Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits
Abstract
1. Introduction
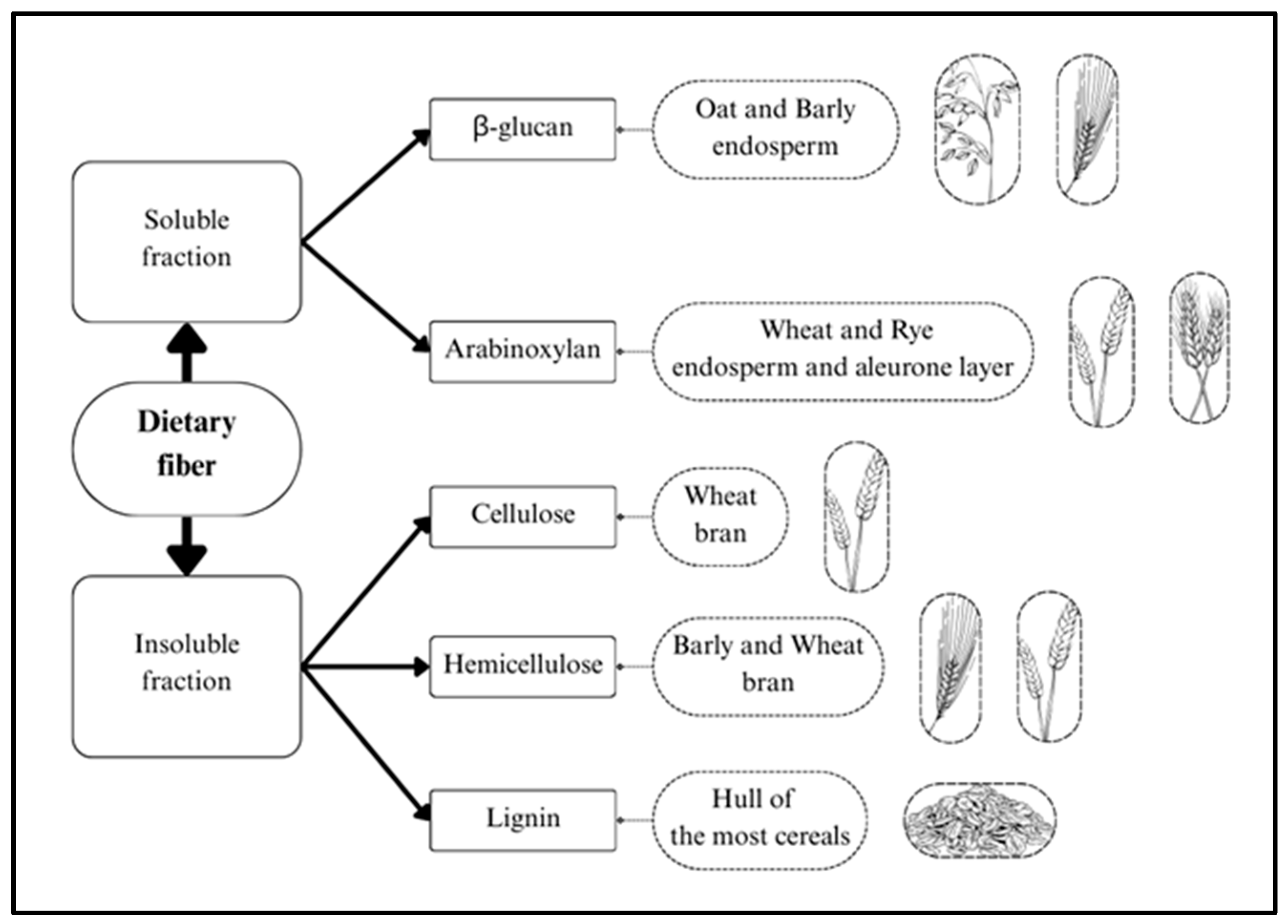
2. Dietary Fiber in Whole Grains
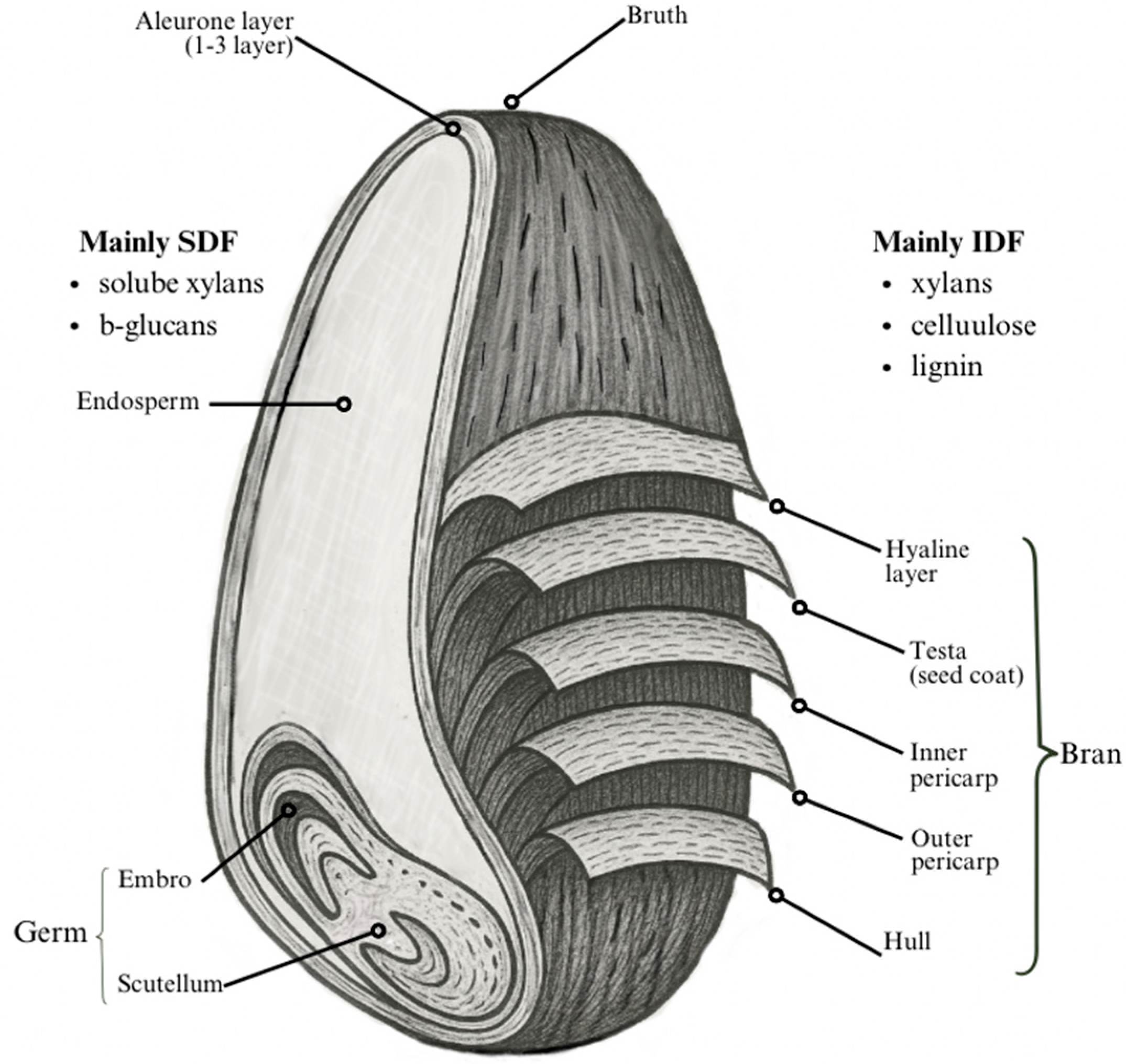
2.1. Structure of Whole Grains
2.2. Dietary Fiber Composition of Selected Whole Grains
3. Physicochemical Properties of Dietary Fiber
3.1. Solubility
3.2. Water-Holding Capacity (WHC)
3.3. Oil-Holding Capacity (OHC)
3.4. Viscosity and Gel Formation
3.5. Bile-Acid-Binding Capacity
3.6. Swelling Ability
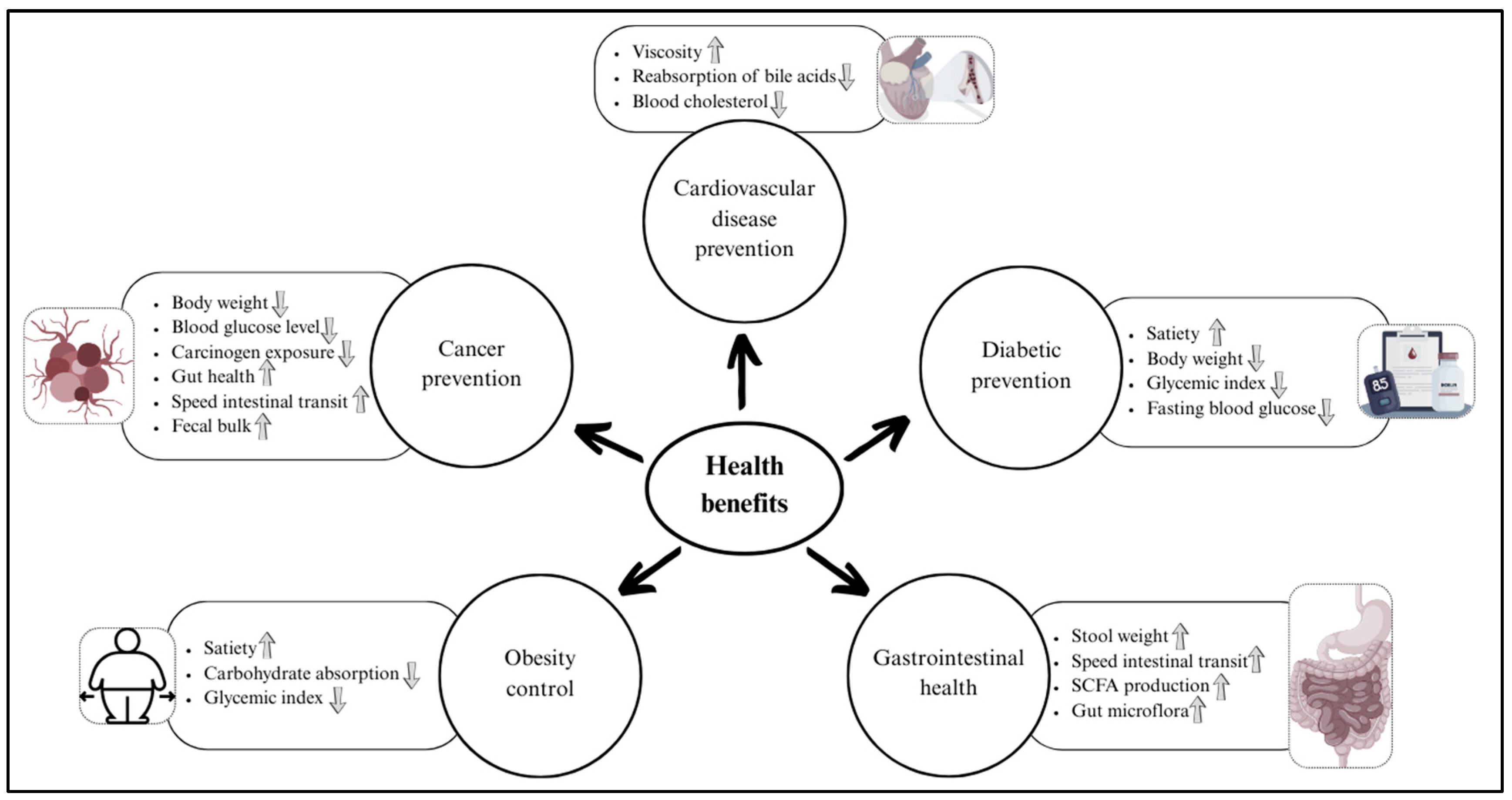
4. Health Benefits of Whole-Grain Dietary Fiber
4.1. Prevention of Cardiovascular Disease
4.2. Prevention of Type 2 Diabetes Mellitus
4.3. Control of Obesity
4.4. Gastrointestinal Health
4.5. Prevention of Cancers
| Dietary Fiber Source | Study Type | Health Benefit | Research Findings | Reference |
|---|---|---|---|---|
| Rye | Human intervention | Cardiovascular disease prevention | Total and LDL cholesterol levels were lower (−0.06 and −0.09 mmol/L, respectively; p < 0.05) after patients had consumed whole-grain rye with lignan supplements for 4 weeks. | [111] |
| Diabetic control | Rye kernel bread decreased blood glucose levels (0–120 min, p = 0.001), serum insulin response time (0–120 min, p < 0.05), and fasting FFA concentrations (p < 0.05). | [112] | ||
| Rye-based foods decreased postprandial glucose and insulin responses. | [113] | |||
| Obesity control | Participants who consumed a rye-based diet for the 12-week period lost 1.08 kg of body weight and 0.54% more body fat than the group that consumed refined wheat (95% confidence interval (CI): 0.36; 1.80, p < 0.01 and 0.05; 1.03, p = 0.03, respectively). | [114] | ||
| Gastrointestinal health | Induced some changes in gut microbiota composition, including increased abundance of the butyrate-producing Agathobacter. | [115] | ||
| Oat | Human intervention | Cardiovascular disease prevention | There was a significant reduction in office systolic blood pressure (oSBP; p < 0.001) and office diastolic blood pressure (oDBP; p < 0.028) in the group that consumed oat bran (30 g/day of oat bran contains 8.9 g of dietary fiber) compared to the control group after a 3-month period. | [58] |
| Consumption of oat dietary fiber reduces levels of systemic chronic inflammation after two weeks post-treatment. | [116] | |||
| Diabetic control | Adhering to a diet enriched with 5 g of oat β glucan for 12 weeks can help improve glycemic control, increase the feeling of satiety, and promote changes in the gut microbiota profile. | [117] | ||
| The results demonstrated that a hypocaloric oat-based diet led to a significant reduction in total insulin dosage and HbA1c levels in insulin-treated outpatients with type 2 diabetes. | [118] | |||
| Obesity control | Oat β-glucan intervention increased the abundance of Lactobacillus and Bifidobacterium. These microbiota alterations contributed to an increase in 7-ketodeoxycholic acid levels and enhanced bile acid synthesis. | [119] | ||
| Ageing control | A decrease in levels of the protein Eotaxin-1, an aging-related chemokine, independent of a person’s gender, body mass index, or age. | [116] | ||
| Wheat | Human intervention | Cardiovascular disease prevention | Total and LDL cholesterol levels in 40 men with a metabolic syndrome risk profile were lowered by −0.09 mmol/L (at p < 0.05) after they had consumed a wheat-based diet for 4 weeks. | [111] |
| Obesity control | The study found that consumption of resistant starch-enriched wheat rolls significantly increased fasting and peak concentrations of peptide YY3–36 (PYY3–36), a hormone associated with satiety, while decreasing peak concentrations and iAUC of glucose-dependent insulinotropic peptide (GIP), which is involved in hunger regulation. | [120] | ||
| Gastrointestinal health | The study found that consuming 15 g/day of wheat bran extract increases fecal Bifidobacterium quantities and softens stool without having major effects on energy metabolism in healthy humans with slow GI transit. | [121] | ||
| In vivo study | Gastrointestinal health | The study found that high amylose wheat (HAW) consumption led to an increase in fecal bacterial loads and gastrointestinal health in mice. | [122] | |
| Corn | Human intervention | Cardiovascular disease prevention | Whole-grain corn flour significantly decreased LDL cholesterol levels over time (−10.4 ± 3.6 mg/dL, p = 0.005) and marginally decreased total cholesterol levels (−9.2 ± 3.9 mg/dL, p = 0.072) over time. | [123] |
| Brown rice | Human intervention | Diabetic control | The researchers observed improved endothelial function, without changes in HbA1c levels | [124] |
| Whole grains | Human intervention | Diabetic control | Higher intake of whole-grain fiber was positively associated with better β-cell function, insulin sensitivity, and postprandial glycemic control. | [125] |
| A systematic review found that increasing whole-grain fiber intake improves glycemic control and reduces cardiometabolic risk factors in individuals with prediabetes, type 1, or type 2 diabetes. The results obtained suggest increasing daily fiber intake by 15 g or to a total of 35 g per day can lower the risk of premature mortality and enhance diabetes management. | [126] |
