Elucidation of Nutritional Quality, Antinutrients, and Protein Digestibility of Dehulled and Malted Flours Produced from Three Varieties of Bambara Groundnut (Vigna subterranean)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Production of Dehulled and Malted Bambara Groundnut Flour
2.3. Proximate Composition Analysis of Bambara Groundnut Flours
2.4. Amino Acid Profile Analysis of Bambara Groundnut Flours
2.5. Mineral Analysis of Bambara Groundnut Flours
2.6. Fatty Acid Profile Analysis of Bambara Groundnut Flours
2.7. Antinutritional Factors Analysis of Bambara Groundnut Flours
2.8. In Vitro Protein Digestibility Analysis of Bambara Groundnut Flours
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Bambara Groundnut Flours
3.2. Amino Acid Profile of Bambara Groundnut Flours
3.3. Mineral Content of Bambara Groundnut Flours
3.4. Fatty Acid Composition of Bambara Groundnut Flours
3.5. Antinutritional Factors of Bambara Groundnut Flours


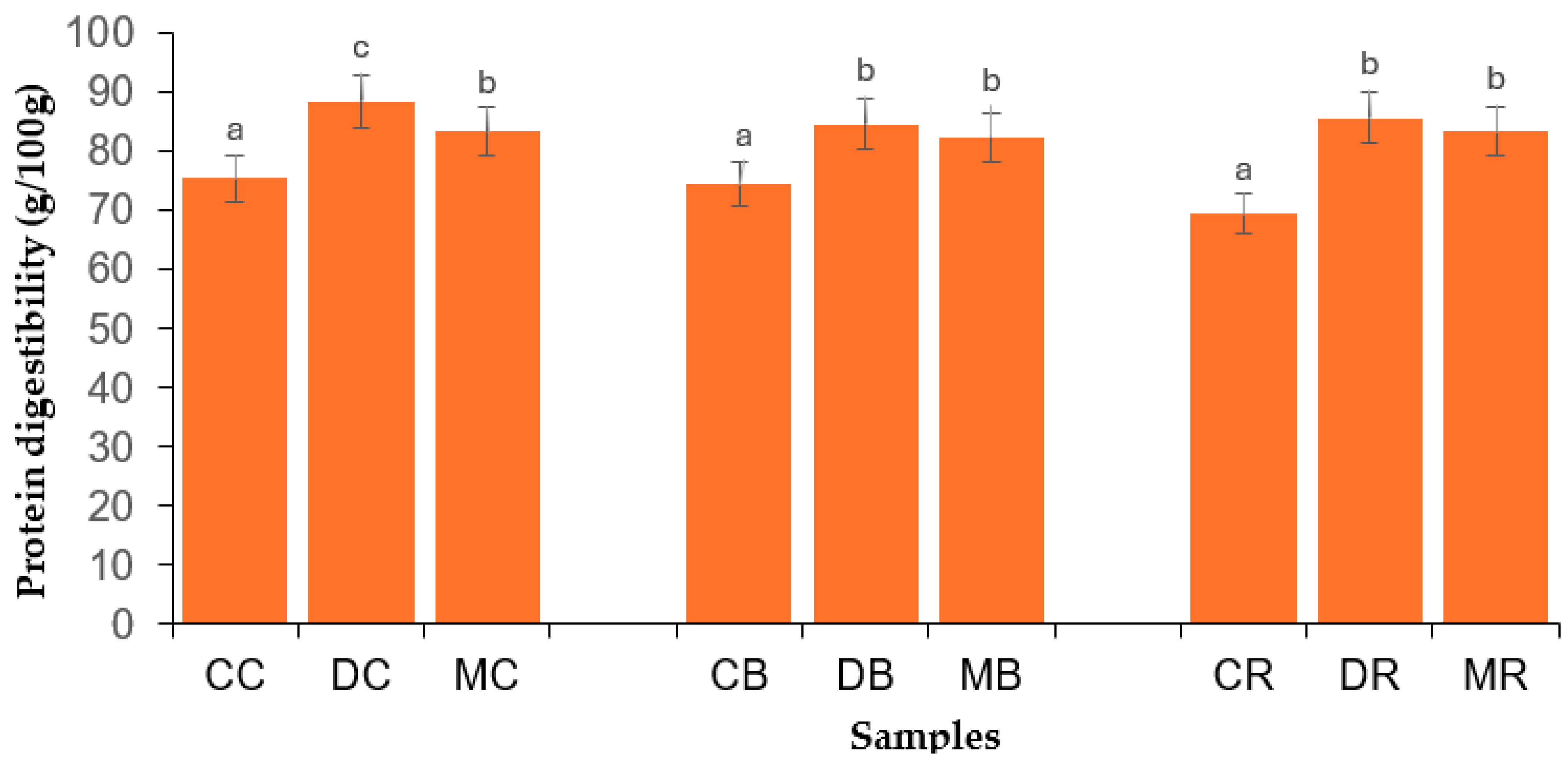
3.6. Protein Digestibility of Bambara Groundnut Flours
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT—Food Sci. Technol. 2021, 140, 110749. [Google Scholar] [CrossRef]
- Kaptso, K.G.; Njintang, Y.N.; Nguemtchouin, M.M.; Scher, J.; Hounhouigan, J.; Mbofung, C.M. Physicochemical and micro-structural properties of flours, starch and proteins from two varieties of legumes: Bambara groundnut (Vigna subterranea). J. Food Sci. Technol. 2014, 52, 4915–4924. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Pinho, O.; Pinto, E.; Casal, S.; Mota, C.; Ferreira, I.M.P.L.V.O. Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata L. Walp) and Bambara groundnuts (Vigna subterranea L. Verdc) from Mozambique. J. Food Meas. 2016, 11, 442–450. [Google Scholar] [CrossRef]
- Ramatsetse, K.E.; Ramashia, S.E.; Mashau, M.E. A review on health benefits, antimicrobial and antioxidant properties of Bambara groundnut (Vigna subterranean). Int. J. Food Prop. 2023, 26, 91–107. [Google Scholar] [CrossRef]
- Okafor, J.N.C.; Jideani, V.A.; Mervin Meyer, M.; Le Roes-Hill, M. Bioactive components in Bambara groundnut (Vigna subterraenea (L.) Verdc) as a potential source of nutraceutical ingredients. Heliyon 2022, 3, e09024. [Google Scholar] [CrossRef] [PubMed]
- Oyeyinka, A.T.; Pillay, K.; Tesfay, S.; Siwela, M. Physical, nutritional and antioxidant properties of Zimbabwean Bambara groundnut and effects of processing methods on their chemical properties. Int. J. Food Sci. Technol. 2017, 52, 2238–2247. [Google Scholar] [CrossRef]
- Adetokunboh, A.H.; Obilana, A.O.; Jideani, V.A. Enzyme and antioxidant activities of malted Bambara groundnut as affected by steeping and sprouting times. Foods 2022, 11, 783. [Google Scholar] [CrossRef]
- William, A.; George, N.; O’Reilly, P.J.; Sean, M.; Aryo, F.; Halimi, A. Adoption of Bambara groundnut production and its effects on farmer’s welfare in Northern Ghana. Afr. J. Agric. Res. 2016, 11, 583–594. [Google Scholar]
- Mazahib, A.M.; Nuha, M.O.; Salawa, I.S.; Babiker, E.E. Some nutritional attributes of Bambara groundnut as influenced by domestic processing. Int. Food Res. J. 2013, 20, 1165–1171. [Google Scholar]
