Brown Seaweed Byproduct Extracts Improve Intestinal Motility and Auto-Inflammation in Mice with Loperamide-Induced Constipation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Marine Byproduct Extracts
2.2. Analysis of the Composition of Marine Byproduct Extracts
2.3. Analysis of Sugar Components in Marine Byproduct Extracts
2.4. Animals and Experimental Design
2.5. Fecal Weight and Water Content
2.6. Intestinal Transit Rate
2.7. Biochemical Analysis
2.8. Assessment of Gut Microbiota Growth
2.9. Statistical Analysis
3. Results
3.1. Composition of Marine Byproduct Extracts
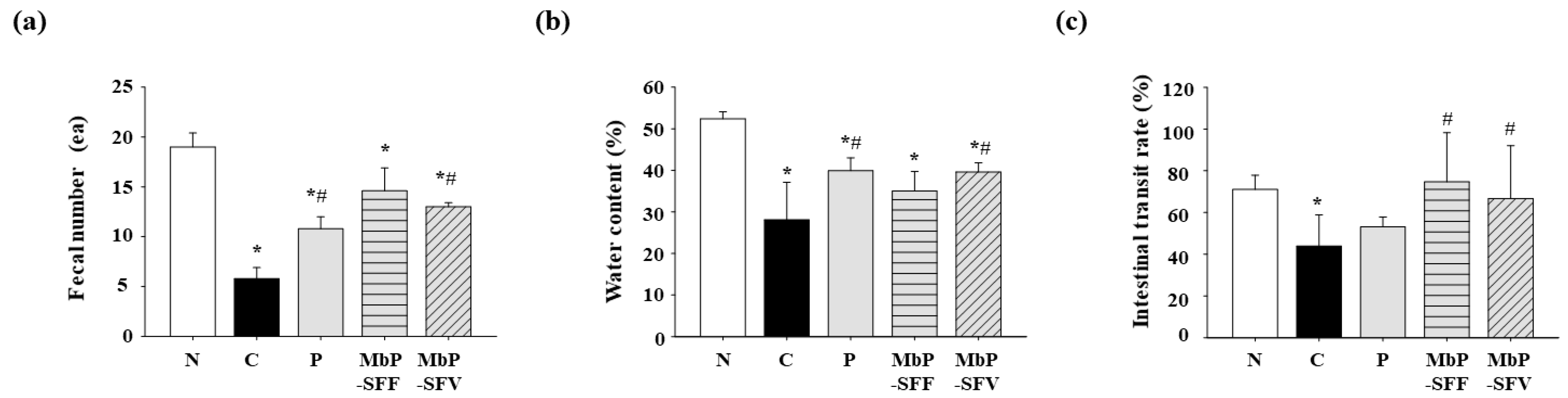
3.2. Effects of Marine Byproduct Extracts on Fecal Excretion and the Small Intestinal Transit
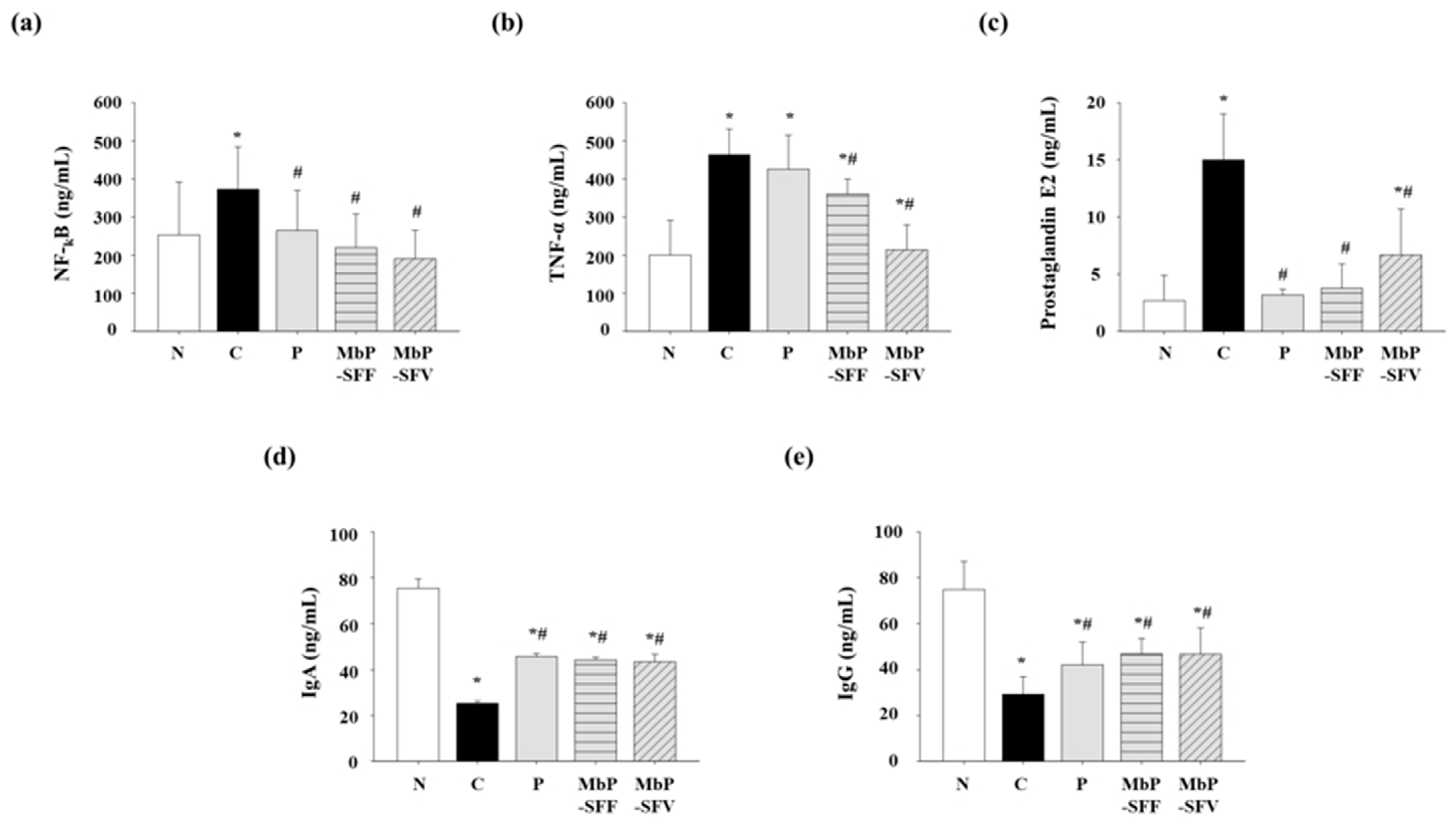
3.3. Effects of Marine Byproduct Extracts on the Levels of Inflammatory Factors and Immune Response
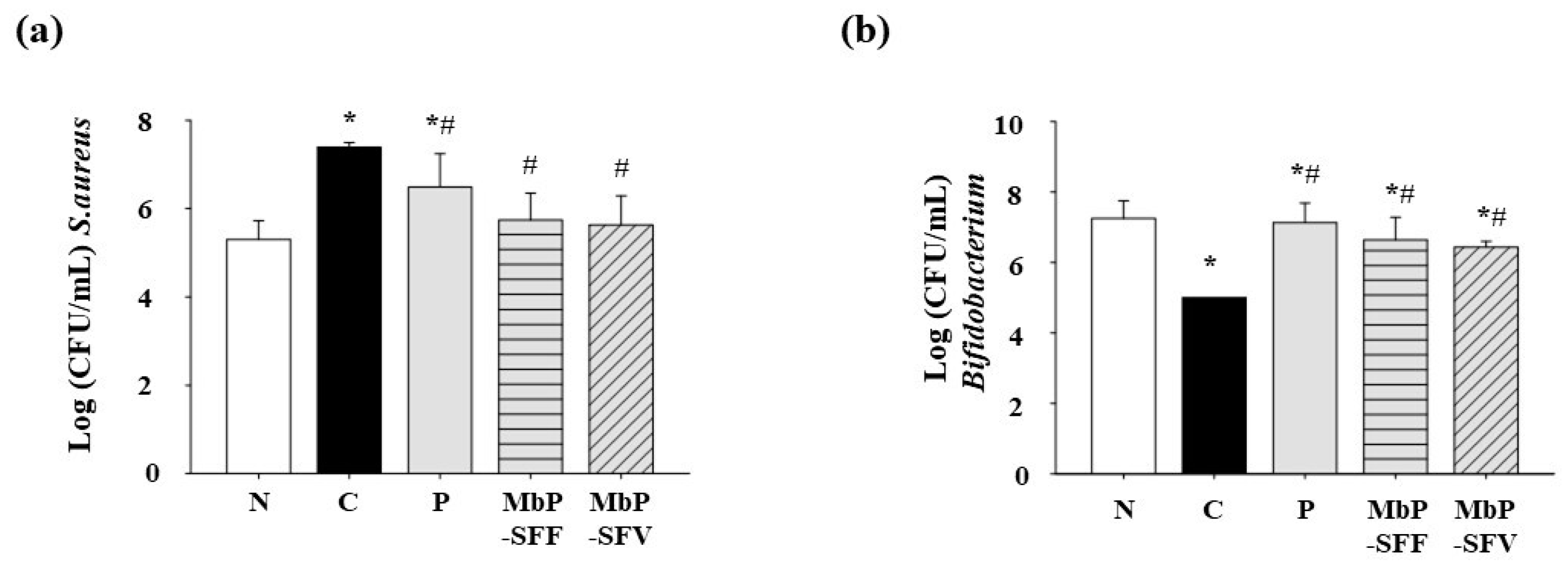
3.4. Effects of Marine Byproduct Extracts on the Intestinal Microbiota
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.I.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luthuli, S.; Wu, Q.; Wu, M.; Choi, J.-I.; Tong, H. Pharmaceutical and Nutraceutical Potential Applications of Sargassum fulvellum. BioMed Res. Int. 2020, 2020, 2417410. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Shon, M.-S.; Moon, H.H.; Han, C.-H.; Choi, H.-J.; Han, B.K. Hangover Relieving through Algae and Plant Complex Extract using Lava Sea Water. Food Eng. Prog. 2018, 22, 110–117. [Google Scholar] [CrossRef]
- Wang, X.; Huang, C.; Fu, X.; Jeon, Y.-J.; Mao, X.; Wang, L. Bioactivities of the Popular Edible Brown Seaweed Sargassum fusiforme: A Review. J. Agric. Food Chem. 2023, 71, 16452–16468. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, S.; Hou, L.; Jiang, H.; Wu, Y.; Wu, M.; Zhang, X. Laxative effect of Sargassum fusiforme sodium alginate in constipation model mice. Food Res. Dev. 2019, 40, 12–16. [Google Scholar]
