Abstract
Metabolic syndrome has emerged as a significant global public health concern worldwide, characterized by a cluster of interrelated risk factors such as hypertension, hyperlipidemia, hyperglycemia, and abdominal obesity. In recent years, functional foods containing bioactive phytochemicals have attracted considerable scientific interest as potential therapeutic approaches for metabolic syndrome management. Xanthones, a class of naturally occurring tricyclic phenolic compounds abundant in various fruits and medicinal plants, demonstrate diverse biological activities relevant to metabolic health. This comprehensive review examines the dietary sources of xanthones, their bioactivity, and their promising role as functional food components for mitigating metabolic syndrome. The underlying mechanisms of action include modulation of lipid metabolism, improvement of insulin signaling pathways, potent anti-inflammatory and antioxidant effects, and modulation of glucose metabolism. Additionally, we discuss the stability and processing considerations of xanthones in food products. These findings highlight the development of xanthone-enriched functional foods and nutraceuticals as dietary interventions for metabolic syndrome prevention and management. This review comprehensively covers all relevant studies published up to the present without time restrictions.
1. Introduction
The definition of metabolic syndrome (MetS) has evolved over the years due to the challenges in establishing universally accepted diagnostic criteria. Nevertheless, MetS is broadly characterized by a cluster of metabolic abnormalities, including hypertension, central obesity, insulin resistance, and dyslipidemia [1,2,3]. The prevalence of MetS is rising globally, paralleling increases in obesity and type 2 diabetes (T2DM). In the United States, approximately one third of adults have MetS, with the prevalence of T2DM reaching 12.2% among adults, and even higher rates in seniors and certain ethnic groups. In China, urban MetS prevalence rose from 8% in 1992 to an estimated 15.5% in 2017. Globally, obesity rates have doubled in over 70 countries since 1980, contributing to the growing burden of MetS. While exact global MetS data remain limited, it is estimated that over one quarter of the world population—more than one billion people—are affected [4,5]. Effectively managing this multifaceted condition necessitates therapeutic strategies that simultaneously target its various components.
Xanthones, a class of polyphenolic compounds predominantly found in plants such as Garcinia, Calophyllum, Hypericum, Platonia, Mangifera, Gentiana, and Swertia, have attracted considerable scientific interest due to for their diverse pharmacological activities [6]. Their structural diversity and bioactive potential make them promising candidates for therapeutic intervention against metabolic syndrome and its associated pathologies [7,8,9]. Due to their presence in commonly consumed edible plants and demonstrated bioactivities, xanthones represent a promising category of functional food components that may contribute to the prevention and management of metabolic syndrome, thereby warranting a focused and comprehensive review [10]. However, previous reviews have predominantly focused on either the structural diversity or the biological activity of naturally occurring xanthones, often limiting their scope to specific genera like as Garcinia and Mangifera, or a select few well-studied compounds like α/γ-mangostin and mangiferin [6,11,12]. Given these limitations, a comprehensive overview examining edible plant-derived xanthones and their multifactorial roles in managing MetS is needed (Figure 1).
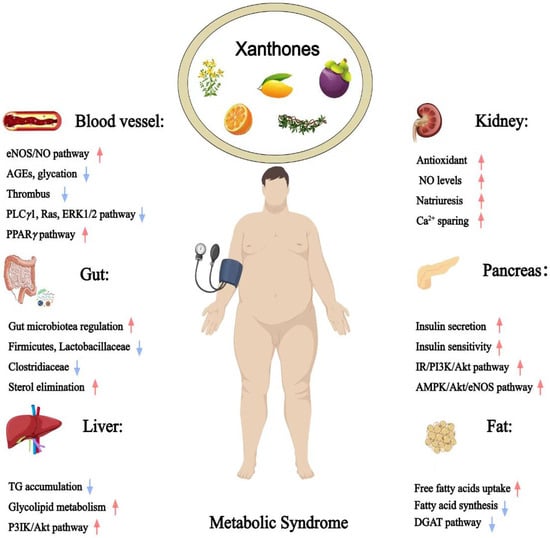
Figure 1.
Therapeutic potential of xanthones against metabolic syndrome (Red arrows = activate; blue arrows = inhibit).
This review aims to provide a thorough analysis of the therapeutic potential and mechanistic insights of edible plant-derived xanthones in managing MetS. By exploring their multifactorial roles in alleviating the core components of MetS, we seek to elucidate the mechanisms underlying their beneficial effects. The review is systematically structured to address each component of metabolic syndrome individually, covering their impact on hypertension, lipid metabolism and obesity, and glucose homeostasis. First, we examine the role of xanthones in modulating hypertension, discussing evidence for their vasodilatory, antithrombotic, endothelial function-enhancing, and diuretic effects, which collectively contribute to blood pressure reduction and decreased cardiovascular risk. Next, we evaluate their impact on hyperlipidemia and obesity, emphasizing their ability to regulate lipid and energy metabolism, inhibit fatty acid synthesis, and prevent atherosclerosis. Subsequent sections explore the anti-hyperglycemic properties of xanthones, detailing their mechanisms in enhancing insulin secretion and sensitivity, delaying carbohydrate digestion and absorption, promoting glucose uptake, and increasing glucose excretion. By integrating mechanistic insights with pharmacological evidence, this review aims to advance the understanding of how edible plant-derived xanthones serve as effective therapeutic agents for metabolic syndrome.
2. Materials and Methods
A comprehensive and systematic literature search was conducted across multiple electronic databases, including Web of Science, PubMed, ScienceDirect, and Scopus, covering all publications up to April 2025. The search employed an advanced strategy combining the term in the topic “xanthone” with each of the following keywords using the Boolean operator AND: “metabolic syndrome”, “hypertension”, “hyperlipidemia”, “hyperglycemia”, “obesity”, “endothelial dysfunction”, “atherosclerosis”, “vasodilation”, “platelet aggregation”, “antithrombotic”, “dyslipidemia”, “hypoglycemic”, “antihyperglycemic”, “antidiabetic”, “insulin resistance”, “antihypertensive”, and “atherosclerosis”. This approach ensured retrieval of studies specifically examining the role of xanthones in metabolic and cardiovascular-related conditions. The initial search yield 1284 potentially relevant studies. Following the application of the predefined exclusion criteria, 176 studies eligible for inclusion in this review were left. Figures were prepared using InDraw and MedPeer platform (https://www.medpeer.cn; accessed on 16 May 2024).
2.1. Inclusion Criteria
- (1)
- Studies published in peer-reviewed journals.
- (2)
- Studies investigating the pharmacological properties and mechanisms of edible plant-derived naturally occurring xanthones in relation to metabolic syndrome.
- (3)
- Studies published in the English language.
2.2. Exclusion Criteria
- (1)
- Studies on synthetic xanthones which are not naturally occurring in plants.
- (2)
- Studies on xanthone extracts without clarifying the components and structure.
- (3)
- Studies published in a language other than English.
- (4)
- Review articles, meta-analyses, case reports, and patents.
The search strategy included articles published up to April 2025. Additionally, the reference lists of relevant articles were carefully examined to identify any supplementary studies that might have been missed in the initial search. Strict inclusion and exclusion criteria were established to ensure only high-quality studies were included in this review.
3. Xanthones Against Hypertension
3.1. Vasodilatory Effects
The methanol extract of Hypericum revolutum Vahl, containing euxanthone (1) and 2,3,4-trimethoxy-xanthone (2) (Figure 2 and Table 1), demonstrated endothelium-dependent vasodilatory effects in isolated aortae. This activity was mediated by enhanced endothelial nitric oxide release, as evidenced by diminished efficacy upon endothelium removal or nitric oxide synthase inhibition with L-NAME [13,14]. Compounds 9-Xanthone (3), 1-hydroxyxanthone (4), 4-hydroxyxanthone (5), 1-hydroxy-8-methoxyxanthone (6), 1,3-dihydroxy-7-methoxyxanthone (7), 2,6,8-trihydroxy-1-methoxyxanthone (8), and 4-methoxyxanthone (9) demonstrated concentration-dependent vasorelaxant effects in endothelium-intact mouse aortic rings. Structural analysis suggested that a hydroxy group at the C-1 position attenuated vasodilation, whereas a hydroxy group at C-4 and an increased number of hydroxy groups enhanced xanthone-mediated vasorelaxation [15,16,17]. 1-Hydroxy-2,3,5-trimethoxyxanthone (10) isolated from Halenia elliptica induced relaxation in rat coronary artery rings via endothelium-dependent nitric oxide pathways and calcium channel inhibition. In contrast, 1,5-dihydroxy-2,3-dimethoxyxanthone (11) elicited endothelium-independent vasorelaxation through potassium channel modulation and partial inhibition of calcium influx [18,19]. Further investigations on six major xanthones 1-hydroxy-2,3,5-trimethoxy-xanthone (10), 1-hydroxy-2,3,4,7-tetramethoxyxanthone (12), 1-hydroxy-2,3,4,5-tetramethoxy-xanthone (13), 1,7-dihydroxy-2,3,4,5-tetramethoxyxanthone (14), 1,5-dihydroxy-2,3-dimethoxyxanthone (11), and 1,7-dihydroxy-2,3-dimethoxyxanthone (15) isolated from Halenia elliptica revealed vasodilatory activity in pre-contracted arteries through both endothelium-dependent and independent mechanisms [20]. Euxanthone (1) promoted vasodilation by inhibiting protein kinase C-activated calcium-sensitive mechanisms, independent of intracellular calcium release or voltage-operated calcium channels. It also induced concentration-dependent relaxation of high potassium and norepinephrine-induced contractions [21,22]. Gentiacaulein (16) and gentiakochianin (17), isolated from the methanolic extract of the roots of Gentiana kochiana, exhibited a vasorelaxant activity in rat aortic preparations pre-contracted with 3 µM norepinephrine [23]. 3-Demethyl-2-geranyl-4-prenylbellidifoline (18), a natural xanthone from Garcinia achachairu, reduced blood pressure in spontaneously hypertensive rats via endothelium-dependent vasorelaxation mediated by nitric oxide pathways and potassium channels [24]. Compounds 2,7-dihydroxy-1-methoxyxanthone (19), 1-methoxy-2,3-methylenedioxyxanthone (20), 7-hydroxy-1-methoxyxanthone (21), and euxanthone (1), isolated from the roots of Polygala caudata, displayed dose-dependent vasodilatory activity in Wistar rat thoracic aorta rings [25]. α-Mangostin (22) significantly improved endothelium-dependent vasodilation in diabetic mice by suppressing the acid sphingomyelinase/ceramide pathway and promoting the eNOS/NO pathway in the aorta [26]. Additionally, it reduced blood pressure and early nephropathy markers in spontaneously hypertensive rats, potentially by downregulating Ang II and inhibiting EMT via the TGF-β signaling pathway [27]. γ-Mangostin (23) induced concentration-dependent vasorelaxation in rat aortic rings precontracted with methoxamine, mediated by the NO-cGMP pathway and potassium channel activation [28].
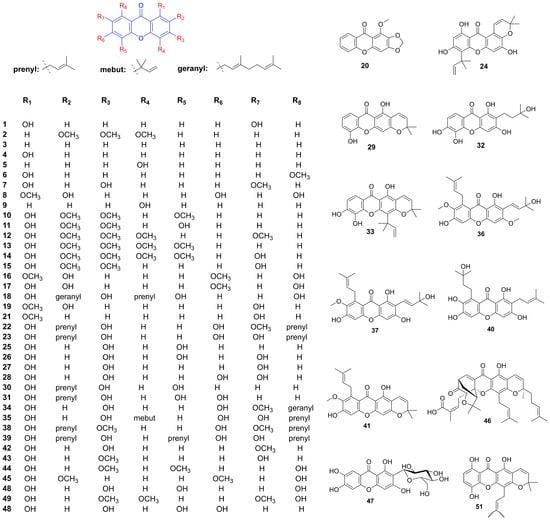
Figure 2.
Structure of xanthones related to hypertension improvement.

Table 1.
Pharmacological activities of xanthones and its mechanism of action.
3.2. Antiplatelet Aggregation Effects
Cudraxanthone B (24) effectively inhibited collagen-induced platelet aggregation, calcium ion mobilization, fibrinogen binding, fibronectin adhesion, and clot retraction without inducing cytotoxicity. It exhibited significant suppression of human platelet activation and thrombus formation [29]. 1,3,5-Trihydroxyxanthone (25) and 1,3,5,7-tetrahydroxyxanthone (26), isolated from Garcinia cantleyana var. cantleyana twigs, demonstrated selective inhibitory activity against ADP-induced platelet aggregation, with inhibition rates of 82.9 ± 8.8% and 90.4 ± 5.6%, respectively [30]. α-and γ-mangostin (22, 23) demonstrated concentration-dependent inhibition of platelet aggregation induced by collagen, thrombin, and ADP at low concentrations (1–10 μM) [31]. γ-Mangostin (23) also functions as a competitive antagonist for 5-HT2A receptors in vascular smooth muscles and platelets. It selectively inhibited 5-HT-induced contractions in rabbit aorta and attenuated the perfusion pressure response of rat coronary artery to 5-HT. Additionally, γ-mangostin (23) inhibited ADP-amplified platelet aggregation [32]. Nine xanthones—4-hydroxyxanthone (5), 1,3,7-trihydroxyxanthone (27), 1,3,6,7-tetrahydroxyxanthone (28), 6-deoxyjacareubin (29), 2-(3-methylbut-2-enyl)-1-,3,5-trihydroxyxanthone (30), 2-(3-methylbut-2-enyl)-1,3,5,6-tetrahydroxyxanthone (31), 2-(3-hydroxy-3-methylbutyl)-1,3,5,6-tetrahydroxyxanthone (32), macluraxanthone (33), and rubraxanthone (34)—inhibited platelet aggregation induced by arachidonic acid, collagen, and adenosine diphosphate, with IC50 values ranging from 47.0 ± 3.5 μM to 247.8 ± 6.3 μM. The presence of a prenyl group at C-2 enhanced their antiplatelet activity, whereas cyclization or hydroxylation of the prenyl group increased the IC50 value [33]. Cudratricusxanthone A (35) exhibited antithrombotic activities by inhibiting fibrin polymerization, platelet aggregation, and the activities of thrombin and activated factor X. It significantly prolonged partial thromboplastin activation time, prothrombin time, and in vivo bleeding time [34]. Euxanthone (1), extracted from Garcinia hombroniana with methanol, exhibited significant and selective inhibition of ADP-induced platelet aggregation, with an IC50 value of 5.7 µm [35].
