, antibiotic resistance and residues in Tema Metropolis, Ghana. J. Agric. Food Sci. 2022, 20, 135–153. [Google Scholar] [CrossRef]
- Hinchliffe, S.; Bard, A.; Chan, K.W.; Adam, K.; Bruce, A.; Reyher, K.; Buller, H. Regulating antimicrobial resistance: Market intermediaries, poultry and the audit lock-in. Agric. Hum. Values 2024, 41, 801–814. [Google Scholar] [CrossRef]
- Nguyen, H. Antibiotic Resistance in Livestock: A Literature Review of Its Consequences and International Effort on Human Antimicrobial Agents Resistant Crisis; Master’s Thesis; Augsburg University: Minneapolis, MN, USA, 2024; 1615p, Available online: https://idun.augsburg.edu/etd/1615 (accessed on 29 June 2025).
- Yang, D.; Heederik, D.J.J.; Scherpenisse, P.; Van Gompel, L.; Luiken, R.E.C.; Wadepohl, K.; Skarżyńska, M.; Van Heijnsbergen, E.; Wouters, I.M.; Greve, G.D.; et al. Antimicrobial resistance genes aph(3′)-III, erm(B), sul2 and tet(W) abundance in animal faeces, meat, production environments and human faeces in Europe. J. Antimicrob. Chemother. 2022, 77, 1883–1893. [Google Scholar] [CrossRef]
- Hughes, A.; Roe, E.; Hocknell, S. Food supply chains and the antimicrobial resistance challenge: On the framing, accomplishments and limitations of corporate responsibility. Environ. Plan A 2021, 53, 1373–1390. [Google Scholar] [CrossRef]
- Mc Nulty, K.; Soon, J.M.; Wallace, C.A.; Nastasijevic, I. Antimicrobial resistance monitoring and surveillance in the meat chain: A report from five countries in the European Union and European Economic Area. Trends Food Sci. Technol. 2016, 58, 1–13. [Google Scholar] [CrossRef]
- DANMAP. Summary. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; Statens Serum Institut: Copenhagen, Denmark, 2023; ISBN 978-87-93565-81-4. Available online: https://www.danmap.org/-/media/institutter/foedevareinstituttet/danmap-site/report-2023/opdaterede-materialer-for-2023/summary_danmap_low_version-1.pdf (accessed on 29 June 2025).
- Anses. Resapath—French Surveillance Network for Antimicrobial Resistance in Bacteria from Diseased Animals: 2022 Annual Report; Report, 53; Anses: Lyon et Ploufragan-Plouzané-Niort, France, 2023; Available online: https://www.anses.fr/en/system/files/LABO-Ra-Resapath2022EN.pdf (accessed on 29 June 2025).
- NethMap. Consumption of Antimicrobial Agents and Antimicrobial Resistance Among Medically Important Bacteria in the Netherlands. 2019. Available online: https://www.rivm.nl/bibliotheek/rapporten/2024-0117.pdf (accessed on 29 June 2025).
- Swedres-Svarm. Sales of Antibiotics and Occurrence of Antibiotic Resistance in Sweden. Solna/Uppsala 2024. Available online: https://www.sva.se/media/amupibfr/swedres-svarm-2024-webb.pdf (accessed on 29 June 2025).
- NORM/NORM-VET. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Ås/Oslo 2024. ISSN:1502-2307 (print)/1890–9965 (electronic). 2023. Available online: https://www.fhi.no/contentassets/4e24fb63a3754577a94c42b6c8cc89c4/norm-norm-vet-2023-komplett.pdf (accessed on 29 June 2025).
- Urdahl, A.M.; Norström, M.; Sunde, M.; Grøntvedt, C.A. The Surveillance Programme for Methicillin Resistant Staphylococcus aureus in Pigs in Norway 2023; Surveillance Program Report; Veterinærinstituttet, Norwegian Veterinary Institute: Oslo, Norway, 2024. [Google Scholar]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://iris.who.int/bitstream/handle/10665/341666/9789240027336-eng.pdf?sequence=1 (accessed on 29 June 2025).
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Dekker, D.M. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; WHO Regional Office for Europe. Surveillance of Antimicrobial Resistance in Europe 2023: Executive Summary; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-ECDC-WHO-executive-summary-2023-data.pdf (accessed on 29 June 2025).
- European Centre for Disease Prevention and Control; Aïch, N.; Latour, K.; Ricchizzi, E.; Suetens, C.; Hopkins, S.; Kolman, J.; Högberg, L.D. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Long-Term Care Facilities—2023–2024. 2025. Available online: https://data.europa.eu/doi/10.2900/5735023 (accessed on 29 June 2025).
