The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Sample Preparation
2.2. Methods
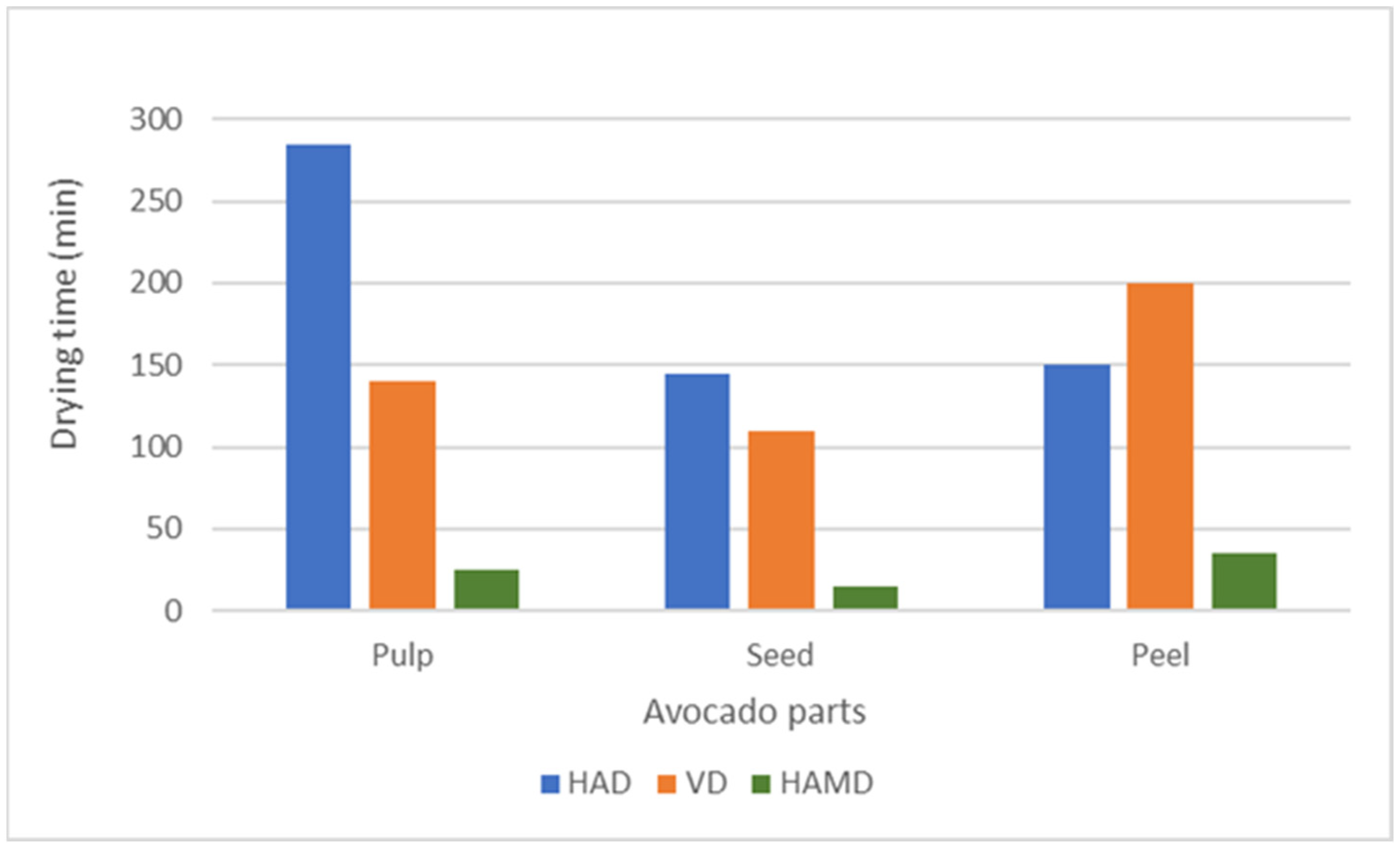
2.2.1. Drying Processes
2.2.2. Fatty Acids Determination
2.2.3. Tocopherols Determination
2.2.4. Fatty Acid Profile Functionality Evaluation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition in Fresh Avocado Parts
3.2. Fatty Acid Composition in Dried Pulp
3.3. Fatty Acid Composition in Dried Seed
3.4. Fatty Acid Composition in Dried Peel
3.5. Fatty Acid Composition of Avocado and Health
3.6. Tocopherol Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurtado-Fernández, E.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Avocado fruit—Persea americana. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., Sousa de Brito, E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 37–48. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT—Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 23 April 2024).