3.3. Endothelial Protective Effects
The deposition of advanced glycation end-products (AGEs) in tissues can lead to severe complications, including endothelial dysfunction, cardiovascular disorders, and atherosclerosis. Eight xanthones—mangostanaxanthones III (36) and IV (37), β-mangostin (38), garcinone E (39), rubraxanthone (34), α-mangostin (22), garcinone C (40), and 9-hydroxycalabaxanthone (41)—isolated from Garcinia mangostana L., demonstrated potent, dose-dependent inhibition of protein glycation induced by both sugar (ribose) and methylglyoxal [36]. Among these, α-mangostin (22) exhibited protective effects against diabetic vascular complications by reducing apoptosis in HUVECs and improving retinal microvascular health. It also displayed antihyperglycemic, antioxidant, anti-inflammatory, and antiglycation properties. Furthermore, it mitigates isoproterenol-induced myocardial necrosis through antioxidant mechanisms and modulation of the nitric oxide pathway [37,38,39,40]. Isogentisin (42), derived from Gentiana lutea, protected human vascular endothelial cells against death induced by cigarette smoke, H2O2, and UV exposure. It activated cellular repair functions, reduces protein oxidation, and stabilizes the microtubule system, contributing to cell shape maintenance [41]. Gentisin (43), also from Gentiana lutea, was identified as a novel inhibitor of vascular smooth muscle cell (VSMC) proliferation, with an IC50 value of 7.84 µM. Additionally, 1-hydroxy-2,3,4,5-tetramethoxyxanthone (13), swerchirin (44), and methylswertianin (45) exhibited moderate anti-proliferative activity against VSMC proliferation, with IC50 values ranging from 10.2 to 12.5 µM, and were confirmed to be non-toxic [42]. Gambogic acid (46) Inhibited proliferation, migration, and DNA synthesis in PDGF-BB-stimulated rat aortic VSMCs by inducing G0/G1 phase arrest and downregulating cell cycle regulators. It blocked PDGFR-β and related signaling pathways, and reversed TGF-β1-induced fibrotic transitions via the VASH-2/VASH-1 and TGF-β1/Smad3 pathways [43,44]. Mangiferin (47) enhanced endothelial function by enhancing exosome secretion from perivascular adipose tissue (PVAT) to attenuate inflammation via NF-κB. It protected HUVECs against doxorubicin (DOX)-induced oxidative stress by upregulating Nrf2 through the PI3K/AKT pathway and ameliorates endoplasmic reticulum (ER) stress-related damage. Additionally, mangiferin exerts anti-atherosclerotic effects by reducing lipid accumulation and inflammatory responses in high-fat diet (HFD)-induced mice and improves endothelial cell responses to ox-LDL via the PTEN/Akt/eNOS pathway. Under hyperglycemic conditions, mangiferin activated Nrf2 and promoted angiogenesis and endothelial resilience, while in hyperuricemic rats, it alleviated hypertension and endothelial dysfunction [45,46,47,48,49,50]. Demethylbellidifolin (48), a xanthone derivative from Swertia davidi Franch, attenuated ox-LDL-induced monocyte adhesion to endothelial cells, reduced TNF-α and asymmetric dimethylarginine (ADMA) levels, and enhanced dimethylarginine dimethylaminohydrolase (DDAH) activity. It demonstrated antioxidant properties by inhibiting LDL oxidation and scavenging free radicals, while improving endothelium-dependent vasodilation and reducing lipid peroxidation [51,52]. Daviditin A (49) effectively preserved endothelium-dependent relaxation and mitigated lysophosphatidylcholine-induced endothelial dysfunction. It reduced lactate dehydrogenase release, malondialdehyde levels, and ADMA concentration, while increasing NO content and maintaining DDAH activity in ECV304 cells [53].
3.4. Diuretic Effects
1,3,5,6-Tetrahydroxyxanthone (50) exhibited diuretic activity along with a Ca2+-sparing effect, and reduced crystal formation in urine. It also modulated renal antioxidant and nitric oxide levels in hypertensive rats. Furthermore, 1,3,5,6-Tetrahydroxyxanthone showed synergistic enhancement of diuretic activity when co-administered with hydrochlorothiazide [54,55]. The diuretic effects of two natural prenylated xanthones, 3-demethyl-2-geranyl-4-prenylbellidypholine (DGP) (18) and 1,5,8-trihydroxy-4′,5′-dimethyl-2H-pyrano (2,3:3,2)-4-(3-methylbut-2-enyl) xanthone (TDP) (51), were evaluated in both normotensive and hypertensive rats. DGP exhibited potassium-sparing and diuretic properties, whereas TDP induced diuresis and increased urinary sodium, chloride, and calcium levels. When combined with hydrochlorothiazide, both DGP and TDP exhibited enhanced diuretic effects. Additionally, TDP showed protective effects against urinary calcium oxalate crystal formation [56]. DGP exhibits prolonged diuretic and nephroprotective effects in rats, promoting natriuresis, Ca2+ sparing, and antioxidant activity in spontaneously hypertensive rats [57].
4. Xanthones Against Hyperlipidemia and Obesity
4.1. Promoting Lipid Metabolism
Mangiferin (47) promoted cholesterol efflux and attenuates atherogenesis by activating the PPARγ-LXRα-ABCA1/G1 pathway, and improves fatty acid metabolism in HepG2 cells by enhancing β-hydroxybutyrate levels, free fatty acid (FFA) uptake, and oxidation via the AMPK pathway [58,59]. In non-alcoholic fatty liver disease (NAFLD) models, mangiferin (47) mitigated liver injury, insulin resistance, and glucose intolerance by modulating glucolipid metabolism through AMPK activation and NLRP3 inflammasome inhibition [60]. In HFD-induced mice, it reduced body weight, triglycerides, and total cholesterol while suppressing inflammation and enhancing autophagy via the AMPK/mTOR pathway [61]. In hyperlipidemic hamsters, mangiferin (47) reduced body and liver weight, visceral fat, serum triglycerides, and free fatty acids by upregulating lipid oxidation genes and downregulating lipogenesis genes [62]. When combined with exercise in KK-Ay mice, it lowered blood cholesterol and triglyceride levels, while in fructose-fed spontaneously hypertensive rats, it reduced hepatic triglyceride content by inhibiting diacylglycerol acyltransferase (DGAT-2) [63,64]. Furthermore, in human mesenchymal stem cells (hMSCs), mangiferin (47) suppressed adipocyte differentiation and lipid accumulation by downregulating key adipogenic genes [65]. Moreover, mangiferin (47) also exhibited antioxidant properties, suppressing SREBP-mediated lipogenesis, and inhibits AGE-induced oxidative stress. In HFD mice, it enhanced mitochondrial capacity, thermogenesis, glucose, and insulin profiles, shifting fuel utilization from fatty acids to carbohydrates [66]. In cultured myotubes, it increased glucose and pyruvate oxidation, ATP production, and targets pyruvate dehydrogenase [67]. Neomangiferin (52) effectively ameliorated HFD-induced NAFLD in rats by decreasing body weight, liver fat, serum lipids, and glucose levels while enhancing serum HDL cholesterol and hepatic antioxidant levels, These effects are mediated by the upregulation of PPARα and CPT1a, and the downregulation of FATP2 and ACSL1 [68]. 6′-O-acetyl mangiferin (53) significantly reduced intracellular lipid and triglyceride accumulation in 3T3-L1 preadipocytes by activating AMPK [69]. In Apoe (−/−) mice, α-mangostin (22) attenuated atherosclerotic progression by reducing body weight gain, improving lipid profiles, and promoting M2 macrophage polarization. In Sprague Dawley rats, it alleviated HFD-induced hepatic steatosis by decreasing plasma-free fatty acids and liver triglycerides, improving antioxidant activity, and restoring mitochondrial function. Its potent antioxidant effects were demonstrated by prolonging the lag phase of LDL oxidation and reducing oxidative stress markers [70,71,72]. In HFD-induced obese mice, α-mangostin (22) improved metabolic profiles and obesity-related parameters by decreasing body, liver, and fat weights, as well as glucose, insulin, triglycerides, cholesterol, and fatty acid levels. Concurrently, it increased adiponectin, reducing inflammatory markers, improving glucose tolerance, and upregulating hepatic AMPK, SirT1, and PPARγ [73,74]. In diet-induced metabolic syndrome rats, it reduces body weight, abdominal fat, and improves liver and cardiovascular function by decreasing inflammation, collagen deposition, and reducing adipocyte size [75]. Additionally, α-mangostin (22) demonstrated anti-inflammatory and anti-adipogenic effects in 3T3-L1 preadipocytes by suppressing NF-κB and adiponectin, while γ-mangostin (23) reduced adipogenesis and inflammation by inhibiting Nrf2 activity and PPARγ expression [76]. Several xanthones isolated from Garcinia mangostana (Figure 3), including α-mangostin (22), β-mangostin (38), γ-mangostin (23), 1-isomangostin (54), gartanin (55), garcinone D (56), 9-hydroxycalabaxanthone (41), smeathxanthone A (57), tovophyllin A (58), 8-deoxygartanin (59), mangostanin (60), and 1,7-dihydroxy-3-methoxy-2-(3-methylbut-2-enyl) xanthen-9-one (61) exhibited inhibitory activities against pancreatic lipase with IC50 ranging from 5.0 to 34.5 µM (compared to orlistat IC50 3.9 µM) [77]. 3,4,5,6-Tetrahydroxyxanthone (62) ameliorated dyslipidemia in ApoE (−/−) mice by lowering plasma and hepatic lipids, while increasing HDL cholesterol via modulation of Angptl3 and LPL. It also improved erythrocyte deformability and reduced oxidative stress markers, MDA, and ADMA, indicating potential benefits for lipid metabolism and vascular health [78,79]. A high-throughput screening identified 1,3,5,8-tetrahydroxyxanthone (63) as an agonist that enhances the interaction between ABCA1 and apoA-I, promoting cholesterol efflux in ABCA1-expressing cells and THP-1 macrophages, as confirmed by flow cytometry, Western blot, and 3H cholesterol efflux assays. This compound also dose-dependently inhibited lipid accumulation in 3T3-L1 adipocytes by reducing PPARγ and C/EBPα expression while activating Hedgehog signaling components Gli1 and Smo [80,81]. Bellidifolin (64) significantly improves HFD-induced obesity and lipid disorders by reducing the abundance of Firmicutes, Lactobacillaceae, and Clostridiaceae in the gut microbiota, while enhancing bile acid biosynthesis and excretion to promote lipid metabolism [82].
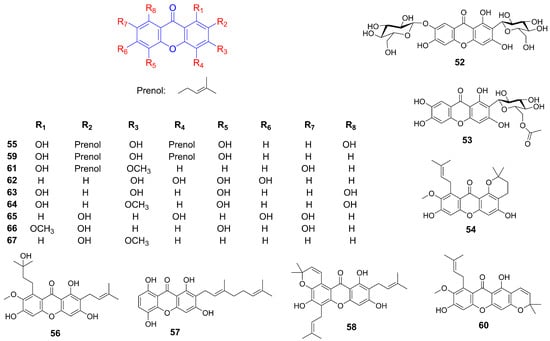
Figure 3.
Structure of additional xanthones related to hyperlipidemia improvement.
4.2. Inhibiting Fatty Acid Synthesis
Mangiferin (47) supplementation (150 mg/day for 12 weeks) significantly improved serum lipid profiles in overweight hyperlipidemic patients by reducing serum TG and FFA, while enhancing lipoprotein lipase activity and markers of fatty acid oxidation including L-carnitine and β-hydroxybutyrate. The reduction in lowering serum TG levels may result from either the inhibition of TG synthesis or the acceleration of TG decomposition [83]. Quantitative proteomic analysis revealed that mangiferin (47) significantly modulated 87 out of 865 liver proteins in mice fed a high-fat diet, specifically enhancing proteins involved in mitochondrial biogenesis and oxidative metabolism while downregulating those associated with lipogenesis. These findings suggested that mangiferin promotes energy expenditure while suppressing fat synthesis [84]. Garcinone E (39), a bioactive compound derived from Garcinia mangostana, demonstrated concentration-dependent inhibition of fatty acid synthase (FAS) with an IC50 of 3.3 μM. Mechanistic studies demonstrated competitive inhibition relative to acetyl-CoA, mixed competitive and noncompetitive inhibition with malonyl-CoA, and noncompetitive inhibition regarding NADPH. Notably, its irreversible inhibition of FAS highlight garcinone E’s potential as a therapeutic agent for obesity and cancer, where FAS plays a crucial role [85]. Natural inhibitors of FAS are emerging as promising candidates for cancer and obesity treatment. A bioassay-guided chemical investigation of Garcinia mangostana hulls led to the isolation of several xanthones, including α-mangostin (22), β-mangostin (38), γ-mangostin (23), 9-hydroxycalabaxanthone (41), garcinone E (39), 1,3,7-trihydroxyxanthone (27), and 2,4,6,7-tetrahydroxyxanthone (65). These compounds exhibited potent FAS inhibitory activity, with IC50 values ranging from 1.24 ± 0.05 to 40.98 ± 0.64 μM [86]. Additionally, the ethanolic extract of Garcinia mangostana peel inhibited human recombinant DGAT1, DGAT2, and pancreatic lipase (PL) activities in vitro. Among the tested bioactive compounds, α-mangostin (22), γ-mangostin (23), gartanin (55), and 8-deoxygartanin (59) were identified as potent inhibitors of DGAT enzymes [87]. Furthermore, α-mangostin (22) induced apoptosis in 3T3-L1 cells, suppressed FAS activity, inhibited lipid accumulation, and promoted lipolysis by targeting the ketoacyl synthase domain of FAS [88].
