Effects of Dietary GABA Levels on Growth, Muscle Quality, and Liver Lipid Profile: Insights from Lipidomics in Juvenile Yellowfin Seabream Acanthopagrus latus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish Husbandry
2.3. Sample Collection
2.4. Analysis of Nutritional Compositions and Texture Parameters
2.5. Analysis of Lipidomic AMD Fatty Acid Profile
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Whole Body and Muscle Proximate Composition
3.3. Muscle Amino Acid Composition
3.4. Muscle Texture
3.5. Results of Lipidomics Analysis of the Liver and Muscle

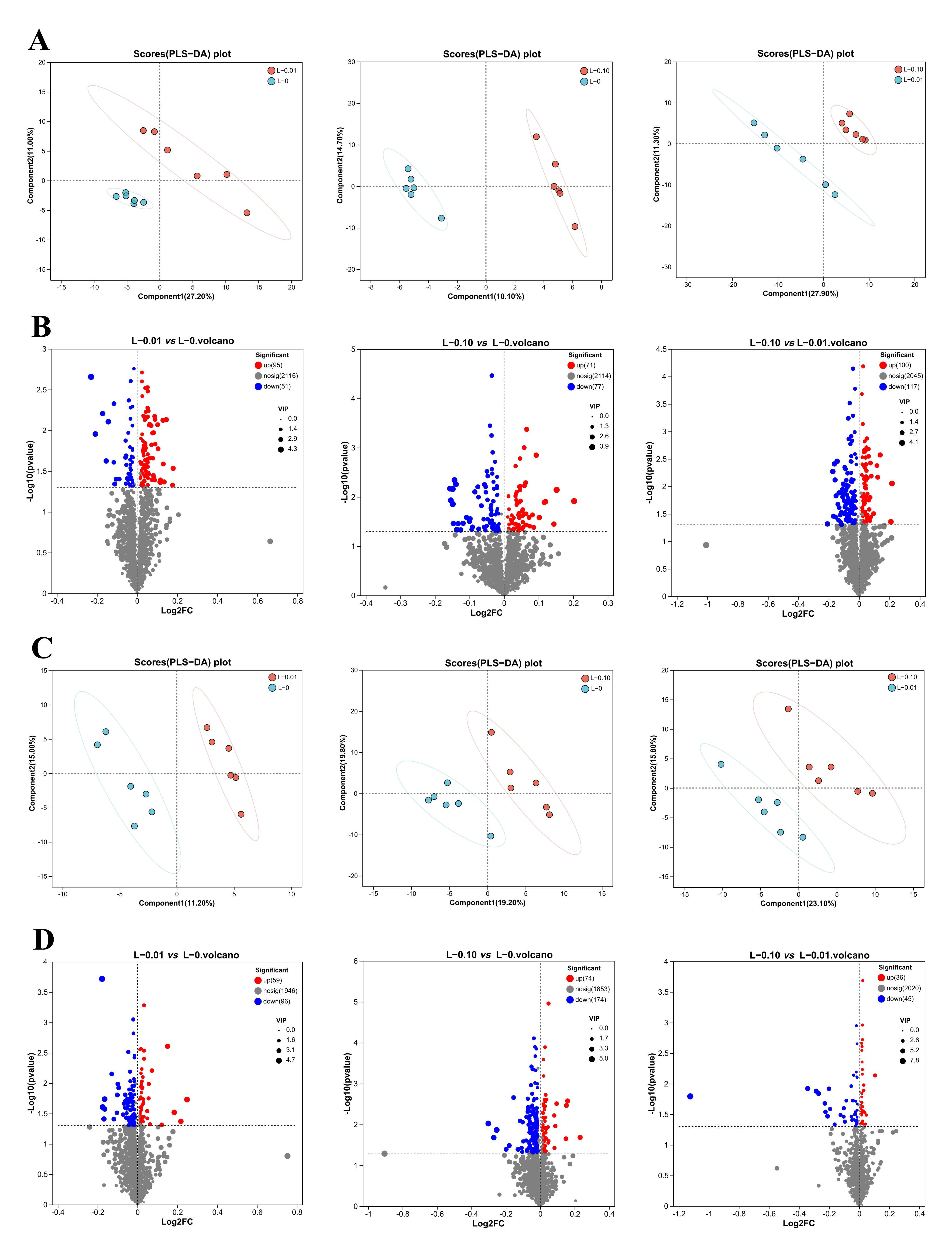
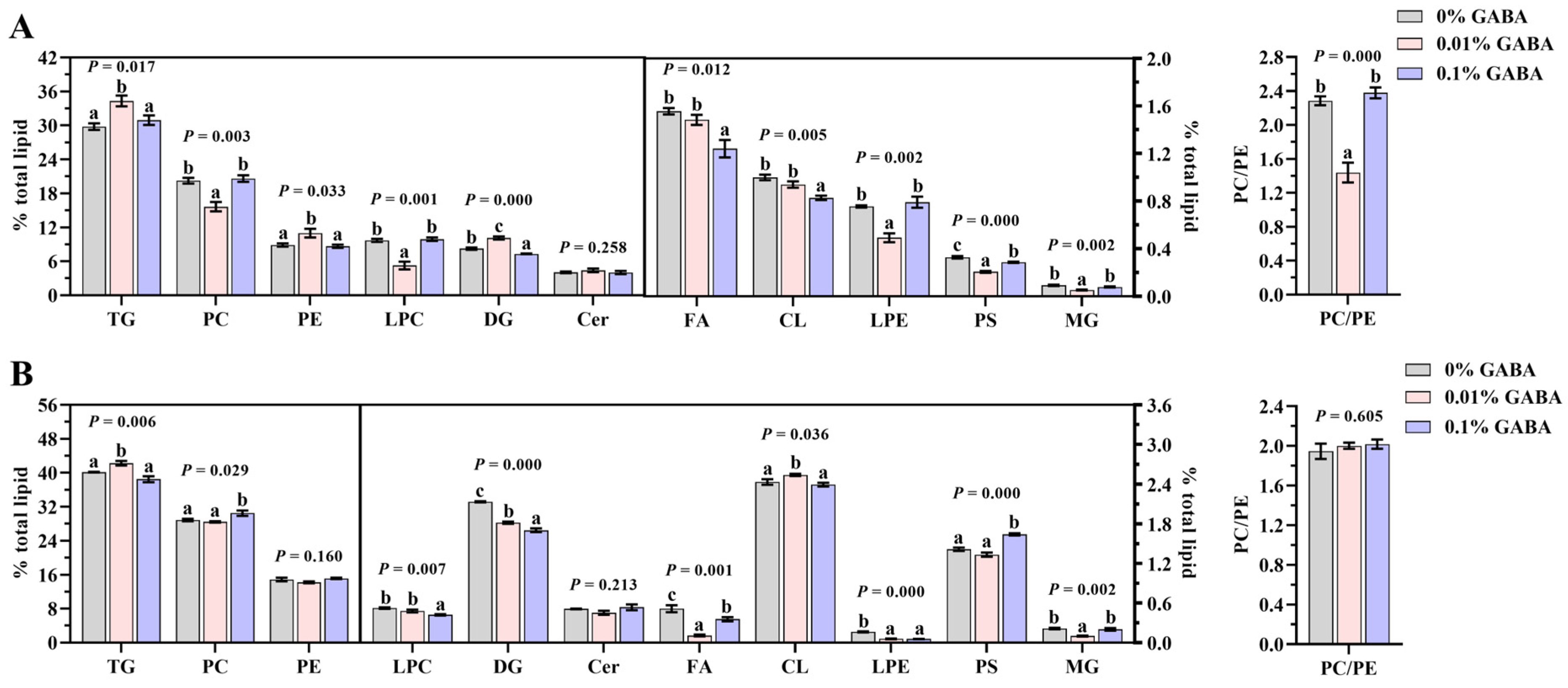
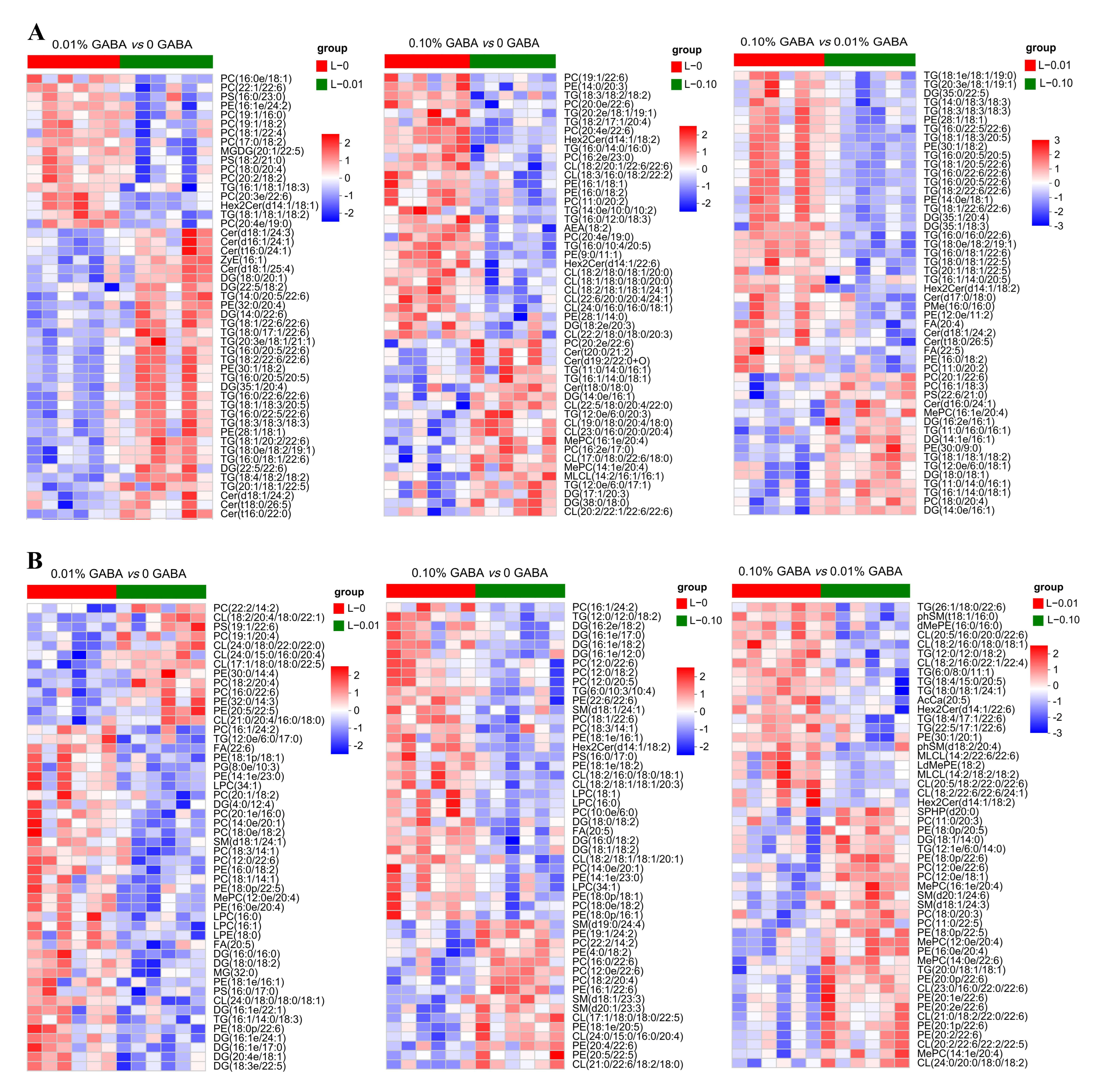
3.6. Fatty Acid Profile of the Liver and Muscle
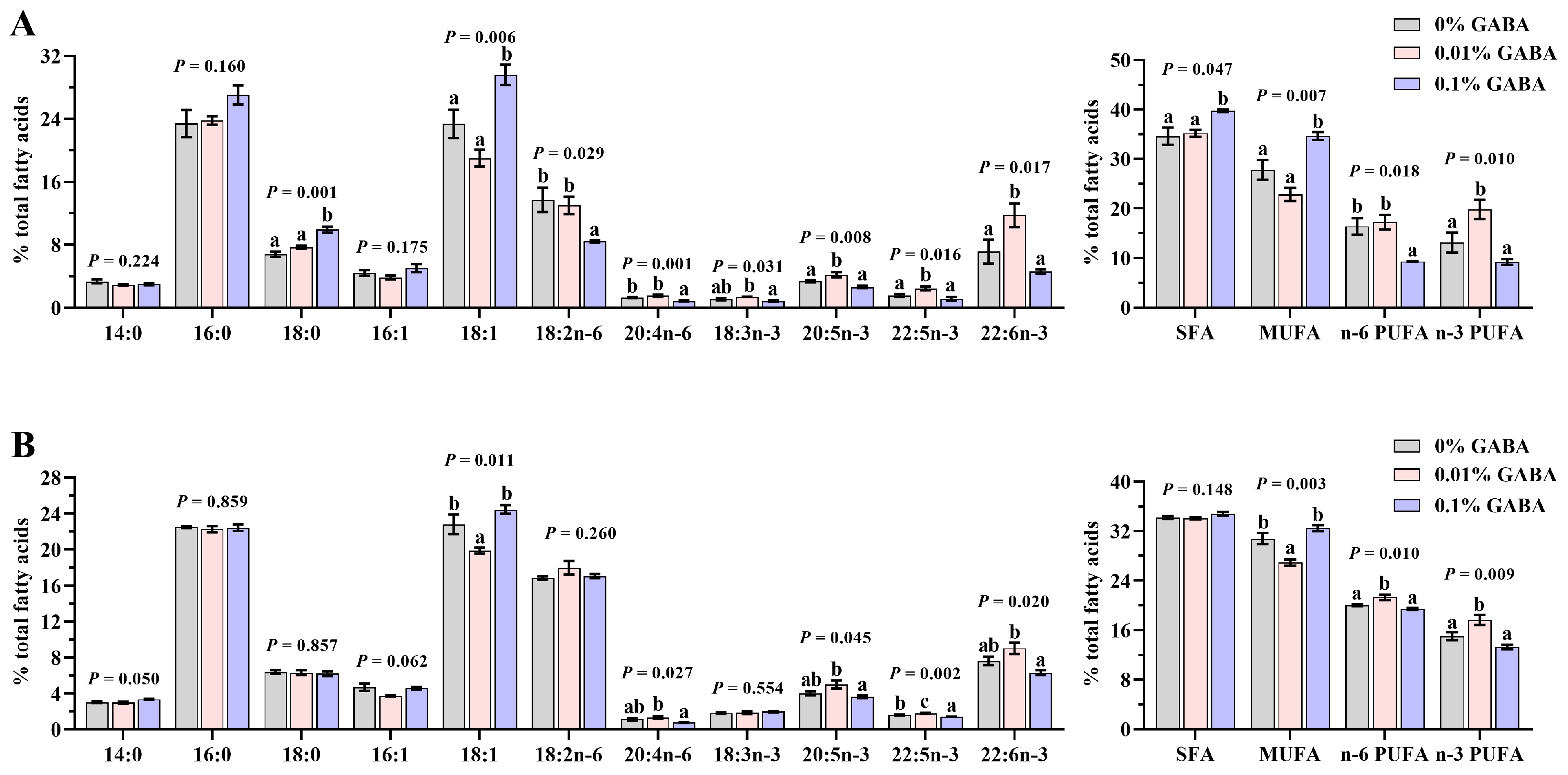
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | linolenic acid |
| ARA | arachidonic acid |
| Cer | ceramide |
| CL | cardiolipin |
| CMR | cooked meat rate |
| DHA | docosahexaenoic acid |
| DGs | diacylglycerols |
| DPA | docosapentaenoic acid |
| EAAs | essential amino acids |
| EPA | eicosapentaenoic acid |
| FAs | fatty acids |
| FAAs | flavor amino acids |
| FCR | feed conversion ratio |
| FI | feed intake |
| GABA | gamma-aminobutyric acid |
| HCl | hydrochloric acid |
| HPLC | high performance liquid chromatography |
| LA | linoleic acid |
| LC-MS | liquid chromatography–mass spectrometry |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| MGs | monoacylglycerols |
| MUFAs | monounsaturated fatty acids |
| NEAA | non-essential amino acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PLS-DA | partial least squares discriminant analysis |
| PUFAs | polyunsaturated fatty acids |
| PS | phosphatidylserine |
| SEM | standard error of the mean |
| SFAs | saturated fatty acids |
| SUR | survival rate |
| SGR | specific growth rate |
| TG | triglyceride |
| VIP | variable importance in projection |
| WGR | weight gain rate |
References
- Lee, S.E.; Lee, Y.; Lee, G.H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res. 2019, 42, 1031–1039. [Google Scholar] [CrossRef]
