Impact of Traditional Food Processing Techniques on Mineral Bioaccessibility in Ghanaian Fermented Millet-Based Koko and Zoomkoom
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sampling Procedure
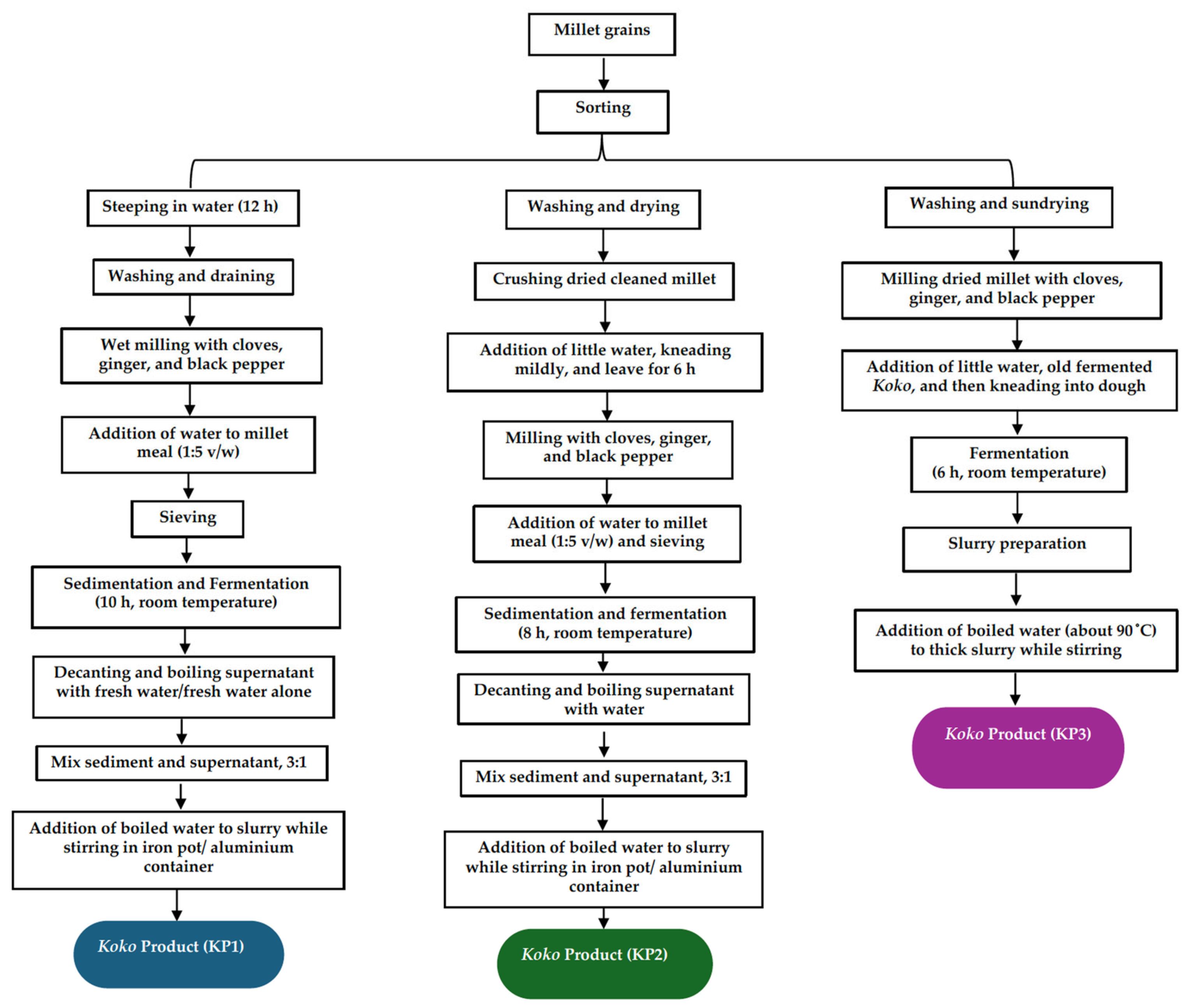
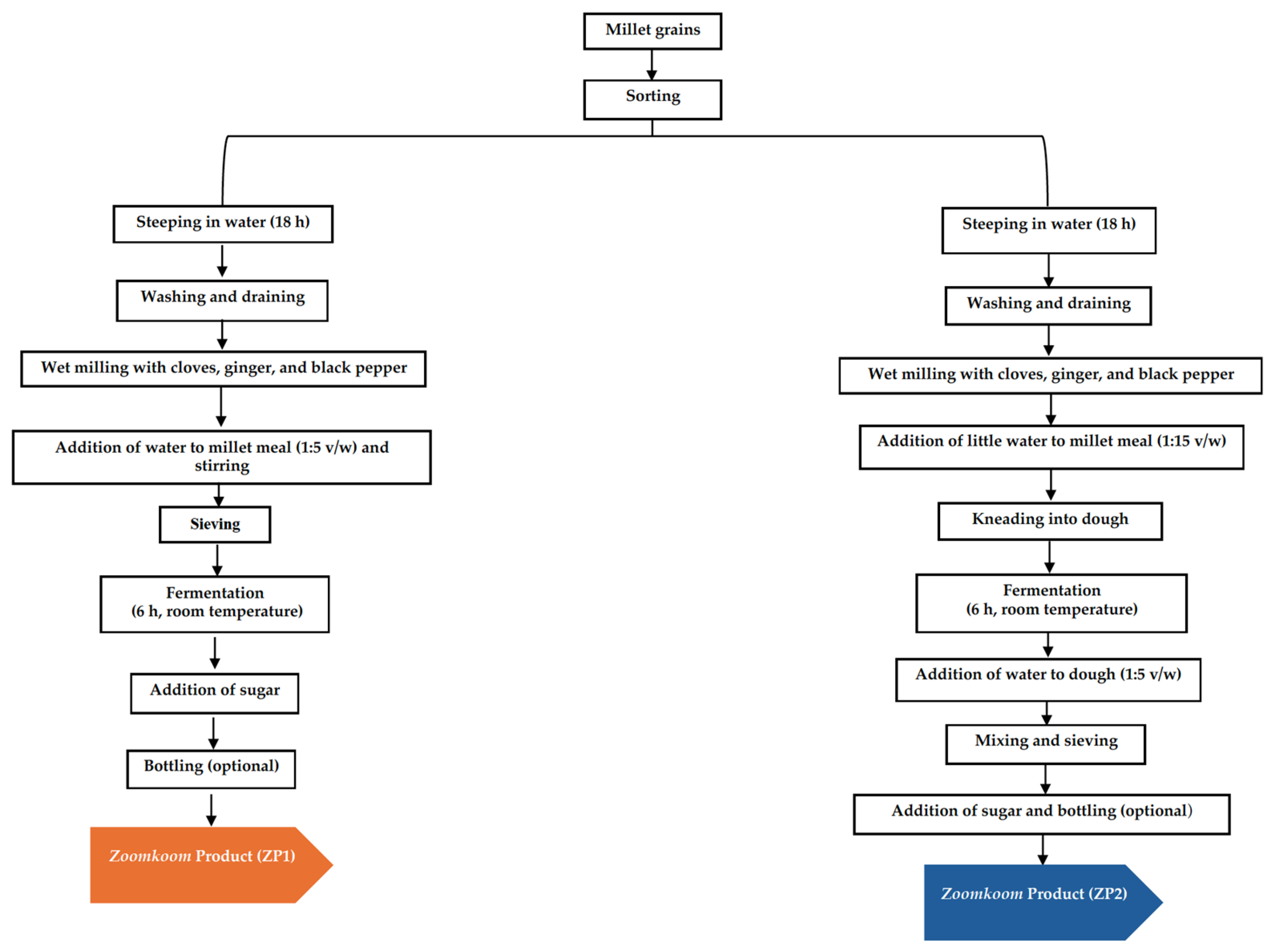
Traditional Production of Pearl Millet into Koko and Zoomkoom
2.3. Physicochemical Analysis
2.3.1. pH
2.3.2. Total Acidity (Ta)
2.4. Analytical Method
2.4.1. Phytate Extraction and Determination
2.4.2. Determination of Total Minerals
2.4.3. Bioaccessibility of Minerals
2.4.4. Percentage of Recommended Nutrient Intake (RNI)
2.4.5. Estimation of Phytate: Minerals Mole Ratios
2.5. Statistical Analysis
3. Results and Discussion
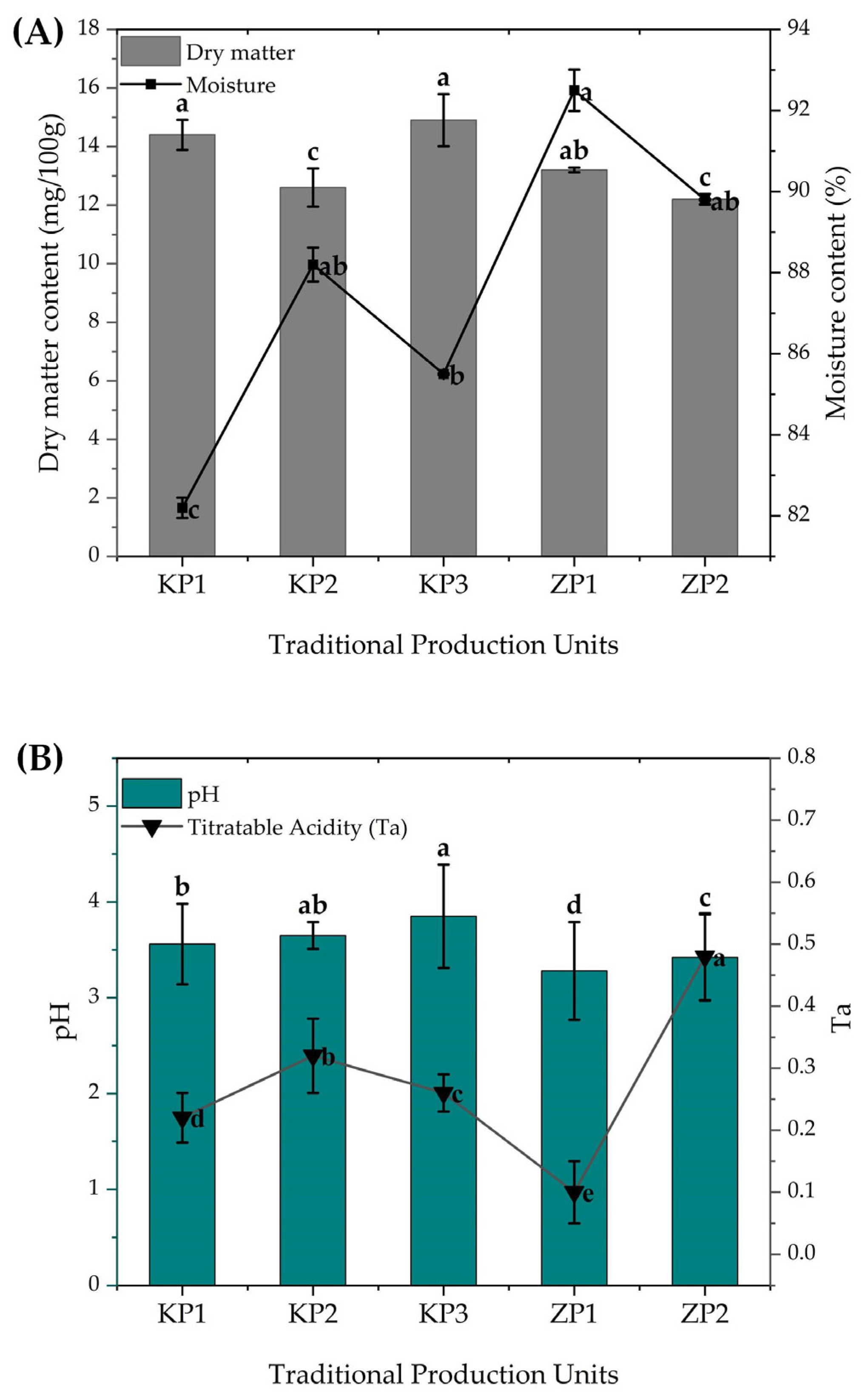
3.1. Physicochemical Properties of Koko and Zoomkoom
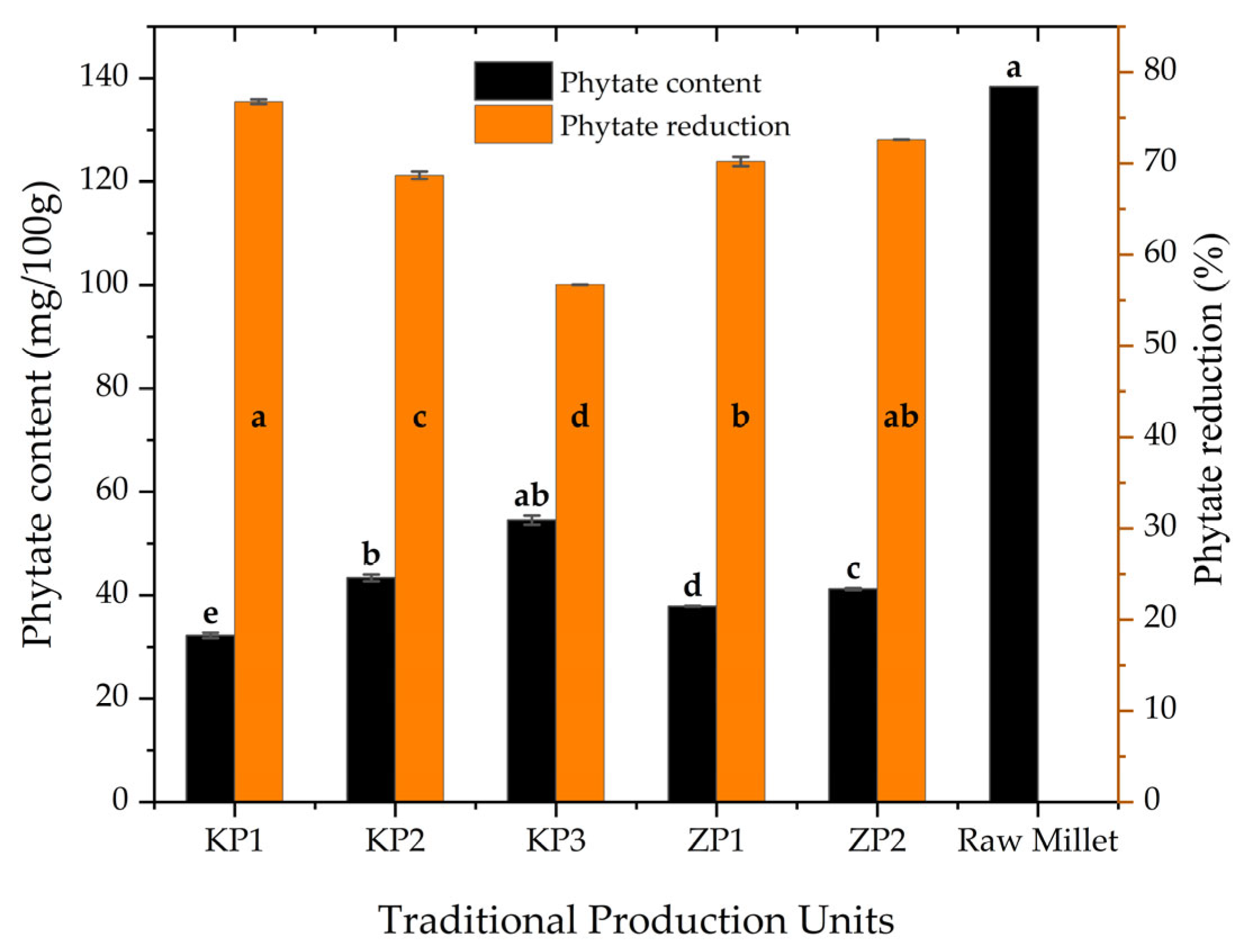
3.2. Phytate Content
3.3. Total Ca, Fe, and Zn
3.4. Recommended Nutrient Intake
3.5. Phytate: Mineral Molar Ratios
3.6. In Vitro Bioaccessibility of Minerals
3.7. Correlation Among Minerals, Phytate, Mineral Bioavailability, and Bioaccessibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RNI | Recommended nutrient intake |
| BA | Bioaccessibility |
| CV | Coefficient of Variation |
| IVS | In vitro solubility |
References
- Choudhary, C.; Vignesh, S.; Chidanand, D.V.; Baskara, N. Effect of Different Processing Methods on Nutrient, Phytochemicals Composition, and Microbial Quality of Pearl Millet. Food Humanit. 2025, 4, 100513. [Google Scholar] [CrossRef]
- Tonapi, V.A.; Thirunavukkarasu, N.; Gupta, S.K.; Gangashetty, P.I.; Yadav, O.P. Pearl Millet in the 21st Century; Springer Nature: Singapore, 2024. [Google Scholar] [CrossRef]
- Sundaresan, T.; Joshi, J.; Rao, P.S. Characterisation and Multivariate Analysis of Changes in Quality Attributes of Microwave-Treated Pearl Millet Flour. J. Cereal Sci. 2025, 121, 104086. [Google Scholar] [CrossRef]
- Meena, K.K.; Meena, S.; Joshi, M.; Dhotre, A.V. Nutritional and Functional Exploration of Pearl Millet and its Processing and Utilization: An overview. Food Humanit. 2024, 3, 100334. [Google Scholar] [CrossRef]
- Taylor, J.R.N. Millet Pearl: Overview. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1–4, pp. 190–198. [Google Scholar] [CrossRef]