- Núñez-Núñez, M.; Navarro, M.D.; Palomo, V.; Rajendran, N.B.; Del Toro, M.D.; Voss, A.; Sharland, M.; Sifakis, F.; Tacconelli, E.; Rodríguez-Baño, J. The methodology of surveillance for antimicrobial resistance and healthcare-associated infections in Europe (SUSPIRE): A systematic review of publicly available information. Clin. Microbiol. Infect. 2018, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- European Sales and Use of Antimicrobials for Veterinary Medicine (ESUAvet). Annual Surveillance Report for 2023’ (EMA/CVMP/ESUAVET/80289/2025). 2025. Available online: https://www.ema.europa.eu/en/documents/report/european-sales-use-antimicrobials-veterinary-medicine-annual-surveillance-report-2023_en.pdf (accessed on 29 June 2025).
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-annual-epidemiological-report-EARS-Net-2023.pdf (accessed on 29 June 2025).
- FDA Requests Public Comments to Inform Development of National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan for 2026–2030. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/national-antimicrobial-resistance-monitoring-system (accessed on 29 June 2025).
- CIPARS. Canadian Integrated Program for Antimicrobial Resistance Surveillance Executive Summary: Key and Integrated Findings; CIPARS: Ottawa, ON, Canada, 2022; ISBN 978-0-660-70100-4. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/drugs-health-products-canadian-integrated-program-antimicrobial-resistance-surveillance-2022-executive-summary/canadian-integrated-program-antimicrobial-resistance-surveillance-2022-executive-summary-en.pdf (accessed on 29 June 2025).
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Jaja, I.F.; Oguttu, J.; Jaja, C.-J.I.; Green, E. Prevalence and distribution of antimicrobial resistance determinants of Escherichia coli isolates obtained from meat in South Africa. PLoS ONE 2020, 15, e0216914. [Google Scholar] [CrossRef]
- Sweileh, W.M. Global research activity on antimicrobial resistance in food-producing animals. Arch. Public Health 2021, 79, 49. [Google Scholar] [CrossRef] [PubMed]
- Timme, R.E.; Wolfgang, W.J.; Balkey, M.; Venkata, S.L.G.; Randolph, R.; Allard, M.; Strain, E. Optimizing open data to support one health: Best practices to ensure interoperability of genomic data from bacterial pathogens. One Health Outlook 2020, 2, 20. [Google Scholar] [CrossRef]
- Pei, R.; Zhang, L.; Duan, C.; Gao, M.; Feng, R.; Jia, Q.; Huang, Z. Investigation of Stress Response Genes in Antimicrobial Resistant Pathogens Sampled from Five Countries. Processes 2021, 9, 927. [Google Scholar] [CrossRef]
- Li, K.; Zheng, J.; Deng, T.; Peng, J.; Daniel, D.; Jia, Q.; Huang, Z.Y. An Analysis of Antimicrobial Resistance of Clinical Pathogens from Historical Samples for Six Countries. Processes 2019, 7, 964. [Google Scholar] [CrossRef]
- Yang, K.; Wang, A.; Fu, M.; Wang, A.; Chen, K.; Jia, Q.; Huang, Z.Y. Investigation of Incidents and Trends of Antimicrobial Resistance in Foodborne Pathogens in Eight Countries from Historical Sample Data. Int. J. Environ. Res. Public Health 2020, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.G.; Huang, W.L.; Chen, A.; Rehmet, M.; Jin, C.; Huang, Z.Y. Comparison of Antimicrobial Resistance Detected in Environmental and Clinical Isolates from Historical Data for the US. Biomed. Res. Int. 2020, 2020, 4254530. [Google Scholar] [CrossRef]
