Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Human LDL Isolation
2.3. LDL Oxidation Assay
2.3.1. Measurement of Conjugated Diene Formation
2.3.2. Measurement of LDL-Tryptophan (TrP) Fluorescence
2.4. Cell Culture Assays
2.4.1. Cell Culture and oxLDL Preparation
2.4.2. Cell Viability Assay
2.4.3. Measurement of Reactive Species (RS) Production
2.4.4. Dihydrorhodamine (DHR) Oxidation
2.4.5. High-Resolution Respirometry of Intact Cells
2.4.6. Cellular Reduced Glutathione (GSH) Levels
2.4.7. Nitrite Assay
2.4.8. Inducible Nitric Oxide Synthase (iNOS) Protein Expression
2.4.9. Nrf2 and Nuclear Factor Kappa B (NF-κB) Protein Expression
2.4.10. Foam Cell Formation Assay
2.5. Statistical Analysis
3. Results
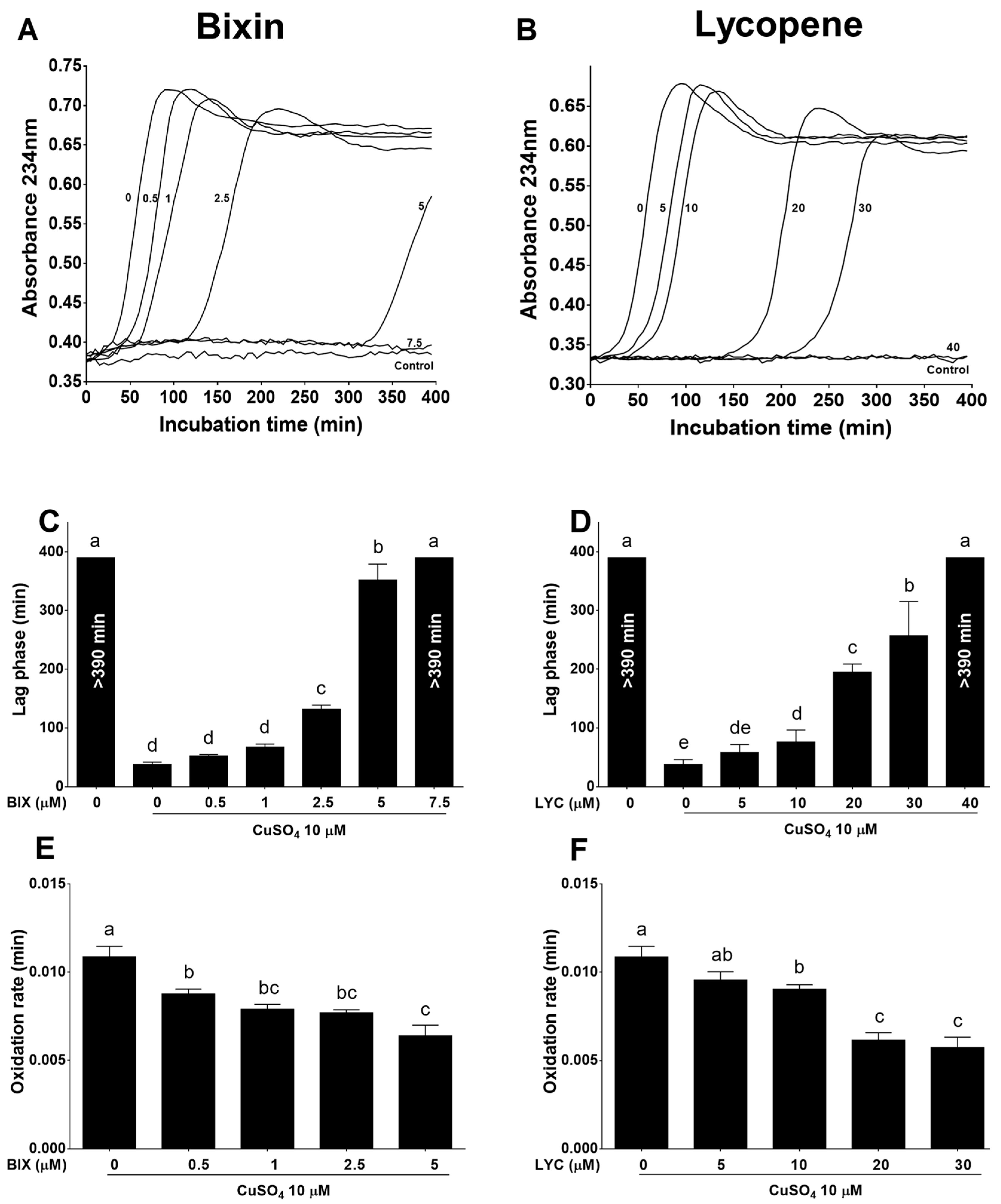
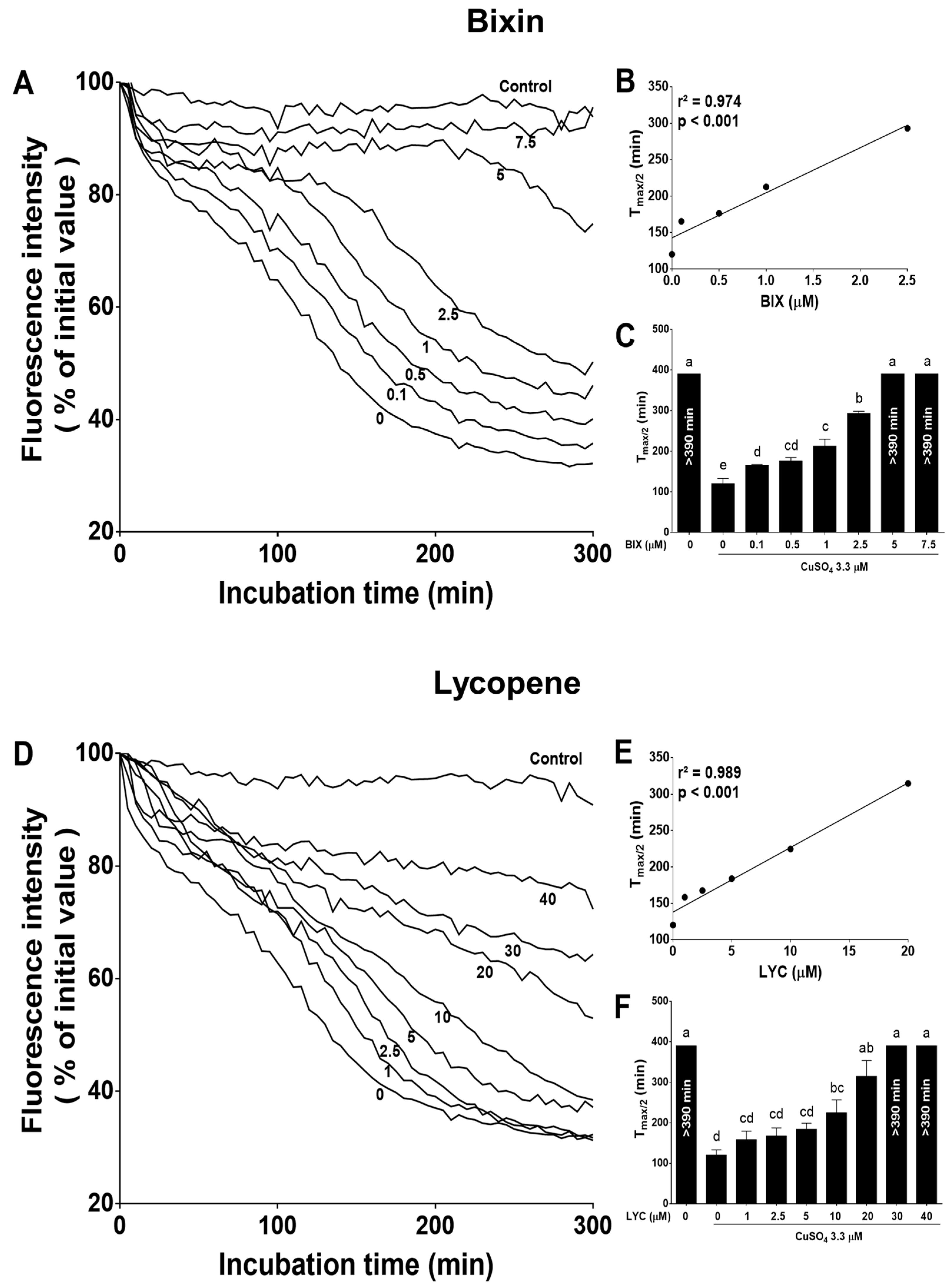
3.1. Bixin Is More Potent Than Lycopene in Inhibiting LDL Oxidation
3.2. Effect of Bixin and Lycopene on Macrophage Cell Viability
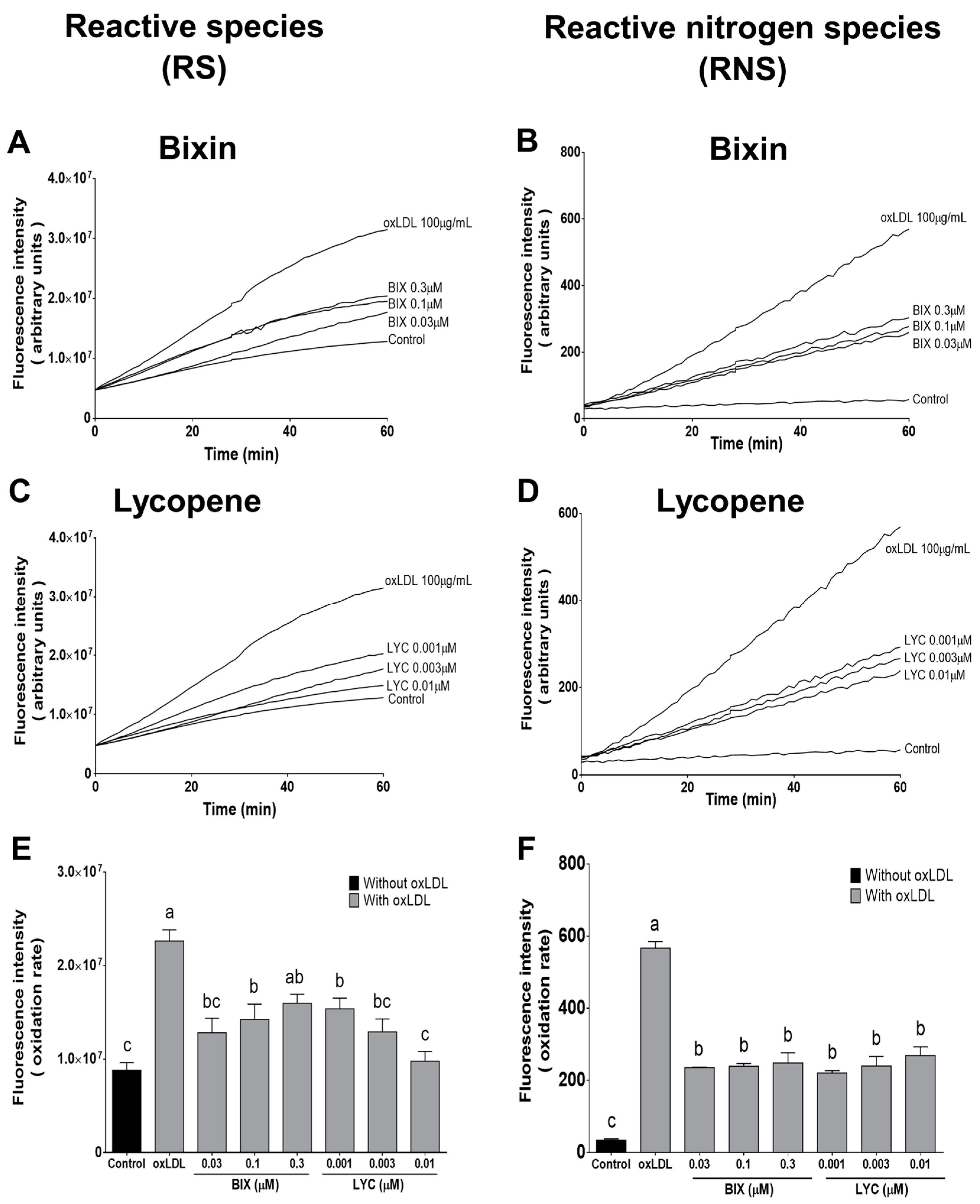
3.3. Bixin and Lycopene Prevent the Generation of Oxidative Species Triggered by oxLDL in Macrophage Cells
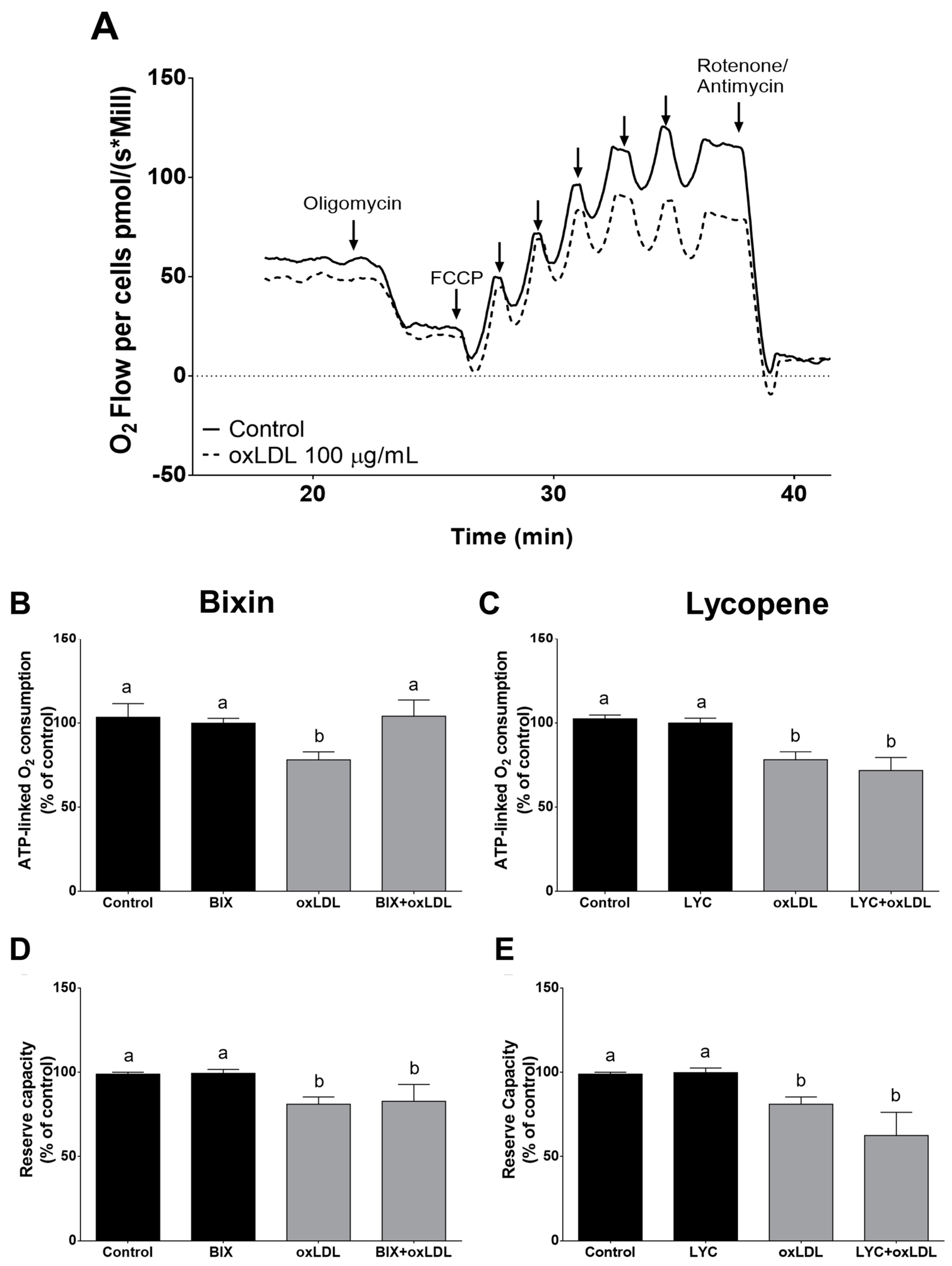
3.4. Effect of Bixin in oxLDL-Induced Mitochondrial Dysfunction
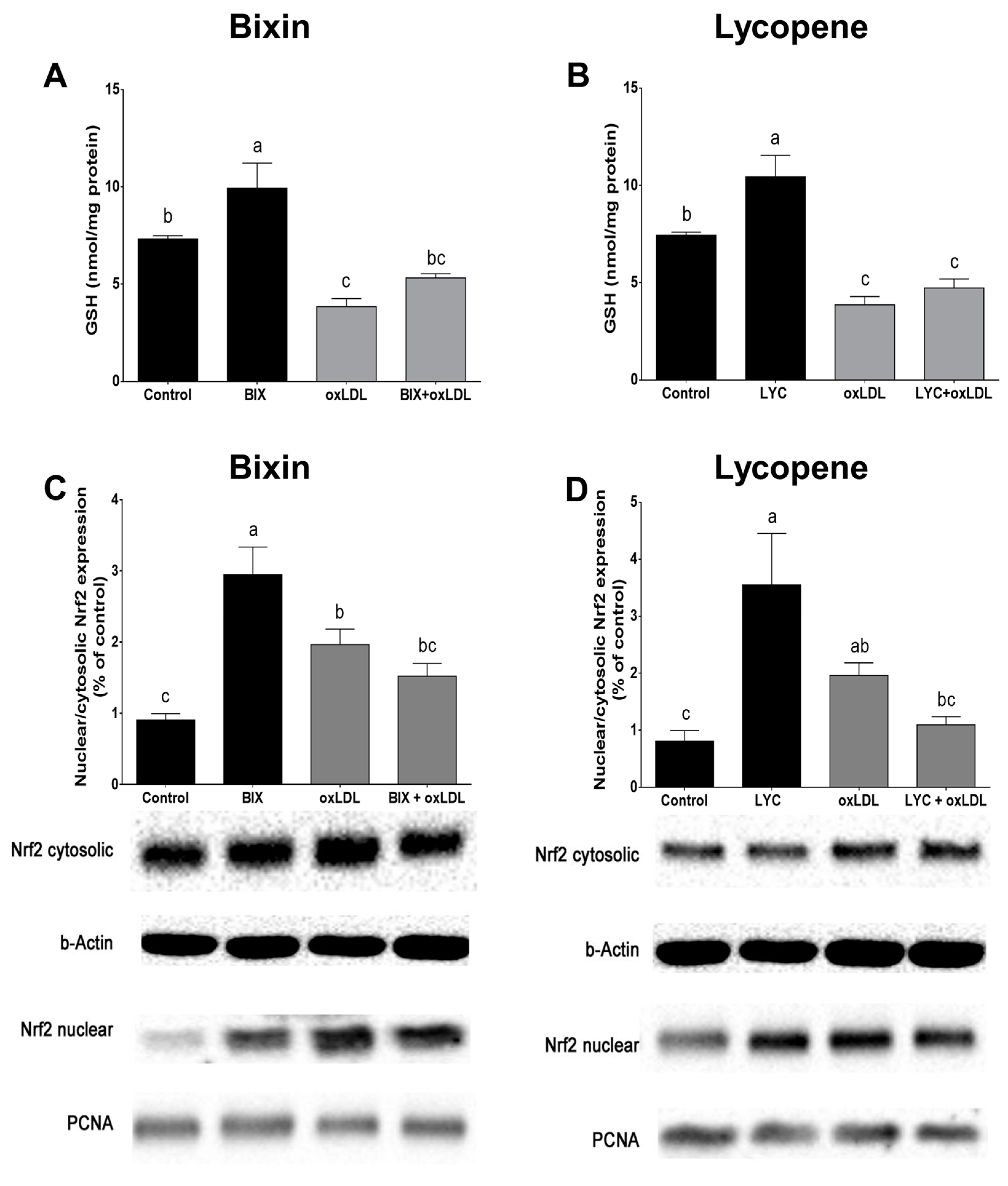
3.5. Bixin Increases GSH Content and Activates Nrf2 in Macrophage Cells
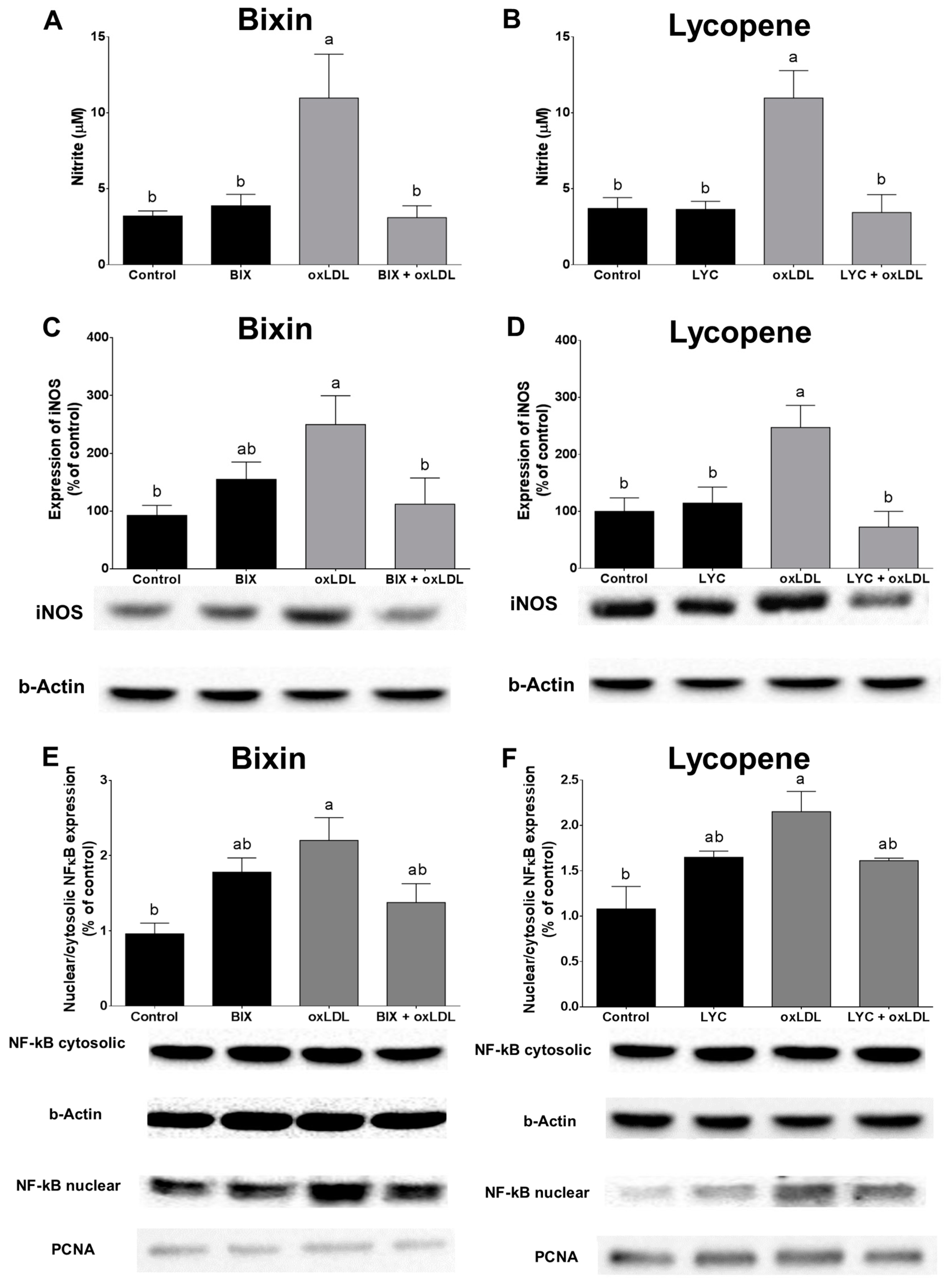
3.6. Bixin and Lycopene Block •NO Production and NF-κB Activation Triggered by oxLDL
3.7. Bixin and Lycopene Reduced oxLDL-Induced Foam Cells Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, P.K. Inflammation, Infection and Atherosclerosis. Trends Cardiovasc. Med. 2019, 29, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent Insights into the Cellular Biology of Atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized Low-Density Lipoprotein