- Dastgerdi, A.H.; Sharifi, M.; Soltani, N. GABA administration improves liver function and insulin resistance in offspring of type 2 diabetic rats. Sci. Rep. 2021, 11, 23155. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.H.; Liu, C.; Li, G.Y.; Qiao, X.Z.; Gan, Y.J.; Zhang, C.C.; Deng, Y.C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, H. Gamma-aminobutyric acid signaling in damage response, metabolism, and disease. Int. J. Mol. Sci. 2023, 24, 4584. [Google Scholar] [CrossRef]
- Nasreen, Z.; Jameel, T.; Hasan, A.; Parveen, N.; Sadasivudu, B. Glutamate decarboxylase and GABA aminotransferase levels in different regions of rat brain on the onset of leptazol induced convulsions. Neurochem. Res. 2012, 37, 202–204. [Google Scholar] [CrossRef]
- Tu, J.; Jin, Y.C.; Zhuo, J.; Cao, X.T.; Liu, G.H.; Du, H.J.; Liu, L.; Wang, J.; Xiao, H. Exogenous GABA improves the antioxidant and anti-aging ability of silkworm (Bombyx mori). Food Chem. 2022, 383, 132400. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhao, J.B.; Hu, J.H.; He, H.X.; Wei, Y.H.; Ji, L.B.; Ma, X. Gama-aminobutyric acid (GABA) alleviates hepatic inflammation via GABA receptors/TLR4/NF-κBpathways in growing-finishing pigs generated by super-multiparous sows. Anim. Nutr. 2022, 9, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Chen, H.Y.; Bao, D.P.; Shin, T.Y.; Zhong, Y.J.; Zhang, X.; Wu, Y.Y. Recent advances of γ-aminobutyric acid: Physiological and immunity function, enrichment, and metabolic pathway. Front. Nutr. 2022, 9, 1076223. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.; Balasubramanian, R.; Sampathrajan, V.; Veerasamy, R.; Appachi, S.V.; Kumar, K.K. Transforming tomatoes into GABA-rich functional foods through genome editing: A modern biotechnological approach. Funct. Integr. Genom. 2025, 25, 27. [Google Scholar] [CrossRef]
- Bai, X.L.; Zhang, L.; Liang, H.L.; Huang, D.Y.; Ren, M.C.; Mi, H.F. Dietary γ-aminobutyric acid promotes growth and immune system performance and improves erythropoiesis and angiogenesis in gibel carp (Carassius auratus gibelio). Animals 2025, 15, 125. [Google Scholar] [CrossRef]
