Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Material and Processing Conditions
2.2. Reagents and Standards
2.3. Physicochemical Analysis on Fermentation Brines
2.4. Olives Composition
2.4.1. Sample Pre-Treatment
2.4.2. Moisture
2.4.3. Total Lipids
2.4.4. Tocopherols Content
2.4.5. HPLC Analysis of Polyphenols
2.4.6. Total Phenolic Content
2.4.7. Chloride Content
2.5. Starter Culture Origin and Preparation
2.6. Microbial Analysis
2.7. Texture Analyses
2.8. Consumer Testing
2.9. Statistical Analyses
3. Results and Discussion
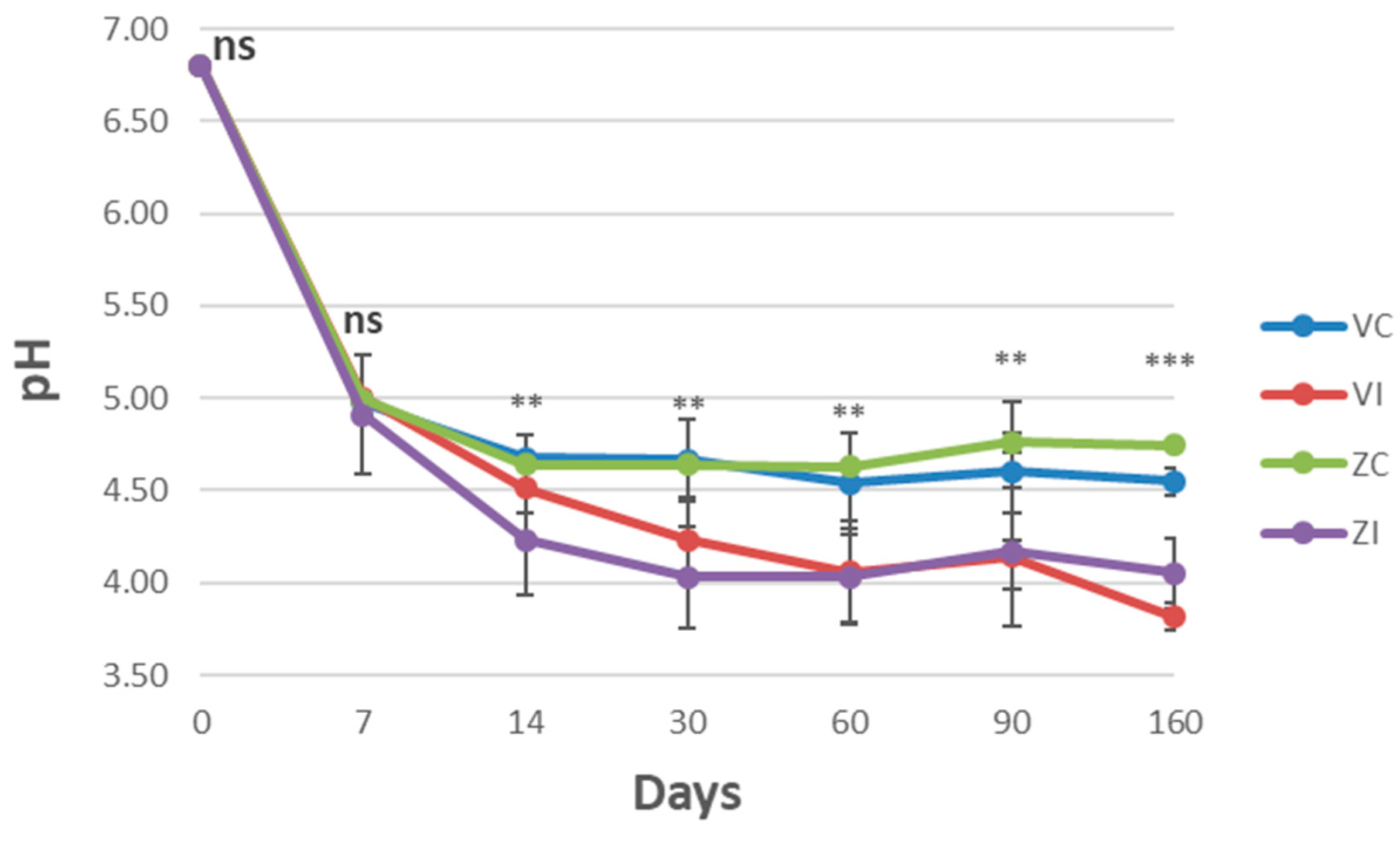
3.1. Chemical–Physical Characteristics
3.2. Olives Composition
3.3. Polyphenolic Profile
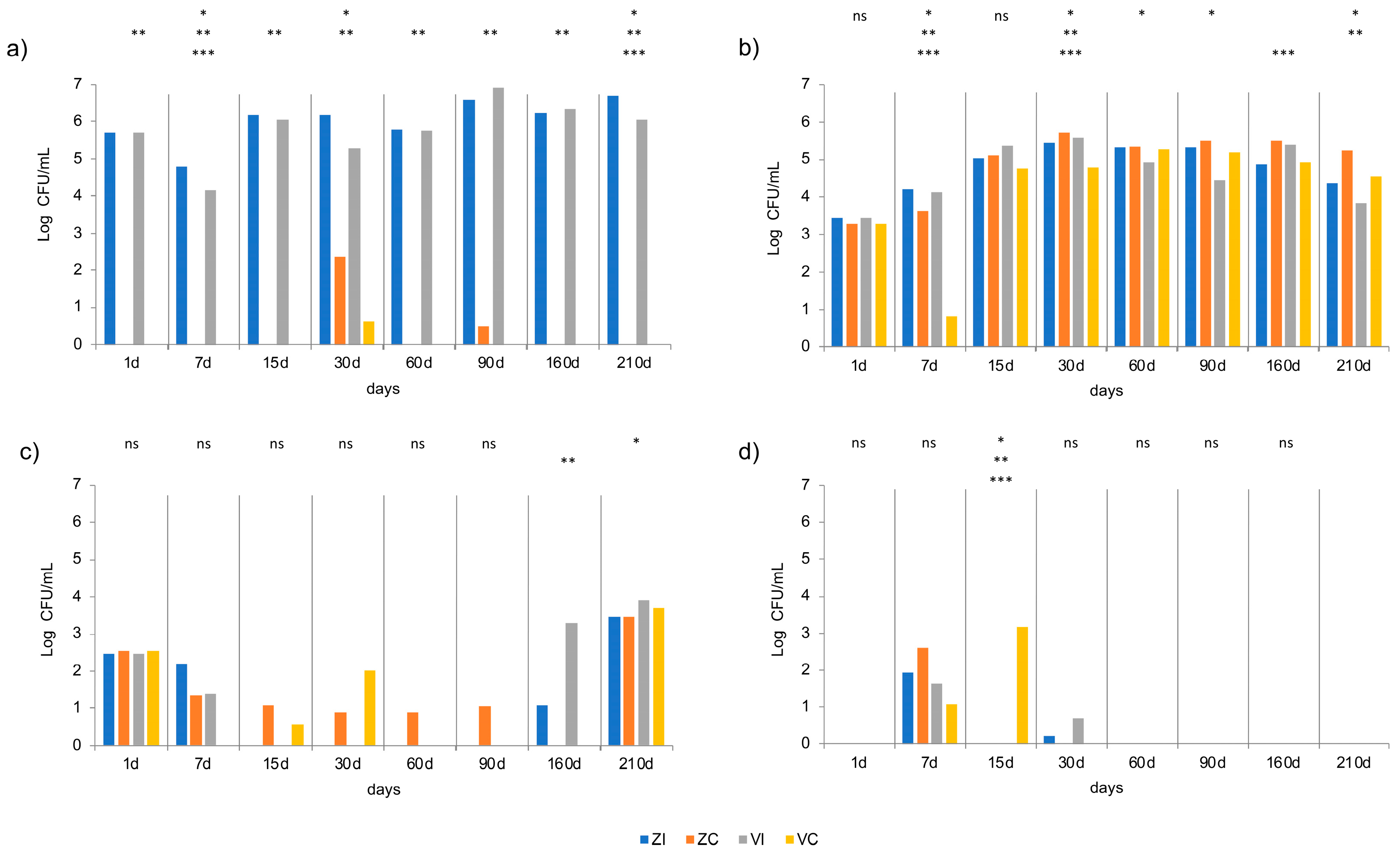
3.4. Microbial Analyses
3.5. Texture Analyses
3.6. Consumer Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and Multiple Inoculum of Lactiplantibacillus Plantarum Strains in Table Olive Lab-Scale Fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.J.E. Table Olive Fermentation Using Starter Cultures with Multifunctional Potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Tufariello, M.; Durante, M.; Grieco, F.; Ramires, F.A.; Mita, G.; Tasioula-Margari, M.; Logrieco, A.F. Physico-Chemical Characterization of Natural Fermentation Process of Conservolea and Kalamàta Table Olives and Developement of a Protocol for the Pre-Selection of Fermentation Starters. Food Microbiol. 2015, 46, 368–382. [Google Scholar] [CrossRef]
- Argyri, A.A.; Panagou, E.Z.; Tassou, C.C. Probiotics from the olive microbiota. In Probiotics, Prebiotics, and Synbiotics. Bioactive Foods in Health Promotion; Academic Press: Cambridge, MA, USA, 2016; pp. 371–389. [Google Scholar]
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiology 2013, 4, 143. [Google Scholar] [CrossRef]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of Starter Cultures to Table Olive Fermentation: An Overview on the Experimental Studies. Front. Microbiol. 2012, 3, 248. [Google Scholar] [CrossRef] [PubMed]