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Linnemann, A.R. Hard-to-cook phenomenon in Bambara groundnut (Vigna subterranea (L.) Verdc.) processing: Options to improve its role in providing food security. Food Rev. Int. 2017, 33, 167–194. [Google Scholar] [CrossRef]
- Diedericks, C.F.; Venema, P.; Mubaiwa, J.; Jideani, V.A.; van der Linden, E. Effect of processing on the microstructure and composition of Bambara groundnut (Vigna subterranea (L.) Verdc.) seeds, flour and protein isolate. Food Hydrocoll. 2020, 108, 1–11. [Google Scholar] [CrossRef]
- Okpuzor, J.; Ogbunugafor, H.A.; Okafor, U.; Sofidiya, M.O. Identification of protein types in Bambara nut seeds: Perspectives for dietary protein supply in developing countries. Exper. Clin. Sci. Int. J. 2010, 9, 17–28. [Google Scholar]
- Unigwe, A.E.; Doria, E.; Adebola, P.; Gerrano, A.S.; Pillay, M. Anti-nutrient analysis of 30 Bambara groundnut (Vigna subterranea) accessions in South Africa. J. Crop Improv. 2018, 32, 208–224. [Google Scholar] [CrossRef]
- Tan, X.L.; Azam-Ali, S.; Goh, E.V.; Mustafa, M.; Chai, H.H.; Ho, W.K.; Mayes, S.; Mabhaudhi, T.; Azam-Ali, S.; Massawe, F. Bambara groundnut: An underutilized leguminous crop for global food security and nutrition. Front. Nutr. 2020, 7, 601496. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef]
- Wang, N. Effect of variety and crude protein content on dehulling quality and on the resulting chemical composition of red lentil (Lens culinaris). J. Sci. Food Agric. 2008, 88, 885–890. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Elucidation of chickpea hydration, the effect of soaking temperature, and extent of germination on characteristics of malted flour. J. Food Sci. 2022, 87, 2197–2210. [Google Scholar] [CrossRef]
- Mudau, M.; Adebo, O.A. Effect of traditional and novel processing technologies on the thermo-pasting, microstructural, nutritional, and antioxidant properties of finger millet and Bambara groundnut flours. Int. J. Food Sci. Technol. 2025, 60, vvae037. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Modi, A.T. Growth, phenological and yield responses of a Bambara groundnut (Vigna subterranea (L.) Verdc.) landrace to imposed water stress under field conditions. S. Afr. J. Plant. Soil. 2013, 30, 69–79. [Google Scholar] [CrossRef]
- Pretorius, B.; Otto, M.; Schönfeldt, H.C. Antinutrients and metabolomic compounds of Bambara groundnut (Vigna subterranean) as affected by traditional processing by smallholder farmers. J. Food Sci. 2023, 88, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Bond, W.J.; Midgley, J.J. The worst drought in 50 years in a South African savannah: Limited impact on vegetation. Afr. J. Ecol. 2019, 57, 490–499. [Google Scholar] [CrossRef]
- Yahaya, D.; Seidu, O.A.; Tiesaah, C.H.; Iddrisu, M.B. The role of soaking, steaming, and dehulling on the nutritional quality of Bambara groundnuts (Vigna subterranea (L) Verdc.). Front. Sustain. Food Syst. 2022, 6, 887311. [Google Scholar] [CrossRef]
- Akpapunam, M.A.; Igbedioh, S.O.; Aremo, I. Effect of malting time on chemical composition and functional properties of soybean and Bambara groundnut flours. Int. J. Food Sci. Nutr. 1996, 47, 27–33. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- AOAC. Official methods of analysis. In Association of Official Analytical Chemists, 17th ed.; Scientific Research Academic Publisher: Arlington, VA, USA, 2002. [Google Scholar]