4.3. Anti-Atherosclerosis Effect
1-Methoxy-2,5,7-trihydroxyxanthone (66) isolated from the roots of Lindera fruticose demonstrated LDL antioxidant activity with an IC50 value of 25.5 µM (positive control, probucol, IC50 = 4.3 µM). Given that LDL cholesterol oxidation is a critical step in atherosclerotic lesion formation, this compound shows potential therapeutic relevance [89]. Cudratricusxanthone A (35), derived from the root bark of Cudrania tricuspidata, demonstrated antiproliferative effects on PDGF-BB-stimulated vascular smooth muscle cells. Its mechanism involves suppression of DNA synthesis, cell proliferation, PDGFRβ phosphorylation, and downstream signaling pathways including PLCγ1, Ras, and ERK1/2 [90]. 3-Methoxy-2-hydroxyxanthone (67), isolated from Calophyllum flavoramulum Hend. & Wyatt-Sm, exhibited potent anti-AGEs activity with an IC50 value of 0.06 mM, suggesting its utility in mitigating advanced glycation end product-related pathologies [91]. Mangiferin (47) alleviated TMAO-induced atherosclerosis in mice by attenuating inflammation, reducing plasma cholesterol levels, and modulating gut microbiota composition. These effects collectively diminish aortic lesions and enhance the elimination of sterols [92].
4.4. Promoting Energy Metabolism
Gambogic acid (46) activated AMPK by binding to the AMPK α subunit, independent of upstream kinases, and enhancing phosphorylation of AMPK α and its substrate ACC across multiple cell lines. This activation was uniquely mediated by intracellular ROS, with levels modulated by thiol antioxidants, suggesting its potential as a novel direct AMPK activator for metabolic diseases management [93]. Mangiferin (47) enhances brown fat markers, induces UCP1, and upregulates thermogenic regulators (PGC1α, PRDM16, PPAR-α/β). It promotes mitochondrial biogenesis and function by activating AMPK and β3-AR-dependent PKA-p38 MAPK-CREB signaling, while suppressing mitophagy [95,96]. α-Mangostin (22) mitigated oxidative stress and metabolic disorders by enhancing AMPK phosphorylation [94]. γ-mangostin (23) was identified as an active compound that induced UCP-3 gene expression in L6 cells, functioning as a dual agonist for PPARδ and PPARα. Additionally, it upregulated acyl-CoA synthase and carnitine palmitoyl-transferase 1A gene expression in HepG2 cells, indicating its potential as a preventive agent for metabolic syndrome [97], as shown in Figure 4.
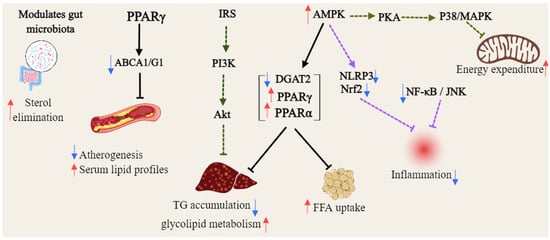
Figure 4.
General overview of the mechanism of xanthones against hyperlipidemia (Red arrows = activate; blue arrows = inhibit; dashed lines = modulation).
5. Xanthones Against Hyperglycemia
5.1. Increasing Insulin Secretion
Supplementation with α-mangostin (22) in diabetic rats significantly reduced fasting blood glucose, HbA1c, cholesterol, and triglyceride levels, while enhancing insulin secretion and sensitivity, as well as improving glucose tolerance. Mechanistically, α-mangostin stimulated insulin release in INS-1 cells by activating the IR, Pdx1, PI3K, Akt, and ERK pathways, while suppressing IRS-1 phosphorylation [98,99]. In a 28-day study, gentiakochianin (17) and 1,2-dihydroxy-6-methoxyxanthone-8-O-β-D-xylopyranosyl (68) isolated from Swertia corymbosa demonstrated significant antidiabetic and antihyperlipidemic effects, markedly reducing blood glucose, glycosylated hemoglobin, and lipid profiles (Figure 5). These compounds also improved liver and kidney function markers while elevating plasma insulin levels [100]. Mangiferin (47) demonstrated significant improvements in glycemic control, glucose tolerance, serum, and insulin levels in diabetic models. It also promoted β-cell hyperplasia, proliferation, and reduced apoptosis. Furthermore, treatment with a hydroalcoholic Bombax ceiba leaf extract and its constituent mangiferin (47) improved glucose regulation, lipid metabolism, and oxidative stress markers in streptozotocin-induced diabetic rats, likely through increased insulin release, antioxidant activity, and hypolipidemic effects [101,102]. A 30-day mangiferin (47) treatment (40 mg/kg) in diabetic rats ameliorated blood glucose levels, enhanced insulin secretion, and improved nonenzymatic antioxidant capacity (vitamins C, E, ceruloplasmin, reduced glutathione). Histological analysis revealed regenerated islet cells and normalized pancreatic ultrastructure, confirming β-cell protection [103]. The combination of sitagliptin with mangiferin (47) synergistically improved glucose tolerance, insulin, and GLP-1 levels in diabetic rats, highlighting its potential for metabolic control. In aged, partially pancreatectomized mice, mangiferin reduced blood glucose, enhanced glucose tolerance, increased insulin production, and promoted β-cell proliferation, while suppressing apoptosis via the modulation of cyclins and cyclin-dependent kinases [104,105]. Crude swerchirin (44) from Swertia chirayita induced a 60% reduction in blood glucose levels in fed rats by potentiating insulin release from pancreatic islets, as evidenced by both in vivo and in vitro studies. Notably, swerchirin treatment depleted β-granules and insulin in pancreatic islets while simultaneously promoting glucose uptake and glycogen synthesis in muscle cells [106].
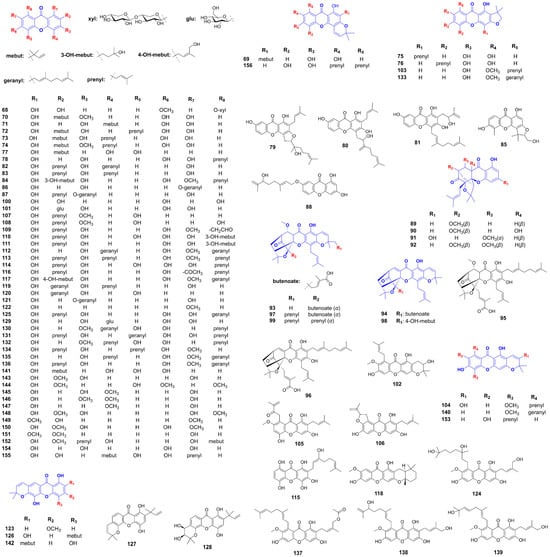
Figure 5.
Structure of additional xanthones related to hyperglycemia improvement.
5.2. Enhancing Insulin Sensitivity
In Wistar-Kyoto rats fed a fructose diet, mangiferin (47) reduced plasma insulin and non-esterified fatty acid (NEFA) levels, mitigating adipose tissue insulin resistance without affecting fructose intake, fat accumulation, or hypertension [107]. In HFD mice, it alleviated endoplasmic reticulum (ER) stress, suppressed NLRP3 inflammasome activation, enhanced AMPK activity, and restored insulin-mediated Akt and eNOS phosphorylation, thereby improving endothelial function and presenting a potential therapeutic approach for preventing endothelial insulin resistance [108]. For gestational diabetes, mangiferin (47) improved glucose and lipid metabolism, reduced placental oxidative stress, increased antioxidant levels, and attenuated inflammation and ER stress [109]. In KK-Ay mice, administration of mangiferin (47) at a 30 mg/kg dose decreased serum insulin levels, enhanced glucose uptake, and promoted fatty acid oxidation [110]. In palmitic acid-treated cells, mangiferin (47) enhanced insulin-stimulated glucose uptake while reducing intracellular glucose, FFA, and TG levels in by upregulating p-Akt, GLUT2, and GLUT4 expression, and by activating fatty acid oxidation via the PPAR pathway [111]. In hypoxic 3T3-L1 adipocytes, it counteracts insulin resistance by inhibiting HIF-1α [112]. In diabetic nephropathy models, mangiferin (47) provided renoprotective effects by inhibiting the MAPK/NF-κB axis, activating the phosphorylated IRS-1/PI3K/Akt pathway, and mitigating renal ferroptosis through enhanced antioxidant activity and reduced pro-ferroptotic lipid production [113]. In type 2 diabetes models, mangiferin (47) improved biochemical parameters, modulated lipid metabolism, and ameliorated obesity, hyperglycemia, insulin resistance, and dyslipidemia. It restored hepatic glycogen levels, normalized serum TNF-α and adiponectin concentrations, and demonstrated efficacy comparable to rosiglitazone [114,115]. When combined with metformin, mangiferin (47) targeted the insulin-dependent Akt pathway, whereas its co-administration with gliclazide affected the insulin-independent AMPK pathway [116]. Additionally, mangiferin (47) and neomangiferin (52) significantly reduced blood glucose levels and improved hyperinsulinemia in non-insulin-dependent diabetes mellitus models, highlighting their potential to enhance insulin sensitivity [117]. α-Mangostin (22) and γ-mangostin (23), derived from mangosteen, reduced LPS-induced inflammation and counteracted aging-related metabolic disorders in mice by reducing pro-inflammatory cytokines, promoting macrophage polarization toward anti-inflammatory phenotypes, and suppressing microRNA-155-5p secretion. These compounds exhibit anti-inflammatory effects in human adipocytes and macrophages by inhibiting MAPK, NF-κB, and AP-1 pathways, thereby reducing LPS-induced inflammatory gene expression and enhancing insulin sensitivity [118,119,120]. Chemical investigation of Cudrania tricuspidata roots yielded nine xanthones, including cudratricusxanthone N (69), 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone (70), cudratricusxanthone L (71), cudratricusxanthone A (35), cudraxanthone L (72), macluraxanthone B (73), cudracuspixanthone A (74), cudraxanthone D (75), and cudraxanthone M (76). These prenylated xanthones exhibited dose-dependent PTP1B inhibitory activity with IC50 values ranging from 1.9 to 4.6 μM [121]. 12b-Hydroxy-des-D-garcigerin A (77) improved glucose consumption in insulin-resistant HepG2 cells by increasing hexokinase and pyruvate kinase activities while upregulating key insulin signaling components, including InsR, IRS-1, phosphorylated PI3K, and phosphorylated AKT, without affecting AMPK phosphorylation at Thr172 [125]. 1,3,6,7-Tetrahydroxy-8-prenylxanthone (78) isolated from Garcinia mangostana attenuated LPS-induced inflammation in macrophages and adipocytes by activating MAPKs and NF-κB pathways and enhancing sirtuin 3 expression [126]. Methylswertianin (45) and bellidifolin (64) significantly improved hyperglycemia, lipid profiles, and insulin signaling in streptozotocin-induced type 2 diabetic mice by upregulating InsR-α, IRS-1, and PI3K expression [127]. Bellidifolin (64), obtained from Swertia japonica, exhibited potent hypoglycemic effects and reduced blood triglyceride levels in diabetic rats, while stimulating glucose uptake in Rat 1 fibroblasts expressing human insulin receptors [128]. Twelve xanthones—including cratoxanthone E (79), cratoxanthone F (80), cratoxanthone A (81), cochinechinone A (82), 1,3,7-trihydroxy-2,4-diisoprenylxanthone (83), α-mangostin (22), γ-mangostin (23), cratoxylone (84), cochinchinone Q (85), 7-geranyloxy-1,3-dihydroxyxanthone (86), pruniflorone S (87), and cochinxanthone A (88)—isolated from Cratoxylum cochinchinense demonstrated dual inhibition of PTP1B (IC50 2.4–52.5 µM) and α-glucosidase (IC50 1.7–72.7 µM) [122]. Four new caged xanthones—cochinchinoxanthone A (89), B (90), C (91), and D (92) —from Cratoxylum cochinchinense roots acted as competitive PTP1B inhibitors [123]. Additionally, the methanol extract of Garcinia hanburyi gum resin, containing prenylated caged xanthones, such as gambogic acid (46), moreollic acid (93), morellic acid (94), 10-methoxygambogenic acid (95), gambogenic acid (96), gambogoic acid (97), morellinol (98), and 10-methoxygambogin (99), exhibited dose-dependent PTP1B inhibition (IC50 0.47–70.25 μM), with gambogic acid, demonstrating superior potency to the positive control, ursolic acid (IC50 15.5 μM) [124].