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Lye, H.S.; Ong, M.K.; Teh, L.K.; Chang, C.C.; Wei, L.K. Avocado. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 67–93. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fuentes, E.; Cádiz-Gurrea, M.d.l.L.; Rodriguez, L.; Villegas-Aguilar, M.d.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Biological Evaluation of Avocado Residues as a Potential Source of Bioactive Compounds. Antioxidants 2022, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2020, 37, 619–655. [Google Scholar] [CrossRef]
- Santos, C.D.M.; Pagno, C.H.; Costa, T.M.H.; Faccin, D.J.L.; Flôres, S.H.; Cardozo, N.S.M. Biobased polymer films from avocado oil extraction residue: Production and characterization. J. Appl. Polym. Sci. 2016, 133, 43957. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of avocado processing industry by-product: A review on future prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Cheikhyoussef, N.; Cheikhyoussef, A. Avocado (Persea Americana) Wastes: Chemical Composition, Biological Activities and Industrial Applications. In Mediterranean Fruits Bio-Wastes Chemistry, Functionality and Technological Applications; Ramadan, M.F., Farag, M.A., Eds.; Springer: Berlin, Germany, 2022; pp. 699–719. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional ingredient from avocado peel: Microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 2020, 352, 129300. [Google Scholar] [CrossRef]
- Grassino, A.N.; Karlović, S.; Šošo, L.; Dujmić, F.; Sabolović, M.B.; Marelja, M.; Brnčić, M. Influence of Different Drying Processes on the Chemical and Texture Profile of Cucurbita maxima Pulp. Foods 2024, 13, 520. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Marelja, M.; Dujmić, F.; Ježek, D.; Škegro, M.; Bosiljkov, T.; Karlović, S.; Brnčić, M. Vacuum drying in Food Industry. Hrvat. Časopis Za Prehrambenu Tehnol. Biotehnol. I Nutr. 2020, 15, 94–101. [Google Scholar] [CrossRef]
- Castro, A.; Mayorga, E.; Moreno, F. Mathematical modelling of convective drying of fruits: A review. J. Food Eng. 2018, 223, 152–167. [Google Scholar] [CrossRef]
- Ali, A.; Oon, C.C.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdylo, A.; Turkiewicz, I.P.; Szumny, A.; Łyczko, J. Volatile and polyphenol composition, anti-oxidant, anti-diabetic and anti-aging properties, and drying kinetics as affected by convective and hybrid vacuum microwave drying of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 151, 112463. [Google Scholar] [CrossRef]
- Radojčin, M.; Pavkov, I.; Kovačević, D.B.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of Selected Drying Methods and Emerging Drying Intensification Technologies on the Quality of Dried Fruit: A Review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A. Microwave-convective drying of food materials: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 59, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.S.; Sunil, C.; Negi, A.; Pare, A. Effect of microwave assisted hot-air drying temperatures on drying kinetics of dried black gram papad (Indian snack food): Drying characteristics of black gram papad. Appl. Food Res. 2022, 2, 100144. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- ISO 15884; Milk Fat—Preparation of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 15885; Milk Fat—Determination of the Fatty Acid Composition by Gas-Liquid Chromathography. International Organization for Standardization: Geneva, Switzerland, 2002.