- Mudau, M.; Ramashia, S.E.; Mashau, M.E. Mineral content, functional, thermo-pasting, and microstructural properties of spontaneously fermented finger millet flours. Foods 2022, 11, 2474. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Shin, M.-J.; Lee, S.; Yi, J.; Jeon, Y.-a.; Wang, X.; Desta, K.T. Nutrient levels, bioactive metabolite contents, and antioxidant capacities of faba beans as affected by dehulling. Foods 2023, 12, 4063. [Google Scholar] [CrossRef] [PubMed]
- Haji, A.; Teka, T.A.; Bereka, T.Y.; Astatkie, T.; Woldemariam, H.W.; Urugo, M.M. Effect of processing methods on the nutrient, antinutrient, functional, and antioxidant properties of pigeon pea (Cajanus cajan (L.) Millsp.) flour. J. Agric. Food Res. 2024, 18, 101493. [Google Scholar] [CrossRef]
- Agbaire, P.O. Nutritional and anti-nutritional levels of some local vegetables (Vernomia anydalira, Manihot esculenta, Teiferia occidentalis, Talinum triangulare, Amaranthus spinosus) from Delta State, Nigeria. J. Appl. Sci. Env. Manag. 2011, 15, 625–628. [Google Scholar]
- Chinma, C.E.; Ezeocha, V.C.; Adedeji, O.E.; Ayo-Omogie, H.N.; Oganah-Ikujenyo, B.C.; Anumba, N.L.; Enimola, G.E.; Adegoke, D.O.; Alhassan, R.; Adebo, O.A. Germinated Bambara groundnut (Vigna subterranea) flour as an ingredient in wheat bread: Physicochemical, nutritional, and sensory properties of bread. J. Food Sci. 2023, 88, 2368–2384. [Google Scholar] [CrossRef]
- Mao, H.; Yuan, S.; Li, Q.; Zhao, X.; Zhang, X.; Liu, H.; Yu, M.; Wang, M. Influence of germination on the bioactivity, structural, functional and volatile characteristics of different chickpea flours. Food Chem. X 2024, 21, 101195. [Google Scholar] [CrossRef]
- Pal, R.S.; Bhartiya, A.; ArunKumar, R.; Kant, L.; Aditya, J.P.; Bisht, J.K. Impact of dehulling and germination on nutrients, antinutrients, and antioxidant properties in horsegram. J. Food Sci. Technol. 2016, 53, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.V.; Yen, N.T.H.; Phi, N.T.L.; Tien, N.P.H.; Trung, N.T.T. Nutritional composition, enzyme activities and bioactive compounds of mung bean (Vignaradiata L.) germinated under dark and light conditions. LWT 2020, 133, 110100. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.G.; Codină, G.G. Physical, physiological and minerals changes of different legumes types during the germination process. Food Technol. 2020, 9, 844–863. [Google Scholar] [CrossRef]
- Lakshmipathy, K.; Buvaneswaran, M.; Rawson, A.; Chidanand, D.V. Effect of dehulling and germination on the functional properties of grass pea (Lathyrus sativus) flour. Food Chem. 2024, 449, 139265. [Google Scholar] [CrossRef] [PubMed]
- Sofi, S.A.; Singh, J.; Muzaffar, K.; Mir, S.A.; Dar, B.N. Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. J. Food Meas. 2020, 14, 2380–2392. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 292–1299. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira, C.; de Oliveira, R.M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT-Food Sci. Technol. 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; Pérez-Carrillo, E.; Romo-López, I.; Ramírez-Valdez, L.E.