5.3. Slowing Digestion and Absorption
Several xanthones derived from Swertia mussotii, including 1,3,7,8-tetrahydroxyxanthone (100), 1,3,5,8-tetrahydroxyxanthone (63), and 2,3,6,8-tetrahydroxyxanthone-7C-(β-D-glucoside) (101), exhibited notable antioxidant activity and α-glucosidase inhibition. Specifically, 1,3,5,8-tetrahydroxyxanthone (63) displayed potent dual inhibition of α-glucosidase (IC50 = 5.2 ± 0.3 μM) and aldose reductase (IC50 = 88.6 ± 1.6 nM) [129]. α-Mangostin (22) exhibited strong antioxidant properties alongside inhibitory effects on α-amylase and α-glucosidase. Mechanistic studies indicated that α-mangostin (22) reversibly interacts with these enzymes, particularly through hydrogen bonding with α-glucosidase [142]. Similarly, γ-mangostin (23), a xanthone derived from Garcinia mangostana, demonstrated significant antihyperglycemic effects in diet-induced diabetic mice, effectively lowering blood glucose levels while exhibiting superior inhibitory activity against α-amylase and α-glucosidase compared to acarbose, without hepatotoxic or nephrotoxic effects [143]. The ethanol extract of Garcinia mangostana fruit case, rich in xanthones, showed potent α-glucosidase inhibition (IC50 = 3.2 μg/mL). Isolated xanthones—including β-mangostin (38), 9-hydroxycalabaxanthone (41), mangostanol (102), mangostenone F (103), allanxanthone E (104), α-mangostin (22), mangostingone (105), garcinone D (56), γ-mangostin (23), mangosenone G (106), cudraxanthone (107), 1,5,8-trihydroxy-3-methoxy-2-(3-methylbut-2-enyl)xanthone (108), 8-deoxygartanin (59), gartanin (55), smeathxanthone A (57), and oxoethylmangostine (109)—demonstrated moderate-to-high α-glucosidase inhibition, with IC50 values ranging from 1.5 to 63.5 μM [130]. Further studies identified five xanthones—mangostanaxanthone VIIII [1,3,5,6,7-pentahydroxy-2-(3-methylbut-2-enyl)-8-(3-hydroxy-3-methylbut-1-enyl)-xanthone] (110), mangostanaxanthone I (111), mangostanaxanthone II (112), γ-mangostin (23), and mangostanaxanthone VII (113)—from the acetone fraction of Garcinia mangostana pericarps, showing α-amylase inhibition ranging from 56.2% to 86.55% [146]. Additionally, 8-deoxygartanin (59), α-mangostin (22), and β-mangostin (23) exhibited polyfunctional inhibition against pancreatic lipase, α-amylase, and α-glucosidase, with IC50 values ranging from 31.6 ± 2.6 to 333.5 ± 4.5 μM [144]. A newly identified xanthone, garcixanthone D [1,3,5,6,7-pentahydroxy-2,8-bis(3-methylbut-2-enyl)-xanthone] (114), along with garcinone E (39) from Garcinia mangostana, exhibited the highest α-amylase inhibition at 85.6% and 93.8%, respectively [147,148]. Another novel xanthone, garcimangostin A (115), along with known compounds garcixanthone A (116), gartanin (55), γ-Mangostin (23), and garcinone C (40), also inhibited α-amylase, with inhibition percentages ranging from 54.9% to 94.1% [149]. Screening of Garcinia cowa leaves revealed that α-mangostin (22) and cowanol (117) displayed significant α-glucosidase inhibition, with IC50 values of 15.0 μM and 18.0 μM, respectively [131]. Six xanthones—garciniacowone L (118), 2-geranyl-1,3,7-trihydroxy-4-(3,3-dimethylallyl)-xanthone (119), mangostinone (120), cochinchinone G (121), 1-hydroxy-7-methoxyxanthone (122), and forbexanthone (123)—isolated from Garcinia cowa Roxb. ex Choisy exhibited α-glucosidase inhibition (IC50 = 85.1 ± 0.3 to 188.8 ± 0.6 μM), comparable to acarbose (IC50 = 76.7 ± 1.4 μM) [132]. Similarly, garcicowanones C (124), cowanol (117), norcowanin (125), and garcinone D (56) from Garcinia cowa exhibited α-glucosidase inhibition (IC50 = 17.2 ± 0.3 to 211.2 ± 3.4 μM, acarbose, 257.3 ± 4.8 μM) [133]. Four xanthones—subelliptenone H (126), 12b-hydroxy-des-D-garcigerin A (76), garciniaxanthone B (127), and garcigerin A (128)—isolated from Garcinia forbesii, inhibited α-glucosidase and α-amylase with an IC50 value ranging from 10.8 ± 0.04 to 139.4 ± 1.21 μM (Acarbose, 4.0 ± 0.32 μM) [145]. The water extract of Anemarrhena asphodeloides rhizoma (AA) significantly reduced blood glucose levels in KK-Ay mice, primarily due to mangiferin (47) and neomangiferin (52). Mangiferin (47) exhibited strong α-glucosidase inhibition (IC50 = 36.84 μg/mL) in both in silico and in vitro studies [134,135]. Furthermore, when combined with metformin and gliclazide, mangiferin (47) significantly alleviated renal injury in type II diabetic rats by reducing serum glucose, urea, and creatinine levels, while modulating inflammation and oxidative stress-related markers [136]. Mangiferin (47) also inhibited DPPH radicals and α-glucosidase [137,138]. Xanthone-enriched fractions from Cyclopia genistoides, along with mangiferin (47) and isomangiferin (129), showed potent α-glucosidase inhibition (IC50 = 43.3 µg/mL) and synergistic effects when combined with acarbose [139]. From Garcinia oblongifolia, oblongixanthones C (130), F (131), G (132), and H (133), 1,3,6-trihydroxy-7-methoxy-2,5-bis(3-methylbut-2-enyl)xanthon (134), isocowanin (135), cowanin (136), cowanol (117), rubraxanthone (34), cowagarcinone E (137), and norcowanin (125) exhibited significant α-glucosidase (IC50 = 1.7 to 53.5 μM) and PTP1B inhibition (IC50 = 14.1 to 44.2 μM) [151]. Two additional xanthones, oblongixanthones I (138) and J (139), showed moderate α-glucosidase (IC50 = 258.7 ± 49.3 μM and 187.1 ± 27.5 μM) and PTP1B inhibition (IC50 = 93.9 ± 12.3 μM and 64.1 ± 5.8 μM) [152]. Among 18 xanthones isolated from Garcinia nigrolineata Planch. ex, cowagarcinone E (137) exhibited the strongest α-glucosidase inhibitory activity (IC50 = 25.8 ± 0.2 µM), while mangostanin (60) and fuscaxanthone A (140) showed the highest α-amylase inhibitory activity (IC50 = 124.8 ± 0.7 µM) and glycation inhibition activity (IC50 = 44.4 ± 1.1 µM), respectively [150]. From Garcinia xanthochymus bark, subelliptenone F (141) and xanthochymusxanthone B (142) significantly inhibited α-glucosidase (IC50 = 4.1 ± 0.3 μM) and PTP1B (IC50 = 8.0 ± 0.6 μM) [153]. Securidaca inappendiculata Hassk., traditionally used for fractures and rheumatoid arthritis, contains xanthones with antidiabetic properties. Eleven xanthones—1,3,7-trihydroxylxanthone (27), 1,3,7-trihydroxyl-2-methoxylxanthone (143), 1,5-dihydroxyl-2,6,8-trimethoxylxanthone (144), 1,3,7-trihydroxyl-4-methoxylxanthone (145), 1,7-dihydroxyl-3,4-dimethoxylxanthone (146), 1,7-dihydroxyl-4-methoxylxanthone (147), euxanthone (1), 1,3,7-trihydroxyl-2,8-dimethoxylxanthone (148), 2-hydroxyl-1,7-dimethoxylxanthone (149), 1,3,6-trihydroxyl-2,7-dimethoxylxanthone (150), and 7-hydroxyl-1,2-dimethoxylxanthone (151)—inhibited α-glucosidase (IC50 = 3.2 to 77.3 μg/mL), with free hydroxyl groups playing a key role in activity [140]. Garceduxanthone (152) and 7-prenyljacareubin (153) isolated from the leaves of Garcinia paucinervis showed moderate a-glucosidase inhibition (IC50 = 8.90 ± 3.35 and 29.36 ± 0.81 μM, respectively), compared to acarbose (IC50 = 2.88 ± 0.85 μM) [141].
5.4. Enhancing Glucose Uptake
Mangiferin (47), isolated from the leaves of Salacia oblonga, was administered to streptozotocin-induced diabetic rats and significantly reduced blood glucose levels, normalized lipid profiles, and improved oxidative stress biomarkers over a 15-day period. Molecular docking and gene expression analyses confirmed that mangiferin activates the PPARγ/GLUT4 signaling pathway, suggesting its therapeutic potential in enhancing glucose uptake and ameliorating diabetes symptoms [154]. Additionally, Salacia oblonga extract, particularly its active component mangiferin (47), enhanced glucose uptake by 50% in muscle L6-myotubes and 3T3-adipocytes. This effect was mediated through increased GLUT4 content, activation of GLUT4 transcription, and facilitation of its translocation via the activation of AMPK. The process was modulated through PPARγ, as evidenced by inhibition with the antagonist GW9662, indicating dual independent pathways for glucose transport enhancement [155]. In streptozotocin-induced diabetic rats, mangiferin (47) also demonstrated significant antidiabetic, antihyperlipidemic, and antiatherogenic effects by reducing fasting plasma glucose, total cholesterol, triglycerides, and LDL-C levels while increasing HDL-C levels and improving glucose tolerance [156]. Targeted metabolomics and transcriptomics studies demonstrated that mangiferin (47) enhanced glycolytic flux and mitochondrial oxidative capacity. These studies reported increases in glycolytic metabolites and key tricarboxylic acid cycle intermediates, such as α-ketoglutarate and fumarate. Mangiferin also upregulated succinate dehydrogenase, improving ATP production, and induced the expression of mitochondrial genes and their transcription factors, suggesting its role in accelerating metabolic processes [157]. Furthermore, mangiferin (47) from Salacia chinensis, administered at a dose of 40 mg/kg body weight daily for 30 days, significantly reduced blood glucose and improved biochemical markers including urea, uric acid, and creatinine in streptozotocin-induced diabetic rats. It also decreased toxicological parameters such as AST, ALT, and ALP while enhancing hematological profiles [158]. Norathyriol (154) and mangiferin (47) significantly enhanced glucose consumption in L6 myotubes, with norathyriol (154) exhibiting a more pronounced effect, particularly in insulin-resistant cells. Both compounds acted synergistically with insulin and their antidiabetic activity was mediated through the upregulation of AMPK phosphorylation [159]. α-Mangostin (22) inhibited adipogenic differentiation, reduced intracellular fat accumulation, enhanced glucose uptake, and altered gene expression related to PPARγ, GLUT4, and leptin [160]. Garcinia xanthochymus is a folk medicine widely used in southwestern China. Compounds 12b-hydroxy-des-d-garcigerrin (76), 1,2,5,6-tretrahydroxy-4-(1,1-dimethyl-2-propenyl)-7-(3-methyl-2-butenyl) xanthone (155), and 1,5,6-trihydroxy-7,8-di(3-methyl-2-butenyl)-6′,6′-dimethylpyrano (2′,3′:3,4) xanthone (156) were isolated from G. xanthochymus and significantly stimulate glucose uptake in skeletal muscle cells. The first two compounds induced GLUT4 translocation through activation of PI3K/Akt and AMPK pathways [161].
5.5. Effects Against Inflammation and Oxidative Stress
α-Mangostin (22) reversed high glucose-induced inhibition of proliferation and migration in HUVECs. RNA-seq analysis revealed that it restored the expression of H19 and HE4 lncRNAs, which were down-regulated under hyperglycemic conditions. Further studies demonstrated that H19 modulates HE4 expression via miR-140, suggesting a novel H19/miR-140/HE4 regulatory pathway for α-mangostin’s potential therapeutic impact in diabetes mellitus [162]. γ-Mangostin (23) treatment significantly reduced fasting blood glucose, cholesterol, SGOT, and SGPT levels, while mitigating hepatocyte damage in STZ-induced diabetic BALB/C mice [176]. Mangiferin (47), first isolated from the root bark of Salacia reticulata in 1985, exhibited multiple therapeutic effects in diabetic models. In diabetic rats, it ameliorated cardiomyopathy by reducing myocardial enzyme levels (CK-MB, LDH), inflammatory mediators (TNF-α, IL-1β), and reactive oxygen species (ROS), while suppressing the AGE/RAGE pathway and NF-κB nuclear translocation [163,164]. In ischemia–reperfusion (IR) injury models, mangiferin enhanced cardiac function, restored antioxidant defenses, and reduced inflammation and apoptosis by modulating the AGE-RAGE/MAPK pathways—specifically inhibiting JNK and p38 activation while upregulating ERK1/2 [165]. Additionally, it also improved renal function in diabetic nephropathy by enhancing glyoxalase 1 (Glo-1) activity, reducing albuminuria, and alleviating oxidative stress [166]. In STZ-induced diabetic rats, mangiferin (47) restored the suppressed hypoxic ventilatory response and normalized oxidative and inflammatory stress markers [167]. Network pharmacology analysis identified 37 key targets, including TNF, EGF, and PTGS2, implicating mangiferin (47) in critical pathways such as PI3K-Akt and NF-κB [168]. Furthermore, it alleviated cognitive deficits in diabetic rats by increasing Glo-1 activity and glutathione levels while reducing oxidative stress and inflammation in the hippocampus [169]. Mangiferin (47) also attenuated diabetic nephropathy by decreasing serum AGEs and malondialdehyde, promoting antioxidant enzyme activity and inhibiting glomerular extracellular matrix expansion [170]. In STZ-induced diabetic Wistar rats, it provided significant cardiorenal protection against oxidative damage, reducing glycosylated hemoglobin and CPK levels comparably to insulin treatment [171]. Moreover, mangiferin (47) mitigated nephrotoxicity by inhibiting PKC isoforms, MAPKs, and NF-κB activation [172]. In H9C2 cells, it protected against H2O2-induced ischemia–reperfusion injury by enhancing antioxidant capacity and metabolic pathways, revealing key enzyme targets and mechanistic insights into its cardioprotective effects [173]. The hypoglycemic activity of the alcoholic extract of Swertia japonica is primarily attributed to its xanthones, with bellidifolin (78) and swerchirin (44) significantly reducing blood glucose in STZ-induced diabetic rats [174]. Swerchirin (44), isolated from Swertia chirayita, exhibited potent glucose-lowering effects across various rat models, with an ED50 of 23.1 mg/kg for a 40% reduction in blood glucose [175], as shown in Figure 6.