- Campus, M.; Sedda, P.; Cauli, E.; Piras, F.; Comunian, R.; Paba, A.; Daga, E.; Schirru, S.; Angioni, A.; Zurru, R.; et al. Evaluation of a Single Strain Starter Culture, a Selected Inoculum Enrichment, and Natural Microflora in the Processing of Tonda Di Cagliari Natural Table Olives: Impact on Chemical, Microbiological, Sensory and Texture Quality. LWT-Food Sci. Technol. 2015, 64, 671–677. [Google Scholar] [CrossRef]
- Campus, M.; Degirmencioglu, N.; Comunian, R. Technologies and Trends to Improve Table Olive Quality and Safety. Front. Microbiol 2018, 9, 617. [Google Scholar] [CrossRef]
- Comunian, R.; Ferrocino, I.; Paba, A.; Daga, E.; Campus, M.; Di Salvo Cauili, E.; Piras, F.; Zurru, R.; Cocolin, L. Evolution of microbiota during spontaneous and inoculated Tonda di Cagliari table olives fermentation and impact on sensory characteristics. LWT–Food Sci. Technol. 2017, 84, 64–72. [Google Scholar] [CrossRef]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Alam, M.K.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178. [Google Scholar] [CrossRef]
- Giavalisco, M.; Lavanga, E.; Ricciardi, A.; Zotta, T. Starter Cultures for the Production of Fermented Table Olives: Current Status and Future Perspectives. Fermentation 2024, 10, 351. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Cortés-Delgado, A.; Sánchez, A.H.; López-López, A.; Montaño, A. Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products. Fermentation 2024, 10, 439. [Google Scholar] [CrossRef]
- Erdemir Tiras, Z.S.; Kalkan Yildirim, H. Application of Mixed Starter Culture for Table Olive Production. Grasas Y Aceites 2021, 72, e405. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; Arias-Calderón, R.; Pérez-Nevado, F. Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives. Foods 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B. Abnormal Fermentations in Table-Olive Processing: Microbial Origin and Sensory Evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Campus, M.; Cauli, E.; Scano, E.; Piras, F.; Comunian, R.; Paba, A.; Daga, E.; Di Salvo, R.; Sedda, P.; Angioni, A.; et al. Towards Controlled Fermentation of Table Olives: LAB Starter Driven Process in an Automatic Pilot Processing Plant. Food Bioproc. Tech. 2017, 10, 1063–1073. [Google Scholar] [CrossRef]