5. Strategies for Enhancing the Physiochemical and Functional Properties of Whole-Grain Dietary Fiber
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCDs | Non-Communicable Diseases |
| IDF | Insoluble Dietary Fiber |
| SDF | Soluble Dietary Fiber |
| TDF | Total Dietary Fiber |
| WHC | Water-Holding Capacity |
| OHC | Oil-Holding Capacity |
| CA | Cholic Acid |
| CDCA | Chenodeoxycholic Acid |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| CVD | Cardiovascular Disease |
| SCFAs | Short-Chain Fatty Acids |
| T2DM | Type 2 Diabetes Mellitus |
| GI | Glycemic Index |
| WHO | World Health Organization |
| BMIBody | Mass Index |
| IBD | Inflammatory Bowel Disease |
References
- Zhang, S.; Xu, X.; Cao, X.; Liu, T. The Structural Characteristics of Dietary Fibers from Tremella Fuciformis and Their Hypolipidemic Effects in Mice. Food Sci. Hum. Wellness 2023, 12, 503–511. [Google Scholar] [CrossRef]
- Prasadi, N.P.V.; Joye, I.J. Dietary Fiber from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Whole Grain. Health Promotion and Disease Prevention Knowledge Gateway. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/whole-grain_en (accessed on 14 November 2023).
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D. Global, Regional and National Consumption of Major Food Groups in 1990 and 2010: A Systematic Analysis Including 266 Country-Specific Nutrition Surveys Worldwide. BMJ Open 2015, 5, e008705. [Google Scholar] [CrossRef]
- European Commission. Whole Grain Intake Across European Countries. Health Promotion and Disease Prevention Knowledge Gateway. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/whole-grain-5_en (accessed on 10 March 2021).
- Van der Kamp, J.-W.; Jones, J.M.; Miller, K.B.; Ross, A.B.; Seal, C.J.; Tan, B.; Beck, E.J. Consensus, Global Definitions of Whole Grain as a Food Ingredient and of Whole-Grain Foods Presented on Behalf of the Whole Grain Initiative. Nutrients 2021, 14, 138. [Google Scholar] [CrossRef]
- Khalid, A.; Hameed, A.; Tahir, M.F. Wheat Quality: A Review on Chemical Composition, Nutritional Attributes, Grain Anatomy, Types, Classification, and Function of Seed Storage Proteins in Bread Making Quality. Front. Nutr. 2023, 10, 1053196. [Google Scholar] [CrossRef]
- Cheng, W.; Sun, Y.; Fan, M.; Li, Y.; Wang, L.; Qian, H. Wheat Bran, as the Resource of Dietary Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7269–7281. [Google Scholar] [CrossRef]
- Liu, T.; Zhen, X.; Lei, H.; Li, J.; Wang, Y.; Gou, D.; Zhao, J. Investigating the Physicochemical Characteristics and Importance of Insoluble Dietary Fiber Extracted from Legumes: An In-Depth Study on Its Biological Functions. Food Chem. X 2024, 22, 101424. [Google Scholar] [CrossRef]
- Rakha, A.; Saulnier, L.; Åman, P.; Andersson, R. Enzymatic Fingerprinting of Arabinoxylan and β-Glucan in Triticale, Barley and Tritordeum Grains. Carbohydr. Polym. 2012, 90, 1226–1234. [Google Scholar] [CrossRef]
- Li, W.; Xu, R.; Qin, S.; Song, Q.; Guo, B.; Li, M.; Zhang, Y.; Zhang, B. Cereal Dietary Fiber Regulates the Quality of Whole Grain Products: Interaction between Composition, Modification and Processing Adaptability. Int. J. Biol. Macromol. 2024, 274, 133223. [Google Scholar] [CrossRef]
- Saroj, R.; Kaur, S.; Malik, M.A.; Puranik, V.; Kaur, D. Thermal Processing of Wheat Bran: Effect on the Bioactive Compounds and Dietary Fiber. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100433. [Google Scholar] [CrossRef]
- Zhong, J.; Xie, H.; Wang, Y.; Xiong, H.; Zhao, Q. Nanofibrillated Cellulose Derived from Rice Bran, Wheat Bran, Okara as Novel Dietary Fibers: Structural, Physicochemical, and Functional Properties. Int. J. Biol. Macromol. 2024, 273, 132902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, L.F.; Bialobzyski, S.; Zijlstra, R.T.; Beltranena, E. Energy and Nutrient Digestibility and Effect of Increasing the Dietary Inclusion of Hull-Less Oats Replacing Wheat Grain on Growth Performance of Weanling Pigs. Anim. Feed. Sci. Technol. 2024, 318, 116139. [Google Scholar] [CrossRef]
- Kang, Z.; Meng, N.; Liu, M.; Liu, Y.; Jiang, P.; Qiao, C.-C.; Tan, B. Enhancement of Physicochemical, in Vitro Hypoglycemic, Hypolipidemic, Antioxidant and Prebiotic Properties of Oat Bran Dietary Fiber: Dynamic High Pressure Microfluidization. Food Biosci. 2024, 61, 104983. [Google Scholar] [CrossRef]
- Kaur, S.; Bhardwaj, R.D.; Kapoor, R.; Grewal, S.K. Biochemical Characterization of Oat (Avena sativa L.) Genotypes with High Nutritional Potential. LWT 2019, 110, 32–39. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Nadeem, M.; Sultan, M.; Sajjad, U.; Hamid, K.; Qureshi, T.M.; Javaria, S. Techno-Functional Characteristics, Mineral Composition and Antioxidant Potential of Dietary Fiber Extracted by Sonication from Different Oat Cultivars (Avena sativa). Future Foods 2024, 9, 100349. [Google Scholar] [CrossRef]
- Boukid, F. Corn (Zea mays L.) Arabinoxylan to Expand the Portfolio of Dietary Fibers. Food Biosci. 2023, 56, 103181. [Google Scholar] [CrossRef]