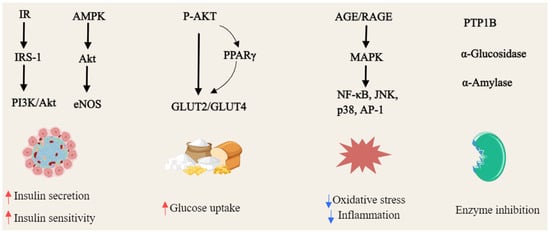
Figure 6.
General overview of the mechanism of xanthones against hyperglycemia (Red arrows = activate; blue arrows = inhibit).
6. Conclusions and Perspectives
In summary, edible plant-derived naturally occurring xanthones exhibit promising therapeutic potential in addressing various aspects of metabolic syndrome, including hypertension, hyperlipidemia, obesity, and hyperglycemia. Their pharmacological effects are mediated through diverse and interconnected molecular pathways including eNOS/NO/cGMP, AMPK/DGAT, PPARγ, AMPK/PKA/p38 MAPK, AMPK/Akt/eNOS, and insulin receptor-related signaling (IR/IRS-1/PI3K/Akt). These mechanisms underlie observed biological activities including vasodilation, anti-platelet aggregation, endothelial protection, modulation of lipid metabolism, enhancement of energy expenditure, improved insulin secretion and sensitivity, and facilitation of glucose uptake and metabolism. The antioxidative and anti-inflammatory properties of xanthones may also contribute to mitigating oxidative stress and inflammation associated with metabolic dysregulation. Structural characteristics, including hydroxylation patterns and prenylation, significantly influence their efficacy in regulating vascular tone, inhibiting key enzymes (such as fatty acid synthase, protein tyrosine phosphatase 1B, and carbohydrate-digesting enzymes), and activating cellular protective mechanisms. Among the xanthones reviewed, compounds such as α-mangostin and mangiferin have been most extensively studied, demonstrating consistent effects in both in vitro and in vivo models, as well as limited clinical investigations. These studies suggest potential benefits on endothelial function, lipid accumulation, insulin resistance, and diabetic complications.
Recent advances in delivery systems, including nanotechnology-based approaches, offer possible strategies to improve the bioavailability and pharmacokinetic profiles of xanthones, which could enhance their therapeutic utility. However, further research is needed to clarify their safety, efficacy, and mechanisms at molecular and systemic levels. Integrative approaches such as multi-omics and network pharmacology may help dissect the complex interactions underlying their activity. Moreover, the integration of emerging artificial intelligence (AI) and machine learning techniques offers a powerful opportunity to advance the identification of novel molecular targets, predict compound–target interactions, and elucidate complex mechanisms of action. AI-driven computational modeling and virtual screening can accelerate structure–activity relationship analyses and facilitate the design of optimized xanthone derivatives with improved efficacy and selectivity. Combining AI approaches with experimental validation presents a promising strategy to overcome traditional bottlenecks in drug discovery and to enhance the precision of translational research on bioactive natural products. Additionally, optimizing structure–activity relationships and formulation technologies could support the development of more potent and selective derivatives. Critically, well-designed clinical trials are essential to substantiate the therapeutic potential of xanthones in metabolic syndrome management, including determination of effective dosing, safety profiles, and possible drug interactions. Examining potential synergistic effects with established treatments and lifestyle interventions might also provide more comprehensive management strategies.
In conclusion, substantial evidence underscores the potential of naturally derived xanthones as versatile agents capable of modulating key pathophysiological pathways in metabolic syndrome. Their incorporation into future therapeutic strategies holds promise for advancing the clinical management of this multifaceted condition. However, realizing this potential requires sustained multidisciplinary research to bridge experimental findings with clinical translation, ultimately enabling the development of safe, effective, and accessible xanthone-based interventions for metabolic health improvement.
Author Contributions
Conceptualization, D.S., A.J. and S.-X.Q.; methodology, S.C.; software, Y.W. and H.L.; validation, A.J., D.S. and S.-X.Q.; formal analysis, F.L.; investigation, B.L.; data curation, H.L.; resources, J.L.; writing—original draft preparation, D.S. and A.J.; writing—review and editing, D.S., A.J. and S.-X.Q.; visualization, J.L. and L.Z.; funding acquisition, D.S. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Key Research and Development Program in the Xinjiang Uygur Autonomous Region (No. 2023B02028-2), and Tianchi Talent Project in Xinjiang Uygur Autonomous Region (No. 51052300521). Science and Technology Projects in Guangzhou (No. 2024E04J0037), National Natural Science Foundation of China (No. 82073733), Innovation Leading Team Project of Guangzhou City (No. 202009020004).
Data Availability Statement
The data supporting this review are derived from previously reported studies and data sets, which have been cited. The processed data are available from the first author upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Abbreviations
The following abbreviations are used in this manuscript:
| ADMA | Asymmetric dimethylarginine |
| ADP | Adenosine diphosphate |
| AGEs | Advanced glycation end-products |
| Ang II | Angiotensin II |
| cGMP | Cyclic guanosine monophosphate |
| DDAH | Dimethylarginine dimethylaminohydrolase |
| DGAT | Diacylglycerol acyltransferase |
| DGAT-2 | Diacylglycerol acyltransferase |
| EMT | Epithelial-to-mesenchymal transition |
| eNOS | Endothelial Nitric Oxide Synthase |
| ER | Endoplasmic reticulum |
| FAS | Fatty acid synthase |
| FFA | Free fatty acids |
| GLUT4 | Glucose transporter 4 |
| HFD | High fat diet |
| hMSCs | Human mesenchymal stem cells |
| HUVEC | Human umbilical vein endothelial cells |
| LPC | Lysophosphatidylcholine |
| MetS | Metabolic syndrome |
| NAFLD | Non-alcoholic fatty liver disease |
| NEFA | Non-esterified fatty acid |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ox-LDL | Oxidized low-density lipoprotein |
| PDGF | Platelet-derived growth factor |
| PL | Pancreatic lipase |
| PTEN | Phosphatase and tension homologue |
| PTP1B | Human Protein Tyrosine Phosphatase 1B |
| ROS | Reactive oxygen species |
| TG | Triglycerides |
| TGF-β | Transforming growth factor-β |
| VSMC | Vascular smooth muscle cell |
| VASH | Vasohibin |
| 5-HT | 5-Hydroxytryptamine |
References
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K.B. Chapter 6—Epidemiology of metabolic syndrome: Global scenario. In Metabolic Syndrome; Mukhopadhyay, S., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 59–71. [Google Scholar]
- El-Seedi, R.H.; Ibrahim, M.S.H.; Yosri, N.; Ibrahim, A.A.M.; Hegazy, F.M.-E.; Setzer, N.W.; Guo, Z.; Zou, X.; Refaey, S.M.; Salem, E.S.; et al. Naturally Occurring Xanthones; Biological Activities, Chemical Profiles and In Silico Drug Discovery. Curr. Med. Chem. 2024, 31, 62–101. [Google Scholar] [CrossRef]
- Tousian Shandiz, H.; Razavi, B.M.; Hosseinzadeh, H. Review of Garcinia mangostana and its Xanthones in Metabolic Syndrome and Related Complications. Phytother. Res. 2017, 31, 1173–1182. [Google Scholar] [CrossRef]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Gunter, N.V.; Teh, S.S.; Jantan, I.; Law, K.P.; Morita, H.; Mah, S.H. Natural xanthones as modulators of the Nrf2/ARE signaling pathway and potential gastroprotective agents. Phytother. Res. 2025, 39, 1721–1734. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Xiang, G.L.; Guo, S.; Xing, N.; Du, Q.Y.; Qin, J.; Gao, H.M.; Zhang, Y.; Wang, S.H. Mangiferin, a potential supplement to improve metabolic syndrome: Current status and future opportunities. Am. J. Chin. Med. 2024, 52, 355–386. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Timraz, N.Z.; Ibrahim, S.R.M.; El-Halawany, A.M.; Malebari, A.M.; Shehata, I.A.; El-Bassossy, H.M. Nitric-oxide-mediated vasodilation of bioactive compounds isolated from Hypericum revolutum in rat aorta. Biology 2021, 10, 541. [Google Scholar] [CrossRef]
- Timraz, N.; El-Bassossy, H.; Ibrahim, S.; El-Halawany, A.; Shehata, I.A.; Aljohani, O.; Abdallah, H. Vasodilating effect of Hypericum revolutum (Vahl) (Clusiaceae) methanol extract in rats. Trop. J. Pharm. Res. 2021, 20, 1003–1007. [Google Scholar] [CrossRef]
- Capettini, L.S.; Campos, L.V.; Dos Santos, M.H.; Nagem, T.J.; Lemos, V.S.; Cortes, S.F. Vasodilator and antioxidant effect of xanthones isolated from Brazilian medicinal plants. Planta Med. 2009, 75, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.F.; Pereira, A.C.; Capettini, L.S.; Santos, M.H.; Nagem, T.J.; Lemos, V.S.; Cortes, S.F. Mechanism of the vasodilator effect of mono-oxygenated xanthones: A structure-activity relationship study. Planta Med. 2013, 79, 1495–1500. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Kang, J.J. Mechanism of vasorelaxation of thoracic aorta caused by xanthone. Eur. J. Pharmacol. 1997, 336, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.-G.; Wang, M.-Z.; Che, C.-T.; Yeung, J.H.K. Mechanisms of the vasorelaxant effect of 1-hydroxy-2, 3, 5-trimethoxy-xanthone, isolated from a Tibetan herb, Halenia elliptica, on rat coronary artery. Life Sci. 2007, 81, 1016–1023. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.-G.; Wang, M.-Z.; Che, C.-T.; Yeung, J.H.K. Mechanisms of the vasorelaxant effect of 1, 5-dihydroxy-2, 3-dimethoxy-xanthone, an active metabolite of 1-hydroxy-2, 3, 5-trimethoxy-xanthone isolated from a Tibetan herb, Halenia elliptica, on rat coronary artery. Life Sci. 2008, 82, 91–98. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.-G.; Wang, M.-Z.; Che, C.-T.; Yeung, J.H.K. Vasodilatory actions of xanthones isolated from a Tibetan herb, Halenia elliptica. Phytomedicine 2009, 16, 1144–1150. [Google Scholar] [CrossRef]
- Câmara, D.V.; Lemos, V.S.; Santos, M.H.; Nagem, T.J.; Cortes, S.F. Mechanism of the vasodilator effect of Euxanthone in rat small mesenteric arteries. Phytomedicine 2010, 17, 690–692. [Google Scholar] [CrossRef]
- Fang, L.H.; Mu, Y.M.; Lin, L.L.; Xiao, P.G.; Du, G.H. Vasorelaxant effect of euxanthone in the rat thoracic aorta. Vasc. Pharmacol. 2006, 45, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Chericoni, S.; Testai, L.; Calderone, V.; Flamini, G.; Nieri, P.; Morelli, I.; Martinotti, E. The xanthones gentiacaulein and gentiakochianin are responsible for the vasodilator action of the roots of Gentiana kochiana. Planta Med. 2003, 69, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Mariano, L.N.B.; da Silva, R.d.C.V.; Niero, R.; Cechinel Filho, V.; da Silva-Santos, J.E.; de Souza, P. Vasodilation and Blood Pressure-Lowering Effect of 3-Demethyl-2-Geranyl-4-Prenylbellidifoline, a Xanthone Obtained from Garcinia achachairu, in Hypertensive Rats. Plants 2024, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Huang, F.; Chen, S.B.; Yang, D.J.; Chen, S.L.; Yang, J.S.; Xiao, P.G. Xanthones from the roots of Polygala caudata and their antioxidation and vasodilatation activities in vitro. Planta Med. 2005, 71, 372–375. [Google Scholar] [CrossRef]
- Jiang, M.; Huang, S.; Duan, W.; Liu, Q.; Lei, M. Alpha-mangostin improves endothelial dysfunction in db/db mice through inhibition of aSMase/ceramide pathway. J. Cell. Mol. Med. 2021, 25, 3601–3609. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, J.; Gao, L.; Lin, H.; Yang, Z.; Liu, X.; Niu, Y. α-Mangostin reduces hypertension in spontaneously hypertensive rats and inhibits EMT and fibrosis in Ang II-induced HK-2 cells. Int. J. Med. Sci. 2024, 21, 1681–1688. [Google Scholar] [CrossRef]
- Tep-Areenan, P.; Suksamrarn, S. Mechanisms of vasorelaxation to gamma-mangostin in the rat aorta. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2012, 95 (Suppl. S12), S63–S68. [Google Scholar]
- Shin, J.-H.; Irfan, M.; Rhee, M.H.; Kwon, H.-W. Antiplatelet effect of cudraxanthone B is related to inhibition of calcium mobilization, αIIbβ3 activation, and clot retraction. Appl. Biol. Chem. 2021, 64, 4. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C. Benzophenones and xanthones from Garcinia cantleyana var. cantleyana and their inhibitory activities on human low-density lipoprotein oxidation and platelet aggregation. Phytochemistry 2012, 80, 58–63. [Google Scholar] [CrossRef]