- Sab, C.; Romero, C.; Brenes, M.; Montaño, A.; Ouelhadj, A.; Medina, E. Industrial Processing of Algerian Table Olive Cultivars Elaborated as Spanish Style. Front. Microbiol. 2021, 12, 729436. [Google Scholar] [CrossRef] [PubMed]
- Paba, A.; Chessa, L.; Daga, E.; Campus, M.; Bulla, M.; Angioni, A.; Sedda, P.; Comunian, R. Do Best-Selected Strains Perform Table Olive Fermentation Better than Undefined Biodiverse Starters? A Comparative Study. Foods 2020, 9, 135. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Classification of Textural Characteristics. J. Food Sci. 1963, 28, 385–389. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The Texturometer—A New Instrument for Objective Texture Measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food; Springer: New York, NY, USA, 2010. [Google Scholar]
- Fadda, C.; Del Caro, A.; Sanguinetti, A.M.; Piga, A. Texture and Antioxidant Evolution of Naturally Green Table Olives as Affected by Different Sodium Chloride Brine Concentrations. Grasas Y Aceites 2014, 65, e002. [Google Scholar] [CrossRef]
- Bok, F.; Moog, H.C.; Brendler, V. The Solubility of Oxygen in Water and Saline Solutions. Front. Nucl. Eng. 2023, 2, 1158109. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Pérez-Nevado, F.; Montaño, A.; Pleite, R.; Martín-Vertedor, D. The Effect of Olive Fruit Maturation in Spanish Style Fermentation with a Controlled Temperature. LWT–Food Sci. Technol. 2018, 91, 40–47. [Google Scholar] [CrossRef]
- Sakouhi, F.; Harrabi, S.; Absalon, C.; Sbei, K.; Boukhchina, S.; Kallel, H. α-Tocopherol and Fatty Acids Contents of Some Tunisian Table Olives (Olea europea L.): Changes in Their Composition during Ripening and Processing. Food Chem. 2008, 108, 833–839. [Google Scholar] [CrossRef]
- Hassapidou, M.N.; Balatsouras, G.D.; Manoukas, A.G. Effect of processing upon the tocopherol and tocotrienol composition of table olives. Food Chem. 1994, 50, 111–114. [Google Scholar] [CrossRef]
- Degirmencioglu, N. Modern Techniques in the Production of Table Olives. In Products from Olive Tree; IntechOpen: London, UK, 2016. [Google Scholar]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant Phenolic Compounds Loss during the Fermentation of Chétoui Olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Hamid abadi Sherahi, M.; Shahidi, F.; Yazdi, F.T.; Hashemi, S.M.B. Effect of Lactobacillus Plantarum on Olive and Olive Oil Quality during Fermentation Process. LWT–Food Science. Technol. 2018, 89, 572–580. [Google Scholar] [CrossRef]