- Ambati, K.; Sucharitha, K.V. Pearl Millet: Biology, Functional Potential and Sustainable Utilization. In Millets: The Multi-Cereal Paradigm for Food Sustainability; Thakur, M., Ed.; World Sustainability Series; Springer: Berlin/Heidelberg, Germany, 2024; Volume 15, pp. 37–48. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Tsusaka, T.W.; Tripathi, D.; Upadhyay, S.; Kavishwar, A.; Jalagam, A.; Sharma, N.; Nedumaran, S. Acceptance and Impact of Millet-Based Mid-Day Meal on the Nutritional Status of Adolescent School Going Children in a Peri Urban Region of Karnataka State in India. Nutrients 2019, 11, 2077. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Serba, D.D.; Perumal, R.; Tesso, T.T.; Min, D. Status of Global Pearl Millet Breeding Programs and the Way Forward. Crop Sci. 2017, 57, 2891–2905. [Google Scholar] [CrossRef]
- Boncompagni, E.; Orozco-Arroyo, G.; Cominelli, E.; Gangashetty, P.I.; Grando, S.; Kwaku Zu, T.T.; Daminati, M.G.; Nielsen, E.; Sparvoli, F. Antinutritional Factors in Pearl Millet Grains: Phytate and Goitrogens Content Variability and Molecular Characterization of Genes Involved in their Pathways. PLoS ONE 2018, 13, e0198394. [Google Scholar] [CrossRef]
- Raes, K.; Knockaert, D.; Struijs, K.; Van Camp, J. Role of Processing on Bioaccessibility of Minerals: Influence of Localization of Minerals and Anti-nutritional Factors in the Plant. Trends Food Sci. Technol. 2014, 37, 32–41. [Google Scholar] [CrossRef]
- Nosratpour, M.; Jafari, S.M. Bioavailability of minerals (Ca, Mg, Zn, K, Mn, Se) in food products. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 148–153. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ali, A.; Ain, H.B.U.; Kausar, S.; Khalil, A.A.; Aadil, R.M.; Zeng, X.A. Bioaccessibility mechanisms, fortification strategies, processing impact on bioavailability, and therapeutic potentials of minerals in cereals. Future Foods 2024, 10, 100425. [Google Scholar] [CrossRef]
- Cilla, A.; Barberá, R.; López-García, G.; Blanco-Morales, V.; Alegría, A.; Garcia-Llatas, G. Impact of processing on mineral bioaccessibility/bioavailability. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 209–239. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef]
- Brouns, F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Abah, C.R.; Ishiwu, C.N.; Obiegbuna, J.E.; Oladejo, A.A. Nutritional Composition, Functional Properties and Food Applications of Millet Grains. Asian Food Sci. J. 2020, 14, 9–19. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Kehinde, B.; Ohomeng-Boahen, G.; Kojo Aduampong Mantey, J.; Decardi-Nelson, T.; Agyei, D. The Role of Indigenous and Traditional Foods in Achieving Food Security in Africa: A Bibliometric Snapshot and Farm-to-fork Perspective. CABI Rev. 2024, 15, 37–48. [Google Scholar] [CrossRef]