- Liu, Y.; Park, J.-M.; Chang, K.-H.; Chin, Y.-W.; Lee, M.-Y. α- and γ-mangostin cause shape changes, inhibit aggregation and induce cytolysis of rat platelets. Chem.-Biol. Interact. 2015, 240, 240–248. [Google Scholar] [CrossRef]
- Chairungsrilerd, N.; Furukawa, K.; Ohta, T.; Nozoe, S.; Ohizumi, Y. γ-Mangostin, a novel type of 5-hydroxytryptamine 2A receptor antagonist. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998, 357, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Mohd Yasin, Y.H.; Jalil, J.; Murad, S.; Idris, M.S. Antiplatelet aggregation activity of compounds isolated from Guttiferae species in human whole blood. Pharm. Biol. 2009, 47, 1090–1095. [Google Scholar] [CrossRef]
- Yoo, H.; Ku, S.K.; Lee, W.; Kwak, S.; Baek, Y.D.; Min, B.W.; Jeong, G.S.; Bae, J.S. Antiplatelet, anticoagulant, and profibrinolytic activities of cudratricusxanthone A. Arch. Pharmacal Res. 2014, 37, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Saputri, F.C.; Jantan, I. Inhibitory Activities of Compounds from the Twigs of Garcinia hombroniana Pierre on Human Low-density Lipoprotein (LDL) Oxidation and Platelet Aggregation. Phytother. Res. 2012, 26, 1845–1850. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Mangostanaxanthones III and IV: Advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. J. Nat. Med. 2017, 71, 216–226. [Google Scholar] [CrossRef]
- Luo, Y.; Lei, M. α-Mangostin protects against high-glucose induced apoptosis of human umbilical vein endothelial cells. Biosci. Rep. 2017, 37, BSR20170779. [Google Scholar] [CrossRef]
- Jariyapongskul, A.; Areebambud, C.; Suksamrarn, S.; Mekseepralard, C. a-Mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. BioMed Res. Int. 2015, 2015, 785826. [Google Scholar] [CrossRef]
- Sampath, P.D.; Kannan, V. Mitigation of mitochondrial dysfunction and regulation of eNOS expression during experimental myocardial necrosis by alpha-mangostin, a xanthonic derivative from Garcinia mangostana. Drug Chem. Toxicol. 2009, 32, 344–352. [Google Scholar] [CrossRef]
- Eisvand, F.; Rezvani, K.; Hosseinzadeh, H.; Razavi, B.M. Alpha-mangostin decreases high glucose-induced damage on human umbilical vein endothelial cells by increasing autophagic protein expression. Iran. J. Basic Med. Sci. 2024, 27, 90–96. [Google Scholar] [CrossRef]
- Schmieder, A.; Schwaiger, S.; Csordas, A.; Backovic, A.; Messner, B.; Wick, G.; Stuppner, H.; Bernhard, D. Isogentisin—A novel compound for the prevention of smoking-caused endothelial injury. Atherosclerosis 2007, 194, 317–325. [Google Scholar] [CrossRef]
- Waltenberger, B.; Liu, R.; Atanasov, A.G.; Schwaiger, S.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. Nonprenylated Xanthones from Gentiana lutea, Frasera caroliniensis, and Centaurium erythraea as Novel Inhibitors of Vascular Smooth Muscle Cell Proliferation. Molecules 2015, 20, 20381–20390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Ye, C.; Lin, Y.; Cheang, T.-Y.; Wang, M.; Zhang, H.; Wang, S.; Zhang, L.; Wang, S. Gambogic Acid Induces G0/G1 Cell Cycle Arrest and Cell Migration Inhibition Via Suppressing PDGF Receptor β Tyrosine Phosphorylation and Rac1 Activity in Rat Aortic Smooth Muscle Cells. J. Atheroscler. Thromb. 2010, 17, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, G.; Ji, Y.; Zhua, H.; Lv, C.; Jiang, W. Protective role of gambogic acid in experimental pulmonary fibrosis in vitro and in vivo. Phytomedicine 2016, 23, 350–358. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Liu, B.; Huang, F.; Li, Y. Exosomes derived from mangiferin-stimulated perivascular adipose tissue ameliorate endothelial dysfunction. Mol. Med. Rep. 2019, 19, 4797–4805. [Google Scholar] [CrossRef]
- Ismail, M.B.; Rajendran, P.; AbuZahra, H.M.; Veeraraghavan, V.P. Mangiferin Inhibits Apoptosis in Doxorubicin-Induced Vascular Endothelial Cells via the Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4259. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metab. Clin. Exp. 2015, 64, 428–437. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, D.-L.; Jia, M.; Hao, W.-H.; Li, Y.-J. Mangiferin inhibits high-fat diet induced vascular injury via regulation of PTEN/AKT/eNOS pathway. J. Pharmacol. Sci. 2018, 137, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, R.; Ramkumar, K.M. Mangiferin alleviates hyperglycemia-induced endothelial impairment via Nrf2 signaling pathway. Eur. J. Pharmacol. 2022, 936, 175359. [Google Scholar] [CrossRef]
- Yang, H.; Bai, W.; Gao, L.; Jiang, J.; Tang, Y.; Niu, Y.; Lin, H.; Li, L. Mangiferin alleviates hypertension induced by hyperuricemia via increasing nitric oxide releases. J. Pharmacol. Sci. 2018, 137, 154–161. [Google Scholar] [CrossRef]
- Jiang, D.-J.; Jiang, J.-L.; Tan, G.-S.; Huang, Z.-Z.; Deng, H.-W.; Li, Y.-J. Demethylbellidifolin Inhibits Adhesion of Monocytes to Endothelial Cells via Reduction of Tumor Necrosis Factor alpha and Endogenous Nitric Oxide Synthase Inhibitor Level. Planta Med. 2003, 69, 1150–1152. [Google Scholar] [CrossRef]
- Jiang, D.J.; Jiang, J.L.; Zhu, H.Q.; Tan, G.S.; Liu, S.Q.; Xu, K.P.; Li, Y.J. Demethylbellidifolin preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. J. Ethnopharmacol. 2004, 93, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.J.; Jiang, J.L.; Tan, G.S.; Du, Y.H.; Xu, K.P.; Li, Y.J. Protective effects of daviditin A against endothelial damage induced by lysophosphatidylcholine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 367, 600–606. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Boeing, T.; da Silva, R.d.C.M.V.d.A.F.; Cechinel-Filho, V.; Niero, R.; da Silva, L.M.; de Souza, P.; de Andrade, S.F. 1,3,5,6-Tetrahydroxyxanthone, a natural xanthone, induces diuresis and saluresis in normotensive and hypertensive rats. Chem. Biol. Interact. 2019, 311, 108778. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Boeing, T.; Cechinel Filho, V.; Niero, R.; Mota da Silva, L.; de Souza, P. 1,3,5,6-tetrahydroxyxanthone promotes diuresis, renal protection and antiurolithic properties in normotensive and hypertensive rats. J. Pharm. Pharmacol. 2021, 73, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Bolda Mariano, L.N.; Boeing, T.; Cechinel-Filho, V.; Niero, R.; Mota da Silva, L.; de Souza, P. The acute diuretic effects with low-doses of natural prenylated xanthones in rats. Eur. J. Pharmacol. 2020, 884, 173432. [Google Scholar] [CrossRef] [PubMed]
- Mariano, L.N.B.; Boeing, T.; Filho, V.C.; Niero, R.; da Silva, L.M.; de Souza, P. 3-Demethyl-2-geranyl-4-prenylbellidifoline, a natural xanthone with diuretic and kidney protective properties. J. Pharm. Pharmacol. 2024, 76, 106–114. [Google Scholar] [CrossRef]
- Ren, K.; Li, H.; Zhou, H.F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.L.; Zhao, G.J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging 2019, 11, 10992–11009. [Google Scholar] [CrossRef]
- Niu, Y.; Li, S.; Na, L.; Feng, R.; Liu, L.; Li, Y.; Sun, C. Mangiferin Decreases Plasma Free Fatty Acids through Promoting Its Catabolism in Liver by Activation of AMPK. PLoS ONE 2012, 7, e30782. [Google Scholar] [CrossRef]
- Yong, Z.; Ruiqi, W.; Hongji, Y.; Ning, M.; Chenzuo, J.; Yu, Z.; Zhixuan, X.; Qiang, L.; Qibing, L.; Weiying, L.; et al. Mangiferin Ameliorates HFD-Induced NAFLD through Regulation of the AMPK and NLRP3 Inflammasome Signal Pathways. J. Immunol. Res. 2021, 2021, 4084566. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.-Y.; Wang, L.; Teng, T.; Zhou, M.; Wang, S.-G.; Tian, Y.-Z.; Du, L.; Yin, X.-X.; Sun, Y. Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed. Pharmacother. 2017, 96, 328–335. [Google Scholar] [CrossRef]
- Guo, F.; Huang, C.; Liao, X.; Wang, Y.; He, Y.; Feng, R.; Li, Y.; Sun, C. Beneficial effects of mangiferin on hyperlipidemia in high-fat-fed hamsters. Mol. Nutr. Food Res. 2011, 55, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Iwamoto, N.; Kato, M.; Ichiki, H.; Kubo, M.; Komatsu, Y.; Ishida, T.; Okada, M.; Tanigawa, K. The suppressive effect of mangiferin with exercise on blood lipids in type 2 diabetes. Biol. Pharm. Bull. 2001, 24, 1091–1092. [Google Scholar] [CrossRef]
- Xing, X.; Li, D.; Chen, D.; Zhou, L.; Chonan, R.; Yamahara, J.; Wang, J.; Li, Y. Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A:diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats: A link to amelioration of fatty liver. Toxicol. Appl. Pharmacol. 2014, 280, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Subash-Babu, P.; Alshatwi, A.A. Evaluation of Antiobesity Effect of Mangiferin in Adipogenesis-Induced Human Mesenchymal Stem Cells by Assessing Adipogenic Genes. J. Food Biochem. 2015, 39, 28–38. [Google Scholar] [CrossRef]
- Mahali, S.K.; Verma, N.; Manna, S.K. Advanced Glycation End Products Induce Lipogenesis: Regulation by Natural Xanthone through Inhibition of ERK and NF-κB. J. Cell. Physiol. 2014, 229, 1972–1980. [Google Scholar] [CrossRef]
- Apontes, P.; Liu, Z.; Su, K.; Benard, O.; Youn, D.Y.; Li, X.; Li, W.; Mirza, R.H.; Bastie, C.C.; Jelicks, L.A.; et al. Mangiferin Stimulates Carbohydrate Oxidation and Protects Against Metabolic Disorders Induced by High-Fat Diets. Diabetes 2014, 63, 3626–3636. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Han, N.; Liu, Z.; Xiao, B.; Yin, J. Beneficial effects of neomangiferin on high fat diet-induced nonalcoholic fatty liver disease in rats. Int. Immunopharmacol. 2015, 25, 218–228. [Google Scholar] [CrossRef]
- Sim, M.-O.; Lee, H.J.; Jeong, D.E.; Jang, J.-H.; Jung, H.-K.; Cho, H.-W. 6′-O-acetyl mangiferin from Iris rossii Baker inhibits lipid accumulation partly via AMPK activation in adipogenesis. Chem. Biol. Interact. 2019, 311, 108755. [Google Scholar] [CrossRef]
- Shibata, M.-A.; Harada-Shiba, M.; Shibata, E.; Tosa, H.; Matoba, Y.; Hamaoka, H.; Iinuma, M.; Kondo, Y. Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism. Int. J. Mol. Sci. 2019, 20, 1722. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Chung, P.-C.; Owaga, E.E.; Tsai, I.J.; Wang, P.-Y.; Tsai, J.-I.; Yeh, T.-S.; Hsieh, R.-H. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab. 2016, 13, 88. [Google Scholar] [CrossRef]
- Williams, P.; Ongsakul, M.; Proudfoot, J.; Croft, K.; Beilin, L. Mangostin Inhibits the Oxidative Modification of Human Low Density Lipoprotein. Free Radic. Res. 1995, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.M.; Huh, J.H.; Lee, E.S.; Kwon, M.H.; Lee, B.R.; Ko, H.-J.; Chung, C.H. α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS ONE 2017, 12, e0179204. [Google Scholar] [CrossRef]
- Choi, Y.H.; Bae, J.K.; Chae, H.-S.; Kim, Y.-M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.-W. α-Mangostin Regulates Hepatic Steatosis and Obesity through SirT1-AMPK and PPARγ Pathways in High-Fat Diet-Induced Obese Mice. J. Agric. Food. Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef] [PubMed]
- John, O.D.; Mouatt, P.; Panchal, S.K.; Brown, L. Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats. Nutrients 2021, 13, 319. [Google Scholar] [CrossRef]
- Shen, Q.; Chitchumroonchokchai, C.; Thomas, J.L.; Gushchina, L.V.; DiSilvestro, D.; Failla, M.L.; Ziouzenkova, O. Adipocyte reporter assays: Application for identification of anti-inflammatory and antioxidant properties of mangosteen xanthones. Mol. Nutr. Food Res. 2014, 58, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-S.; Kim, E.-Y.; Han, L.; Kim, N.-R.; Lam, B.; Paik, J.H.; Yoon, K.D.; Choi, Y.H.; Chin, Y.-W. Xanthones with pancreatic lipase inhibitory activity from the pericarps of Garcinia mangostana L. (Guttiferae). Eur. J. Lipid Sci. Technol. 2016, 118, 1416–1421. [Google Scholar] [CrossRef]
- Xiao, H.-B.; Sun, Z.-L.; Lu, X.-Y.; Li, D.-Z.; Xu, J.-P.; Hu, Y.-P. Beneficial effect of 3,4,5,6-tetrahydroxyxanthone on dyslipidemia in apolipoprotein E-deficient mice. Can. J. Physiol. Pharmacol. 2008, 86, 815–826. [Google Scholar] [CrossRef]