- Kachouri, F.; Hamdi, M. Use Lactobacillus Plantarum in Olive Oil Process and Improvement of Phenolic Compounds Content. J. Food Eng. 2006, 77, 746–752. [Google Scholar] [CrossRef]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian Table Olives: Effect of Selected Microbial Starters on Polyphenols Composition, Antioxidant Activities and Bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Goulas, V.; Xenofontos, E.; Vouras, C.; Nikoloudakis, N.; Tsaltas, D. Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation. Foods 2020, 9, 17. [Google Scholar] [CrossRef]
- Kaltsa, A.; Papaliaga, D.; Papaioannou, E.; Kotzekidou, P. Characteristics of Oleuropeinolytic Strains of Lactobacillus Plantarum Group and Influence on Phenolic Compounds in Table Olives Elaborated under Reduced Salt Conditions. Food Microbiol. 2015, 48, 58–62. [Google Scholar] [CrossRef]
- Iorizzo, M.; Lombardi, S.J.; Macciola, V.; Testa, B.; Lustrato, G.; Lopez, F.; De Leonardis, A. Technological Potential of Lactobacillus Strains Isolated from Fermented Green Olives: In Vitro Studies with Emphasis on Oleuropein-Degrading Capability. Sci. World J. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, C.L.; Todaro, A.; Pino, A.; Pitino, I.; Corona, O.; Mazzaglia, A.; Caggia, C. Giarraffa and Grossa Di Spagna Naturally Fermented Table Olives: Effect of Starter and Probiotic Cultures on Chemical, Microbiological and Sensory Traits. Food Res. Int. 2014, 62, 1154–1164. [Google Scholar] [CrossRef]
- Vega Leal-Sánchez, M.; Ruiz-Barba, J.L.; Sánchez, A.H.; Rejano, L.; Jiménez-Díaz, R.; Garrido, A. Fermentation Profile and Optimization of Green Olive Fermentation Using Lactobacillus Plantarum LPCO10 as a Starter Culture. Food Microbiol. 2003, 20, 421–430. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Boziaris, I.S.; Stamatiou, A.; Nychas, G.J.E. Microbial Association and Acidity Development of Unheated and Pasteurized Green-Table Olives Fermented Using Glucose or Sucrose Supplements at Various Levels. Food Microbiol. 2005, 22, 117–124. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Waldron, K.W.; Delgadillo, I.; Selvendran, R.R. Effect of Processing on Cell Wall Polysaccharides of Green Table Olives. J. Agric. Food Chem. 1996, 44, 2394–2401. [Google Scholar] [CrossRef]