- Montoya-Arroyo, A.; Toro-González, C.; Sus, N.; Warner, J.; Esquivel, P.; Jiménez, V.M.; Frank, J. Vitamin E and carotenoid profiles in leaves, stems, petioles and flowers of stinging nettle (Urtica leptophylla Kunth) from Costa Rica. J. Sci. Food Agric. 2022, 102, 6340–6348. [Google Scholar] [CrossRef] [PubMed]
- Chauveau-Duriot, B.; Doreau, M.; Nozière, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Kurilich, A.C.; Juvik, J.A. Simultaneous quantification of carotenoids and tocopherols in corn kernel extracts by hplc. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 2925–2934. [Google Scholar] [CrossRef]
- Mclaughlin, P.; Weihrauch, J.L. Vitamin E content of foods. J. Am. Diet. Assoc. 1979, 75, 647–665. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Lourenço, S.; Lopes, A.; Andrade, C.; Câmara, J.S.; Castilho, P.; Perestrelo, R. Evaluation of Fatty Acids Profile as a Useful Tool towards Valorization of By-Products of Agri-Food Industry. Foods 2021, 10, 2867. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Mierliță, D. Effects of diets containing hemp seeds or hemp cake on fatty acid composition and oxidative stability of sheep milk. S. Afr. J. Anim. Sci. 2018, 48, 504–515. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Mugendi, B.; Njoroge, P.W.; Wanjiru, S.N. A Review of Fatty Acid Components in Avocado. IOSR-JAC 2023, 16, 18–27. [Google Scholar] [CrossRef]
- Galvão, M.d.S.; Narain, N.; Nigam, N. Influence of different cultivars on oil quality and chemical characteristics of avocado fruit. Food Sci. Technol. 2014, 34, 539–546. [Google Scholar] [CrossRef]
- Gonçalves, D.; Gouveia, C.S.; Ferreira, M.J.; Ganança, J.F.; Pinto, D.C.G.; de Carvalho, M.A.P. Comparative analysis of antioxidant and fatty acid composition in avocado (Persea americana Mill.) fruits: Exploring regional and commercial varieties. Food Chem. 2024, 442, 138403. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Béjar, A.A.; Yahia, E.M.; Ornelas-Paz, J.d.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Escalante-Minakata, P. Effect of cultivar on the content of selected phytochemicals in avocado peels. Food Res. Int. 2020, 140, 110024. [Google Scholar] [CrossRef]
- Babiker, E.E.; Ahmed, I.A.M.; Uslu, N.; Özcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Almusallam, I.A. Influence of Drying Methods on Bioactive Properties, Fatty Acids and Phenolic Compounds of Different Parts of Ripe and Unripe Avocado Fruits. J. Oleo Sci. 2021, 70, 589–598. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Uslu, N.; Özcan, M.M.; Babiker, E.E.; Ghafoor, K.; Ahmed, I.M.; Alsawmahi, O.N. Effects of drying process on oil quality, the bioactive properties and phytochemical characteristics of avocado (Fuerte) fruits harvested at two different maturity stages. J. Food Process. Preserv. 2021, 45, e15368. [Google Scholar] [CrossRef]
- Chimsook, T.; Assawarachan, R. Effect of Drying Methods on Yield and Quality of the Avocado Oil. Key Eng. Mater. 2017, 735, 127–131. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Mendonça, C.R.B.; Borges, C.D.; Crizel-Cardozo, M.M.; dos Santos, M.A.Z.; Otero, D.M.; Zambiazi, R.C. Margarida avocado oil: Effect of processing on quality, bioactive compounds and fatty acid profile. Food Chem. Adv. 2024, 4, 100617. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral contents and Fatty Acid Composition of Different Parts of Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018, 243, 414–419. [Google Scholar] [CrossRef]