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A. Effect of dehulling and germination on physicochemical and pasting properties of black beans (Phaseolus vulgaris L.). Cereal Chem. 2017, 94, 98–103. [Google Scholar] [CrossRef]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-chemical and functional properties of legume protein, starch, and dietary fiber—A review. Legume Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Moktan, K.; Ojha, P. Quality evaluation of physical properties, antinutritional factors, and antioxidant activity of bread fortified with germinated horse gram (Dolichus uniflorus) flour. Food Sci. Nutr. 2016, 4, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Aguilar-Arteaga, K.; Castro-Rosas, J.; Falfán-Cortés, R.N.; Navarro-Cortez, R.O.; Gómez-Aldapa, C.A. Common bean (Phaseolus vulgaris L.) seed germination improves the essential amino acids profile, flavonoid content and expansion index. Czech J. Food Sci. 2023, 41, 73–77. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Ann. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Rodriguez, C.; Frias, J.; Vidal-Valverde, C.; Hernandez, A. Correlation between some nitrogen fractions, lysine, histidine, tyrosine, and ornithine contents during the germination of peas, beans, and lentils. Food Chem. 2008, 108, 245–252. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Rosas-Ordoñez, L.; Ramírez-Rodrigues, M.M.; Ramírez-Rodrigues, M.A.; Pereira, T.S.S. Enhanced protein digestibility and amino acid profile of a novel legume (Inga paterno) seed flours: Evaluation of proximal composition changes by sprouting. Appl. Biosci. 2025, 4, 15. [Google Scholar] [CrossRef]
- Dong, Y.; Tong, C.; Li, Q.; Zhang, L.; Gao, Y.; Yu, X. Dynamics of composition, structure, and metabolism of three energy substances in flaxseed (Linum usitatissimum L.) during germination. Food Chem. 2023, 410, 135344. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1102–1156. [Google Scholar]
- Rocha, M.; Licausi, F.; Araujo, W.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by water logging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef]
- Yoshari, R.M.; Astawan, M.; Prangdimurti, E.; Wresdiyati, T. The production process of Tempe protein isolate from germinated soybeans and its potential as an antidiabetic. Food Res. 2023, 7, 71–79. [Google Scholar] [CrossRef]
- Lupton, J.R.; Brooks, J.A.; Butte, N.F.; Caballero, B.; Flatt, S.K. Fried, Dietary Reference Intakes for Energy, Carbohydrate, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy Press: Washington, DC, USA, 2005; Volume 5, pp. 589–768. [Google Scholar]
- Tonesk, X.; Buchanan, R.G. An AAMC pilot study by 10 medical schools of clinical evaluation of students. J. Med. Educ. 1998, 62, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Rai, P. Role of essential amino acids in protein synthesis and muscle growth. J. Bioche. Resea. 2023, 6, 92–96. [Google Scholar]
- Bains, K.; Uppal, V.; Kaur, H. Optimization of germination time and heat treatments for enhanced availability of minerals from leguminous sprouts. J. Food Sci. Technol. 2014, 51, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Ndidi, U.S.; Ndidi, C.U.; Olagunju, A.; Muhammad, A.; Billy, F.G.; Okpe, O. Proximate, antinutrients and mineral composition of raw and processed (boiled and roasted) Sphenostylis stenocarpa seeds from Southern Kaduna, Northwest Nigeria. ISRN Nutr. 2014, 2014, 280837. [Google Scholar] [CrossRef] [PubMed]