- Servili, M.; Minnocci, A.; Veneziani, G.; Taticchi, A.; Urbani, S.; Esposto, S.; Sebastiani, L.; Valmorri, S.; Corsetti, A. Compositional and Tissue Modifications Induced by the Natural Fermentation Process in Table Olives. J. Agric. Food Chem. 2008, 56, 6389–6396. [Google Scholar] [CrossRef]
- Hernández, A.; Martin, A.; Aranda, E.; Pérez-Nevado, F.; Córdoba, M.G. Identification and characterization of yeast isolated from the elaboration of seasoned green table olives. Food Microbiology 2007, 24, 346–351. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Yeasts in table oliveprocessing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and Metabolome of Un-Started and Started Greek-Type Fermentation of Bella Di Cerignola Table Olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Settanni, L.; Corona, O.; la Croce, F.; Caruso, T.; Moschetti, G.; Francesca, N. An Innovative Method to Produce Green Table Olives Based on “Pied de Cuve” Technology. Food Microbiol. 2015, 50, 126–140. [Google Scholar] [CrossRef]
| Factors | Samples | Moisture | Total Lipids | Tocopherols | NaCl |
|---|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (mg/kg) | (w/w) | ||
| ZC 7 d | 65.7 ± 1.5 | 16.5 ± 6.4 | 9.6 ± 3.9 | 1.1 ± 3.4 | |
| ZI 7 d | 65.1 ± 1.9 | 13.5 ± 17.1 | 11.2 ± 1.5 | 0.9 ± 14.4 | |
| VC 7 d | 64.4 ± 0.65 | 13.7 ± 8.7 | 11.7 ± 9.1 | 0.9 ± 7.3 | |
| VI 7 d | 65.8 ± 1.0 | 16.0 ± 6.2 | 18.1 ± 13.0 | 1.0 ± 12.6 | |
| Temperature (T) | ns | ns | * | ||
| Inoculum (I) | ns | ns | * | ||
| T × I | ns | ns | * | ||
| ZC 14 d | 64.1 ± 0.4 | 18.8 ± 4.6 | 13.7 ± 9.5 | 1.2 ± 8.9 | |
| ZI 14 d | 64.4 ± 2.9 | 17.0 ± 5.1 | 17.6 ± 8.6 | 1.0 ± 9.9 | |
| VC 14 d | 64.8 ± 0.8 | 17.6 ± 5.4 | 14.8 ± 4.2 | 1.2 ± 9.8 | |
| VI 14 d | 65.2 ± 1.1 | 13.5 ± 17.1 | 17.7 ± 1.1 | 1.2 ± 11.0 | |
| Temperature (T) | ns | ns | ns | ||
| Inoculum (I) | ns | * | * | ||
| T × I | ns | * | ns | ||