- Caponio, F.; Pasqualone, A.; Gomes, T. Changes in the fatty acid composition of vegetable oils in model doughs submitted to conventional or microwave heating. Int. J. Food Sci. Technol. 2003, 38, 481–486. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rodriguez-Estrada, M.T.; Vittadini, E.; Pellegrini, N. Microwave heating of different vegetable oils: Relation between chemical and thermal parameters. LWT 2010, 43, 1104–1112. [Google Scholar] [CrossRef]
- Dostálová, J.; Hanzlík, P.; Réblová, Z.; Pokorný, J. Oxidative changes of vegetable oils during microwave heating. Czech J. Food Sci. 2005, 23, 230–239. [Google Scholar] [CrossRef]
- Pop, F. Effect of Microwave Heating on Quality and Fatty Acids Composition of Vegetable Oils. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 43–52. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2010, 44, 1231–1237. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical and possible successor. Prostag. Leukotr. Ess. 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; Silva, L.d.O.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shi, A.; Liu, L.; Fauconnier, M.L.; Wang, Q. The Effect of Microwave Pretreatment on Micronutrient Contents, Oxidative Stability and Flavor Quality of Peanut Oil. Molecules 2018, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Uslu, N.; Al-Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.; Yıldız, M.U.; Alswahmi, O.N.; Özcan, M.M. Tocopherol Contents of Pulp Oils Extracted from Ripe and Unripe Avocado Fruits Dried by Different Drying Systems. J. Oleo Sci. 2021, 70, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Alibas, I.; Köksal, N. Convective, vacuum and microwave drying kinetics of mallow leaves and comparison of color and ascorbic acid values of three drying methods. Food Sci. Technol. 2014, 34, 358–364. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Horuz, E.; Bozkurt, H.; Karataş, H.; Maskan, M. Effects of hybrid (microwave-convectional) and convectional drying on drying kinetics, total phenolics, antioxidant capacity, vitamin C, color and rehydration capacity of sour cherries. Food Chem. 2017, 230, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, S.-H.M.; Sturm, B.; Nasirahmadi, A. Effects of hot-air and hybrid hot air-microwave drying on drying kinetics and textural quality of nectarine slices. Heat Mass Transf. 2018, 54, 915–927. [Google Scholar] [CrossRef]
| Fatty Acid | Pulp | Seed | Peel |
|---|---|---|---|
| C14:0 | - | 0.38 ± 0.004 A | 0.12 ± 0.002 B |
| C16:0 | 20.34 ± 0.22 B | 29.45 ± 0.60 A | 19.90 ± 0.44 B |
| C16:1 | 8.58 ± 0.14 A | 8.72 ± 0.33 A | 7.60 ± 0.11 B |
| C17:0 | - | - | - |
| C17:1 | - | - | - |
| C18:0 | 0.45 ± 0.03 B | - | 0.55 ± 0.02 A |
| C18:1 | 57.93 ± 0.52 A | 41.28 ± 0.67 B | 56.79 ± 0.50 A |
| C18:2 | 8.44 ± 0.22 C | 14.95 ± 0.40 A | 11.62 ± 0.34 B |
| C18:3 | - | - | 1.33 ± 0.01 A |
| C20:0 | - | 1.28 ± 0.13 A | 0.13 ± 0.007 B |
| C20:1 | - | - | 0.21 ± 0.01 A |