- Ma, J.M.; Yang, Y.B.; Ding, H.; Tan, Q.Y.; Song, Y.J.; Shen, H.C.; Ai, Q.H.; Zhao, C.Y.; Dewangan, N.K.; Xu, C. γ-aminobutyric acid improves the growth performance, food intake and glucose homeostasis of Micropterus salmoides fed high-carbohydrate diets. Aquac. Rep. 2024, 38, 102350. [Google Scholar] [CrossRef]
- Li, C.Q.; Tian, Y.; Ma, Q.Y.; Zhang, B.L. Dietary gamma-aminobutyric acid ameliorates growth impairment and intestinal dysfunction in turbot (Scophthalmus maximus L.) fed a high soybean meal diet. Food Funct. 2022, 13, 290. [Google Scholar] [CrossRef]
- Lin, Y.J.; Li, X.N.; Chen, X.M.; Chen, J.M.; Jin, X.Y.; Sun, J.X.; Niu, X.T.; Kong, Y.D.; Li, M.; Wang, G.Q. γ-aminobutyric acid effectively modulate growth performance, physiological response of largemouth bass (Micropterus salmoides) under combined stress of flow velocity and density. Aquac. Nutr. 2024, 2024, 9180554. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Bron, J.E.; Tocher, D.R. Determination of n-3 HUFA content in Atlantic salmon flesh based on the lipid content, morphometric measurements and blood fatty acid composition: A modeling approach. J. Appl. Ichthyol. 2009, 25, 120–123. [Google Scholar] [CrossRef]
- .Gogus, U.; Smith, C. N-3 omega fatty acids: A review of current knowledge. Int. J. Food Sci. Technol. 2010, 45, 417–436. [Google Scholar] [CrossRef]
- Zhang, G.R.; Xu, C.; You, C.H.; Wang, S.Q.; Xie, D.Z.; Hasan, A.K.M.M.; Ma, Y.C.; Li, Y.Y. Effects of dietary vitamin E on growth performance, antioxidant capacity and lipid metabolism of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2021, 27, 2205–2217. [Google Scholar] [CrossRef]