- Han, E.J.; Kim, M.J.; Shin, E.-j.; Ahn, G. Laxative Effects of Sargassum fulvellum on Loperamide-Induced Constipation in Mice. J. Chitin Chitosan 2021, 26, 16–21. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 59–74. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.; Candy, D.C.; Catto-Smith, A.G.; Clayden, G.; Loening-Baucke, V.; Di Lorenzo, C.; Nurko, S.; Staiano, A. The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 273–275. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.-Y.; Xu, D.-Q.; Yue, S.-J.; Fu, R.-J.; Yang, J.; Xing, L.-M.; Tang, Y.-P. Action Mode of Gut Gotility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef]
- Tack, J.; Muller-Lissner, S.; Stanghellini, V.; Boeckxstaens, G.; Kamm, M.A.; Simren, M.; Galmiche, J.P.; Fried, M. Diagnosis and Treatment of Chronic Constipation- A European Perspective. Neurogastroenterol. Motil. 2011, 23, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal Microbiota in Human Health and Disease: The Impact of Probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Kirgizov, I.; Sukhorukov, A.; Dudarev, V.; Istomin, A. Hemostasis in Children with Dysbacteriosis in Chronic Constipation. Clin. Appl. Thromb. Hemost. 2001, 7, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.; Konovitch, E.A.; Maximova, I.D. Alterations inThe Colonic Flora and Intestinal Permeability and Evidence of Immune Activation in Chronic Constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.-B. Intestinal microbiota and chronic constipation. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk Between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chai, M.; Wang, J.; Qiangqing, Y.; Wang, G.; Zhang, H.; Zhao, J.; Chen, W. Bifidobacterium Longum Relieves Constipation by Regulating the Intestinal Barrier of Mice. Food Funct. 2022, 13, 5037–5049. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S. Lactobacillus reuteri Induces Gut Intraepithelial CD4+ CD8αα+ T Cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Dai, J.; Wu, Y.; Chen, S.-w.; Zhu, S.; Yin, H.-p.; Wang, M.; Tang, J. Sugar Compositional Determination of Polysaccharides from Dunaliella salina by Modified RP-HPLC Method of Precolumn Derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-Performance Liquid Chromatography of Reducing Carbohydrates as Strongly Ultraviolet-Absorbing and Electrochemically Sensitive 1-Phenyl-3-methyl5-pyrazolone Derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B. The role of Loperamide in Gastrointestinal Disorders. Rev. Gastroenterol. Disord. 2008, 8, 15–20. [Google Scholar] [PubMed]
- Kim, J.-E.; Lee, Y.-J.; Kwak, M.-H.; Jun, G.; Koh, E.-K.; Song, S.-H.; Seong, J.-E.; Kim, J.W.; Kim, K.-B.; Kim, S. Metabolomics Approach to Serum Biomarker for Loperamide-Induced Constipation in SD rats. Lab. Anim. Res. 2014, 30, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, Isolation, Purification and Biological Activities of Sargassum fusiforme Polysaccharides: A Review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan Isolated from Invasive Sargassum horneri inhibit LPS-induced Inflammation via Blocking NF-κB and MAPK Pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.S.; Kovalev, V.; Savchenko, O.; Ziganshina, O. Physical–chemical Properties, Physiological Activity, and Usage of Alginates, thePolysaccharides of Brown Algae. Russ. J. Marine Biol. 2001, 27, S53–S64. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.; Hsieh, Y.S.