- Elobuike, C.S.; Idowu, M.A.; Adeola, A.A.; Henry, A.; Bakare, H.A. Nutritional and functional attributes of mungbean (Vigna radiata [L] Wilczek) flour as affected by sprouting time. Legume Sci. 2021, 3, e100. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The role of minerals in the optimal functioning of the immune system. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Hassan, S.; Imran, M.; Ahmad, N.; Khan, M.K. Lipids characterization of ultrasound and microwave processed germinated sorghum. Lipids Health Dis. 2017, 16, 125. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived a-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef]
- Akkad, R.; Kharraz, E.; Han, J.; House, J.D.; Curtis, J.M. Characterisation of the volatile flavour compounds in low and high tannin faba beans (Vicia faba var. minor) grown in Alberta, Canada. Food Res. Int. 2019, 120, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile compounds in pulses: A review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef] [PubMed]
- Moongngarm, A.; Saetung, N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010, 122, 782–788. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legume Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumari, N.; Saxena, D.C.; Singh, S. Effect of germination on fatty acid profile, amino acid profile and minerals of amaranth (Amaranthus spp.) grain. J. Food Meas. 2022, 16, 1777–1786. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef]
- Ojha, P.; Bhurtel, Y.; Karki, R.; Subedi, U. Processing effects on anti-nutritional factors, phytochemicals, and functional properties of horse gram (Macrotyloma uniflorum) flour. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1080–1086. [Google Scholar] [CrossRef]
- Murugkar, D.A.; Gulati, P.; Gupta, C. Effect of sprouting on physical properties and functional and nutritional components of multi-nutrient mixes. Int. J. Food Nutr. Sci. 2013, 2, 2–15. [Google Scholar]
- Mang, D.Y.; Abdou, A.B.; Njintang, N.Y.; Manejo, E.J.; Djiogue, E.; Bernard, D.C.; Joel Scher, J.; Mbofung, M.C. Effect of dehulling and boiling on the physico-chemical, functional and pasting properties of two varieties of Mucuna bean (Mucuna pruriens L.) flours. J. Food Meas. 2015, 9, 435–447. [Google Scholar] [CrossRef]
- Embaby, H.E.-S. Effect of soaking, dehulling, and cooking methods on certain antinutrients and in vitro protein digestibility of bitter and sweet lupin seeds. Food Sci. Biotechnol. 2010, 19, 1055–1062. [Google Scholar]
- Wang, Y.; Xie, Y.; Wang, A.; Wang, J.; Wu, X.; Wu, Y.; Fu, Y.; Sun, H. Insights into interactions between food polyphenols and proteins: An updated overview. J. Food Process. Preserv. 2022, 46, e16597. [Google Scholar] [CrossRef]
- El-Safy, F.; Salem, R.H.A.; Ensaf Mukhtar, Y.Y. The Impact of soaking and germination on chemical composition, carbohydrate fractions, digestibility, antinutritional factors and minerals content of some legumes and cereals grain seeds. Alex. Sci. Exch. J. 2013, 34, 499–513. [Google Scholar]
- Carbone, J.W.; Pasiakos, S.M. Dietary protein and muscle mass: Translating science to application and health benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
| Samples | Moisture (%) | Fat (%) | Ash (%) | Protein (%) | Crude Fiber (%) | Carbohydrates (%) |