- Bi, C.P.; Yin, J.J.; Yang, W.; Shi, B.M.; Shan, A.S. Effects of dietary γ-aminobutyric acid supplementation on antioxidant status, blood hormones and meat quality in growing-finishing pigs undergoing transport stress. J. Anim. Physiol. Anim. Nutr. 2019, 104, 590–596. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, D.; Wu, X.T.; Feng, Y.Y.; Ni, Y.D. Dietary γ-Aminobutyric acid supplementation inhibits high-fat diet-induced hepatic steatosis via modulating gut microbiota in broilers. Microorganisms 2022, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, G.H.; Hoang, T.H.; Kim, Y.M.; Jang, G.H.; Seok, C.H.; Gwak, Y.G.S.; Lim, J.; Kim, J.; Chae, H.J. GABA and fermented Curcuma longa L. extract enriched with GABA ameliorate obesity through Nox4-IRE1α sulfonation-RIDD-SIRT1 decay axis in high-fat diet-induced obese mice. Nutrients 2022, 14, 1680. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorn, M.; He, S.; Chang, C.H.; Koh, Y.C.; Yang, M.J.; Pan, M.H. High gamma-aminobutyric acid (GABA) oolong tea alleviates high-fat diet-induced metabolic disorders in mice. ACS Omega 2023, 8, 33997–34007. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Ma, J.R.; Kong, L.M.; Huang, Z.F.; Wang, X.J.; Quan, F.Q.; Zhao, X.; Yi, Z.Y.; Lin, H.; Liu, L.H.; Zhao, Y.T.; et al. Effects of dietary puerarin on growth, digestive enzyme, antioxidant capacity, immune and liver health of Acanthopagrus latus. Aquac. Rep. 2024, 37, 102261. [Google Scholar] [CrossRef]
- Nong, X.L.; Zhu, K.C.; Guo, H.Y.; Liu, B.S.; Zhang, N.; Zhang, Q.; Zhang, D.C. Effects of Density Stress During Transportation on the Antioxidant Activity and Immuno-Related Gene Expression in Yellowfin Seabream (Acanthopagrus latus Houttuyn, 1782). Genes 2024, 15, 1479. [Google Scholar] [CrossRef]
- Gunathilaka, B.E.; Jeong, S.M.; Kim, K.W.; Lee, S.; Hur, S.W.; You, S.G.; Lee, S.M. Evaluation of gamma aminobutyric acid and sodium butyrate in juvenile red seabream (Pagrus major) diets containing graded levels of fish meal and soy protein concentrate. Animals 2024, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Farris, N.W.; Hamidoghli, A.; Bae, J.; Won, S.; Choi, W.; Biro, J.; Lee, S.; Bai, S.C. Dietary Supplementation with γ-aminobutyric acid improves growth, digestive enzyme activity, non-specific immunity and disease resistance against Streptococcus iniae in juvenile olive flounder, Paralichthys olivaceus. Animals 2022, 12, 248. [Google Scholar] [CrossRef]