| C20:2 | - | - | - |
| C20:3 | - | 0.14 ± 0.02 A | - |
| C20:5 | - | 1.06 ± 0.04 A | - |
| C21:0 | - | 0.28 ± 0.02 A | - |
| C22:0 | 1.25 ± 0.04 A | 0.25 ± 0.03 B | - |
| C22:1 | 0.48 ± 0.02 A | 0.35 ± 0.04 B | 0.11 ± 0.004 B |
| C22:2 | - | - | 1.30 ± 0.09 A |
| C23:0 | - | - | - |
| C24:0 | - | - | - |
| C24:1 | 2.53 ± 0.02 A | 0.19 ± 0.003 C | 0.33 ± 0.08 B |
| SFA | 22.04 ± 0.27 A | 31.64 ± 0.69 A | 20.7 ± 0.25 C |
| MUFA | 69.52 ± 0.34 A | 50.54 ± 0.64 C | 65.04 ± 0.48 B |
| PUFA | 8.44 ± 0.21 C | 16.15 ± 0.43 A | 14.25 ± 0.38 B |
| TUFA | 77.96 ± 0.55 A | 66.69 ± 0.68 B | 79.29 ± 0.9 A |
| Pulp | |||
|---|---|---|---|
| Fatty Acid | HAD | VD | HAMD |
| C14:0 | 0.08 ± 0.005 B | 0.10 ± 0.004 A | 0.02 ± 0.001 C |
| C16:0 | 26.15 ± 0.48 B | 30.18 ± 0.51 A | 26.01 ± 0.35 B |
| C16:1 | 12.23 ± 0.32 B | 12.11 ± 0.38 B | 13.02 ± 0.41 A |
| C17:0 | - | - | 0.47 ± 0.02 A |
| C17:1 | - | - | 0.15 ± 0.01 A |
| C18:0 | 0.6 ± 0.03 B | 0.69 ± 0.05 A | 0.60 ± 0.06 A |
| C18:1 | 44.42 ± 0.94 A | 42.81 ± 0.47 B | 44.01 ± 1.06 A |
| C18:2 | 15.44 ± 0.61 A | 10.19 ± 0.23 C | 13.95 ± 0.33 B |
| C18:3 | 0.76 ± 0.01 A | 0.57 ± 0.02 B | 0.79 ± 0.04 A |
| C20:0 | 0.08 ± 0.01 B | 0.19 ± 0.02 A | - |
| C20:1 | 0.13 ± 0.01 B | 0.16 ± 0.01 A | - |
| C20:2 | - | - | - |
| C20:3 | - | - | - |
| C20:5 | - | 1.15 ± 0.06 A | - |
| C21:0 | - | - | - |
| C22:0 | - | 0.08 ± 0.01 A | - |
| C22:1 | - | 0.38 ± 0.04 A | - |
| C22:2 | - | - | 0.54 ± 0.02 A |
| C23:0 | - | 0.19 ± 0.006 A | - |
| C24:0 | - | - | 0.26 ± 0.007 A |
| C24:1 | - | 1.00 ± 0.02 A | - |
| SFA | 26.91 ± 0.46 B | 31.43 ± 0.48 A | 26.89 ± 0.42 B |
| MUFA | 56.78 ± 0.98 A | 56.46 ± 0.65 A | 57.14 ± 1.01 A |
| PUFA | 16.20 ± 0.56 A | 11.91 ± 0.25 C | 15.28 ± 0.48 B |
| TUFA | 72.98 ± 1.43 A | 68.37 ± 0.82 B | 72.42 ± 1.45 A |
| Seed | |||
|---|---|---|---|
| Fatty Acid | HAD | VD | HAMD |
| C14:0 | - | 0.48 ± 0.02 B | 1.19 ± 0.04 A |
| C16:0 | 15.19 ± 0.31 C | 19.27 ± 0.27 B | 24.77 ± 0.34 A |
| C16:1 | 3.87 ± 0.12 C | 4.85 ± 0.11 B | 11.06 ± 0.14 A |
| C17:0 | - | - | 0.63 ± 0.02 A |
| C17:1 | - | - | 0.21 ± 0.006 A |
| C18:0 | 0.62 ± 0.02 C | 0.82 ± 0.02 B | 1.46 ± 0.02 A |
| C18:1 | 34.42 ± 1.04 B | 33.56 ± 0.71 B | 42.33 ± 0.60 A |
| C18:2 | 42.45 ± 0.81 A | 39.83 ± 0.73 B | 13.67 ± 0.38 C |
| C18:3 | 0.68 ± 0.04 B | - | 0.99 ± 0.11 A |
| C20:0 | 0.63 ± 0.02 A | - | - |
| C20:1 | 1.08 ± 0.02 A | - | - |
| C20:2 | - | - | - |
| C20:3 | - | - | - |
| C20:5 | - | - | - |
| C21:0 | 1.07 ± 0.04 A | - | - |
| C22:0 | - | - | - |
| C22:1 | - | - | 0.59 ± 0.02 A |
| C22:2 | - | - | 1.18 ± 0.03 A |
| C23:0 | - | - | - |
| C24:0 | - | - | 0.76 ± 0.02 A |
| C24:1 | - | - | - |
| SFA | 17.51 ± 0.39 C | 20.57 ± 0.31 B | 28.81 ± 0.44 A |
| MUFA | 39.37 ± 1.18 B | 38.41 ± 0.82 B | 54.19 ± 0.77 A |
| PUFA | 43.13 ± 0.85 A | 39.83 ± 0.73 B | 15.84 ± 0.41 C |
| TUFA | 82.50 ± 2.03 A | 78.24 ± 1.55 B | 70.03 ± 1.18 C |
| Peel | |||
|---|---|---|---|
| Fatty Acid | HAD | VD | HAMD |
| C14:0 | - | - | 0.20 ± 0.01 A |
| C16:0 | 21.78 ± 0.24 B | 25.59 ± 0.25 A | 21.88 ± 0.79 B |
| C16:1 | 9.96 ± 0.11 B | 10.68 ± 0.22 A | 10.71 ± 0.53 A |
| C17:0 | - | - | - |
| C17:1 | - | - | 0.13 ± 0.01 A |
| C18:0 | 0.78 ± 0.02 A | 0.72 ± 0.02 B | 0.63 ± 0.03 C |