and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Salvayre, R.; Negre-Salvayre, A.; Camaré, C. Oxidative Theory of Atherosclerosis and Antioxidants. Biochimie 2016, 125, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Lönn, M.E.; Dennis, J.M.; Stocker, R. Actions of “Antioxidants” in the Protection against Atherosclerosis. Free Radic. Biol. Med. 2012, 53, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress—Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and Lycopene Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Serum Lycopene Concentrations and Carotid Atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2003, 77, 133–138. [Google Scholar] [CrossRef]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A Regular Lycopene Enriched Tomato Sauce Consumption Influences Antioxidant Status of Healthy Young-Subjects: A Crossover Study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
- Erdman, J.W.; Ford, N.A.; Lindshield, B.L. Are the Health Attributes of Lycopene Related to Its Antioxidant Function? Arch. Biochem. Biophys. 2009, 483, 229–235. [Google Scholar] [CrossRef]
- Ulbricht, C.; Windsor, R.C.; Brigham, A.; Bryan, J.K.; Conquer, J.; Costa, D.; Giese, N.; Guilford, J.; Higdon, E.R.B.B.; Holmes, K.; et al. An Evidence-Based Systematic Review of Annatto (Bixa orellana L.) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2012, 9, 57–77. [Google Scholar] [CrossRef]
- Levy, L.W.; Regalado, E.; Navarrete, S.; Watkins, R.H. Bixin and Norbixin in Human Plasma: Determination and Study of the Absorption of a Single Dose of Annatto Food Color. Analyst 1997, 122, 977–980. [Google Scholar] [CrossRef]
