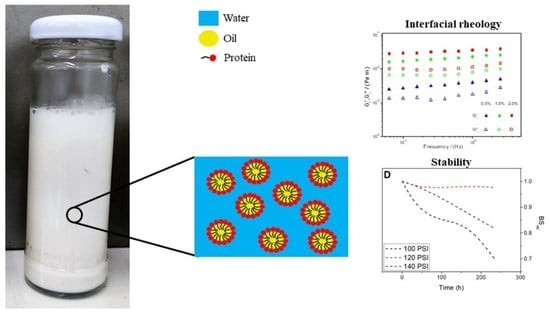
Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition of Protein Isolate
2.3. Amino Acid Composition
2.4. ζ-Potential
2.5. Solubility
2.6. Surface Tension
2.7. Interfacial Rheology
2.8. Preparation of the Nanoemulsion
2.8.1. Pre-Emulsion Preparation
2.8.2. Emulsion Preparation
2.9. Nanoemulsion Characterization
2.9.1. BackScattering
2.9.2. Microstructure
2.9.3. Rheology
2.10. Statistical Analysis
3. Results & Discussion
3.1. Chemical Composition of the Protein Isolate
3.2. Characterization of the Aqueous Phase
3.2.1. ζ-Potential and Solubility
3.2.2. Surface Tension
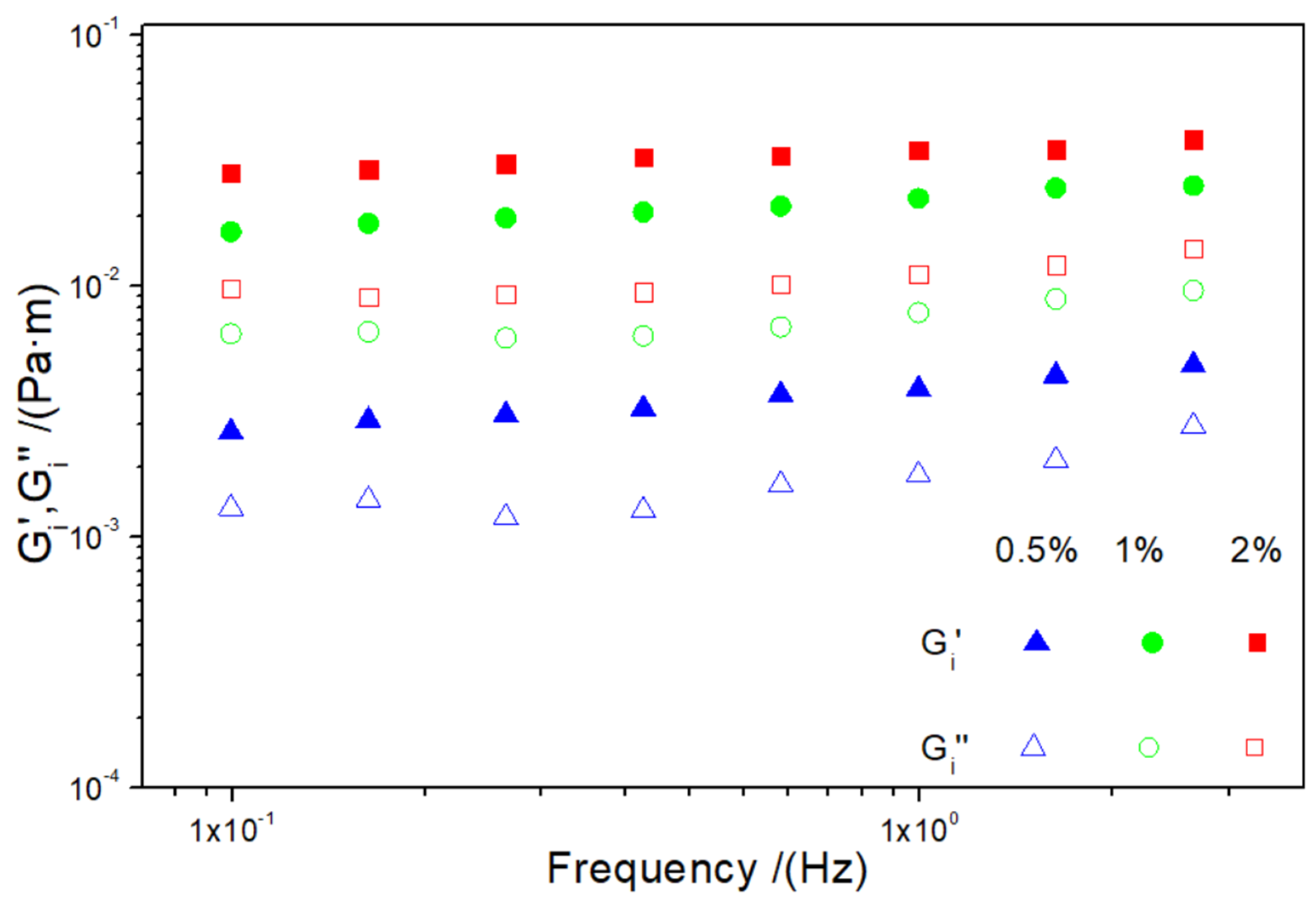
3.2.3. Interfacial Rheology
3.3. Characterization of the Nanoemulsions
3.3.1. Backscattering
3.3.2. Droplet Size Distribution
3.3.3. Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brouwer, I.D.; van Liere, M.J.; de Brauw, A.; Dominguez-Salas, P.; Herforth, A.; Kennedy, G.; Lachat, C.; Omosa, E.B.; Talsma, E.F.; Vandevijvere, S.; et al. Reverse Thinking: Taking a Healthy Diet Perspective towards Food Systems Transformations. Food Secur. 2021, 13, 1497–1523. [Google Scholar] [CrossRef]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Ross, S. Functional Foods: The Food and Drug Administration Perspective. Am. J. Clin. Nutr. 2000, 71, 1735S–1738S. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Das, M. Functional Foods: An Overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Kasote, D.; Tiozon, R.N.; Sartagoda, K.J.D.; Itagi, H.; Roy, P.; Kohli, A.; Regina, A.; Sreenivasulu, N. Food Processing Technologies to Develop Functional Foods With Enriched Bioactive Phenolic Compounds in Cereals. Front. Plant Sci. 2021, 12, 771276. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of Active Ingredients in Functional Foods: From Research Stage to Commercial Food Products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Varvara, R.-A.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemeş, S.-A.; Mitrea, L.; Martău, G.A.; Ciont, C.; Călinoiu, L.F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients: Characterization, Applicability, and Human Health Benefits. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Tomašević, I.; Putnik, P.; Valjak, F.; Pavlić, B.; Šojić, B.; Bebek Markovinović, A.; Bursać Kovačević, D. 3D Printing as Novel Tool for Fruit-Based Functional Food Production. Curr. Opin. Food Sci. 2021, 41, 138–145. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Bush, L.; Stevenson, L.; Lane, K.E. The Oxidative Stability of Omega-3 Oil-in-Water Nanoemulsion Systems Suitable for Functional Food Enrichment: A Systematic Review of the Literature. Crit. Rev. Food Sci. Nutr. 2019, 59, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-Term Stability of Food-Grade Nanoemulsions from High Methoxyl Pectin Containing Essential Oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, Structure, and Physical Properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Friberg, S.; Larsson, K.; Sjoblom, J. (Eds.) Food Emulsions; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203913222. [Google Scholar]
- Carrera-Sánchez, C.; Félix, M.; Bengoechea, C.; Romero, A.; Guerrero, A. Development of Complex Interfaces for the Encapsulation of Bioactive Ingredients to Promote Healthy and Nutritional Food Products. In Delivering Functionality in Foods; Springer: Cham, Switzerland, 2022; pp. 69–104. [Google Scholar]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in Natural Emulsifiers for Utilization in Food Emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Hoffmann, H.; Reger, M. Emulsions with Unique Properties from Proteins as Emulsifiers. Adv. Colloid Interface Sci. 2014, 205, 94–104. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.D.A.; Aquino, A.; Das Neves, M.A.; Conte-Junior, C.A. Nanoemulsions: Using Emulsifiers from Natural Sources Replacing Synthetic Ones—A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E. Interfacial Antioxidants: A Review of Natural and Synthetic Emulsifiers and Coemulsifiers That Can Inhibit Lipid Oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura Datos de Alimentación y Agricultura. Available online: https://www.fao.org (accessed on 29 November 2022).