| C18:1 | 47.47 ± 0.34 A | 44.53 ± 0.66 B | 41.71 ± 0.89 C |
| C18:2 | 17.99 ± 0.29 A | 16.12 ± 0.43 B | 17.49 ± 0.64 A |
| C18:3 | 1.72 ± 0.09 C | 2.10 ± 0.08 B | 3.08 ± 0.05 A |
| C20:0 | - | - | 0.17 ± 0.02 A |
| C20:1 | - | - | 0.16 ± 0.01 A |
| C20:2 | 0.11 ± 0.01 A | - | - |
| C20:3 | - | - | - |
| C20:5 | - | - | 0.07 ± 0.01 A |
| C21:0 | - | - | - |
| C22:0 | - | - | 0.25 ± 0.01 A |
| C22:1 | - | - | - |
| C22:2 | 0.19 ± 0.01 C | 0.26 ± 0.01 B | 2.67 ± 0.07 A |
| C23:0 | - | - | 0.04 ± 0.01 A |
| C24:0 | - | - | 0.32 ± 0.01 A |
| C24:1 | - | - | 0.12 ± 0.01 A |
| SFA | 22.56 ± 0.28 C | 26.31 ± 0.26 A | 23.49 ± 0.8 B |
| MUFA | 57.43 ± 0.46 A | 55.21 ± 0.65 B | 52.83 ± 0.98 C |
| PUFA | 20.01 ± 0.3 B | 18.48 ± 0.4 C | 23.31 ± 0.56 A |
| TUFA | 77.44 ± 0.73 A | 73.69 ± 0.99 B | 76.14 ± 1.34 A |
| TUFA/SFA | PUFA/SFA | H/H Ratio | IA | ||
|---|---|---|---|---|---|
| Pulp | Fresh | 3.54 | 0.38 | 3.26 | 0.26 |
| HAD | 2.71 | 0.60 | 2.31 | 0.36 | |
| VD | 2.17 | 0.38 | 1.77 | 0.45 | |
| HAMD | 2.69 | 0.57 | 2.28 | 0.36 | |
| Seed | Fresh | 2.11 | 0.51 | 1.93 | 0.46 |
| HAD | 4.71 | 2.46 | 4.91 | 0.19 | |
| VD | 3.80 | 1.94 | 3.72 | 0.25 | |
| HAMD | 2.43 | 0.55 | 2.24 | 0.38 | |
| Peel | Fresh | 3.83 | 0.69 | 3.55 | 0.26 |
| HAD | 3.43 | 0.89 | 3.10 | 0.28 | |
| VD | 2.80 | 0.70 | 2.46 | 0.35 | |
| HAMD | 3.24 | 0.99 | 2.94 | 0.30 |
| α-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | Vitamin E Activity | |
|---|---|---|---|---|---|
| Peel | 57.57 ± 1.18 a | 43.23 ± 1.04 a | 82.42 ± 1.55 a | 183.2 ± 3.77 a | 62.72 ± 1.30 a |
| Pulp | 24.57 ± 1.01 b | 26.26 ± 0.77 b | 6.57 ± 0.29 b | 57.41 ± 2.07 b | 27.26 ± 1.08 b |
| Seed | 13.61 ± 0.32 c | - | - | 13.61 ± 0.32 c | 13.61 ± 0.32 c |
| α-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | Vitamin E Activity | |
|---|---|---|---|---|---|
| HAD | 69.53 ± 0.91 c | 55.13 ± 1.00 b | 129.14 ± 0.76 a | 253.8 ± 3.43 b | 76.33 ± 1.01 c |
| VD | 108.81 ± 1.48 a | 64.80 ± 0.94 a | 83.01 ± 0.60 b | 256.62 ± 3.02 a | 116.12 ± 1.54 a |
| HAMD | 91.11 ± 1.91 b | 47.10 ± 0.33 c | 130.24 ± 1.10 a | 268.45 ± 3.23 a | 97.12 ± 2.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marović, R.; Badanjak Sabolović, M.; Brnčić, M.; Ninčević Grassino, A.; Kljak, K.; Voća, S.; Karlović, S.; Rimac Brnčić, S. The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes. Foods 2024, 13, 2003. https://doi.org/10.3390/foods13132003
Marović R, Badanjak Sabolović M, Brnčić M, Ninčević Grassino A, Kljak K, Voća S, Karlović S, Rimac Brnčić S. The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes. Foods. 2024; 13(13):2003. https://doi.org/10.3390/foods13132003
Chicago/Turabian StyleMarović, Roko, Marija Badanjak Sabolović, Mladen Brnčić, Antonela Ninčević Grassino, Kristina Kljak, Sandra Voća, Sven Karlović, and Suzana Rimac Brnčić. 2024. "The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes" Foods 13, no. 13: 2003. https://doi.org/10.3390/foods13132003
APA StyleMarović, R., Badanjak Sabolović, M., Brnčić, M., Ninčević Grassino, A., Kljak, K., Voća, S., Karlović, S., & Rimac Brnčić, S. (2024). The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes. Foods, 13(13), 2003. https://doi.org/10.3390/foods13132003