- Tao, S.; Park, S.L.; Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Systemic Administration of the Apocarotenoid Bixin Protects Skin against Solar UV-Induced Damage through Activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Somacal, S.; Nichelle, S.M.; Rampelotto, C.; Robalo, S.S.; Roehrs, M.; Emanuelli, T. Short-Term Bixin Supplementation of Healthy Subjects Decreases the Susceptibility of LDL to Cu2+ -Induced Oxidation Ex Vivo. J. Nutr. Metab. 2019, 2019, 9407069. [Google Scholar] [CrossRef]
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto Carotenoids Attenuate Oxidative Stress and Inflammatory Response after High-Calorie Meal in Healthy Subjects. Food Res. Int. 2017, 100, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; Lima, J.L.F.C.; Bragagnolo, N. In Vitro Scavenging Capacity of Annatto Seed Extracts against Reactive Oxygen and Nitrogen Species. Food Chem. 2011, 127, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Devasagayam, T.P.; Kaiser, S.; Sies, H. Carotenoids, Tocopherols and Thiols as Biological Singlet Molecular Oxygen Quenchers. Biochem. Soc. Trans. 1990, 18, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.O.; Antunes, L.M.G.; Bianchi, M.L.P. Bixin and Lycopene Modulation of Free Radical Generation Induced by Cisplatin–DNA Interaction. Food Chem. 2009, 113, 1113–1118. [Google Scholar] [CrossRef]
- Anantharaman, A.; Hemachandran, H.; Mohan, S.; Ayyathan, D.M.; Kumar, D.T.; Doss, C.G.P.; Siva, R. Induction of Apoptosis by Apocarotenoids in B16 Melanoma Cells through ROS-Mediated Mitochondrial-Dependent Pathway. J. Funct. Foods 2016, 20, 346–357. [Google Scholar] [CrossRef]
- Mimura, J.; Itoh, K. Role of Nrf2 in the Pathogenesis of Atherosclerosis. Free Radic. Biol. Med. 2015, 88, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Somacal, S.; Figueiredo, C.G.; Quatrin, A.; Ruviaro, A.R.; Conte, L.; Augusti, P.R.; Roehrs, M.; Denardin, I.T.; Kasten, J.; da Veiga, M.L.; et al. The Antiatherogenic Effect of Bixin in Hypercholesterolemic Rabbits Is Associated to the Improvement of Lipid Profile and to Its Antioxidant and Anti-Inflammatory Effects. Mol. Cell. Biochem. 