- Peisker, M. Manufacturing of Soy Protein Concentrate for Animal Nutrition. In Feed Manufacturing in the Mediterranean Region. Improving Safety: From Feed to Food; Brufau, J., Ed.; Cahiers Options Méditerranéennes; CIHEAM: Zaragoza, Spain, 2001; Volume 54, pp. 103–107. [Google Scholar]
- Ahmad, N.; Sahrin, N.; Talib, N.; Abdul Ghani, F. Characterization of Energy Content in Food Waste by Using Thermogravimetric Analyser (TGA) and Elemental Analyser (CHNS-O). J. Phys. Conf. Ser. 2019, 1349, 012140. [Google Scholar] [CrossRef]
- Zarnowski, R.; Suzuki, Y. Expedient Soxhlet Extraction of Resorcinolic Lipids from Wheat Grains. J. Food Compos. Anal. 2004, 17, 649–663. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005; ISBN 9780935584752. [Google Scholar]
- Felix, M.; Perez-Puyana, V.; Romero, A.; Guerrero, A. Development of Thermally Processed Bioactive Pea Protein Gels: Evaluation of Mechanical and Antioxidant Properties. Food Bioprod. Process. 2017, 101, 74–83. [Google Scholar] [CrossRef]
- Sarkar, A.; Kamaruddin, H.; Bentley, A.; Wang, S. Emulsion Stabilization by Tomato Seed Protein Isolate: Influence of PH, Ionic Strength and Thermal Treatment. Food Hydrocoll. 2016, 57, 160–168. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of Pea Protein-Based Bioplastics Processed by Injection Moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Li, Q.; Niu, J. Surface Tension, Interfacial Tension and Emulsification of Sodium Dodecyl Sulfate Extended Surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 201–208. [Google Scholar] [CrossRef]
- Wu, N.; Dai, J.; Micale, F.J. Dynamic Surface Tension Measurement with a Dynamic Wilhelmy Plate Technique. J. Colloid Interface Sci. 1999, 215, 258–269. [Google Scholar] [CrossRef]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2006, 70, R54–R66. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Sanchez, C.C.; Guerrero, A. Modelling the Non-Linear Interfacial Shear Rheology Behaviour of Chickpea Protein-Adsorbed Complex Oil/Water Layers. Appl. Surf. Sci. 2019, 469, 792–803. [Google Scholar] [CrossRef]
- Brennan, J.G.; Grandison, A.S. (Eds.) Food Processing Handbook; Wiley: Hoboken, NJ, USA, 2011; ISBN 9783527324682. [Google Scholar]
- Berk, Z. Mixing. In Food Process Engineering and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 175–194. [Google Scholar]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and Stability Evaluation of β-Carotene Nanoemulsions Prepared by High Pressure Homogenization under Various Emulsifying Conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Phipps, L.W. The Fragmentation of Oil Drops in Emulsions by a High-Pressure Homogenizer. J. Phys. D Appl. Phys. 1975, 8, 448–462. [Google Scholar] [CrossRef]
- Mengual, O. TURBISCAN MA 2000: Multiple Light Scattering Measurement for Concentrated Emulsion and Suspension Instability Analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Cerimedo, M.S.; Iriart, C.H.; Candal, R.J.; Herrera, M.L. Stability of Emulsions Formulated with High Concentrations of Sodium Caseinate and Trehalose. Food Res. Int. 2010, 43, 1482–1493. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of High-Pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Stojanović, Z.S.; Marković, S.; Uskoković, D. Determination of Particle Size Distributions by Laser Diffraction. In Technics—New Materials (Special Edition); Union of Engineers and Technicians of Serbia: Belgrade, Serbia, 2012; pp. 11–20. [Google Scholar]
- Pearson, A.M. Soy proteins. In Developments in Food Proteins; BJF Hudson: London, UK; Englewood, NJ, USA, 1983; pp. 67–108. [Google Scholar]
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, Cereals, Seeds and Legumes Proteins Derived Emulsifiers as a Source of Plant Protein Beverages: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef]