- Atter, A.; Diaz, M.; Tano-Debrah, K.; Kunadu, A.P.H.; Mayer, M.J.; Colquhoun, I.J.; Nielsen, D.S.; Baker, D.; Narbad, A.; Amoa-Awua, W. Microbial Diversity and Metabolite Profile of Fermenting Millet in the Production of Hausa koko, a Ghanaian Fermented Cereal Porridge. Front. Microbiol. 2021, 12, 681983. [Google Scholar] [CrossRef]
- Ibrahim, T.; Abdulai, A.M.; Ibrahim, A.R.; Yahaya, S.; Fadila, A.; Yakubu, A. Removal of Iron Fillings from Corn Flour: Recipe for Sustainable Development. Int. J. Agric. Econ. 2021, 6, 227. [Google Scholar] [CrossRef]
- Jideani, V.A.; Nkama, I.; Agbo, E.B.; Jideani, I.A. Survey of Fura Production in Some Northern States of Nigeria. Plant Foods Hum. Nutr. 2001, 56, 23–36. [Google Scholar] [CrossRef]
- Korley, F.D.; Lamptey, F.; Nkansah, E.; Yeboah, A.A.; Akyereko, G.; Wireko-Manu, Y.G. Hausa Koko: Consumer Knowledge and Preparation Methods. J. Sci. Technol. 2024, 42, 40–53. [Google Scholar]
- Lei, V.; Jakobsen, M. Microbiological Characterization and Probiotic Potential of Koko and Koko Sour Water, African Spontaneously Fermented Millet Porridge and Drink. J. Appl. Microbiol. 2004, 96, 384–397. [Google Scholar] [CrossRef]
- Annor, G.A.; Tano Debrah, K.; Essen, A. Mineral and Phytate Contents of Some Prepared Popular Ghanaian Foods. Springerplus 2016, 5, 581. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists International (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2011; p. 2590. [Google Scholar]
- Karimou, R.; Noumavo, A.D.P.; Aboudou, K.; Boya, B.; Assouma, F.F.; Salami, H.A.; Konmy, B.B.S.; Houngbo, H.Y.; Adjanohoun, A.; Baba-Moussa, L.; et al. Nutritional and Microbial Qualities of Fermented Cereal-Based Porridges Produced in Northern Benin. J. Food Qual. 2024, 2024, 7200190. [Google Scholar] [CrossRef]
- Feng, S.; Xiang, S.; Bian, X.; Li, G. Quantitative Analysis of Total Acidity in Aqueous Lactic Acid Solutions by Direct Potentiometric Titration. Microchem. J. 2020, 157, 105049. [Google Scholar] [CrossRef]
- Paul, V.; Singh, A.; Pandey, R. Determination of Titrable Acidity (Ta). Res. Gate 2010, 23–25, 2010. [Google Scholar] [CrossRef]
- Pandey, V.; Krishnan, V.; Basak, N.; Hada, A.; Punjabi, M.; Jolly, M.; Lal, S.K.; Singh, S.B.; Sachdev, A. Phytic Acid Dynamics During Seed Development and it’s Composition in Yellow and Black Indian soybean (Glycine max L.) Genotypes Through a Modified Extraction and HPLC Method. J. Plant Biochem. Biotechnol. 2016, 25, 367–374. [Google Scholar] [CrossRef]