- Temu, V.; Kim, H.; Hamidoghli, A.; Park, M.; Won, S.; Oh, M.; Han, J.; Bai, S.C. Effects of dietary gamma-aminobutyric acid in juvenile Nile tilapia, Orechromis niloticus. Aquaculture 2019, 507, 475–480. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Lin, H.B.; Yu, X.Y.; Fang, J.X.; Lu, Y.H.; Liu, P.; Xing, Y.G.; Wang, Q.; Che, Z.M.; He, Q. Flavor compounds in pixian broad-bean paste: Non-volatile organic acids and amino acids. Molecules 2018, 23, 1299. [Google Scholar] [CrossRef]
- Li, M.M.; Zhang, M.; Ma, Y.C.; Ye, R.K.; Li, Y.Y. Dietary supplementation with n-3 high unsaturated fatty acids decreases serum lipid levels and improves flesh quality in the marine teleost golden pompano Trachinotus ovatus. Aquaculture 2019, 516, 734632. [Google Scholar] [CrossRef]
- Ye, R.K.; Zheng, J.; Li, M.M.; Chen, H.Y.; Ma, Y.C.; Xie, D.Z.; Ning, L.J.; Sun, L.H.; Wang, Y.; Li, Y.Y. Effects of liquid and powdered fat on growth, health and muscle quality of juvenile GIFT Oreochromis niloticus. J. Fish. China 2019, 43, 2197–2208. [Google Scholar]
- Zhang, G.R.; Xu, J.Z.; Chen, F.; Guan, J.F.; Su, N.N.; Gao, X.; Xie, D.Z.; Li, Y.Y. Dietary fatty acid composition modulates growth in the marine teleost Trachinotus ovatus by regulating the hepatic triglyceride to (phosphatidylcholine + phosphatidylethanolamine) ratio via the Ampk/Srebp signaling pathway. Aquaculture 2025, 607, 742660. [Google Scholar] [CrossRef]
- Li, M.M.; Zhu, M.X.; Chai, W.Q.; Wang, Y.H.; Song, Y.H.; Liu, B.X.; Cai, C.Y.; Song, Y.Z.; Sun, X.; Xue, P.; et al. Determination of the heterogeneity of intramuscular fat and visceral adipose tissue from dezhou donkey by lipidomics and transcriptomics profiling. Front. Nutr. 2021, 8, 746684. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Available online: https://www.R-project.orgR Foundation for Statistical Computing: Vienna, Austria, 2020; (accessed on 8 January 2025).