- Xiao, H.-B.; Lu, X.-Y.; Li, Y.-J.; Xu, J.-P.; Chao-Tan; Sun, Z.-L. Effect of 3,4,5,6-tetrahydroxyxanthone on erythrocyte deformability in apolipoprotein E-deficient mice. J. Asian Nat. Prod. Res. 2009, 11, 643–651. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Xu, Y.; Feng, T.; Jiang, W.; Li, Z.; Hong, B.; Xie, Z.; Si, S. IMB2026791, a Xanthone, Stimulates Cholesterol Efflux by Increasing the Binding of Apolipoprotein A-I to ATP-Binding Cassette Transporter A1. Molecules 2012, 17, 2833–2854. [Google Scholar] [CrossRef]
- Zhou, Y.; Kim, J.T.; Qiu, S.; Lee, S.B.; Park, H.J.; Soon, M.J.; Lee, H.J. 1,3,5,8-Tetrahydroxyxanthone suppressed adipogenesis via activating Hedgehog signaling in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2022, 31, 1473–1480. [Google Scholar] [CrossRef]
- Jumai, A.; Chen, S.; Wu, Y.; Liu, F.; Li, B.; Zhu, B.; Zhao, L.; Liu, K.; Zhang, Q.; Qiu, S.-X. Bellidifolin, a constituent from edible Mongolic Liver Tea (Swertia diluta), promotes lipid metabolism by regulating intestinal microbiota and bile acid metabolism in mice during high fat diet-induced obesity. Food Biosci. 2025, 68, 106562. [Google Scholar] [CrossRef]
- Na, L.; Zhang, Q.; Jiang, S.; Du, S.; Zhang, W.; Li, Y.; Sun, C.; Niu, Y. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: A double-blind randomized controlled trial. Sci. Rep. 2015, 5, 10344. [Google Scholar] [CrossRef]
- Lim, J.; Liu, Z.; Apontes, P.; Feng, D.; Pessin, J.E.; Sauve, A.A.; Angeletti, R.H.; Chi, Y. Dual Mode Action of Mangiferin in Mouse Liver under High Fat Diet. PLoS ONE 2014, 9, e90137. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, D.; Gao, X.; Wu, H. Inhibitory effects of garcinone E on fatty acid synthase. RSC Adv. 2018, 8, 8112–8117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Z.; Quan, X.F.; Tian, W.X.; Hu, J.M.; Wang, P.C.; Huang, S.Z.; Cheng, Z.Q.; Liang, W.J.; Zhou, J.; Ma, X.F.; et al. Fatty acid synthase inhibitors of phenolic constituents isolated from Garcinia mangostana. Bioorg. Med. Chem. Lett. 2010, 20, 6045–6047. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-B.; Moon, D.-O.; Oh, E.S.; Song, Y.N.; Park, J.-Y.; Ryu, H.W.; Kim, D.-Y.; Chin, Y.-W.; Lee, H.-S.; Lee, S.U.; et al. Garcinia mangostana Suppresses Triacylglycerol Synthesis in Hepatocytes and Enterocytes. J. Med. Food 2023, 26, 732–738. [Google Scholar] [CrossRef]
- Quan, X.; Wang, Y.; Ma, X.; Liang, Y.; Tian, W.; Ma, Q.; Jiang, H.; Zhao, Y. α-Mangostin Induces Apoptosis and Suppresses Differentiation of 3T3-L1 Cells via Inhibiting Fatty Acid Synthase. PLoS ONE 2012, 7, e33376. [Google Scholar] [CrossRef]
- Song, M.-C.; Nigussie, F.; Jeong, T.-S.; Lee, C.-Y.; Regassa, F.; Markos, T.; Baek, N.-I. Phenolic Compounds from the Roots of Lindera fruticosa. J. Nat. Prod. 2006, 69, 853–855. [Google Scholar] [CrossRef]
- Kim, T.-J.; Han, H.-J.; Hong, S.-S.; Hwang, J.-H.; Hwang, B.-Y.; Yoo, H.-S.; Jin, Y.-R.; Lee, J.-J.; Yu, J.-Y.; Lee, K.-H.; et al. Cudratricusxanthone A isolated from the root bark of Cudrania tricuspidata inhibits the proliferation of vascular smooth muscle cells through the suppression of PDGF-receptor beta tyrosine kinase. Biol. Pharm. Bull. 2007, 30, 805–809. [Google Scholar] [CrossRef]
- Ferchichi, L.; Derbré, S.; Mahmood, K.; Touré, K.; Guilet, D.; Litaudon, M.; Awang, K.; Hadi, A.H.A.; Le Ray, A.M.; Richomme, P. Bioguided fractionation and isolation of natural inhibitors of advanced glycation end-products (AGEs) from Calophyllum flavoramulum. Phytochemistry 2012, 78, 98–106. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Ma, K.Y.; Chen, Z.-Y. Mangiferin alleviates trimethylamine-N-oxide (TMAO)-induced atherogenesis and modulates gut microbiota in mice. Food Funct. 2023, 14, 9212–9225. [Google Scholar] [CrossRef]
- Zhao, B.; Shen, H.; Zhang, L.; Shen, Y. Gambogic acid activates AMP-activated protein kinase in mammalian cells. Biochem. Biophys. Res. Commun. 2012, 424, 100–104. [Google Scholar] [CrossRef]
- Ardakanian, A.; Ghasemzadeh Rahbardar, M.; Omidkhoda, F.; Razavi, B.M.; Hosseinzadeh, H. Effect of alpha-mangostin on olanzapine-induced metabolic disorders in rats. Iran. J. Basic Med. Sci. 2022, 25, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kim, Y.-S. Mangiferin induces the expression of a thermogenic signature via AMPK signaling during brown-adipocyte differentiation. Food Chem. Toxicol. 2020, 141, 111415. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kim, Y.-S. PINK1–PRKN mitophagy suppression by mangiferin promotes a brown-fat-phenotype via PKA-p38 MAPK signalling in murine C3H10T1/2 mesenchymal stem cells. Metab. Clin. Exp. 2020, 107, 154228. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Gamo, K.; Miyachi, H.; Iinuma, M.; Kawada, T.; Takahashi, N.; Akao, Y.; Tosa, H. γ-Mangostin from Garcinia Mangostana Pericarps as a Dual Agonist That Activates Both PPARα and PPARδ. Biosci. Biotechnol. Biochem. 2013, 77, 2430–2435. [Google Scholar] [CrossRef]
- Mekseepralard, C.; Areebambud, C.; Suksamrarn, S.; Jariyapongskul, A. Effects of Long-Term Alpha-mangostin Supplementation on Hyperglycemia and Insulin Resistance in Type 2 Diabetic Rats Induced by High Fat Diet and Low Dose Streptozotocin. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2015, 98, S23–S30. [Google Scholar]
- Lee, D.; Kim, Y.-M.; Jung, K.; Chin, Y.-W.; Kang, K.S. Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage. Int. J. Mol. Sci. 2018, 19, 1484. [Google Scholar] [CrossRef]
- Mahendran, G.; Manoj, M.; Murugesh, E.; Sathish Kumar, R.; Shanmughavel, P.; Rajendra Prasad, K.J.; Narmatha Bai, V. In vivo anti-diabetic, antioxidant and molecular docking studies of 1, 2, 8-trihydroxy-6-methoxy xanthone and 1, 2-dihydroxy-6-methoxyxanthone-8-O-β-d-xylopyranosyl isolated from Swertia corymbosa. Phytomedicine 2014, 21, 1237–1248. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, C.-Y.; Zhang, B.; Liu, Y.-D.; Lu, B.-M.; Shi, Z.; An, N.; Zhao, L.-K.; Zhang, J.-J.; Bao, J.-K.; et al. Mangiferin Facilitates Islet Regeneration and β-Cell Proliferation through Upregulation of Cell Cycle and β-Cell Regeneration Regulators. Int. J. Mol. Sci. 2014, 15, 9016–9035. [Google Scholar] [CrossRef]
- Bhargava, S.; Shah, M.B. Evaluation of efficacy of Bombax ceiba extract and its major constituent, mangiferin in streptozotocin (STZ)-induced diabetic rats. J. Complement. Integr. Med. 2021, 18, 311–318. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Arulselvan, P.; Muniappan, B.P.; Fakurazi, S.; Kandasamy, M. Mangiferin from Salacia chinensis Prevents Oxidative Stress and Protects Pancreatic β-Cells in Streptozotocin-Induced Diabetic Rats. J. Med. Food 2013, 16, 719–727. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, D.; Fan, K.; Yu, B.; Xiao, W.; Ma, J.; Jin, W.; Tan, Y.; Wu, J. Combination of Mangiferin and Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Improves Impaired Glucose Tolerance in Streptozotocin-Diabetic Rats. Pharmacology 2012, 90, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, X.; Lei, T.; Liu, Y.; Huai, G.; Sun, M.; Deng, S.; Yang, H.; Tong, R.; Wang, Y. Mangiferin induces islet regeneration in aged mice through regulating p16INK4a. Int. J. Mol. Med. 2018, 41, 3231–3242. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.M.; Bajpai, M.B.; Murthy, P.S.R.; Mukherjee, S.K. Mechanism of blood sugar lowering by a swerchirin-containing hexane fraction (SWI) of Swertia chirayita. Indian J. Exp. Biol. 1993, 31, 178–181. [Google Scholar] [PubMed]
- Zhou, L.; Pan, Y.; Chonan, R.; Batey, R.; Rong, X.; Yamahara, J.; Wang, J.; Li, Y. Mitigation of Insulin Resistance by Mangiferin in a Rat Model of Fructose-Induced Metabolic Syndrome Is Associated with Modulation of CD36 Redistribution in the Skeletal Muscle. J. Pharmacol. Exp. Ther. 2016, 356, 74–84. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Song, J.; Hou, F.; Ma, X.; Liu, B.; Huang, F. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur. J. Nutr. 2018, 57, 1563–1575. [Google Scholar] [CrossRef]
- Sha, H.; Zeng, H.; Zhao, J.; Jin, H. Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur. J. Pharmacol. 2019, 859, 172522. [Google Scholar] [CrossRef]
- Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kao, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; et al. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine 2001, 8, 85–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yuan, H.; Guan, H.; Li, Y.; Niu, Y. Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPARα: Mangiferin Improved Insulin Resistance. J. Diabetes Res. 2019, 2019, 2052675. [Google Scholar] [CrossRef]
- Yang, C.Q.; Xu, J.H.; Yan, D.D.; Liu, B.L.; Liu, K.; Huang, F. Mangiferin ameliorates insulin resistance by inhibiting inflammation and regulatiing adipokine expression in adipocytes under hypoxic condition. Chin. J. Nat. Med. 2017, 15, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pu, Z.; Gao, J.; Liu, C.; Xing, J.; Lang, W.; Chen, J.; Yuan, C.; Zhou, C. “Multiomics” Analyses Combined with Systems Pharmacology Reveal the Renoprotection of Mangiferin Monosodium Salt in Rats with Diabetic Nephropathy: Focus on Improvements in Renal Ferroptosis, Renal Inflammation, and Podocyte Insulin Resistance. J. Agric. Food. Chem. 2023, 71, 358–381. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, Y.; Tan, Y.; Zhang, X.; Wang, R.; Chen, D.; Wang, Z.; Zhong, X. Lipidomics of the erythrocyte membrane and network pharmacology to explore the mechanism of mangiferin from Anemarrhenae rhizoma in treating type 2 diabetes mellitus rats. J. Pharm. Biomed. Anal. 2023, 230, 115386. [Google Scholar] [CrossRef]
- Saleh, S.; El-Maraghy, N.; Reda, E.; Barakat, W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: Role of adiponectin and TNF-α. An. Acad. Bras. Cienc. 2014, 86, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Mani, S.; Malarvizhi, R.; Nithya, P.; Vasanthi, H.R. Antidiabetic effect of mangiferin in combination with oral hypoglycemic agents metformin and gliclazide. Phytomedicine 2019, 59, 152901. [Google Scholar] [CrossRef]
- Ichiki, H.; Miura, T.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Tanigawa, K.; Okada, M. New antidiabetic compounds, mangiferin and its glucoside. Biol. Pharm. Bull. 1998, 21, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chitchumroonchokchai, C.; Chuang, C.-C.; West, T.; Kennedy, A.; McIntosh, M. Xanthones from Mangosteen Prevent Lipopolysaccharide-Mediated Inflammation and Insulin Resistance in Primary Cultures of Human Adipocytes12. J. Nutr. 2009, 139, 1185–1191. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Kalpravidh, R.W.; Chuang, C.-C.; Overman, A.; Martinez, K.; Kennedy, A.; McIntosh, M. Xanthones from Mangosteen Inhibit Inflammation in Human Macrophages and in Human Adipocytes Exposed to Macrophage-Conditioned Media1,2. J. Nutr. 2010, 140, 842–847. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.Y.; Lu, X.Q.; Li, Z.Q.; Wang, C.M.; Leung, C.H.; Wang, Y.T.; Peng, C.; Lin, L.G. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging 2019, 11, 11084–11110. [Google Scholar] [CrossRef]
- Quang, T.H.; Ngan, N.T.; Yoon, C.-S.; Cho, K.-H.; Kang, D.G.; Lee, H.S.; Kim, Y.-C.; Oh, H. Protein Tyrosine Phosphatase 1B Inhibitors from the Roots of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef]
- Li, Z.P.; Song, Y.H.; Uddin, Z.; Wang, Y.; Park, K.H. Inhibition of protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase by xanthones from Cratoxylum cochinchinense, and their kinetic characterization. Bioorg. Med. Chem. 2018, 26, 737–746. [Google Scholar] [CrossRef]
- Li, Z.P.; Lee, H.-H.; Uddin, Z.; Song, Y.H.; Park, K.H. Caged xanthones displaying protein tyrosine phosphatase 1B (PTP1B) inhibition from Cratoxylum cochinchinense. Bioorg. Chem. 2018, 78, 39–45. [Google Scholar] [CrossRef]