- Lehrfeld, J. High-Performance Liquid Chromatography Analysis of Phytic Acid on a pH-Stable, Macroporous Polymer Column. Cereal Chem. 1989, 66, 510–515. [Google Scholar]
- Chiocchetti, G.M.; De Nadai Fernandes, E.A.; Wawer, A.A.; Fairweather-Tait, S.; Christides, T. In Vitro Iron Bioavailability of Brazilian Food-Based by-Products. Medicines 2018, 5, 45. [Google Scholar] [CrossRef]
- Pupin, L.; da Santos, V.S.; dos Santos Neto, J.P.; De Fusco, D.O.; de Teixeira, G.H.A. Is the Bioaccessibility of Minerals Affected by the Processing Steps of Juçara Fruit (Euterpe edulis Mart.)? Learn. Technol. 2018, 91, 14–25. [Google Scholar] [CrossRef]
- WFP/GHS; World Food Programme. Fill the Nutrient Gap Ghana: Full Report October 2016; World Food Programme: Rome, Italy, 2016. [Google Scholar]
- Famuyide, O.Y.; Lubaale, J.; Ndiaye, C.; Duodu, K.G.; Taylor, J.R.N. Effect of Extrusion Cooking in Combination with Food-to-Food Fortification on the Mineral Bioaccessibility of African-Type Pearl Millet-Based Porridge. NFS J. 2024, 34, 100165. [Google Scholar] [CrossRef]
- Igwe, C.U.; Ibegbulem, C.O.; Nwaogu, L.A.; Ujowundu, C.O.; Okwu, G.N. Calcium, Zinc and Phytate Interrelationships in Four Lesser-Known African Seeds Processed into Food Condiments. J. Adv. Chem. 2013, 12, 288–294. [Google Scholar] [CrossRef]
- Adeyeye, E.I.; Olaleye, A.A.; Aremu, M.O.; Atere, J.O.; Idowu, O.T. Sugar, Antinutrient and Food Properties Levels in Raw, Fermented and Germinated Pearl Millet Grains. FUW Trends Sci. Technol. 2020, 5, 745–758. [Google Scholar] [CrossRef]
- Shi, C.; Zhe, G.; Ding, X.; Meng, Q.; Li, J.; Deng, L. Effect of Cooking Conditions on Iron Release from Pots and Development of Kinetic Models for Iron Supplementation in Nips. Curr. Res. Food Sci. 2024, 9, 100830. [Google Scholar] [CrossRef]
- Harusekwi Julien, S. Development of Fermented Corn and Rapoko Blend Instant Porridge. Int. J. Nutr. Food Sci. 2016, 5, 246. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, Water Activity, Water Structure and the Stability of Foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Krishnan, R.; Meera, M.S. Assessment of Inhibitory Factors on Bioaccessibility of Iron and Zinc in Pearl Millet (Pennisetum glaucum (L.) R. Br.) Cultivars. J. Food Sci. Technol. 2017, 54, 4378–4386. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Gibson, R.S. Traditional Food-Processing and Preparation Practices to Enhance the Bioavailability of Micronutrients in Plant-Based Diets1. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef]