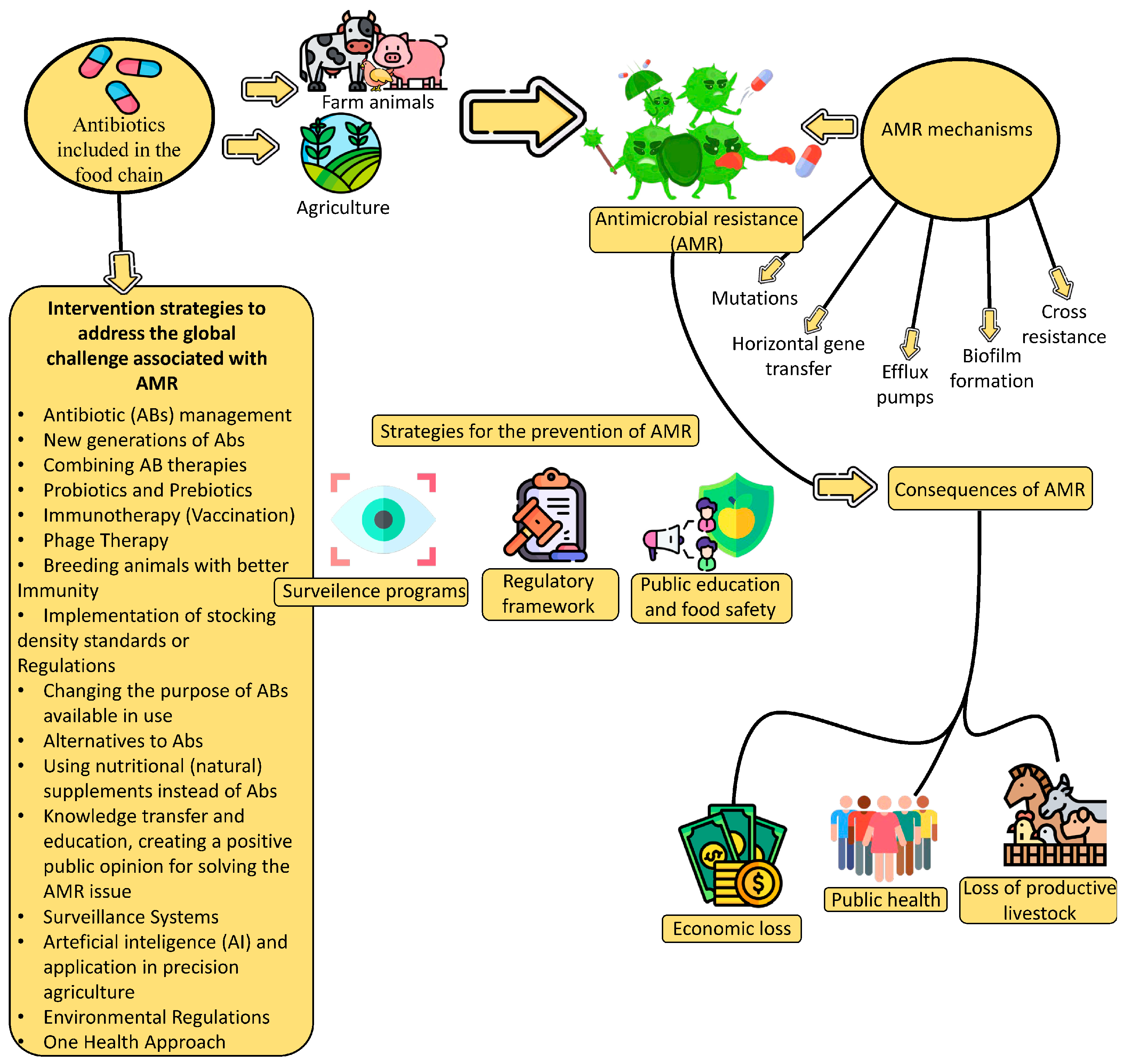
| Strategy | Approach | Flaws (Disadvantages) | Strengths (Benefits) | |
|---|---|---|---|---|
| 1. | Antibiotic (ABs) management | Targeted use of ABs, therapeutic administration and dosage must be optimized, ethical, and traceable. | Changes in the habits of patients and healthcare professionals are necessary. | The strategy reduces the emergence of resistance, maintaining AB efficacy at a desirable, justified level. |
| 2. | New generations of ABs | Research and development in the creation of new ABs to eliminate existing and prevent new bacterial mechanisms that lead to AMR. | It is expensive, the development and registration of new ABs is lengthy, and there is a potential for cross-resistance to occur. | Reduces or prevents the emergence of AMR to available ABs already in use. |
| 3. | Combining AB therapies | Treatment of infections using multiple ABs with different mechanisms of action. | Dosage is complicated, risk of AB antagonism and side effects. | Potential synergistic effects increase efficiency and effectiveness and reduce the AMR. |
| 4. | Probiotics and Prebiotics | Promoting the growth of beneficial bacteria to outcompete harmful strains. | Limited knowledge of optimal strains, challenges in colonization and persistence. | Supports healthy microbiota, reduces space for pathogens. |
| 5. | Immunotherapy (Vaccination) | Developing and boosting the immune response to fight against infections. | Live attenuated vaccines carry the risk of returning virulence and pathogenicity. Killed vaccines are safe but create weaker immunity with the need for revaccination; they are often specific for certain infections, i.e., only for certain strains (genotypes) of the virus; potential for the development of autoimmune processes. | Effective against various types and strains of microorganisms, long-term protection is possible. |
| 6. | Phage Therapy | Using bacteriophages (viruses that multiply extremely quickly in bacteria and lead to their disintegration) to target specific bacterial strains. | Insufficient knowledge about phage-bacterial interactions, regulatory issues. | Highly targeted perspective, can be expeditiously modified. |
| 7. | Breeding animals with better immunity | Animals with better immunity are easier to gain, healthier, and more resistant to breeding or infectious diseases. | Complex and uncertain, long-term creation is primarily achieved by genetic engineering. | Lower mortality, improved animal health and welfare, lower costs of ABs purchasing and vet services |
| 8. | Implementation of stocking density standards or regulations | Prevention of the occurrence of various diseases, especially respiratory ones, which require metaphylaxis or mass therapy of sick or suspected sick animals with antibiotics | Reduced capacity of facilities for housing animals. | Lower costs of ABs purchasing and vet services; healthier animals, less difficult occurrence of infections and/or transfer/spread of infectious agents (especially in respiratory infections). |
| 9. | Changing the purpose of ABs available in use | Identifying non-AB agents with AM power. | Limited options and number of ABs, dose optimization is a reality, as are side effects. | Lower costs due to potentially faster development. |