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Jang, M.; Hong, J.T.; Hwang, D.Y. Loperamide-induced constipation activates inflammatory signaling pathways in the mid colon of SD rats via complement C3 and its receptors. Curr. Mol. Med. 2022, 22, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D’Angelo, G.; Di Rienzo, T.A.; Bibbò, S.; Migneco, A.; Gasbarrini, A. The Effect of Lactobacillus reuteri Supplementation in Adults with Chronic Functional Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gastrointestin. Liver Dis. 2014, 23, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Carmagnola, S.; Anderloni, A.; Andorno, S.; Ballarè, M.; Balzarini, M.; Montino, F.; Orsello, M.; Pagliarulo, M.; Sartori, M. The Use of Probiotics in Healthy Volunteers with Evacuation Disorders and Hard Stools: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Gastroenterol. 2010, 44, S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. 2022, 20, 1–10. [Google Scholar] [CrossRef]
- Kang, D.-W.; DiBaise, J.K.; Ilhan, Z.E.; Crowell, M.D.; Rideout, J.R.; Caporaso, J.G.; Rittmann, B.E.; Krajmalnik-Brown, R. Gut Microbial and Short-Chain Fatty Acid Profiles in Adults with Chronic Constipation Before and After Treatment with Lubiprostone. Anaerobe 2015, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan Oligosaccharides Attenuate Loperamide-Induced Constipation Through Regulation of Gut Microbiota in Mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Mokhtare, M.; Alimoradzadeh, R.; Agah, S.; Mirmiranpour, H.; Khodabandehloo, N. The Association Between Modulating Inflammatory Cytokines and Constipation of Geriatrics in Iran. Middle East J. Dig. Dis. 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
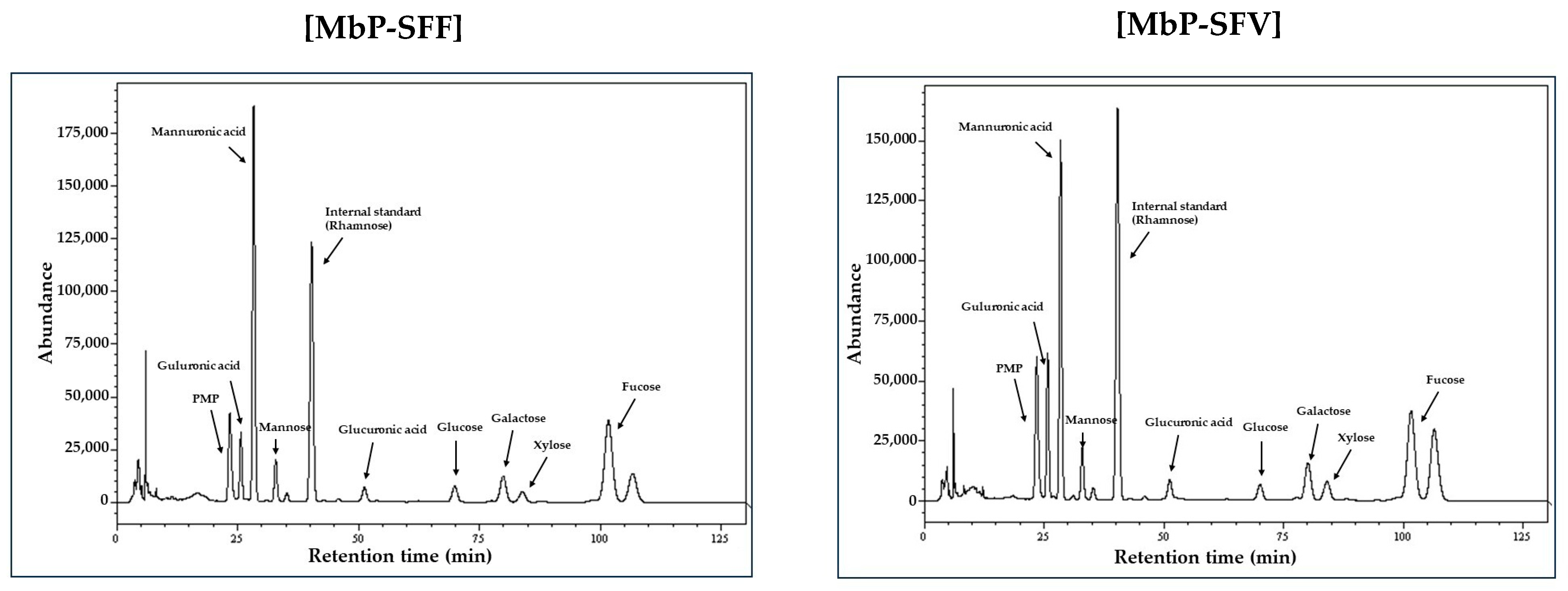