|---|---|---|---|---|---|---|
| Cream Control | 6.55 ± 0.03 a | 6.56 ± 0.03 b | 2.88 ± 0.16 b | 19.37 ± 0.06 a | 4.78 ± 0.17 b | 60.86 ± 0.23 c |
| Dehulled | 7.42 ± 0.04 b | 7.84 ± 0.10 c | 2.39 ± 0.01 a | 21.10 ± 0.17 c | 3.26 ± 0.16 a | 57.99 ± 0.18 a |
| Malted | 8.71 ± 0.09 c | 5.21 ± 0.03 a | 2.33 ± 0.27 a | 20.07 ± 0.06 b | 5.22 ± 0.18 c | 58.46 ± 0.40 b |
| Brown Control | 6.01 ± 0.06 a | 5.94 ± 0.04 b | 2.98 ± 0.12 c | 18.97 ± 0.06 a | 7.71 ± 0.26 b | 58.39 ± 0.23 b |
| Dehulled | 7.76 ± 0.07 b | 7.17 ± 0.04 c | 2.34 ± 0.01 b | 21.87 ± 0.06 c | 5.23 ± 0.35 a | 55.63 ± 0.12 a |
| Malted | 8.71 ± 0.20 c | 5.14 ± 0.03 a | 2.26 ± 0.13 a | 19.73 ± 0.12 b | 8.28 ± 0.66 c | 55.88 ± 0.21 a |
| Red Control | 6.20 ± 0.12 a | 5.82 ± 0.04 b | 3.08 ± 0.04 b | 18.63 ± 0.06 a | 5.93 ± 0.47 b | 60.34 ± 0.13 c |
| Dehulled | 7.82 ± 0.01 b | 7.16 ± 0.03 c | 2.78 ± 0.12 a | 21.20 ± 0.00 c | 3.98 ± 0.29 a | 57.06 ± 0.08 a |
| Malted | 8.65 ± 0.05 c | 5.12 ± 0.08 a | 2.70 ± 0.07 a | 19.53 ± 0.15 b | 6.95 ± 0.24 c | 57.68 ± 0.27 b |
| BGN Variety | Essential Amino Acids | |||||||||
| Samples | Valine | Leucine | Isoleucine | Methionine | Threonine | Phenylalanine | Lysine | Histidine | Tryptophan | |
| Cream Control | 2.68 ± 0.54 a | 6.55 ± 1.13 a | 1.84 ± 0.39 a | 0.94 ± 0.07 a | 4.80 ± 0.62 a | 6.53 ± 0.43 a | 4.41 ± 0.45 a | 2.18 ± 0.01 a | 3.05 ± 0.30 b | |
| Dehulled | 4.22 ± 0.95 b | 9.79 ± 1.18 c | 2.95 ± 0.76 b | 1.28 ± 0.23 b | 5.86 ± 0.36 b | 8.59 ± 0.62 c | 5.84 ± 0.17 c | 2.35 ± 0.26 b | 2.58 ± 0.37 a | |
| Malted | 3.96 ± 0.73 b | 8.93 ± 1.36 b | 2.64 ± 0.42 b | 1.33 ± 0.02 b | 5.51 ± 0.52 b | 7.17 ± 0.45 b | 4.98 ± 0.18 b | 2.66 ± 0.18 c | 2.53 ± 0.27 a | |
| Brown Control | 3.73 ± 0.36 a | 8.45 ± 0.87 a | 2.55 ± 0.25 a | 1.33 ± 0.06 a | 4.76 ± 0.11 a | 7.16 ± 0.14 a | 5.20 ± 0.29 a | 2.39 ± 0.22 a | 3.02 ± 0.11 c | |
| Dehulled | 4.22 ± 0.54 b | 9.61 ± 0.44 b | 2.95 ± 0.35 b | 1.67 ± 0.15 c | 6.36 ± 1.00 b | 8.89 ± 1.13 b | 6.04 ± 0.36 b | 2.39 ± 0.03 a | 2.68 ± 0.16 b | |
| Malted | 4.56 ± 0.44 b | 10.11 ± 0.69 b | 3.01 ± 0.23 b | 1.44 ± 0.06 b | 6.29 ± 0.45 b | 7.85 ± 0.35 b | 5.73 ± 0.25 b | 2.57 ± 0.05 b | 2.19 ± 0.04 a | |
| Red Control | 2.88 ± 0.29 a | 6.94 ± 0.57 a | 1.91 ± 0.18 a | 0.97 ± 0.08 a | 5.09 ± 0.12 a | 6.12 ± 0.17 a | 4.27 ± 0.61 a | 2.07 ± 0.01 a | 3.42 ± 0.17 b | |
| Dehulled | 5.46 ± 0.75 c | 12.21 ± 2.08 c | 3.87 ± 0.76 c | 1.35 ± 0.15 b | 6.30 ± 0.17 b | 9.33 ± 1.89 c | 6.29 ± 0.59 c | 2.81 ± 0.58 b | 2.32 ± 0.00 a | |
| Malted | 3.48 ± 0.05 b | 8.13 ± 0.23 b | 2.34 ± 0.05 b | 1.21 ± 0.02 b | 6.16 ± 0.31 b | 7.09 ± 0.39 b | 5.24 ± 0.73 b | 2.57 ± 0.33 b | 3.86 ± 0.47 c | |
| Total EAA | 35 | 81 | 24 | 12 | 51 | 69 | 48 | 20 | 24 = 364 | |
| Non-essential amino acids | ||||||||||
| BGN variety | Alanine | Glycine | Proline | Serine | Aspartic acid | Cysteine | Glutamic | Asparagine | Arginine | Tyrosine |
| Cream Control | 9.44 ± 1.73 a | 9.91 ± 1.95 a | 9.16 ± 1.75 a | 5.32 ± 0.35 a | 19.10 ± 0.45 a | 0.87 ± 0.04 b | 27.10 ± 0.29 a | 0.11 ± 0.00 a | 1.58 ± 0.30 a | 1.45 ± 0.01 a |
| Dehulled | 12.24 ± 2.26 b | 12.38 ± 2.03 b | 14.95 ± 3.17 b | 6.98 ± 1.00 c | 22.22 ± 0.66 c | 0.78 ± 0.03 a | 30.11 ± 0.16 b | 0.11 ± 0.01 a | 1.89 ± 0.21 a | 1.80 ± 0.22 b |