- Kozlova, L.V.; Nazipova, A.R.; Gorshkov, O.V.; Gilmullina, L.F.; Sautkina, O.V.; Petrova, N.V.; Trofimova, O.I.; Ponomarev, S.N.; Ponomareva, M.L.; Gorshkova, T.A. Identification of Genes Involved in the Formation of Soluble Dietary Fiber in Winter Rye Grain and Their Expression in Cultivars with Different Viscosities of Whole meal Water Extract. Crop J. 2022, 10, 532–549. [Google Scholar] [CrossRef]
- Tagliasco, M.; Font, G.; Renzetti, S.; Capuano, E.; Pellegrini, N. Role of Particle Size in Modulating Starch Digestibility and Textural Properties in a Rye Bread Model System. Food Res. Int. 2024, 190, 114565. [Google Scholar] [CrossRef]
- Pferdmenges, L.E.; Lohmayer, R.; Frommherz, L.; Brühl, L.; Hüsken, A.; Mayer-Miebach, E.; Meinhardt, A.-K.; Ostermeyer, U.; Sciurba, E.; Zentgraf, H.; et al. Overview of the Nutrient Composition of Selected Milling Products from Rye, Spelt and Wheat in Germany. J. Food Compos. Anal. 2025, 140, 107275. [Google Scholar] [CrossRef]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, Phytochemical and Functional Potential of Sorghum: A Review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.-M.; Shin, M.-J.; Yoon, H.; Wang, X.; Lee, Y.; Yi, J.; Jeon, Y.; Desta, K.T. Exploring the Potentials of Sorghum Genotypes: A Comprehensive Study on Nutritional Qualities, Functional Metabolites, and Antioxidant Capacities. Front. Nutr. 2023, 10, 1238729. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Hernández, P.; Chávez González, M.; Ascacio-Valdés, J.; Dávila-Medina, D.; Flores-Naveda, A.; Silva, T.; Ruelas-Chacón, X.; Sepúlveda-Torre, L. Sorghum (Sorghum bicolor L. Moench): Chemical Composition and Its Health Benefits; Asociación Mexicana de Ciencia de los Alimentos: Coahuila, Mexico, 2022; Volume 7. [Google Scholar]
- Kanwar, P.; Yadav, R.B.; Yadav, B.S. Cross-Linking, Carboxymethylation and Hydroxypropylation Treatment to Sorghum Dietary Fiber: Effect on Physicochemical, Micro Structural and Thermal Properties. Int. J. Biol. Macromol. 2023, 233, 123638. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, G.; Ning, Y.; Li, X.; Hu, S.; Zhao, J.; Qu, Y. Production of Cellulosic Ethanol and Value-Added Products from Corn Fiber. Bioresour. Bioprocess. 2022, 9, 81. [Google Scholar] [CrossRef]
- Colasanto, A.; Travaglia, F.; Bordiga, M.; Coïsson, J.D.; Arlorio, M.; Locatelli, M. Impact of Traditional and Innovative Cooking Techniques on Italian Black Rice (Oryza sativa L., Artemide Cv) Composition. Food Res. Int. 2024, 194, 114906. [Google Scholar] [CrossRef]
- Qadir, N.; Wani, I.A. Physicochemical and Functional Characterization of Dietary Fibres from Four Indian Temperate Rice Cultivars. Bioact. Carbohydr. Diet. Fiber 2022, 28, 100336. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Q.; Deng, M.; Jia, X.; Huang, F.; Dong, L.; Zhang, R.; Sun, Z.; Zhang, M. Composition, Structural, Physicochemical and Functional Properties of Dietary Fiber from Different Milling Fractions of Black Rice Bran. LWT 2024, 195, 115743. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical Composition, Structure, Physicochemical and Functional Properties of Rice Bran Dietary Fiber Modified by Cellulase Treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Ma, Q.; Yi, J.; Cai, S. Anti-Diabetic Effects of Different Phenolic-Rich Fractions from Rhus Chinensis Mill. Fruits in Vitro. eFood 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Zhang, R.; Huang, F.; Jia, X.; Dong, L.; Liu, D.; Zhang, M. Structural, Physicochemical and Functional Properties of Dietary Fiber from Black Rice Bran Treated by Different Processing Methods. Food Biosci. 2025, 65, 106025. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, X.; Ao, T.; Chen, Y.; Xie, J.; Gao, X.; Liu, L.; Hu, X.; Yu, Q. The Role of Bound Polyphenols in the Anti-Obesity Effects of Defatted Rice Bran Insoluble Dietary Fiber: An Insight from Multi-Omics. Food Chem. 2024, 459, 140345. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Bhat, R.; Kuang, Y.T. Composition of Amino Acids, Fatty Acids, Minerals and Dietary Fiber in Some of the Local and Import Rice Varieties of Malaysia. Int. Food Res. 2015, 22, 1148–1155. [Google Scholar]
- Bader Ul Ain, H.; Saeed, F.; Ahmed, A.; Asif Khan, M.; Niaz, B.; Tufail, T. Improving the Physicochemical Properties of Partially Enhanced Soluble Dietary Fiber through Innovative Techniques: A Coherent Review. J. Food Process Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]
- Zou, X.; Xu, X.; Chao, Z.; Jiang, X.; Zheng, L.; Jiang, B. Properties of Plant-Derived Soluble Dietary Fibers for Fiber-Enriched Foods: A Comparative Evaluation. Int. J. Biol. Macromol. 2022, 223, 1196–1207. [Google Scholar] [CrossRef]
- Dhillon, B.; Choudhary, G.; Sodhi, N.S. A Study on Physicochemical, Antioxidant and Microbial Properties of Germinated Wheat Flour and Its Utilization in Breads. J. Food Sci. Technol. 2020, 57, 2800–2808. [Google Scholar] [CrossRef]