| ZC 30 d | 64.1 ± 2.0 | 14.5 ± 5.9 | 21.6 ± 4.7 | 1.8 ± 17.2 | |
| ZI 30 d | 64.7 ± 2.7 | 14.9 ± 9.0 | 20.9 ± 2.5 | 1.3 ± 15.7 | |
| VC 30 d | 63.0 ± 0.3 | 15.4 ± 7.9 | 21.7 ± 3.9 | 1.3 ± 12.1 | |
| VI 30 d | 64.7 ± 1.7 | 11.3 ± 11.2 | 16.5 ± 11.2 | 1.1 ± 16.0 | |
| Temperature (T) | ns | ns | ns | ||
| Inoculum (I) | ns | ns | ns | ||
| T × I | ns | ns | ns | ||
| ZC 45 d | 64.7 ± 1.3 | 16.2 ± 7.0 | 38.9 ± 9.3 | 1.8 ± 11.9 | |
| ZI 45 d | 64.4 ± 1.3 | 16.1 ± 3.8 | 30.5 ± 4.1 | 1.7 ± 16.0 | |
| VC 45 d | 65.6 ± 3.1 | 14.6 ± 9.8 | 41.4 ± 0.7 | 1.0 ± 5.7 | |
| VI 45 d | 63.9 ± 2.1 | 14.9 ± 8.8 | 32.1 ± 18.3 | 1.3 ± 14.4 | |
| Temperature (T) | ns | ns | ns | ||
| Inoculum (I) | ns | ns | * | ||
| T × I | ns | ns | ns | ||
| ZC 60 d | 64.8 ± 1.0 | 16.9 ± 16.4 | 39.0 ± 4.4 | 1.9 ± 10.8 | |
| ZI 60 d | 65.7 ± 0.6 | 15.3 ± 10.4 | 37.3 ± 5.8 | 2.2 ± 7.7 | |
| VC 60 d | 63.9 ± 0.8 | 16.6 ± 9.0 | 40.7 ± 15.7 | 1.8 ± 10.4 | |
| VI 60 d | 62.9 ± 1.6 | 17.2 ± 7.5 | 20.5 ± 1.3 | 1.4 ± 9.0 | |
| Temperature (T) | * | ns | * | ||
| Inoculum (I) | ns | ns | * | ||
| T × I | * | ns | * | ||
| ZC 90 d | 65.3 ± 1.6 | 12.0 ± 4.0 | 16.2 ± 8.5 | 2.2 ± 11.4 | |
| ZI 90 d | 67.4 ± 2.1 | 12.0 ± 8.3 | 15.8 ± 4.6 | 2.4 ± 12.8 | |
| VC 90 d | 63.1 ± 0.7 | 13.2 ± 10.4 | 15.3 ± 7.0 | 2.6 ± 5.0 | |
| VI 90 d | 63.2 ± 2.0 | 13.4 ± 0.8 | 16.2 ± 9.4 | 2.4 ± 8.1 | |
| Temperature (T) | * | ns | ns | ||
| Inoculum (I) | ns | ns | ns | ||
| T × I | ns | ns | ns | ||
| ZC 160 d | 67.0 ± 2.9 | 14.6 ± 5.7 | 12.8 ± 5.6 | 1.3 ± 4.9 | |
| ZI 160 d | 67.9 ± 2.2 | 13.5 ± 2.0 | 14.4 ± 7.5 | 1.8 ± 3.2 | |
| VC 160 d | 62.6 ± 1.0 | 16.2 ± 9.5 | 15.8 ± 9.6 | 1.6 ± 5.5 | |
| VI 160 d | 64.8 ± 1.4 | 17.3 ± 1.8 | 12.8 ± 2.1 | 1.0 ± 3.4 | |
| Temperature (T) | * | * | ns | ||
| Inoculum (I) | ns | ns | ns | ||
| T × I | ns | ns | * |
| Sample | ZC | ZI | VC | VI | Factors (7 d) | Factors (160 d) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Time (d) | 7 | 160 | 7 | 160 | 7 | 160 | 7 | 160 | ||||||
| Compounds | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | T | I | T × I | T | I | T × I |
| Hydroxy-tyrosol | 238.3 ± 4.3 | 480.7 ± 7.3 | 322.5 ± 5.1 | 719.2 ± 25.3 | 435.1 ± 10.7 | 326.1 ± 2.8 | 337.8 ± 9.3 | 762.9 ± 9.0 | * | ns | ns | ns | * | ns |