| Nutritional Composition (%) | MbP-SFF | MbP-SFV |
|---|---|---|
| Carbohydrate | 72.45 ± 0.8 | 71.57 ± 1.1 |
| Crude protein | 3.36 ± 0.1 | 4.44 ± 0.4 |
| Crude fat | 0.82 ± 0.7 | 0.98 ± 1.0 |
| Crude fiber | 0.40 ± 0.4 | 0.17 ± 0.1 |
| Crude ash | 22.69 ± 0.2 | 22.53 ± 0.3 |
| Dietary fiber | 66.68 ± 1.2 | 63.02 ± 1.8 |
| Sugar Component (mole %) | MbP-SFF | MbP-SFV |
|---|---|---|
| Guluronic acid | 18.8 ± 0.1 | 32.9 ± 0.3 |
| Mannuronic acid | 52.0 ± 0.7 | 36.9 ± 0.7 |
| Mannose | 2.8 ± 0.1 | 2.8 ± 0.0 |
| Glucuronic acid | 4.3 ± 0.0 | 4.6 ± 0.2 |
| Glucose | 2.1 ± 0.0 | 1.7 ± 0.0 |
| Galactose | 3.4 ± 0.1 | 4.1 ± 0.0 |
| Xylose | 1.6 ± 0.1 | 2.6 ± 0.0 |
| Fucose | 14.9 ± 0.8 | 14.4 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, E.-J.; Shin, K.-S.; Sunwoo, I.Y.; Kim, J.; Choi, W.-Y. Brown Seaweed Byproduct Extracts Improve Intestinal Motility and Auto-Inflammation in Mice with Loperamide-Induced Constipation. Foods 2024, 13, 2037. https://doi.org/10.3390/foods13132037
Koh E-J, Shin K-S, Sunwoo IY, Kim J, Choi W-Y. Brown Seaweed Byproduct Extracts Improve Intestinal Motility and Auto-Inflammation in Mice with Loperamide-Induced Constipation. Foods. 2024; 13(13):2037. https://doi.org/10.3390/foods13132037
Chicago/Turabian StyleKoh, Eun-Jeong, Kwang-Soon Shin, In Yung Sunwoo, Junseong Kim, and Woon-Yong Choi. 2024. "Brown Seaweed Byproduct Extracts Improve Intestinal Motility and Auto-Inflammation in Mice with Loperamide-Induced Constipation" Foods 13, no. 13: 2037. https://doi.org/10.3390/foods13132037
APA StyleKoh, E.-J., Shin, K.-S., Sunwoo, I. Y., Kim, J., & Choi, W.-Y. (2024). Brown Seaweed Byproduct Extracts Improve Intestinal Motility and Auto-Inflammation in Mice with Loperamide-Induced Constipation. Foods, 13(13), 2037. https://doi.org/10.3390/foods13132037