- Zhu, R.; Xu, T.; He, B.; Wang, Y.; Zhang, L.; Huang, L. Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats. Foods 2022, 11, 1716. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Din, A. Physicochemical and Functional Properties of Barley Β-glucan as Affected by Different Extraction Procedures. J. Food Sci. Technol. 2009, 44, 181–187. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wang, Z.; Hao, Y.; Wang, Y.; Yang, Z.; Li, W.; Wang, J. Physicochemical and Functional Properties of Soluble Dietary Fiber from Different Colored Quinoa Varieties (Chenopodium Quinoa Willd). J. Cereal Sci. 2020, 95, 103045. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Guo, X.; Bai, X.; Zhang, J.; Huo, R.; Zhang, Y. Improving the Adsorption Characteristics and Antioxidant Activity of Oat Bran by Superfine Grinding. Food Sci. Nutr. 2023, 11, 216–227. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle Size of Insoluble Dietary Fiber from Rice Bran Affects Its Phenolic Profile, Bioaccessibility and Functional Properties. LWT 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y.; et al. Effects of High-Temperature, High-Pressure, and Ultrasonic Treatment on the Physicochemical Properties and Structure of Soluble Dietary Fibers of Millet Bran. Front. Nutr. 2022, 8, 820715. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yu, T.; Cao, X.; Xia, H.; Wang, S.; Sun, G.; Chen, L.; Liao, W. Effect of Viscous Soluble Dietary Fiber on Glucose and Lipid Metabolism in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis on Randomized Clinical Trials. Front. Nutr. 2023, 10, 1253312. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.C.; Kouhfar, F.; Shojaosadati, S.A. Pectin/Lignocellulose Nanofibers/Chitin Nanofibers Bionanocomposite as an Efficient Biosorbent of Cholesterol and Bile Salts. Carbohydr. Polym. 2021, 261, 117883. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, X.; Xing, X.; Hu, X.; Sun, Y. Comparison of Different Technologies for Dietary Fiber Extraction from Cold-Pressed Corn Germ Meal: Changes in Structural Characteristics, Physicochemical Properties and Adsorption Capacity. J. Cereal Sci. 2025, 121, 104077. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Wang, W.; Huang, C.; Hu, Y.; Johnston, L.; Wang, F. Dietary Supplementation With Fine-Grinding Wheat Bran Improves Lipid Metabolism and Inflammatory Response via Modulating the Gut Microbiota Structure in Pregnant Sow. Front. Microbiol. 2022, 13, 835950. [Google Scholar] [CrossRef]
- Barkas, F.; Adamidis, P.; Koutsogianni, A.-D.; Liamis, G.; Liberopoulos, E. Statin-Associated Side Effects in Patients Attending a Lipid Clinic: Evidence from a 6-Year Study. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, 182–187. [Google Scholar] [CrossRef]
- Kelly, S.A.; Hartley, L.; Loveman, E.; Colquitt, J.L.; Jones, H.M.; Al-Khudairy, L.; Clar, C.; Germanò, R.; Lunn, H.R.; Frost, G.; et al. Whole Grain Cereals for the Primary or Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2017, 2021, CD005051. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Postprandial Hyperlipidemia: Its Pathophysiology, Diagnosis, Atherogenesis, and Treatments. Int. J. Mol. Sci. 2023, 24, 13942. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Bøtker, H.E.; Jensen, J.M.; Maeng, M.; Bentzon, J.F.; Kanstrup, H.; Sørensen, H.T.; Leipsic, J.; Blankstein, R.; et al. Low-Density Lipoprotein Cholesterol Is Predominantly Associated with Atherosclerotic Cardiovascular Disease Events in Patients with Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation 2023, 147, 1053–1063. [Google Scholar] [CrossRef]
- Naumann, S.; Schweiggert-Weisz, U.; Eglmeier, J.; Haller, D.; Eisner, P. In Vitro Interactions of Dietary Fibre Enriched Food Ingredients with Primary and Secondary Bile Acids. Nutrients 2019, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary Fibre in Hypertension and Cardiovascular Disease Management: Systematic Review and Meta-Analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Llanaj, E.; Dejanovic, G.M.; Valido, E.; Bano, A.; Gamba, M.; Kastrati, L.; Minder, B.; Stojic, S.; Voortman, T.; Marques-Vidal, P.; et al. Effect of Oat Supplementation Interventions on Cardiovascular Disease Risk Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2022, 61, 1749–1778. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, C.; Zhang, Z.; Zhu, H.; Liu, Y.; Liu, T.; Ojo, O.; Qiu, J.; Wang, X. Effect of Dietary Fiber (Oat Bran) Supplement in Heart Rate Lowering in Patients with Hypertension: A Randomized DASH-Diet-Controlled Clinical Trial. Nutrients 2022, 14, 3148. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The Effect of Dietary Fiber (Oat Bran) Supplement on Blood Pressure in Patients with Essential Hypertension: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef]
- Mäkelä, N.; Brinck, O.; Sontag-Strohm, T. Viscosity of β-Glucan from Oat Products at the Intestinal Phase of the Gastrointestinal Model. Food Hydrocoll. 2020, 100, 105422. [Google Scholar] [CrossRef]