2015, 403, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, M.; Figueiredo, C.G.; Zanchi, M.M.; Bochi, G.V.; Moresco, R.N.; Quatrin, A.; Somacal, S.; Conte, L.; Emanuelli, T. Bixin and Norbixin Have Opposite Effects on Glycemia, Lipidemia, and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Int. J. Endocrinol. 2014, 2014, 839095. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Goto, T.; Taimatsu, A.; Egawa, K.; Katoh, S.; Kusudo, T.; Sakamoto, T.; Ohyane, C.; Lee, J.-Y.; Kim, Y.-I.; et al. Bixin Regulates MRNA Expression Involved in Adipogenesis and Enhances Insulin Sensitivity in 3T3-L1 Adipocytes through PPARgamma Activation. Biochem. Biophys. Res. Commun. 2009, 390, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takahashi, N.; Kato, S.; Kim, Y.-I.; Kusudo, T.; Taimatsu, A.; Egawa, K.; Kang, M.-S.; Hiramatsu, T.; Sakamoto, T.; et al. Bixin Activates PPARα and Improves Obesity-Induced Abnormalities of Carbohydrate and Lipid Metabolism in Mice. J. Agric. Food Chem. 2012, 60, 11952–11958. [Google Scholar] [CrossRef] [PubMed]
- Kleinveld, H.A.; Hak-Lemmers, H.L.; Stalenhoef, A.F.; Demacker, P.N. Improved Measurement of Low-Density-Lipoprotein Susceptibility to Copper-Induced Oxidation: Application of a Short Procedure for Isolating Low-Density Lipoprotein. Clin. Chem. 1992, 38, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous Monitoring of in Vitro Oxidation of Human Low Density Lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Gieseg, S.P.; Esterbauer, H. Low Density Lipoprotein Is Saturable by Pro-Oxidant Copper. FEBS Lett. 1994, 343, 188–194. [Google Scholar] [CrossRef]
- Giessauf, A.; Steiner, E.; Esterbauer, H. Early Destruction of Tryptophan Residues of Apolipoprotein B Is a Vitamin E-Independent Process during Copper-Mediated Oxidation of LDL. Biochim. Biophys. Acta 1995, 1256, 221–232. [Google Scholar]