- Tan, X.F.; Uddin, Z.; Park, C.; Song, Y.H.; Son, M.; Lee, K.W.; Park, K.H. Competitive protein tyrosine phosphatase 1B (PTP1B) inhibitors, prenylated caged xanthones from Garcinia hanburyi and their inhibitory mechanism. Bioorg. Med. Chem. 2017, 25, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Tian, S.-T.; Hu, X.; Xu, J.; Yang, G.-Z.; Wang, C.-Y. 12b-hydroxy-des-D-garcigerin A enhances glucose metabolism in insulin-resistant HepG2 cells via the IRS-1/PI3-K/Akt cell signaling pathway. J. Asian Nat. Prod. Res. 2016, 18, 1091–1100. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Sun, W.; Chen, X.; Wang, Y.; Sun, Y.; Lin, L. 1,3,6,7-Tetrahydroxy-8-prenylxanthone ameliorates inflammatory responses resulting from the paracrine interaction of adipocytes and macrophages. Br. J. Pharmacol. 2018, 175, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.Y.; Bai, X.; Chen, X.H.; Fang, J.B.; Liu, S.H.; Chen, J.C. Anti-diabetic effect of methylswertianin and bellidifolin from Swertia punicea Hemsl. and its potential mechanism. Phytomedicine 2010, 17, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Kadota, S.; Shimizu, M.; Takata, Y.; Kobayashi, M.; Namba, T. Bellidifolin Stimulates Glucose Uptake in Rat 1 Fibroblasts and Ameliorates Hyperglycemia in Streptozotocin (STZ)-Induced Diabetic Rats. Planta Med. 1995, 61, 402–405. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Luo, C.-T.; Chen, H.; Lin, J.-N.; Ye, C.-L.; Mao, S.-S.; Li, Y.-L. Xanthones from Swertia mussotii as Multitarget-Directed Antidiabetic Agents. ChemMedChem 2014, 9, 1374–1377. [Google Scholar] [CrossRef]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef]
- Phukhatmuen, P.; Raksat, A.; Laphookhieo, S.; Charoensup, R.; Duangyod, T.; Maneerat, W. Bioassay-guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon 2020, 6, e03625. [Google Scholar] [CrossRef]
- Charoensup, R.; Betangah, M.E.; Suthiphasilp, V.; Phukhatmuen, P.; Maneerat, T.; Duangyod, T.; Laphookhieo, S. Antidiabetic properties of garciniacowone L, a new xanthone with an unusual 5,5,8a-trimethyloctahydro-2H-1-benzopyran moiety, and other xanthones from the twig extract of Garcinia cowa Roxb. ex Choisy. J. King Saud Univ.-Sci. 2022, 34, 102201. [Google Scholar] [CrossRef]
- An, N.T.K.; Van Hien, N.; Thi Thuy, N.; Lan Phuong, D.; Gia Bach, H.; Tra, N.T.; Quang Tung, N.; Tham, P.T.; Tai, B.H.; Thu Thuy, T.T. Garcicowanones C-E, three new hydrated-geranylated xanthones from the roots of Garcinia cowa Roxb. ex Choisy, and their α-glucosidase inhibition activities. Nat. Prod. Res. 2023, 37, 3668–3676. [Google Scholar] [CrossRef]
- Miura, T.; Ichiki, H.; Iwamoto, N.; Kato, M.; Kubo, M.; Sasaki, H.; Okada, M.; Ishida, T.; Seino, Y.; Tanigawa, K. Antidiabetic activity of the rhizoma of anemarrhena asphodeloides and active components, mangiferin and its glucoside. Biol. Pharm. Bull. 2001, 24, 1009–1011. [Google Scholar] [CrossRef]
- Sekar, V.; Chakraborty, S.; Mani, S.; Sali, V.K.; Vasanthi, H.R. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot. 2019, 120, 129–134. [Google Scholar] [CrossRef]
- Sekar, V.; Mani, S.; Malarvizhi, R.; Barathidasan, R.; Vasanthi, H.R. Positive interaction of mangiferin with selected oral hypoglycemic drugs: A therapeutic strategy to alleviate diabetic nephropathy in experimental rats. Mol. Biol. Rep. 2020, 47, 4465–4475. [Google Scholar] [CrossRef] [PubMed]
- Phoboo, S.; Pinto, M.D.S.; Barbosa, A.C.L.; Sarkar, D.; Bhowmik, P.C.; Jha, P.K.; Shetty, K. Phenolic-Linked Biochemical Rationale for the Anti-Diabetic Properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytother. Res. 2013, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tokunaga, M.; Hirata, N.; Iwahashi, H.; Naruto, S.; Kubo, M. Studies on palauan medicinal herbs. I. Antidiabetic effect of Ongael, leaves of Phaleria cumingii (MEISN.) F. VILL. Nat. Med. 2004, 58, 278–283. [Google Scholar]
- Miller, N.; Malherbe, C.J.; Joubert, E. In vitro α-glucosidase inhibition by honeybush (Cyclopia genistoides) food ingredient extract—Potential for dose reduction of acarbose through synergism. Food Funct. 2020, 11, 6476–6486. [Google Scholar] [CrossRef]
- Zuo, J.; Ji, C.-L.; Xia, Y.; Li, X.; Chen, J.-W. Xanthones as α-glucosidase inhibitors from the antihyperglycemic extract of Securidaca inappendiculata. Pharm. Biol. 2014, 52, 898–903. [Google Scholar] [CrossRef]
- Jia, C.; Han, T.; Xu, J.; Li, S.; Sun, Y.; Li, D.; Li, Z.; Hua, H. A new biflavonoid and a new triterpene from the leaves of Garcinia paucinervis and their biological activities. J. Nat. Med. 2017, 71, 642–649. [Google Scholar] [CrossRef]
- Wang, Q.; Li, R.; Li, N.; Jia, Y.; Wang, Y.; Chen, Y.; Panichayupakaranant, P.; Chen, H. The antioxidant activities, inhibitory effects, kinetics, and mechanisms of artocarpin and α-mangostin on α-glucosidase and α-amylase. Int. J. Biol. Macromol. 2022, 213, 880–891. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lin, S.-R.; Chen, T.-H.; Ng, H.-S.; Yim, H.-S.; Leong, M.K.; Weng, C.-F. Mangosteen xanthone γ-mangostin exerts lowering blood glucose effect with potentiating insulin sensitivity through the mediation of AMPK/PPARγ. Biomed. Pharmacother. 2021, 144, 112333. [Google Scholar] [CrossRef] [PubMed]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget Action of Xanthones from Garcinia mangostana against α-Amylase, α-Glucosidase and Pancreatic Lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef]
- Wairata, J.; Sukandar, E.R.; Fadlan, A.; Purnomo, A.S.; Taher, M.; Ersam, T. Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines 2021, 9, 1380. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H.; Alshali, K.Z. Mangostanaxanthone VIIII, a new xanthone from Garcinia mangostana pericarps, α-amylase inhibitory activity, and molecular docking studies. Rev. Bras. Farmacogn. 2019, 29, 206–212. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Chang, A.K.; Li, Y.; Tao, X.; Liu, W.; Wang, Z.; Su, W.; Li, Z.; Liang, X. Screening and tissue distribution of protein tyrosine phosphatase 1B inhibitors in mice following oral administration of Garcinia mangostana L. ethanolic extract. Food Chem. 2021, 357, 129759. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. Garcixanthone D, a New Xanthone, and Other Xanthone Derivatives From Garcinia mangostana Pericarps: Their α-Amylase Inhibitory Potential and Molecular Docking Studies. Starch-Stärke 2019, 71, 1800354. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. α-Amylase inhibition of xanthones from Garcinia mangostana pericarps and their possible use for the treatment of diabetes with molecular docking studies. J. Food Biochem. 2019, 43, e12844. [Google Scholar] [CrossRef]
- Phukhatmuen, P.; Suthiphasilp, V.; Rujanapan, N.; Duangyod, T.; Maneerat, T.; Charoensup, R.; Laphookhieo, S. Xanthones from the latex and twig extracts of Garcinia nigrolineata Planch. ex T. Anderson (Clusiaceae) and their antidiabetic and cytotoxic activities. Nat. Prod. Res. 2023, 37, 702–712. [Google Scholar] [CrossRef]
- Trinh, B.T.D.; Quach, T.T.T.; Bui, D.N.; Staerk, D.; Nguyen, L.-H.D.; Jäger, A.K. Xanthones from the twigs of Garcinia oblongifolia and their antidiabetic activity. Fitoterapia 2017, 118, 126–131. [Google Scholar] [CrossRef]
- Bui, D.N.; Nguyen, L.T.T.; Nguyen, L.-T.T.; Ngo, N.T.N.; Tran, P.T.; Nguyen, H.T.; Dang, L.T.N.; Nguyen, L.-H.D.; Trinh, B.T.D. Two new antidiabetic xanthones from the twigs of Garcinia oblongifolia. Nat. Prod. Res. 2023, 37, 2541–2550. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Trinh, B.T.D.; Tran, T.B.; Nguyen, L.-T.T.; Jäger, A.K.; Nguyen, L.-H.D. Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 2017, 27, 3301–3304. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARγ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef]
- Girón, M.D.; Sevillano, N.; Salto, R.; Haidour, A.; Manzano, M.; Jiménez, M.L.; Rueda, R.; López-Pedrosa, J.M. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: Role of mangiferin. Clin. Nutr. 2009, 28, 565–574. [Google Scholar] [CrossRef]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Apontes, P.; Fomenko, E.V.; Chi, N.; Schuster, V.L.; Kurland, I.J.; Pessin, J.E.; Chi, Y. Mangiferin Accelerates Glycolysis and Enhances Mitochondrial Bioenergetics. Int. J. Mol. Sci. 2018, 19, 201. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Arulselvan, P.; Fakurazi, S.; Kandasamy, M. Beneficial effects of mangiferin isolated from Salacia chinensis on biochemical and hematological parameters in rats with streptozotocin-induced diabetes. Pak. J. Pharm. Sci. 2014, 27, 161–167. [Google Scholar] [PubMed]
- Wang, F.; Yan, J.; Niu, Y.; Li, Y.; Lin, H.; Liu, X.; Liu, J.; Li, L. Mangiferin and its aglycone, norathyriol, improve glucose metabolism by activation of AMP-activated protein kinase. Pharm. Biol. 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Taher, M.; Amiroudine, M.; Zakaria, T.; Susanti, D.; Ichwan, S.J.A.; Kaderi, M.A.; Ahmed, Q.U.; Zakaria, Z.A. α-Mangostin Improves Glucose Uptake and Inhibits Adipocytes Differentiation in 3T3-L1 Cells via PPARγ, GLUT4, and Leptin Expressions. Evid.-Based Complement. Altern. Med. 2015, 2015, 740238. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Chen, Y.; Fu, Y.; Shi, K.; Liu, L.; Liu, H.; Xiong, M.; Liu, Q.-H.; Yang, G.; et al. Depsidone and xanthones from Garcinia xanthochymus with hypoglycemic activity and the mechanism of promoting glucose uptake in L6 myotubes. Bioorg. Med. Chem. 2017, 25, 6605–6613. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, Z.; Ling, Y.; Luo, W. LncRNA-H19 acts as a ceRNA to regulate HE4 expression by sponging miR-140 in human umbilical vein endothelial cells under hyperglycemia with or without α-Mangostin. Biomed. Pharmacother. 2019, 118, 109256. [Google Scholar] [CrossRef]
- Karunanayake, E.H.; Sirimanne, S.R. Mangiferin from the root bark of Salacia reticulata. J. Ethnopharmacol. 1985, 13, 227–228. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, D.; Fung, G.; Deng, H.; Chen, L.; Liang, J.; Jiang, Y.; Hu, Y. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can. J. Physiol. Pharmacol. 2015, 94, 332–340. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Zhu, X.; Zhang, L.; Lu, Q.; Wang, J.-Y.; Zhang, F.; Guo, H.; Yin, J.-L.; Yin, X.-X. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2013, 721, 355–364. [Google Scholar] [CrossRef]
- Pokorski, M.; Poździk, M.; Mazzatenta, A. Antioxidant treatment for impaired hypoxic ventilatory responses in experimental diabetes in the rat. Respir. Physiol. Neurobiol. 2018, 255, 30–38. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.J.; Su, X.L.; Xu, T.H. Predicting the Targets of Mangiferin in the Treatment of Diabetes Mellitus on Network Pharmacology and Analysis of its Metabolites in vivo. Pharmacogn. Mag. 2022, 18, 1137–1145. [Google Scholar]
- Liu, Y.-W.; Zhu, X.; Yang, Q.-Q.; Lu, Q.; Wang, J.-Y.; Li, H.-P.; Wei, Y.-Q.; Yin, J.-L.; Yin, X.-X. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology 2013, 228, 585–594. [Google Scholar] [CrossRef]
- Li, X.; Cui, X.; Sun, X.; Li, X.; Zhu, Q.; Li, W. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, 893–899. [Google Scholar] [CrossRef]
- Muruganandan, S.; Gupta, S.; Kataria, M.; Lal, J.; Gupta, P.K. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 2002, 176, 165–173. [Google Scholar] [CrossRef]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin Attenuates Diabetic Nephropathy by Inhibiting Oxidative Stress Mediated Signaling Cascade, TNFα Related and Mitochondrial Dependent Apoptotic Pathways in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2014, 9, e107220. [Google Scholar] [CrossRef]
- Guan, W.; Liu, Y.; Liu, Y.; Wang, Q.; Ye, H.-L.; Cheng, Y.-G.; Kuang, H.-X.; Jiang, X.-C.; Yang, B.-Y. Proteomics Research on the Protective Effect of Mangiferin on H9C2 Cell Injury Induced by H2O2. Molecules 2019, 24, 1911. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Shimizu, M.; Namba, T. Bellidifolin: A Potent Hypoglycemic Agent in Streptozotocin (STZ)-Induced Diabetic Rats from Swertia japonica. Planta Med. 1994, 60, 507–511. [Google Scholar] [CrossRef]
- Bajpai, M.B.; Asthana, R.K.; Sharma, N.K.; Chatterjee, S.K.; Mukherjee, S.K. Hypoglycemic Effect of Swerchirin from the Hexane Fraction of Swertia chirayita. Planta Med. 1991, 57, 102–104. [Google Scholar] [CrossRef]
- Husen, S.A.; Winarni, D.; Salamun; Ansori, A.N.M.; Susilo, R.J.K.; Hayaza, S. Hepatoprotective Effect of Gamma-mangostin for Amelioration of Impaired Liver Structure and Function in Streptozotocin-induced Diabetic Mice. IOP Conf. Ser. Earth Environ. Sci. 2018, 217, 012031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).