- Wei, W.U.; Cheng, F.M.; Liu, Z.H.; Wei, K.S. Difference of Phytic Acid Content and its Relation to Four Protein Composition Contents in Grains of Twenty-nine japonica Rice Varieties from Jiangsu and Zhejiang Provinces, China. Rice Sci. 2007, 14, 311–314. [Google Scholar] [CrossRef]
- Akeem, S.A.; Kolawole, F.L.; Joseph, J.K.; Monday, R.; Kayode, O.; Akintayo, O.A. Traditional Food Processing Techniques and Micronutrients bioavailability of plants and plant-based foods: A review. Ann. Sci. Technol. 2019, 20, 30–41. [Google Scholar]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on Environment and Human Nutrition. A Challenge for Molecular Breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef]
- Hendek Ertop, M.; Bektaş, M. Enhancement of Bioavailable Micronutrients and Reduction of Antinutrients in Foods with Some Processes. Food Health 2018, 4, 159–165. [Google Scholar] [CrossRef]
- Sihag, M.K.; Sharma, V.; Goyal, A.; Arora, S.; Singh, A.K. Effect of Domestic Processing Treatments on Iron, β-carotene, Phytic Acid and Polyphenols of Pearl Millet. Cogent Food Agric. 2015, 1, 1109171. [Google Scholar] [CrossRef]
- Minnis-Ndimba, R.; Kruger, J.; Taylor, J.R.N.; Mtshali, C.; Pineda-Vargas, C.A. Micro-PIXE Mapping of Mineral Distribution in Mature Grain of Two Pearl Millet Cultivars. In Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms; Elsevier: Amsterdam, The Netherlands, 2015; Volume 363, pp. 177–182. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.; Singh, B. Modulation in the Bio-Functional & Technological Characteristics, In Vitro Digestibility, Structural and Molecular Interactions During Bioprocessing of Proso Millet (Panicum miliaceum L.). J. Food Compos. Anal. 2022, 107, 104372. [Google Scholar] [CrossRef]
- Sow, M.S.; Sarr, F.; Mertz, C.; Cisse, M.; Fall, S.M.; Diallo, D. Effects of Fermentation, Germination, Roasting and Mono-Screw Extrusion Cooking on the Phytate and Iron Contents of Millet Souna Produced in Senegal (Pennisetum Glaucum). J. Nutr. Health Food Sci. 2019, 7, 1–7. [Google Scholar]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. Evaluating the Physical and Chemical Contents of Millets Obtained from South Africa and Zimbabwe. CyTA J. Food 2020, 18, 662–669. [Google Scholar] [CrossRef]
- Gabaza, M.; Shumoy, H.; Louwagie, L.; Muchuweti, M.; Vandamme, P.; Du Laing, G.; Raes, K. Traditional Fermentation and Cooking of Finger Millet: Implications on Mineral Binders and Subsequent Bioaccessibility. J. Food Compos. Anal. 2017, 68, 87–94. [Google Scholar] [CrossRef]