- Reyftmann, J.-P.; Santus, R.; Mazière, J.-C.; Morlière, P.; Salmon, S.; Candide, C.; Mazière, C.; Haigle, J. Sensitivity of Tryptophan and Related Compounds to Oxidation Induced by Lipid Autoperoxidation. Application to Human Serum Low- and High-Density Lipoproteins. Biochim. Biophys. Acta Lipids Lipid Metab. 1990, 1042, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Straliotto, M.R.; Hort, M.A.; Fiuza, B.; Rocha, J.B.T.; Farina, M.; Chiabrando, G.; de Bem, A.F. Diphenyl Diselenide Modulates OxLDL-Induced Cytotoxicity in Macrophage by Improving the Redox Signaling. Biochimie 2013, 95, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying Cellular Oxidative Stress by Dichlorofluorescein Assay Using Microplate Reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling Peroxynitrite Formation in Biological Systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Wrona, M.; Patel, K.; Wardman, P. Reactivity of 2′,7′-Dichlorodihydrofluorescein and Dihydrorhodamine 123 and Their Oxidized Forms toward Carbonate, Nitrogen Dioxide, and Hydroxyl Radicals. Free Radic. Biol. Med. 2005, 38, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, B.; Subelzú, N.; Calcerrada, P.; Straliotto, M.R.; Piacenza, L.; Cassina, A.; Rocha, J.B.T.; Radi, R.; De Bem, A.F.; Peluffo, G. Impact of SIN-1-Derived Peroxynitrite Flux on Endothelial Cell Redox Homeostasis and Bioenergetics: Protective Role of Diphenyl Diselenide via Induction of Peroxiredoxins. Free Radic. Res. 2015, 49, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hort, M.A.; Straliotto, M.R.; de Oliveira, J.; Amoêdo, N.D.; da Rocha, J.B.T.; Galina, A.; Ribeiro-do-Valle, R.M.; de Bem, A.F. Diphenyl Diselenide Protects Endothelial Cells against Oxidized Low Density Lipoprotein-Induced Injury: Involvement