| Malted | 13.18 ± 2.58 b | 13.45 ± 2.86 b | 12.86 ± 2.11 b | 5.68 ± 0.08 b | 20.53 ± 0.67 b | 0.77 ± 0.01 a | 29.67 ± 0.13 b | 0.11 ± 0.03 a | 1.72 ± 0.26 a | 1.91 ± 0.54 b |
| Brown Control | 12.29 ± 0.33 a | 13.07 ± 1.45 a | 11.48 ± 0.78 a | 5.71 ± 0.21 a | 20.02 ± 0.23 a | 0.72 ± 0.00 a | 28.96 ± 0.54 a | 0.11 ± 0.01 a | 1.70 ± 0.16 a | 1.50 ± 0.08 a |
| Dehulled | 13.01 ± 0.70 b | 23.34 ± 2.58 c | 14.87 ± 0.85 b | 6.77 ± 0.37 b | 22.89 ± 0.32 b | 0.73 ± 0.01 a | 32.24 ± 0.83 b | 0.10 ± 0.01 a | 2.19 ± 0.29 b | 1.74 ± 0.06 b |
| Malted | 14.89 ± 1.90 b | 15.22 ± 1.58 b | 14.49 ± 0.25 b | 6.37 ± 0.04 ab | 22.36 ± 0.42 b | 0.87 ± 0.01 b | 30.19 ± 0.75 b | 0.11 ± 0.01 a | 2.36 ± 0.25 b | 1.72 ± 0.10 b |
| Red Control | 9.98 ± 0.04 a | 10.74 ± 0.51 a | 9.25 ± 1.06 a | 5.28 ± 0.72 a | 17.84 ± 0.67 a | 0.82 ± 0.02 a | 24.91 ± 1.05 a | 0.10 ± 0.00 a | 1.74 ± 0.36 a | 1.38 ± 0.13 a |
| Dehulled | 17.02 ± 1.39 c | 16.46 ± 0.96 b | 18.57 ± 2.95 c | 7.84 ± 0.87 b | 24.04 ± 0.79 c | 0.90 ± 0.02 b | 33.24 ± 1.82 c | 0.12 ± 0.02 a | 2.47 ± 0.63 b | 2.17 ± 0.71 b |
| Malted | 11.62 ± 0.30 b | 11.91 ± 0.57 a | 11.94 ± 0.25 b | 5.76 ± 0.26 a | 21.81 ± 0.46 b | 0.82 ± 0.00 a | 29.96 ± 1.19 b | 0.12 ± 0.02 a | 2.59 ± 0.25 b | 2.31 ± 0.23 b |
| Total NEAA | 114 | 126 | 118 | 56 | 191 | 7 | 236 | 1 | 18 | 16 = 883 |
| TEAA:TNEAA ratio | 0.41 | |||||||||
| BGN Variety | Calcium | Phosphorus | Magnesium | Potassium | Sulfur | Zinc | Iron |
|---|---|---|---|---|---|---|---|
| Cream | |||||||
| Control | 0.97 ± 0.14 b | 51.52 ± 0.04 a | 2.53 ± 0.01 a | 0.54 ± 0.02 a | 51.47 ± 0.03 a | 0.97 ± 0.03 c | 0.63 ± 0.01 b |
| Dehulled | 0.94 ± 0.01 b | 52.48 ± 0.03 b | 2.99 ± 0.01 b | 0.64 ± 0.02 b | 52.48 ± 0.03 b | 0.94 ± 0.02 b | 0.54 ± 0.01 a |
| Malted | 0.82 ± 0.01 a | 55.82 ± 0.16 c | 4.65 ± 0.01 c | 1.02 ± 0.02 c | 55.87 ± 0.09 c | 0.82 ± 0.04 a | 0.81 ± 0.01 a |
| Brown | |||||||
| Control | 0.88 ± 0.01 b | 50.30 ± 0.08 a | 3.38 ± 0.04 a | 0.82 ± 0.03 a | 50.08 ± 0.13 a | 0.98 ± 0.01 b | 0.81 ± 0.01 a |
| Dehulled | 0.77 ± 0.01 a | 52.19 ± 0.0 b | 3.61 ± 0.02 b | 1.12 ± 0.03 b | 52.23 ± 0.17 b | 0.77 ± 0.01 a | 0.71 ± 0.07 c |
| Malted | 0.77 ± 0.01 a | 54.02 ± 0.06 c | 4.42 ± 0.02 c | 1.44 ± 0.04 c | 54.08 ± 0.23 c | 0.82 ± 0.01 c | 1.12 ± 0.17 b |
| Red | |||||||
| Control | 0.97 ± 0.01 c | 49.48 ± 0.03 a | 3.37 ± 0.02 b | 0.35 ± 0.01 a | 49.46 ± 0.13 a | 0.97 ± 0.01 b | 0.85 ± 0.09 c |
| Dehulled | 0.94 ± 0.01 b | 51.50 ± 0.03 b | 3.61 ± 0.03 c | 0.56 ± 0.02 b | 50.51 ± 0.02 b | 0.98 ± 0.01 b | 0.57 ± 0.10 a |
| Malted | 0.91 ± 0.01 a | 53.56 ± 0.25 c | 3.74 ± 0.03 a | 0.80 ± 0.04 c | 51.67 ± 0.10 c | 0.91 ± 0.01 a | 0.98 ± 0.11 b |
| Saturated Fatty Acids (µg/g) | |||||||
| Sample | Myristic | Palmitic | Margaric | Stearic | Palmitoleic | Arachidic | Behenic |
| Cream Control | 5.42 ± 0.08 a | 235.19 ± 2.99 a | 2.88 ± 0.31 a | 78.35 ± 0.99 b | 2.05 ± 0.07 a | 23.01 ± 0.02 a | 72.96 ± 1.01 a |
| Dehulled | 5.39 ± 0.00 a | 242.61 ± 1.04 b | 2.92 ± 0.02 a | 76.38 ± 0.15 a | 2.04 ± 0.00 a | 23.41 ± 0.04 b | 74.10 ± 0.15 a |
| Malted | 5.39 ± 0.00 a | 242.61 ± 0.08 b | 2.93 ± 0.00 a | 75.95 ± 0.06 a | 2.03 ± 0.00 a | 23.47 ± 0.01 b | 74.01 ± 0.21 a |
| Brown Control | 5.40 ± 2.03 a | 243.99 ± 0.70 b | 2.95 ± 0.09 a | 75.42 ± 4.12 a | 2.12 ± 0.02 b | 24.83 ± 2.70 a | 72.17 ± 2.54 a |