| Tyrosol | 30.2 ± 10.3 | 114.8 ± 3.2 | 38.8 ± 14.9 | 113.1 ± 8.3 | 43.9 ± 3.4 | 143.3 ± 4.7 | 38.1 ± 7.7 | 105.5 ± 5.5 | ns | ns | ns | * | * | * |
| Verbascoside der. a | 166.6 ± 7.9 | 390.1 ± 17.3 | 129.5 ± 5.6 | 297.3 ± 0.1 | 205.7 ± 6.9 | 451.5 ± 12.3 | 136.3 ± 1.7 | 293.3 ± 12.9 | ns | ns | ns | ns | * | ns |
| Verbascoside der. a | 130.9 ± 6.5 | 269.2 ± 3.9 | 125.5 ± 17.5 | 193.2 ± 6.5 | 217.0 ± 11.5 | 411.4 ± 5.6 | 138.0 ± 7.4 | 215.4 ± 8.1 | ns | ns | ns | * | * | ns |
| Verbascoside der. a | 311.5 ± 18.8 | 412.9 ± 3.2 | 349.3 ± 11.9 | 179.7 ± 10.9 | 588.3 ± 2.1 | 27.4 ± 2.2 | 445.4 ± 6.1 | 138.0 ± 5.7 | * | ns | ns | ns | * | * |
| Verbascoside | 256.4 ± 13.4 | 194.4 ± 25.9 | 293.0 ± 4.6 | 396.6 ± 18.7 | nd | 333.4 ± 6.8 | 434.8 ± | 409.0 | * | ns | ns | ns | ns | ns |
| Coumaric acid | nd | nd | nd | nd | 104.1 ± 5.8 | 54.8 ± 8.3 | 118.2 ± 0.5 | 135.2 ± 35.6 | * | ns | ns | * | * | * |
| Rutin | 218.3 ± 6.3 | 85.2 ± 10.2 | 263.3 ± 12.8 | 32.3 ± 5.7 | 368.6 ± 18.4 | nd | 322.6 ± 5.6 | nd | * | ns | ns | ns | * | ns |
| Lutelolin glucoside | 511.4 ± 6.0 | 214.0 ± 17.6 | 563.2 ± 3.4 | 244.6 ± 31.0 | 733.6 ± 14.4 | 277.6 ± 17.1 | 604.1 ± 11.1 | 120.6 ± 2.4 | * | ns | ns | ns | ns | ns |
| Oleuropein | 210.2 ± 22.5 | 99.7 ± 16.3 | 386.9 ± 23.5 | 123.9 ± 12.5 | 525.8 ± 10.9 | 115.7 ± 4.6 | 445.0 ± 17.1 | 74.3 ± 14.0 | * | ns | ns | ns | ns | ns |
| Verbascoside der. a | 105.2 ± 8.2 | 66.9 ± 7.7 | 108.4 ± 9.7 | 89.7 ± 7.6 | 222.3 ± 9.8 | 64.5 ± 2.1 | 210.6 ± 21.7 | 82.6 ± 19.3 | * | ns | ns | ns | ns | ns |
| Sinapic acid der. b | 183.1 ± 2.6 | 160.0 ± 6.8 | 176.9 ± 9.3 | 115.8 ± 7.4 | 302.9 ± 6.9 | 150.2 ± 3.7 | 207.9 ± 6.5 | 147.0 ± 12.7 | * | ns | ns | ns | * | ns |
| Luteolin c | 189.0 ± 5.5 | 342.5 ± 9.0 | 183.1 ± 1.9 | 339.9 ± 14.2 | 146.0 ± 6.6 | 296.2 ± 5.3 | 158.7 ± 25.7 | 304.9 ± 2.7 | ns | ns | ns | ns | ns | ns |
| Apigenin | 2.7 ± 2.2 | 4.4 ± 17.9 | 2.8 ± 4.6 | 4.3 ± 15.6 | 2.1 ± 8.0 | 3.6 ± 2.6 | 2.4 ± 13.8 | 3.6 ± 15.4 | ns | ns | ns | * | ns | ns |
| Total polyp. HPLC | 2553 ± 7.6 | 2834 ± 1.7 | 3158 ± 10.9 | 2534 ± 15.3 | 4079 ± 14.4 | 2659 ± 8.1 | 3271 ± 3.4 | 2664 ± 20.6 | * | ns | * | * | ns | ns |