- Mujinda, G.; Manhokwe, S.; Chawafambira, A.; Mugadza, D.T.; Chagwena, D.T.; Jombo, T.Z. Optimisation of Nutritional Composition of Traditional Porridges Produced from Blended Pearl Millet, Cowpeas, and Wild Loquat and Velvet Wild Medlar Fruits. Food Chem. Adv. 2023, 3, 100478. [Google Scholar] [CrossRef]
- Kiio, J.; Nduati, R.; Kuria, E.; Ochola, S.; Okoth, J. Bioaccessibility of Iron and Zinc in Selected Complementary Foods Fortified with Micronutrient Powders in Kenya. Int. Food Res. J. 2023, 30, 514–523. [Google Scholar] [CrossRef]
- Aparna, T.; Sonali, R.; Sheetal, A.; Ashwini, S.; Sneha, C.; Padmini, S.G.; Shobha, A.U.; Erick, B. Effect of Processing on Total Iron and Zinc, Ionizable Iron, Extractable Zinc, Phytate and Phytate: Mineral Ratios in Pearl Millet. Adv. Food Sci. Eng. 2017, 1, 129–143. [Google Scholar] [CrossRef]
- Afify, A.E.M.R.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Bioavailability of Iron, Zinc, Phytate and Phytase Activity during Soaking and Germination of White Sorghum Varieties. PLoS ONE 2011, 6, e25512. [Google Scholar] [CrossRef]
- Eyzaguirre, R.Z.; Nienaltowska, K.; de Jong, L.E.; Hasenack, B.B.; Nout, M.R. Effect of Food Processing of Pearl Millet (Pennisetum Glaucum) IKMP-5 on the Level of Phenolics, Phytate, Iron and Zinc. J. Sci. Food Agric. 2006, 86, 1391–1398. [Google Scholar] [CrossRef]
- de Jager, I.; Giller, K.E.; Brouwer, I.D. Food and Nutrient Gaps in Rural Northern Ghana: Does Production of Smallholder Farming Households Support Adoption of Food-Based Dietary Guidelines? PLoS ONE 2018, 13, e0204014. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- FAO/IZiNCG. FAO/INFOODS/IZiNCG Global Food Composition Database for Phytate Version 1.0—PhyFoodComp 1.0. 2018; FAO: Rome, Italy, 2018. [Google Scholar]
- Gargari, B.P.; Mahboob, S.; Razavieh, S.V. Content of Phytic Acid and Its Mole Ratio to Zinc in Flour and Breads Consumed In Tabriz, Iran. Food Chem. 2007, 100, 1115–1119. [Google Scholar] [CrossRef]
- Igwe, C.U.; Ibegbulem, C.O.; Nwaogu, L.A.; Ujowundu, C.O.; Ene, A.C. Chemical Composition and Bioavailability of Zinc and Iron in Kunu-zaki, a Chemical Composition and Bioavailability of Zinc and Iron in Kunu-zaki, a Nigerian Traditional Beverage. Int. J. Pharmacol. Phytochem. Ethnomedicine 2016, 3, 9–19. [Google Scholar]