| Dehulled | 5.40 ± 0.02 a | 242.34 ± 0.13 a | 2.94 ± 0.01 a | 76.49 ± 0.42 a | 2.05 ± 0.01 a | 23.78 ± 0.09 a | 73.79 ± 0.37 a |
| Malted | 5.39 ± 0.00 a | 242.51 ± 0.01 a | 2.93 ± 0.09 a | 76.10 ± 0.20 a | 2.04 ± 0.00 a | 23.53 ± 0.02 a | 73.98 ± 0.05 a |
| Red Control | 5.36 ± 1.03 a | 248.11 ± 2.31 b | 2.98 ± 0.26 b | 74.64 ± 2.31 a | 2.00 ± 1.00 a | 23.34 ± 1.51 a | 75.45 ± 6.86 a |
| Dehulled | 5.38 ± 0.01 a | 242.55 ± 2.55 a | 2.93 ± 0.03 a | 75.49 ± 0.49 a | 2.02 ± 0.01 a | 23.44 ± 0.11 a | 73.96 ± 0.69 a |
| Malted | 5.39 ± 0.00 a | 242.41 ± 0.21 a | 2.93 ± 0.10 a | 75.84 ± 0.00 a | 2.03 ± 0.10 a | 23.48 ± 0.00 a | 73.94 ± 0.05 a |
| Unsaturated fatty acids (µg/g) | |||||||
| y-Linolenic | Elaidic | Linolelaidic | α- Linolenic | Eicosenoic | Docosadienoic | Dihomo-γ-linolenic acid | |
| Cream Control | 23.59 ± 0.02 a | 272.10 ± 0.42 a | 440.46 ± 0.86 a | 27.77 ± 0.08 a | 10.21 ± 0.27 a | 20.33 ± 0.43 a | 4.20 ± 0.05 a |
| Dehulled | 23.99 ± 0.04 b | 280.13 ± 0.23 b | 444.52 ± 1.20 b | 29.36 ± 0.10 b | 10.61 ± 0.06 a | 20.70 ± 0.07 a | 4.27 ± 0.00 a |
| Malted | 24.05 ± 0.01 b | 280.35 ± 0.04 b | 443.48 ± 0.02 b | 29.52 ± 0.03 b | 10.62 ± 0.01 a | 20.65 ± 0.00 a | 4.26 ± 0.00 a |
| Brown Control | 21.42 ± 0.70 a | 278.89 ± 0.19 a | 442.68 ± 0.87 a | 31.28 ± 0.64 b | 10.66 ± 0.22 a | 20.49 ± 0.60 a | 4.02 ± 0.21 a |
| Dehulled | 24.38 ± 0.09 b | 280.02 ± 0.60 b | 443.43 ± 0.01 a | 29.72 ± 0.12 a | 10.59 ± 0.01 a | 20.64 ± 0.06 a | 4.22 ± 0.04 a |
| Malted | 24.12 ± 0.02 b | 280.28 ± 0.07 b | 443.48 ± 0.01 a | 29.55 ± 0.03 a | 10.61 ± 0.00 a | 20.65 ± 0.01 a | 4.25 ± 0.01 a |
| Red Control | 23.92 ± 0.08 a | 287.80 ± 2.45 b | 447.12 ± 0.39 b | 30.38 ± 2.69 a | 10.90 ± 0.98 a | 20.97 ± 2.52 a | 4.37 ± 0.36 a |
| Dehulled | 24.02 ± 0.11 b | 280.54 ± 2.98 a | 442.49 ± 3.58 a | 29.61 ± 0.29 a | 10.63 ± 0.10 a | 20.61 ± 0.23 a | 4.26 ± 0.04 a |
| Malted | 24.06 ± 0.06 b | 280.20 ± 0.27 a | 443.00 ± 0.22 a | 29.54 ± 0.03 a | 10.61 ± 0.01 a | 20.63 ± 0.02 a | 4.25 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashau, M.E.; Takalani, T.; Bamidele, O.P.; Ramashia, S.E. Elucidation of Nutritional Quality, Antinutrients, and Protein Digestibility of Dehulled and Malted Flours Produced from Three Varieties of Bambara Groundnut (Vigna subterranean). Foods 2025, 14, 2450. https://doi.org/10.3390/foods14142450
Mashau ME, Takalani T, Bamidele OP, Ramashia SE. Elucidation of Nutritional Quality, Antinutrients, and Protein Digestibility of Dehulled and Malted Flours Produced from Three Varieties of Bambara Groundnut (Vigna subterranean). Foods. 2025; 14(14):2450. https://doi.org/10.3390/foods14142450
Chicago/Turabian StyleMashau, Mpho Edward, Thakhani Takalani, Oluwaseun Peter Bamidele, and Shonisani Eugenia Ramashia. 2025. "Elucidation of Nutritional Quality, Antinutrients, and Protein Digestibility of Dehulled and Malted Flours Produced from Three Varieties of Bambara Groundnut (Vigna subterranean)" Foods 14, no. 14: 2450. https://doi.org/10.3390/foods14142450
APA StyleMashau, M. E., Takalani, T., Bamidele, O. P., & Ramashia, S. E. (2025). Elucidation of Nutritional Quality, Antinutrients, and Protein Digestibility of Dehulled and Malted Flours Produced from Three Varieties of Bambara Groundnut (Vigna subterranean). Foods, 14(14), 2450. https://doi.org/10.3390/foods14142450