of Mitochondrial Function. Biochimie 2014, 105, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R. A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Schulz, K.; Kerber, S.; Kelm, M. Reevaluation of the Griess Method for Determining NO/NO−2 in Aqueous and Protein-Containing Samples. Nitric Oxide 1999, 3, 225–234. [Google Scholar] [CrossRef]
- Koopman, R.; Schaart, G.; Hesselink, M.K. Optimisation of Oil Red O Staining Permits Combination with Immunofluorescence and Automated Quantification of Lipids. Histochem. Cell Biol. 2001, 116, 63–68. [Google Scholar] [CrossRef] [PubMed]
- de Bem, A.F.; Fiuza, B.; Calcerrada, P.; Brito, P.M.; Peluffo, G.; Dinis, T.C.P.; Trujillo, M.; Rocha, J.B.T.; Radi, R.; Almeida, L.M. Protective Effect of Diphenyl Diselenide against Peroxynitrite-Mediated Endothelial Cell Death: A Comparison with Ebselen. Nitric Oxide 2013, 31, 20–30. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R.; Ballinger, S.; Dell’Italia, L.; Zhang, J.; Darley-Usmar, V.M. Integration of Cellular Bioenergetics with Mitochondrial Quality Control and Autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef]
- Young, I.S.; McEneny, J. Lipoprotein Oxidation and Atherosclerosis. Biochem. Soc. Trans. 2001, 29, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Straliotto, M.R.; Mancini, G.; Figueiredo, C.P.; Braga, A.L.; Teixeira, J.B.R.; Bem, A.F. Atheroprotective Action of a Modified Organoselenium Compound: In Vitro Evidence. Acad. Bras. Cienc. 2016, 88, 1953–1965. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Chisté, R.C.; Mercadante, A.Z. Development of a Novel Micro-Assay for Evaluation of Peroxyl Radical Scavenger Capacity: Application to Carotenoids and Structure–Activity Relationship. Food Chem. 2012, 135, 2103–2111. [Google Scholar] [CrossRef]