- Li, Y.Y.; Chen, W.Z.; Sun, Z.W.; Chen, J.H.; Wu, K.G. Effects of n-3 HUFA content in broodstock diet on spawning performance and fatty acid composition of eggs and larvae in Plectorhynchus cinctus. Aquaculture 2005, 245, 263–272. [Google Scholar] [CrossRef]
- Ruenkoed, S.; Nontasan, S.; Phudkliang, J.; Phudinsai, P.; Pongtanalert, P.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Effect of dietary gamma aminobutyric acid (GABA) modulated the growth performance, immune and antioxidant capacity, digestive enzymes, intestinal histology and gene expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 141, 109056. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Won, S.; Choi, W.; Lim, S.G.; Kim, K.W.; Lee, B.J.; Hur, S.W.; Bai, S.C. Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 2020, 525, 735333. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Farris, N.W.; Olowe, O.S.; Choi, W.; Lee, S.; Won, S.; Ohh, M.; Lee, S.; Bai, S.C. Dietary γ-aminobutyric acid (GABA) promotes growth and resistance to vibrio alginolyticus in whiteleg shrimp Litopenaeus vannamei. Aquac. Nutr. 2022, 2022, 9105068. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, C.S.; Du, X.Y.; Xu, H.; Wang, G.Q.; Zhang, D.M. Effects of dietary γ-aminobutyric acid levels on the growth, serum biochemical indexes, immune-related signalling molecules of Jian carp. Aquac. Res. 2020, 52, 1096–1105. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, B.; Xia, Y.Y.; Liao, S.M.; Wang, M.W.; Yin, J.; Wang, J.; Xiao, H.; Qi, M.; Bin, P.; et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct. 2019, 10, 366–378. [Google Scholar] [CrossRef]
- Ma, Y.C.; Li, M.M.; Xie, D.Z.; Chen, S.J.; Dong, Y.W.; Wang, M.; Zhang, G.R.; Zhang, M.; Chen, H.Y.; Ye, R.K.; et al. Fishmeal can be replaced with a high proportion of terrestrial protein in the diet of the carnivorous marine teleost (Trachinotus ovatus). Aquaculture 2020, 519, 734910. [Google Scholar] [CrossRef]
- Xie, S.W.; Li, Y.T.; Zhou, W.W.; Tian, L.X.; Li, Y.M.; Zeng, S.L.; Liu, Y.J. Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquac. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.H.; Liu, J.Q.; Huang, D.Q. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach. Food Control 2019, 100, 305–314. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Jia, W.; Zhang, C.H.; Li, X.; Fan, Y.O.; Wang, H. Characterization and discrimination of Taihe black-boned silky fowl (Gallus gallus domesticus Brisson) muscles using LC/MS-based lipidomics. Food Res. Int. 2018, 109, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, J.G.; Gao, S.; Ning, L.J.; Limbu, S.M.; Qiao, F.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Dietary oils modify lipid molecules and nutritional value of fillet in nile tilapia: A deep lipidomics analysis. Food Chem. 2019, 277, 515–523. [Google Scholar] [CrossRef]
- Chen, F.Q.; Wang, Y.T.; Liu, Y.Y.; Chen, Q.S.; Liu, H.L.; Tian, J.; Wang, M.X.; Ren, C.Y.; Zhao, Q.; Yang, F.J.; et al. Exogenous γ-aminobutyric acid (GABA) provides a carbon skeleton to promote the accumulation of sugar and unsaturated fatty acids in vegetable soybean seeds. Environ. Exp. Bot. 2025, 229, 106052. [Google Scholar] [CrossRef]
- Cai, W.J.; Liu, H.K.; Fu, L.L.; Han, D.; Zhu, X.M.; Jin, J.Y.; Yang, Y.X.; Xie, S.Q. Dietary inosine monophosphate improved liver health and flesh quality of gibel carp (Carassius auratus gibelio) via activating AMPK signalling pathway and enhancing the contents of muscle fat and flavour substance. Front. Mar. Sci. 2022, 9, 940732. [Google Scholar] [CrossRef]
- Rincón, L.; Castro, P.L.; Alvarez, B.; Hernández, M.D.; Alvarez, A.; Claret, A.; Guerrero, L.; Ginés, R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Chen, Y.K.; Zhong, J.; Chen, X.Q.; Li, X.M.; Pu, H.Q.; Chen, B.Y.; Guo, Y.C.; Chen, A.Q.; Li, W.J.; Hu, P.; et al. Dietary astaxanthin alleviates black soldier fly oil-induced negative changes of fatty acids content and muscle quality on Oncorhynchus mykiss via mammalian target of rapamycin and AMP-activated protein kinase pathway. Anim. Nutr. 2024, 19, 313–324. [Google Scholar] [CrossRef]
- Gong, Y.; Weng, M.; Wang, X.E.; Zhang, W.C.; Wang, Z.; Sun, J.; Cao, X.F.; Zhang, J.M.; Zhao, M.X.; Zhang, Z.; et al. Effects of vegetable oil replacement on intramuscular fat deposition and flesh quality of large yellow croaker (Larimichthys crocea) juveniles. Aquaculture 2023, 575, 739731. [Google Scholar] [CrossRef]
- Song, R.; Yao, X.F.; Jing, F.T.; Yang, W.X.; Wu, J.J.; Zhang, H.; Zhang, P.H.; Xie, Y.Y.; Pan, X.W.; Zhao, L.; et al. Effects of five lipid sources on growth, hematological parameters, immunity and muscle quality in juvenile largemouth bass (Micropterus salmoides). Animals 2024, 14, 781. [Google Scholar] [CrossRef]