| 10. | Alternatives to ABs | Creating non-AB treatments (AM peptides or bacteriocins; Plant-based drugs); | Insufficient clinical data support; toxicity and delivery method are still challenging. | Lower risk of AMR occurrence, various mechanisms; natural AM agents are biodegradable, with low allergenic potential. |
| 11. | Using nutritional (natural) supplements instead of ABs | Plant-Derived Substances (polyphenols, alkaloids, and tannins) present a great potential for use, like antimicrobials or as AB resistance modifiers. | May cause side effects, interfere with the effects of other supplements or medications; In inappropriate, high doses, they can be toxic. | Do not normally cause AMR; Different mechanisms of action that can overcome AMR and the reduced side effects. |
| 12. | Knowledge transfer and education, creating a positive public opinion for solving the AMR issue | Promoting good hygiene practice, knowledge of AB use, and understanding of AMR. | Reduces unnecessary and improper AB demand and misuse. | Continued efforts are expected and required: the change in awareness of the problems associated with AMR is gradual, and the impact is difficult to measure and assess. |
| 13. | Surveillance Systems | Monitoring and tracking resistance patterns to inform treatment guidelines. | Resource-intensive, challenges in data sharing and harmonization. | Provides real-time data, guides treatment decisions. |
| 14. | Artificial intelligence (AI) and applications in precision agriculture. | Optimizing AB use in agriculture; early disease detection and diagnosis; preventing AMR with predictions; precise application of treatments in agriculture; improved animal welfare; developing new AM drugs; amplifying surveillance and monitoring. | Insufficient farmer trust, higher investment required, possible only in industrial food production. | Quality and Availability of (Big) Data; Model Explainability; Ethical issues; Fusing with Existing Systems. |
| 15. | Environmental Regulations | Limiting or reducing AB use in agronomy and the food industry to limit AMR transfer. | Regulatory enforcement, global coordination, and economic implications. | Mitigates selection pressure for resistance. |
| 16. | One Health Approach | Coordinating efforts across human, animal, and environmental health to tackle resistance. | A multi-/interdisciplinary approach is necessary for collaboration, challenges in communication and policy alignment. | Addresses complex sources of AMR spread. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurćubić, V.S.; Munjić, M.D.; Dmitrić, M.P.; Živković, S.; Stajić, S.B.; Tomasevic, I. Bacterial Antimicrobial Resistance in Meat Products—Current Concepts. Foods 2025, 14, 2792. https://doi.org/10.3390/foods14162792
Kurćubić VS, Munjić MD, Dmitrić MP, Živković S, Stajić SB, Tomasevic I. Bacterial Antimicrobial Resistance in Meat Products—Current Concepts. Foods. 2025; 14(16):2792. https://doi.org/10.3390/foods14162792
Chicago/Turabian StyleKurćubić, Vladimir S., Matija D. Munjić, Marko P. Dmitrić, Saša Živković, Slaviša B. Stajić, and Igor Tomasevic. 2025. "Bacterial Antimicrobial Resistance in Meat Products—Current Concepts" Foods 14, no. 16: 2792. https://doi.org/10.3390/foods14162792
APA StyleKurćubić, V. S., Munjić, M. D., Dmitrić, M. P., Živković, S., Stajić, S. B., & Tomasevic, I. (2025). Bacterial Antimicrobial Resistance in Meat Products—Current Concepts. Foods, 14(16), 2792. https://doi.org/10.3390/foods14162792