- Deng, T.; Xu, K.; Zhang, L.; Zheng, X. Dynamic Determination of Ox-LDL-Induced Oxidative/Nitrosative Stress in Single Macrophage by Using Fluorescent Probes. Cell Biol. Int. 2008, 32, 1425–1432. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The Role of Oxidative Stress, Antioxidants and Vascular Inflammation in Cardiovascular Disease (a Review). Vasc. Pharmacolology 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.-Y.; Kong, A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Asmis, R.; Begley, J.G. Oxidized LDL Promotes Peroxide-Mediated Mitochondrial Dysfunction and Cell Death in Human Macrophages: A Caspase-3-Independent Pathway. Circ. Res. 2003, 92, 20e–29e. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, Pathophysiology and Development of Therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.L.; Jaramillo, M.L.; Galant, L.S.; Engel, D.; Dafre, A.L.; Teixeira da Rocha, J.B.; Radi, R.; Farina, M.; de Bem, A.F. Diphenyl Diselenide Protects Neuronal Cells against Oxidative Stress and Mitochondrial Dysfunction: Involvement of the Glutathione-Dependent Antioxidant System. Redox Biol. 2019, 20, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Bai, S.-K.; Lee, K.-S.; Namkoong, S.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Yim, S.-V.; Chang, K.; Kwon, Y.-G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IkB Kinase-Dependent NF-KB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene Inhibits Inflammatory Gene Expression in Lipopolysaccharide-Stimulated Macrophages by Suppressing Redox-Based NF-ΚB Activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Takahashi, T.; Kapahi, P.; Delhase, M.; Chen, Y.; Makris, C.; Rothwarf, D.; Baud, V.; Natoli, G.; Guido, F.; et al. Oxidative Stress and Gene Expression: The AP-1 and NF-ΚB Connections. BioFactors 2001, 15, 87–89. [Google Scholar] [CrossRef]