| Total polyp. Folin | 2906 ± 6.3 | 3138 ± 8.6 | 2350 ± 6.4 | 2112 ± 1.3 | 3223 ± 12.3 | 3458 ± 10.1 | 3637 ± 9.9 | 3622 ± 2.6 | * | ns | * | * | * | * |
| TPA Parameter | VC | VI | ZC | ZI | T | I | T × I |
|---|---|---|---|---|---|---|---|
| Hardness (gr) | 1651.2 ± 29.89 | 1771.56 ± 23.26 | 2290.84 ± 24.15 | 2102.44 ± 24.43 | * | ns | * |
| Adhesiveness (g.s) | −1.23 ± 113.01 | −2.42 ± 186.6 | −3.15 ± 2.53 | −2.77 ± 223.5 | * | ns | ns |
| Springiness | 0.74 ± 8.11 | 0.73 ± 10.96 | 0.72 ± 5.56 | 0.69 ± 11.59 | * | * | ns |
| Cohesiveness | 0.63 ± 6.35 | 0.61 ± 8.2 | 0.59 ± 5.08 | 0.58 ± 6.9 | * | * | ns |
| Gumminess | 1032.33 ± 30.05 | 1068.35 ± 22.83 | 1340 ± 17.14 | 1220.72 ± 23.78 | * | ns | * |
| Chewiness | 774.54 ± 33.78 | 786.28 ± 27.2 | 959.99 ± 21.74 | 844.74 ± 28.17 | * | ns | * |
| Resilience | 0.32 ± 12.5 | 0.32 ±12.5 | 0.31 ± 9.68 | 0.29 ± 13.79 | * | ns | ns |
| Sample | Average Hedonic Judgments |
|---|---|
| VI | 5.52 ± 32.56 |
| VC | 5.76 ± 25.66 |
| ZI | 5.80 ± 24.53 |
| ZC | 6.58 ± 20.39 |
| Temperature | * |
| Inoculum | * |
| T × I | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campus, M.; Corrias, F.; Angioni, A.; Arru, N.; Sedda, P.; Addis, M.; Fiori, M.; Paba, A.; Chessa, L.; Comunian, R. Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature. Foods 2025, 14, 2159. https://doi.org/10.3390/foods14132159
Campus M, Corrias F, Angioni A, Arru N, Sedda P, Addis M, Fiori M, Paba A, Chessa L, Comunian R. Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature. Foods. 2025; 14(13):2159. https://doi.org/10.3390/foods14132159
Chicago/Turabian StyleCampus, Marco, Francesco Corrias, Alberto Angioni, Nicola Arru, Piergiorgio Sedda, Margherita Addis, Myriam Fiori, Antonio Paba, Luigi Chessa, and Roberta Comunian. 2025. "Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature" Foods 14, no. 13: 2159. https://doi.org/10.3390/foods14132159
APA StyleCampus, M., Corrias, F., Angioni, A., Arru, N., Sedda, P., Addis, M., Fiori, M., Paba, A., Chessa, L., & Comunian, R. (2025). Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature. Foods, 14(13), 2159. https://doi.org/10.3390/foods14132159