- Jin, G.X.; Zhang, L.; Mai, K.S.; Chen, X.R.; Xu, S.D.; Ai, Q.H. Effects of different dietary lipid sources on growth performance, hepatic lipid deposition and transcriptome response in spotted sea bass (Lateolabrax maculatus). Aquaculture 2023, 566, 739143. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xie, P.S.; Cai, Z.W. Lipid metabolism disorders contribute to hepatotoxicity of triclosan in mice. J. Hazard. Mater. 2019, 384, 121310. [Google Scholar] [CrossRef]
- Ding, R.B.; Tian, K.; Cao, Y.W.; Bao, J.L.; Wang, M.; He, C.; Hu, Y.; Su, H.; Wan, J.B. Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanolmediated oxidative stress. J. Agric. Food Chem. 2015, 63, 2413–2422. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Devlin, C.; Tabas, I.; Vance, D.E. Targeted deletion of hepatic CTP: Phosphocholine cytidylyltransferase α in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004, 279, 47402–47410. [Google Scholar] [CrossRef]
- Jin, H.; Xia, P.K.; Deng, Z.C.; Hou, T.; Li, J.; Li, B. Effects of konjac glucomannan on weight management and liver health: Insights from liver lipidomics in obese and nonobese mice. J. Agric. Food Chem. 2024, 72, 7906–7918. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta Mol. 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Mashek, D.G. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol. Metab. 2021, 50, 101115. [Google Scholar] [CrossRef]
- Han, M.S.; Lim, Y.M.; Quan, W.; Kim, J.R.; Chung, K.W.; Kang, M.; Kim, S.; Park, S.Y.; Han, J.S.; Park, S.Y. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid Res. 2011, 52, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.A.; Bawden, S.J.; Malaikah, S.; Sargeant, J.A.; Stensel, D.J.; Aithal, G.P.; King, J.A. The role of hepatic lipid composition in obesity-related metabolic disease. Liver Int. 2021, 41, 2819–2835. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; Koehler, C.M. The complexity of cardiolipin in health and disease. Trends Biochem. Sci. 2012, 37, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, M.M.; Chen, C.; Chen, J.J.; Tan, Q.S. Effects of dietary gamma aminobutyric acid on growth performance, antioxidant status, and feeding-related gene expression of juvenile grass carp, Ctenopharyngodon idellus. J. World Aquac. Soc. 2017, 47, 820–829. [Google Scholar] [CrossRef]
| Diets | |||
|---|---|---|---|
| Ingredients | L-0 | L-0.01 | L-0.1 |
| Fish meal | 30.00 | 30.00 | 30.00 |
| Chicken meal | 10.40 | 10.40 | 10.40 |
| Soybean meal | 20.50 | 20.50 | 20.50 |
| Ethanol clostridium protein | 5.00 | 5.00 | 5.00 |
| Black soldier fly powder | 5.00 | 5.00 | 5.00 |
| Fish oil | 2.00 | 2.00 | 2.00 |
| Soybean oil | 2.00 | 2.00 | 2.00 |
| Lecithin | 2.00 | 2.00 | 2.00 |
| Cassava starch | 10.00 | 10.00 | 10.00 |
| α-starch | 8.30 | 8.29 | 8.20 |
| Vitamin premix 1 | 1.00 | 1.00 | 1.00 |
| Mineral premix 2 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 |
| Monocalcium phosphate | 1.50 | 1.50 | 1.50 |
| Taurine | 0.80 | 0.80 | 0.80 |
| GABA | 0 | 0.01 | 0.10 |
| Proximate composition | |||
| Moisture | 7.13 | 6.67 | 6.85 |
| Crude protein | 50.30 | 50.46 | 50.46 |
| Crude lipid | 11.04 | 10.77 | 10.94 |
| Ash | 8.98 | 8.88 | 8.52 |
| GABA (mg/kg) | 64.93 | 136.59 | 772.93 |
| Items | Diets | Pooled SEM | p-Value | Regression (p, r2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| L-0 | L-0.01 | L-0.1 | Linear | Quadratic | |||||
| Initial weight (g) | 9.82 | 9.91 | 10.15 | 0.146 | 0.693 | 0.379 | 0.112 | 0.693 | 0.115 |
| Final weight (g) | 28.60 a | 31.57 b | 27.74 a | 0.653 | 0.009 | 0.138 | 0.286 | 0.009 | 0.789 |
| WGR (%) | 191.53 b | 218.86 c | 173.27 a | 7.002 | 0.001 | 0.034 | 0.497 | 0.001 | 0.895 |
| SGR (%/day) | 1.70 b | 1.84 c | 1.60 a | 0.037 | 0.001 | 0.029 | 0.517 | 0.001 | 0.899 |
| FI, (g/fish) | 18.43 b | 14.35 a | 13.91 a | 0.794 | 0.006 | 0.098 | 0.343 | 0.006 | 0.817 |
| FCR | 1.24 b | 0.97 a | 1.00 a | 0.050 | 0.018 | 0.247 | 0.186 | 0.018 | 0.739 |
| SUR (%) | 89.33 | 94.67 | 92.00 | 1.886 | 0.579 | 0.924 | 0.001 | 0.579 | 0.167 |
| Items | Diets | Pooled SEM | p-Value | Regression (p, r2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| L-0 | L-0.01 | L-0.1 | Linear | Quadratic | |||||
| Whole body (%) | |||||||||
| Moisture | 73.15 b | 70.82 a | 71.83 ab | 0.426 | 0.042 | 0.520 | 0.061 | 0.042 | 0.653 |
| Protein | 16.98 a | 17.53 b | 16.68 a | 0.133 | 0.002 | 0.051 | 0.442 | 0.002 | 0.869 |