- Wiesner, P.; Choi, S.-H.; Almazan, F.; Benner, C.; Huang, W.; Diehl, C.J.; Gonen, A.; Butler, S.; Witztum, J.L.; Glass, C.K.; et al. Low Doses of Lipopolysaccharide and Minimally Oxidized Low-Density Lipoprotein Cooperatively Activate Macrophages via Nuclear Factor B and Activator Protein-1: Possible Mechanism for Acceleration of Atherosclerosis by Subclinical Endotoxemia. Circ. Res. 2010, 107, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-Antioxidant Signaling Attenuates NFκB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The Immune Response in Atherosclerosis: A Double-Edged Sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Rios, F.J.O.; Koga, M.M.; Ferracini, M.; Jancar, S. Co-Stimulation of PAFR and CD36 Is Required for OxLDL-Induced Human Macrophages Activation. PLoS ONE 2012, 7, e36632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somacal, S.; Schüler da Silva, L.C.; de Oliveira, J.; Emanuelli, T.; Fabro de Bem, A. Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways. Foods 2024, 13, 2002. https://doi.org/10.3390/foods13132002
Somacal S, Schüler da Silva LC, de Oliveira J, Emanuelli T, Fabro de Bem A. Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways. Foods. 2024; 13(13):2002. https://doi.org/10.3390/foods13132002
Chicago/Turabian StyleSomacal, Sabrina, Luana Caroline Schüler da Silva, Jade de Oliveira, Tatiana Emanuelli, and Andreza Fabro de Bem. 2024. "Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways" Foods 13, no. 13: 2002. https://doi.org/10.3390/foods13132002
APA StyleSomacal, S., Schüler da Silva, L. C., de Oliveira, J., Emanuelli, T., & Fabro de Bem, A. (2024). Bixin, a New Atheroprotective Carotenoid Candidate, Prevents oxLDL-Induced Cytotoxicity and Mitochondrial Dysfunction in Macrophages: Involvement of the Nrf2 and NF-κB Pathways. Foods, 13(13), 2002. https://doi.org/10.3390/foods13132002