- Romero, A.; Beaumal, V.; David-Briand, E.; Cordobés, F.; Guerrero, A.; Anton, M. Interfacial and Oil/Water Emulsions Characterization of Potato Protein Isolates. J. Agric. Food Chem. 2011, 59, 9466–9474. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Maigret, J.-E.; Perez-Puyana, V.; Romero, A.; Lourdin, D. Revaluation of a Soy Protein By-Product in Eco-Friendly Bioplastics by Extrusion. J. Polym. Environ. 2022, 30, 1587–1599. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic Acid as Anticancer Agent: An Overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of β-Alanine Supplementation and High-Intensity Interval Training on Endurance Performance and Body Composition in Men; a Double-Blind Trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Coupland, J.N. The Effect of Surfactants on the Solubility, Zeta Potential, and Viscosity of Soy Protein Isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Kozlowski, L.P. IPC—Isoelectric Point Calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; Abdel-Razek, A.G.; Gromadzka, K.; Badr, A.N. Prevention of Aflatoxin Occurrence Using Nuts-Edible Coating of Ginger Oil Nanoemulsions and Investigate the Molecular Docking Strategy. Plants 2022, 11, 2228. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Kamysz, E.; Sikorska, E.; Puchalski, C.; Mleko, S.; Ozimek, L.; Kowaluk, G.; Gustaw, W.; Wesołowska-Trojanowska, M. Changes of Secondary Structure and Surface Tension of Whey Protein Isolate Dispersions upon PH and Temperature. Czech J. Food Sci. 2014, 32, 82–89. [Google Scholar] [CrossRef]
- Miller, R.; Fainerman, V.B.; Leser, M.E.; Michel, M. Surface Tension of Mixed Non-Ionic Surfactant/Protein Solutions: Comparison of a Simple Theoretical Model with Experiments. Colloids Surf. A Physicochem. Eng. Asp. 2004, 233, 39–42. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, X.; Zeng, M.; He, Z.; Chen, J. Interfacial Rheology and Foaming Properties of Soy Protein and Hydrolysates under Acid Condition. Food Biophys. 2021, 16, 484–491. [Google Scholar] [CrossRef]
- Wilde, P.J. Interfaces: Their Role in Foam and Emulsion Behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Zhao, X. Interfacial Rheology of Alkali PH-Shifted Myofibrillar Protein at O/W Interface and Impact of Tween 20 Displacement. Food Hydrocoll. 2022, 124, 107275. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Optimization of Nano-Emulsions Production by Microfluidization. Eur. Food Res. Technol. 2007, 225, 733–741. [Google Scholar] [CrossRef]
- Tadros, T. Emulsion Formation and Stability; Tadros, T.F., Ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 9783527319916. [Google Scholar]





| wt% Dry Basis | |
|---|---|
| Moisture | 6.0 ± 2.3 a |
| Lipids | 1.0 ± 0.5 b |
| Carbohydrates | 0.1 ± 0.3 b |
| Ashes | 5.0 ± 1.2 a |
| Proteins | 90.0 ± 1.0 c |
| Amino Acids | Concentration (%) |
|---|---|
| * Aspartic acid(Asp) | 8.2 |
| * Threonine (Thr) | 2.5 |
| * Serine (Ser) | 1.0 |
| * Glutamic acid (Glu) | 21.7 |
| * Glycine (Gly) | 7.4 |
| ** Alanine (Ala) | 10.4 |
| * Cysteine (Cys) | 1.7 |
| ** Valine (Val) | 5.9 |
| Methionine (Met) | 0.9 |
| ** Isoleucine (Ile) | 4.4 |
| ** Leucine (Leu) | 8.6 |
| Tryptophan (Trp) | <0.1 |
| * Tyrosine (Tyr) | 3.0 |
| Phenylalanine (Phe) | 4.2 |
| * Histidine (His) | 1.4 |
| * Lysine (Lys) | 5.6 |
| Proline (Pro) | 7.8 |
| * Arginine (Arg) | 5.3 |
| 0.5 wt% | 1.0 wt% | 2.0 wt% | |
|---|---|---|---|
| G′i1 (mPa·m) | 3.71 a | 20.89 b | 33.10 c |
| tan δi1 (-) | 0.437 B | 0.330 A | 0.309 A |
| t (Days) | D3,2 (nm) | D4,3 (nm) | U |
|---|---|---|---|
| 0 | 162 ± 2.1 a | 459 ± 7 A | 1.6 α |
| 3 | 174 ± 1.4 b | 550 ± 6 C | 1.8 α |
| 7 | 173 ± 10 ab | 490 ± 4 B | 1.6 α |
| 19 | 171 ± 14 ab | 922 ± 122 D | 3.6 β |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Criado, D.; Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation. Foods 2023, 12, 507. https://doi.org/10.3390/foods12030507
Castro-Criado D, Jiménez-Rosado M, Perez-Puyana V, Romero A. Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation. Foods. 2023; 12(3):507. https://doi.org/10.3390/foods12030507
Chicago/Turabian StyleCastro-Criado, Daniel, Mercedes Jiménez-Rosado, Víctor Perez-Puyana, and Alberto Romero. 2023. "Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation" Foods 12, no. 3: 507. https://doi.org/10.3390/foods12030507
APA StyleCastro-Criado, D., Jiménez-Rosado, M., Perez-Puyana, V., & Romero, A. (2023). Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation. Foods, 12(3), 507. https://doi.org/10.3390/foods12030507