- Tosh, S.M.; Bordenave, N. Emerging Science on Benefits of Whole Grain Oat and Barley and Their Soluble Dietary Fibers for Heart Health, Glycemic Response, and Gut Microbiota. Nutr. Rev. 2020, 78, 13–20. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Piccolo, B.D.; Marco, M.L.; Kim, E.B.; Goodson, M.L.; Keenan, M.J.; Dunn, T.N.; Knudsen, K.E.B.; Adams, S.H.; Martin, R.J. Obese Mice Fed a Diet Supplemented with Enzyme-Treated Wheat Bran Display Marked Shifts in the Liver Metabolome Concurrent with Altered Gut Bacteria. J. Nutr. 2016, 146, 2445–2460. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- McCarthy, C.; Papada, E.; Kalea, A.Z. The Effects of Cereal β-Glucans on Cardiovascular Risk Factors and the Role of the Gut Microbiome. Crit. Rev. Food Sci. Nutr. 2025, 65, 2489–2505. [Google Scholar] [CrossRef]
- Jama, H.A.; Snelson, M.; Schutte, A.E.; Muir, J.; Marques, F.Z. Recommendations for the Use of Dietary Fiber to Improve Blood Pressure Control. Hypertension 2024, 81, 1450–1459. [Google Scholar] [CrossRef]
- Faraco, G.; Sugiyama, Y.; Lane, D.; Garcia-Bonilla, L.; Chang, H.; Santisteban, M.M.; Racchumi, G.; Murphy, M.; Van Rooijen, N.; Anrather, J.; et al. Perivascular Macrophages Mediate the Neurovascular and Cognitive Dysfunction Associated with Hypertension. J. Clin. Investig. 2016, 126, 4674–4689. [Google Scholar] [CrossRef]
- Rudzka, A.; Zielińska, D.; Neffe-Skocińska, K.; Sionek, B.; Szydłowska, A.; Górnik-Horn, K.; Kołożyn-Krajewska, D. The Role of Intestinal Microbiota and Dietary Fibre in the Regulation of Blood Pressure Through the Interaction with Sodium: A Narrative Review. Microorganisms 2025, 13, 1269. [Google Scholar] [CrossRef]
- Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. J. Clin. Med. 2022, 11, 3557. [Google Scholar] [CrossRef]
- Satheesh Babu, A.K.; Srinivasan, H.; Anandh Babu, P.V. Breaking Bugs: Gut Microbes Metabolize Dietary Components and Modulate Vascular Health. Crit. Rev. Food Sci. Nutr. 2024, 64, 12411–12419. [Google Scholar] [CrossRef]
- Wu, W.; Qiu, J.; Wang, A.; Li, Z. Impact of Whole Cereals and Processing on Type 2 Diabetes Mellitus: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1447–1474. [Google Scholar] [CrossRef]
- Torres, N.; Avila-Nava, A.; Medina-Vera, I.; Tovar, A.R. Dietary Fiber and Diabetes. In Science and Technology of Fibers in Food Systems; Springer: Berlin/Heidelberg, Germany, 2020; pp. 201–218. [Google Scholar]
- Kabthymer, R.H.; Karim, M.N.; Hodge, A.M.; de Courten, B. High Cereal Fibre but Not Total Fibre Is Associated with a Lower Risk of Type 2 Diabetes: Evidence from the Melbourne Collaborative Cohort Study. Diabetes Obes. Metab. 2023, 25, 1911–1921. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of Whole Grain Foods and Risk of Type 2 Diabetes: Results from Three Prospective Cohort Studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef]
- Ghanbari-Gohari, F.; Mousavi, S.M.; Esmaillzadeh, A. Consumption of Whole Grains and Risk of Type 2 Diabetes: A Comprehensive Systematic Review and Dose–Response Meta-analysis of Prospective Cohort Studies. Food Sci. Nutr. 2022, 10, 1950–1960. [Google Scholar] [CrossRef]
- Wehrli, F.; Taneri, P.E.; Bano, A.; Bally, L.; Blekkenhorst, L.C.; Bussler, W.; Metzger, B.; Minder, B.; Glisic, M.; Muka, T.; et al. Oat Intake and Risk of Type 2 Diabetes, Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2560. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Guo, D.; Bai, B.; Bo, T.; Fan, S. Antidiabetic Effect of Millet Bran Polysaccharides Partially Mediated via Changes in Gut Microbiome. Foods 2022, 11, 3406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Zhang, X.-X.; Zhang, R.; Ni, Z.-J.; Elam, E.; Thakur, K.; Cespedes-Acuña, C.L.; Zhang, J.-G.; Wei, Z.-J. Gut Modulation Based Anti-Diabetic Effects of Carboxymethylated Wheat Bran Dietary Fiber in High-Fat Diet/Streptozotocin-Induced Diabetic Mice and Their Potential Mechanisms. Food Chem. Toxicol. 2021, 152, 112235. [Google Scholar] [CrossRef] [PubMed]
- Shah, B. Obesity in Modern Society: Analysis, Statistics, and Treatment Approaches. Int. J. Sci. Res. (IJSR) 2023, 12, 358–360. [Google Scholar] [CrossRef]
- Novelli, G.; Cassadonte, C.; Sbraccia, P.; Biancolella, M. Genetics: A Starting Point for the Prevention and the Treatment of Obesity. Nutrients 2023, 15, 2782. [Google Scholar] [CrossRef]
- Spring, B.; Champion, K.E.; Acabchuk, R.; Hennessy, E.A. Self-Regulatory Behaviour Change Techniques in Interventions to Promote Healthy Eating, Physical Activity, or Weight Loss: A Meta-Review. Health Psychol. Rev. 2021, 15, 508–539. [Google Scholar] [CrossRef]
- Palmeira, A.L.; Marques, M.M.; Sánchez-Oliva, D.; Encantado, J.; Santos, I.; Duarte, C.; Matos, M.; Carneiro-Barrera, A.; Larsen, S.C.; Horgan, G.; et al. Are Motivational and Self-Regulation Factors Associated with 12 Months’ Weight Regain Prevention in the NoHoW Study? An Analysis of European Adults. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 128. [Google Scholar] [CrossRef]
- Khan, J.; Gul, P.; Liu, K. Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer. Foods 2024, 13, 2112. [Google Scholar] [CrossRef]
- Tammi, R.; Männistö, S.; Maukonen, M.; Kaartinen, N.E. Whole Grain Intake, Diet Quality and Risk Factors of Chronic Diseases: Results from a Population-Based Study in Finnish Adults. Eur. J. Nutr. 2024, 63, 397–408. [Google Scholar] [CrossRef]