| Lipid | 5.22 | 6.30 | 5.51 | 0.311 | 0.389 | 0.820 | 0008 | 0.389 | 0.270 |
| Ash | 5.81 b | 5.16 a | 5.43 ab | 0.115 | 0.035 | 0.687 | 0.025 | 0.035 | 0.673 |
| Muscle tissue (%) | |||||||||
| Moisture | 77.87 | 77.36 | 77.79 | 0.115 | 0.144 | 0.615 | 0.038 | 0.144 | 0.476 |
| Protein | 18.58 | 18.65 | 18.48 | 0.047 | 0.407 | 0.238 | 0.192 | 0.407 | 0.259 |
| Lipid | 1.76 b | 2.80 c | 0.97 a | 0.285 | 0.003 | 0.031 | 0.509 | 0.003 | 0.861 |
| Ash | 1.27 b | 0.94 a | 1.31 b | 0.065 | 0.010 | 0.229 | 0.199 | 0.010 | 0.787 |
| Items | Diets | Pooled SEM | p-Value | Regression (p, r2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| L-0 | L-0.01 | L-0.1 | Linear | Quadratic | |||||
| Ala | 4.00 a | 4.37 c | 4.24 b | 0.054 | 0.000 | 0.507 | 0.065 | 0.000 | 0.999 |
| Arg | 3.98 a | 4.33 c | 4.18 b | 0.050 | 0.000 | 0.644 | 0.032 | 0.000 | 0.999 |
| Asp | 6.57 a | 7.40 c | 7.07 b | 0.120 | 0.000 | 0.594 | 0.043 | 0.000 | 1.000 |
| Cys | 0.02 a | 0.01 a | 0.02 b | 0.001 | 0.001 | 0.002 | 0.780 | 0.001 | 0.890 |
| Cystine | 0.34 a | 0.40 b | 0.33 a | 0.012 | 0.014 | 0.171 | 0.250 | 0.014 | 0.757 |
| Glu | 8.70 a | 9.88 c | 9.46 b | 0.172 | 0.000 | 0.513 | 0.063 | 0.000 | 1.000 |
| Gly | 3.49 a | 3.66 c | 3.58 b | 0.025 | 0.000 | 0.706 | 0.022 | 0.000 | 0.988 |
| His | 1.54 a | 1.66 c | 1.61 b | 0.018 | 0.001 | 0.594 | 0.043 | 0.001 | 0.899 |
| Ile | 3.00 a | 3.31 c | 3.11 b | 0.045 | 0.000 | 0.859 | 0.005 | 0.000 | 0.994 |
| Leu | 5.38 a | 5.96 c | 5.70 b | 0.085 | 0.000 | 0.699 | 0.023 | 0.000 | 0.999 |
| Lys | 6.42 a | 7.22 c | 6.84 b | 0115 | 0.000 | 0.762 | 0.014 | 0.000 | 0.999 |
| Met | 1.87 a | 2.19 c | 2.02 b | 0.046 | 0.000 | 0.911 | 0.014 | 0.000 | 1.000 |
| Phe | 3.25 a | 3.57 c | 3.43 b | 0.047 | 0.000 | 0.666 | 0.028 | 0.000 | 0.995 |
| Pro | 2.36 a | 2.56 c | 2.44 b | 0.029 | 0.000 | 0.992 | 0.000 | 0.000 | 0.974 |
| Ser | 2.22 a | 2.40 b | 2.43 c | 0.033 | 0.000 | 0.049 | 0.446 | 0.000 | 0.998 |
| Thr | 2.90 a | 3.29 c | 3.20 b | 0.044 | 0.000 | 0.399 | 0.104 | 0.000 | 1.000 |
| Try | 0.81 b | 0.83 c | 0.80 a | 0.004 | 0.000 | 0.068 | 0.398 | 0.000 | 0.984 |
| Tyr | 2.19 a | 2.41 c | 2.33 b | 0.032 | 0.000 | 0.537 | 0.057 | 0.000 | 0.991 |
| Val | 3.36 a | 3.66 c | 3.47 b | 0.045 | 0.000 | 0.883 | 0.003 | 0.000 | 0.999 |
| EAA | 32.60 a | 36.02 c | 34.37 b | 0.494 | 0.000 | 0.775 | 0.012 | 0.000 | 0.999 |
| NEAA | 29.89 a | 33.08 c | 31.89 b | 0.465 | 0.000 | 0.542 | 0.055 | 0.000 | 1.000 |
| FAA | 22.76 a | 25.30 c | 24.34 b | 0.370 | 0.000 | 0.550 | 0.053 | 0.000 | 1.000 |
| Items | Diets | Pooled SEM | p-Value | Regression (p, r2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| L-0 | L-0.01 | L-0.1 | Linear | Quadratic | |||||
| Hardness (gf) | 96.31 | 108.30 | 107.59 | 2.807 | 0.146 | 0.319 | 0.099 | 0.146 | 0.348 |
| Springiness (mm) | 0.47 a | 0.54 c | 0.52 b | 0.010 | 0.000 | 0.354 | 0.086 | 0.000 | 0.871 |
| Chewiness (mJ) | 24.61 a | 33.78 b | 24.98 a | 1.427 | 0.001 | 0.272 | 0.119 | 0.001 | 0.801 |
| Gumminess (mJ) | 49.45 a | 58.42 c | 52.72 b | 1.338 | 0.000 | 0.876 | 0.004 | 0.000 | 0.958 |
| Cohesiveness | 0.56 | 0.56 | 0.55 | 0.004 | 0.555 | 0.319 | 0.099 | 0.555 | 0.123 |
| Shear force (gf) | 589.26 | 602.76 | 586.75 | 21.34 | 0.956 | 0.867 | 0.003 | 0.956 | 0.010 |
| Cooked meat rate (%) | 89.99 | 87.83 | 88.98 | 0.705 | 0.452 | 0.902 | 0.002 | 0.452 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Yang, Y.; Huang, Z.; Zheng, S.; Feng, X.; Li, J.; Chen, F.; Li, Y. Effects of Dietary GABA Levels on Growth, Muscle Quality, and Liver Lipid Profile: Insights from Lipidomics in Juvenile Yellowfin Seabream Acanthopagrus latus. Foods 2025, 14, 2761. https://doi.org/10.3390/foods14162761
Zhang G, Yang Y, Huang Z, Zheng S, Feng X, Li J, Chen F, Li Y. Effects of Dietary GABA Levels on Growth, Muscle Quality, and Liver Lipid Profile: Insights from Lipidomics in Juvenile Yellowfin Seabream Acanthopagrus latus. Foods. 2025; 14(16):2761. https://doi.org/10.3390/foods14162761
Chicago/Turabian StyleZhang, Guanrong, Yanjian Yang, Zini Huang, Shishi Zheng, Xinyu Feng, Ju Li, Fang Chen, and Yuanyou Li. 2025. "Effects of Dietary GABA Levels on Growth, Muscle Quality, and Liver Lipid Profile: Insights from Lipidomics in Juvenile Yellowfin Seabream Acanthopagrus latus" Foods 14, no. 16: 2761. https://doi.org/10.3390/foods14162761
APA StyleZhang, G., Yang, Y., Huang, Z., Zheng, S., Feng, X., Li, J., Chen, F., & Li, Y. (2025). Effects of Dietary GABA Levels on Growth, Muscle Quality, and Liver Lipid Profile: Insights from Lipidomics in Juvenile Yellowfin Seabream Acanthopagrus latus. Foods, 14(16), 2761. https://doi.org/10.3390/foods14162761