- Liang, J.; Han, B.Z.; Nout, M.J.R.; Hamer, R.J. In Vitro Solubility of Calcium, Iron and Zinc in Relation to Phytic Acid Levels in Rice-Based Consumer Products in China. Int. J. Food Sci. Nutr. 2010, 61, 40–51. [Google Scholar] [CrossRef] [PubMed]

| Sample | Calcium (Ca) | Iron (Fe) | Zinc (Zn) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (mg/100 g DM) | IVS (mg/100 g DM) | BA (%) | Total (mg/100 g DM) | IVS (mg/100 g DM) | BA (%) | Total (mg/100 g DM) | IVS (mg/100 g DM) | BA (%) | |
| Raw millet | 9.0 ± 0.9 aF | ND | ND | 7.9 ± 0.4 bD | 0.9 ± 0.5 aD | 11.4 aD | 2.8 ± 0.7 cB | 0.1 ± 0.6 bE | 6.3 bE |
| koko | |||||||||
| KP1 | 17.2 ± 5.1 aE | ND | ND | 11.9 ± 1.2 bB | 2.6 ± 3.4 aA | 21.8 aA | 2.9 ± 1.5 cB | ND | ND |
| KP2 | 22.9 ± 5.5 aC | ND | ND | 8.0 ± 2.2 bC | 1.4 ± 1.8 aB | 16.9 bB | 1.6 ± 0.6 cC | 0.4 ± 1.9 bD | 23.3 aB |
| KP3 | 18.9 ± 8.28 aD | ND | ND | 17.8 ± 3.6 bA | 1.1 ± 0.7 aC | 6.1 bF | 3.3 ± 6.9 cA | 0.8 ± 0.6 bA | 22.4 aC |
| Average | 19.6 ± 4.9 aB | ND | ND | 12.6 ± 1.8 bA | 1.7 ± 1.6 aA | 14.9 bA | 2.6 ± 2.4 cA | 0.6 ± 1.0 bA | 22.9 aB |
| Range | 17.2–22.9 | ND | ND | 8.24–19 | 1.1–2.6 | 6.1–21.8 | 1.6–2.7 | ND-0.8 | ND-23.3 |
| CV | 27.4 | 346.4 | 346.4 | 69.4/35.2 | 47.8 | 47.8 | 37.7 | 59.4 | 59.4 |
| zoomkoom | |||||||||
| ZP1 | 58.0 ± 0.1 aA | 3.9 ± 0.0 a | 6.8 cA | 4.4 ± 1.1 bF | 0.7 ± 0.2 bF | 15.2 bC | 1.1 ± 0.3 cD | 0.5 ± 0.4 cC | 42.2 aA |
| ZP2 | 38.5 ± 8.7 aB | ND | ND | 6.28 ± 0.3 bE | 0.8 ± 0.6 aE | 8.5 bE | 3.3 ± 0.9 cA | 0.7 ± 0.0 bB | 20.6 aD |
| Average | 48.2 ± 4.4 aA | 3.9 ± 0.0 aA | 8.2 cA | 5.3 ± 0.7 bB | 0.7 ± 0.4 bB | 11.8 bB | 2.2 ± 0.6 cA | 0.6 ± 0.2 cA | 31.4 aA |
| Range | 38.5–58.0 | ND-3.9 | ND-6.8 | 3.6–3.8–4.1 | 0.7–0.8 | 8.5–15.2 | 3.2–3.3 | 0.7–2.1 | 20.6–42.2 |
| CV | 10 | 0.6 | 14.6 | 75 | 69.7 | 69.7 | 77 | 11.3 | ND |
| Processing Procedure | [Ca]:[Phy] | [Fe]:[Phy] | [Phy]:[Zn] | [Ca]*[Phy]:[Zn] | [Fe]:[Zn] |
|---|---|---|---|---|---|
| Raw millet | 0.95 | 1.49 | 4.85 | 1.09 | 3.25 |
| KP1 | 0.12 | 0.23 | 1.09 | 0.47 | 4.74 |
| KP2 | 0.12 | 0.45 | 2.7 | 1.54 | 5.91 |
| KP3 | 0.19 | 0.28 | 1.75 | 0.82 | 6.17 |
| ZP1 | 0.04 | 0.79 | 3.73 | 5.39 | 4.68 |
| ZP2 | 0.06 | 0.51 | 1.12 | 1.07 | 2.18 |
| Critical value | >0.24 | >1.00 | >15 | 0.5 | 4 |
| T-Ca | T-Fe | T-Zn | B-Ca | B-Fe | B-Zn | Phy | [Phy]:[Ca] | [Phy]:[Fe] | [Phy]:[Zn] | |
|---|---|---|---|---|---|---|---|---|---|---|
| T-Ca | 1 | 0.39 ns | −0.12 ns | 0.09 ns | 0.39 ns | −0.10 ns | −0.78 ** | −0.81 ** | −0.75 ** | −0.79 ** |
| T-Fe | 1 | −0.22 ns | 0.62 * | −0.05 ns | 0.26 ns | −0.36 ns | −0.40 ns | −0.40 ns | −0.23 ns | |