- Sanders, L.M.; Zhu, Y.; Wilcox, M.L.; Koecher, K.; Maki, K.C. Effects of Whole Grain Intake, Compared with Refined Grain, on Appetite and Energy Intake: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1177–1195. [Google Scholar] [CrossRef]
- Maki, K.C.; Palacios, O.M.; Koecher, K.; Sawicki, C.M.; Livingston, K.A.; Bell, M.; Nelson Cortes, H.; McKeown, N.M. The Relationship between Whole Grain Intake and Body Weight: Results of Meta-Analyses of Observational Studies and Randomized Controlled Trials. Nutrients 2019, 11, 1245. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.; Liu, T.; Gong, Z.; Zhuo, Q. Association between Whole-Grain Intake and Obesity Defined by Different Anthropometric Indicators and Dose–Response Relationship Analysis among U.S. Adults: A Population-Based Study. Nutrients 2024, 16, 2373. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Bakhshi, B.; Mirmiran, P.; Gaeini, Z.; Hadaegh, F.; Azizi, F. Effect of Weight Change on the Association between Overall and Source of Carbohydrate Intake and Risk of Metabolic Syndrome: Tehran Lipid and Glucose Study. Nutr. Metab. 2023, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Haus, J.M.; Filion, J.; Pagadala, M.R.; Godin, J.-P.; Kochhar, S.; Ross, A.B.; Kirwan, J.P. A Whole-Grain Diet Reduces Peripheral Insulin Resistance and Improves Glucose Kinetics in Obese Adults: A Randomized-Controlled Trial. Metabolism 2018, 82, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Ndou, S.P.; Wang, Y.; Nyachoti, C.M.; Ames, N.P. Barley β-Glucan Increases Fecal Bile Acid Excretion and Short Chain Fatty Acid Levels in Mildly Hypercholesterolemic Individuals. Food Funct. 2018, 9, 3092–3096. [Google Scholar] [CrossRef]
- Pi, X.; Yu, Z.; Yang, X.; Du, Z.; Liu, W. Effects of Zymosan on Short-Chain Fatty Acid and Gas Production in in Vitro Fermentation Models of the Human Intestinal Microbiota. Front. Nutr. 2022, 9, 921137. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, X.; Wei, X.; Liu, X.; Wang, J.; Zheng, X. Improvement of the Functional Properties of Insoluble Dietary Fiber from Corn Bran by Ultrasonic-Microwave Synergistic Modification. Ultrason. Sonochem. 2024, 104, 106817. [Google Scholar] [CrossRef]
- Tamura, K.; Hemsworth, G.R.; Déjean, G.; Rogers, T.E.; Pudlo, N.A.; Urs, K.; Jain, N.; Davies, G.J.; Martens, E.C.; Brumer, H. Molecular Mechanism by Which Prominent Human Gut Bacteroidetes Utilize Mixed-Linkage Beta-Glucans, Major Health-Promoting Cereal Polysaccharides. Cell Rep. 2017, 21, 417–430. [Google Scholar] [CrossRef]
- Sebastià, C.; Folch, J.M.; Ballester, M.; Estellé, J.; Passols, M.; Muñoz, M.; García-Casco, J.M.; Fernández, A.I.; Castelló, A.; Sánchez, A.; et al. Interrelation between Gut Microbiota, SCFA, and Fatty Acid Composition in Pigs. mSystems 2024, 9, e0104923. [Google Scholar] [CrossRef]
- Valido, E.; Stoyanov, J.; Bertolo, A.; Hertig-Godeschalk, A.; Zeh, R.M.; Flueck, J.L.; Minder, B.; Stojic, S.; Metzger, B.; Bussler, W.; et al. Systematic Review of the Effects of Oat Intake on Gastrointestinal Health. J. Nutr. 2021, 151, 3075–3090. [Google Scholar] [CrossRef]
- Procházková, N.; Venlet, N.; Hansen, M.L.; Lieberoth, C.B.; Dragsted, L.O.; Bahl, M.I.; Licht, T.R.; Kleerebezem, M.; Lauritzen, L.; Roager, H.M. Effects of a Wholegrain-Rich Diet on Markers of Colonic Fermentation and Bowel Function and Their Associations with the Gut Microbiome: A Randomised Controlled Cross-over Trial. Front. Nutr. 2023, 10, 1187165. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, K.; Iversen, K.N.; Qu, Z.; Dong, C.; Jin, T.; Hallmans, G.; Åman, P.; Johansson, A.; He, G.; et al. The Effects of Fermented Rye Products on Gut Microbiota and Their Association with Metabolic Factors in Chinese Adults—An Explorative Study. Food Funct. 2021, 12, 9141–9150. [Google Scholar] [CrossRef] [PubMed]
- Faubel, N.; Blanco-Morales, V.; Barberá, R.; Garcia-Llatas, G. Impact of Colonic Fermentation of Plant Sterol-Enriched Rye Bread on Gut Microbiota and Metabolites. Biol. Life Sci. Forum 2023, 26, 87. [Google Scholar]
- Jefferson, A.; Adolphus, K. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-Grain Wheat Consumption Reduces Inflammation in a Randomized Controlled Trial on Overweight and Obese Subjects with Unhealthy Dietary and Lifestyle Behaviors: Role of Polyphenols Bound to Cereal Dietary Fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, Y.; Li, Y.; Chang, X.; Lin, J.; Chen, L.; Lyu, Q.; Chen, X.; Ding, W. Examination of the Bioavailability and Bioconversion of Wheat Bran-Bound Ferulic Acid: Insights into Gastrointestinal Processing and Colonic Metabolites. J. Agric. Food Chem. 2025, 73, 1331–1344. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, Y.; Xue, K.; Kan, J. Use of Dietary Fibers in Reducing the Risk of Several Cancer Types: An Umbrella Review. Nutrients 2023, 15, 2545. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, X.-K.; Tang, Y.-J.; Guan, X.-X.; Guo, Y.; Fan, J.-M.; Cui, L.-L. Association of Whole Grains Intake and the Risk of Digestive Tract Cancer: A Systematic Review and Meta-Analysis. Nutr. J. 2020, 19, 52. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Cortez Lainez, J.; Pelucchi, C.; Negri, E.; Bonzi, R.; Palli, D.; Ferraroni, M.; Zhang, Z.-F.; Yu, G.-P.; Lunet, N.; et al. The Association between Dietary Fiber Intake and Gastric Cancer: A Pooled Analysis of 11 Case–Control Studies. Eur. J. Nutr. 2024, 63, 1857–1865. [Google Scholar] [CrossRef]
- Hullings, A.G.; Sinha, R.; Liao, L.M.; Freedman, N.D.; Graubard, B.I.; Loftfield, E. Whole Grain and Dietary Fiber Intake and Risk of Colorectal Cancer in the NIH-AARP Diet and Health Study Cohort. Am. J. Clin. Nutr. 2020, 112, 603–612. [Google Scholar] [CrossRef]
- Kyrø, C.; Tjønneland, A.; Overvad, K.; Olsen, A.; Landberg, R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J. Nutr. 2018, 148, 1434–1444. [Google Scholar] [CrossRef]
- Makarem, N.; Nicholson, J.M.; Bandera, E.V.; McKeown, N.M.; Parekh, N. Consumption of Whole Grains and Cereal Fiber in Relation to Cancer Risk: A Systematic Review of Longitudinal Studies. Nutr. Rev. 2016, 74, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, W.; Petrick, J.L.; Liao, L.M.; Wang, W.; He, N.; Campbell, P.T.; Zhang, Z.-F.; Giovannucci, E.; McGlynn, K.A.; et al. Higher Intake of Whole Grains and Dietary Fiber Are Associated with Lower Risk of Liver Cancer and Chronic Liver Disease Mortality. Nat. Commun. 2021, 12, 6388. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Šmejkal, K.; Maršík, P.; Zulli, A.; Gönciová, G.; Mojžiš, J.; et al. Young Barley Indicates Antitumor Effects in Experimental Breast Cancer In Vivo and In Vitro. Nutr. Cancer 2016, 68, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, A.; Kulbacka, J.; Harasym, J.; Oledzki, R.; Szewczyk, A.; Saczko, J. High- and Low-Molecular Weight Oat Beta-Glucan Reveals Antitumor Activity in Human Epithelial Lung Cancer. Pathol. Oncol. Res. 2018, 24, 583–592. [Google Scholar] [CrossRef]
- Harasym, J.; Dziendzikowska, K.; Kopiasz, Ł.; Wilczak, J.; Sapierzyński, R.; Gromadzka-Ostrowska, J. Consumption of Feed Supplemented with Oat Beta-Glucan as a Chemopreventive Agent against Colon Cancerogenesis in Rats. Nutrients 2024, 16, 1125. [Google Scholar] [CrossRef]
- Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. [Google Scholar] [CrossRef]
- Eriksen, A.K.; Brunius, C.; Mazidi, M.; Hellström, P.M.; Risérus, U.; Iversen, K.N.; Fristedt, R.; Sun, L.; Huang, Y.; Nørskov, N.P.; et al. Effects of Whole-Grain Wheat, Rye, and Lignan Supplementation on Cardiometabolic Risk Factors in Men with Metabolic Syndrome: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 864–876. [Google Scholar] [CrossRef]
- Sandberg, J.C.; Björck, I.M.E.; Nilsson, A.C. Rye-Based Evening Meals Favorably Affected Glucose Regulation and Appetite Variables at the Following Breakfast; A Randomized Controlled Study in Healthy Subjects. PLoS ONE 2016, 11, e0151985. [Google Scholar] [CrossRef]
- Iversen, K.N.; Jonsson, K.; Landberg, R. The Effect of Rye-Based Foods on Postprandial Plasma Insulin Concentration: The Rye Factor. Front. Nutr. 2022, 9, 868938. [Google Scholar] [CrossRef]
- Iversen, K.N.; Carlsson, F.; Andersson, A.; Michaëlsson, K.; Langton, M.; Risérus, U.; Hellström, P.M.; Landberg, R. A Hypocaloric Diet Rich in High Fiber Rye Foods Causes Greater Reduction in Body Weight and Body Fat than a Diet Rich in Refined Wheat: A Parallel Randomized Controlled Trial in Adults with Overweight and Obesity (the RyeWeight Study). Clin. Nutr. ESPEN 2021, 45, 155–169. [Google Scholar] [CrossRef]
- Iversen, K.N.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.A.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Dioum, E.H.M.; Schneider, K.L.; Vigerust, D.J.; Cox, B.D.; Chu, Y.; Zachwieja, J.J.; Furman, D. Oats Lower Age-Related Systemic Chronic Inflammation (IAge) in Adults at Risk for Cardiovascular Disease. Nutrients 2022, 14, 4471. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.L.; Mujica, V.; Arredondo, M. Effect of Dietary Supplementation with Oat β-Glucan for 3 months in Subjects with Type 2 Diabetes: A Randomized, Double-Blind, Controlled Clinical Trial. J. Funct. Foods 2021, 77, 104311. [Google Scholar] [CrossRef]
- Fiedler, M.; Müller, N.; Kloos, C.; Kramer, G.; Kellner, C.; Schmidt, S.; Wolf, G.; Kuniß, N. A Randomized Controlled Trial to Assess the Feasibility and Practicability of an Oatmeal Intervention in Individuals with Type 2 Diabetes: A Pilot Study in the Outpatient Sector. J. Clin. Med. 2024, 13, 5126. [Google Scholar] [CrossRef]
- Huang, K.; Hong, C.; Huang, Y.; Liu, Y.; Yu, Z.; Li, S.; Guan, X.; Zhao, W. Oat β-Glucan Prevents High Fat Diet Induced Obesity by Targeting Ileal Farnesoid X Receptor-Fibroblast Growth Factor 15 Signaling. Int. J. Biol. Macromol. 2025, 306, 141543. [Google Scholar] [CrossRef]