| T-Zn | 1 | −0.37 ns | −0.48 ns | 0.13 ns | 0.09 ns | 0.16 ns | 0.02 ns | 0.05 ns | ||
| B-Ca | 1 | −0.13 ns | −0.32 | −0.36 ns | −0.34 ns | −0.39 ns | −0.18 ns | |||
| B-Fe | 1 | 0.16 ns | −0.01 ns | −0.08 ns | −0.02 ns | −0.20 ns | ||||
| B-Zn | 1 | 0.13 ns | 0.06 ns | 0.05 ns | −0.03 ns | |||||
| Phy | 1 | 0.99 *** | 0.95 *** | 0.98 *** | ||||||
| [Phy]:[Ca] | 1 | 0.98 *** | 0.96 *** | |||||||
| [Phy]:[Fe] | 1 | 0.96 *** | ||||||||
| [Phy]:[Zn] | 1 |
| T-Ca | T-Fe | T-Zn | B-Ca | B-Fe | B-Zn | Phy | [Phy]:[Ca] | [Phy]:[Fe] | [Phy]:[Zn] | |
|---|---|---|---|---|---|---|---|---|---|---|
| T-Ca | 1 | −0.28 ns | −0.52 ns | 0.81 * | −0.41 ns | −0.14 ns | −0.91 ** | −0.93 *** | 0.28 ns | −0.85 ** |
| T-Fe | 1 | 0.79 * | −0.58 ns | 0.06 ns | 0.49 ns | 0.31 ns | 0.30 ns | 0.82 * | 0.32 ns | |
| T-Zn | 1 | −0.87 ** | 0.41 ns | 0.79 * | 0.36 ns | 0.34 ns | 0.58 ns | 0.25 ns | ||
| B-Ca | 1 | −0.32 ns | −0.69 ns | −0.59 ns | −0.61 ns | −0.22 ns | −0.53 ns | |||
| B-Fe | 1 | 0.10 ns | 0.31 ns | 0.31 ns | −0.19 ns | 0.07 ns | ||||
| B-Zn | 1 | −0.16 ns | −0.16 ns | 0.59 ns | −0.22 ns | |||||
| Phy | 1 | 0.98 *** | −0.26 ns | 0.97 *** | ||||||
| [Phy]:[Ca] | 1 | −0.30 ns | 0.97 *** | |||||||
| [Phy]:[Fe] | 1 | −0.24 ns | ||||||||
| [Phy]:[Zn] | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuni, A.; Alemawor, F.; Mills-Robertson, F.C.; Boateng, E.F.; Owusu-Kwarteng, J. Impact of Traditional Food Processing Techniques on Mineral Bioaccessibility in Ghanaian Fermented Millet-Based Koko and Zoomkoom. Foods 2025, 14, 2126. https://doi.org/10.3390/foods14122126
Wuni A, Alemawor F, Mills-Robertson FC, Boateng EF, Owusu-Kwarteng J. Impact of Traditional Food Processing Techniques on Mineral Bioaccessibility in Ghanaian Fermented Millet-Based Koko and Zoomkoom. Foods. 2025; 14(12):2126. https://doi.org/10.3390/foods14122126
Chicago/Turabian StyleWuni, Alhassan, Francis Alemawor, Felix Charles Mills-Robertson, Evans Frimpong Boateng, and James Owusu-Kwarteng. 2025. "Impact of Traditional Food Processing Techniques on Mineral Bioaccessibility in Ghanaian Fermented Millet-Based Koko and Zoomkoom" Foods 14, no. 12: 2126. https://doi.org/10.3390/foods14122126
APA StyleWuni, A., Alemawor, F., Mills-Robertson, F. C., Boateng, E. F., & Owusu-Kwarteng, J. (2025). Impact of Traditional Food Processing Techniques on Mineral Bioaccessibility in Ghanaian Fermented Millet-Based Koko and Zoomkoom. Foods, 14(12), 2126. https://doi.org/10.3390/foods14122126