- Hughes, R.L.; Horn, W.F.; Wen, A.; Rust, B.; Woodhouse, L.R.; Newman, J.W.; Keim, N.L. Resistant Starch Wheat Increases PYY and Decreases GIP but Has No Effect on Self-Reported Perceptions of Satiety. Appetite 2022, 168, 105802. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, G.D.A.; Emanuel, E.C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W.E.; et al. Effect of Wheat Bran Derived Prebiotic Supplementation on Gastrointestinal Transit, Gut Microbiota, and Metabolic Health: A Randomized Controlled Trial in Healthy Adults with a Slow Gut Transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef]
- Lim, S.M.; Choo, J.M.; Li, H.; O’Rielly, R.; Carragher, J.; Rogers, G.B.; Searle, I.; Robertson, S.A.; Page, A.J.; Muhlhausler, B. A High Amylose Wheat Diet Improves Gastrointestinal Health Parameters and Gut Microbiota in Male and Female Mice. Foods 2021, 10, 220. [Google Scholar] [CrossRef]
- Liedike, B.; Khatib, M.; Tabarsi, B.; Harris, M.; Wilson, S.L.; Ortega-Santos, C.P.; Mohr, A.E.; Vega-López, S.; Whisner, C.M. Evaluating the Effects of Corn Flour Product Consumption on Cardiometabolic Outcomes and the Gut Microbiota in Adults with Elevated Cholesterol: A Randomized Crossover. J. Nutr. 2024, 154, 2437–2447. [Google Scholar] [CrossRef]
- Kondo, K.; Morino, K.; Nishio, Y.; Ishikado, A.; Arima, H.; Nakao, K.; Nakagawa, F.; Nikami, F.; Sekine, O.; Nemoto, K.; et al. Fiber-Rich Diet with Brown Rice Improves Endothelial Function in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. PLoS ONE 2017, 12, e0179869. [Google Scholar] [CrossRef]
- Liu, J.; An, Y.; Yang, N.; Xu, Y.; Wang, G. Longitudinal Associations of Dietary Fiber and Its Source with 48-Week Weight Loss Maintenance, Cardiometabolic Risk Factors and Glycemic Status under Metformin or Acarbose Treatment: A Secondary Analysis of the March Randomized Trial. Nutr. Diabetes 2024, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of Germination Time on the Compositional, Functional and Antioxidant Properties of Whole Wheat Malt and Its End-Use Evaluation in Cookie-Making. Food Chem. 2021, 349, 129125. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.O.; Yousif, S.A.; Ali, A.E.; Hamadnalla, H.M. Effect of Germination on Proximate Composition of Three Grains from Sudan. EAS J. Nutr. Food Sci. 2021, 3, 104–109. [Google Scholar]
- Gong, K.; Chen, L.; Li, X.; Sun, L.; Liu, K. Effects of Germination Combined with Extrusion on the Nutritional Composition, Functional Properties and Polyphenol Profile and Related in Vitro Hypoglycemic Effect of Whole Grain Corn. J. Cereal Sci. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Yi, C.; Qiang, N.; Zhu, H.; Xiao, Q.; Li, Z. Extrusion Processing: A Strategy for Improving the Functional Components, Physicochemical Properties, and Health Benefits of Whole Grains. Food Res. Int. 2022, 160, 111681. [Google Scholar] [CrossRef]
- Boakye, P.G.; Okyere, A.Y.; Annor, G.A. Impact of Extrusion Processing on the Nutritional and Physicochemical Properties of Intermediate Wheatgrass (Thinopyrum intermedium). Cereal Chem. 2023, 100, 628–642. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Ma, S.; Tian, X.; Sun, B.; Huang, J.; Li, L.; Wang, X.; Bao, Q. Effect of Wheat Bran Dietary Fiber on Structural Properties of Wheat Starch after Synergistic Fermentation of Lactobacillus Plantarum and Saccharomyces Cerevisiae. Int. J. Biol. Macromol. 2021, 190, 86–92. [Google Scholar] [CrossRef]
- Liao, A.-M.; Zhang, J.; Yang, Z.-L.; Huang, J.-H.; Pan, L.; Hou, Y.-C.; Li, X.-X.; Zhao, P.-H.; Dong, Y.-Q.; Hu, Z.-Y.; et al. Structural, Physicochemical, and Functional Properties of Wheat Bran Insoluble Dietary Fiber Modified with Probiotic Fermentation. Front. Nutr. 2022, 9, 803440. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved Physicochemical and Functional Properties of Dietary Fiber from Millet Bran Fermented by Bacillus Natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Mustafa, G.; Arshad, M.U.; Saeed, F.; Afzaal, M.; Niaz, B.; Hussain, M.; Raza, M.A.; Nayik, G.A.; Obaid, S.A.; Ansari, M.J.; et al. Comparative Study of Raw and Fermented Oat Bran: Nutritional Composition with Special Reference to Their Structural and Antioxidant Profile. Fermentation 2022, 8, 509. [Google Scholar] [CrossRef]
- Maina, N.H.; Rieder, A.; De Bondt, Y.; Mäkelä-Salmi, N.; Sahlstrøm, S.; Mattila, O.; Lamothe, L.M.; Nyström, L.; Courtin, C.M.; Katina, K.; et al. Process-Induced Changes in the Quantity and Characteristics of Grain Dietary Fiber. Foods 2021, 10, 2566. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyarathna, P.; Mizera, P.; Walkowiak, J.; Dziedzic, K. Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits. Foods 2025, 14, 2447. https://doi.org/10.3390/foods14142447
Ariyarathna P, Mizera P, Walkowiak J, Dziedzic K. Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits. Foods. 2025; 14(14):2447. https://doi.org/10.3390/foods14142447
Chicago/Turabian StyleAriyarathna, Pathumi, Patryk Mizera, Jarosław Walkowiak, and Krzysztof Dziedzic. 2025. "Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits" Foods 14, no. 14: 2447. https://doi.org/10.3390/foods14142447
APA StyleAriyarathna, P., Mizera, P., Walkowiak, J., & Dziedzic, K. (2025). Physicochemical and Functional Properties of Soluble and Insoluble Dietary Fibers in Whole Grains and Their Health Benefits. Foods, 14(14), 2447. https://doi.org/10.3390/foods14142447







