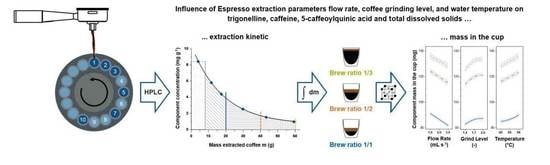
Influence of Flow Rate, Particle Size, and Temperature on Espresso Extraction Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Coffee Beans and Roasting
2.3. Brewing Water
2.4. Coffee Puck Preparation
2.5. Espresso Coffee Preparation
2.6. HPLC Analysis
2.7. Determination of TDS
2.8. Data Processing and Statistical Analysis
2.8.1. Extraction Kinetics Fitting
2.8.2. Calculation of Component Mass in EC Cup
2.8.3. Statistical Analysis
3. Results & Discussion
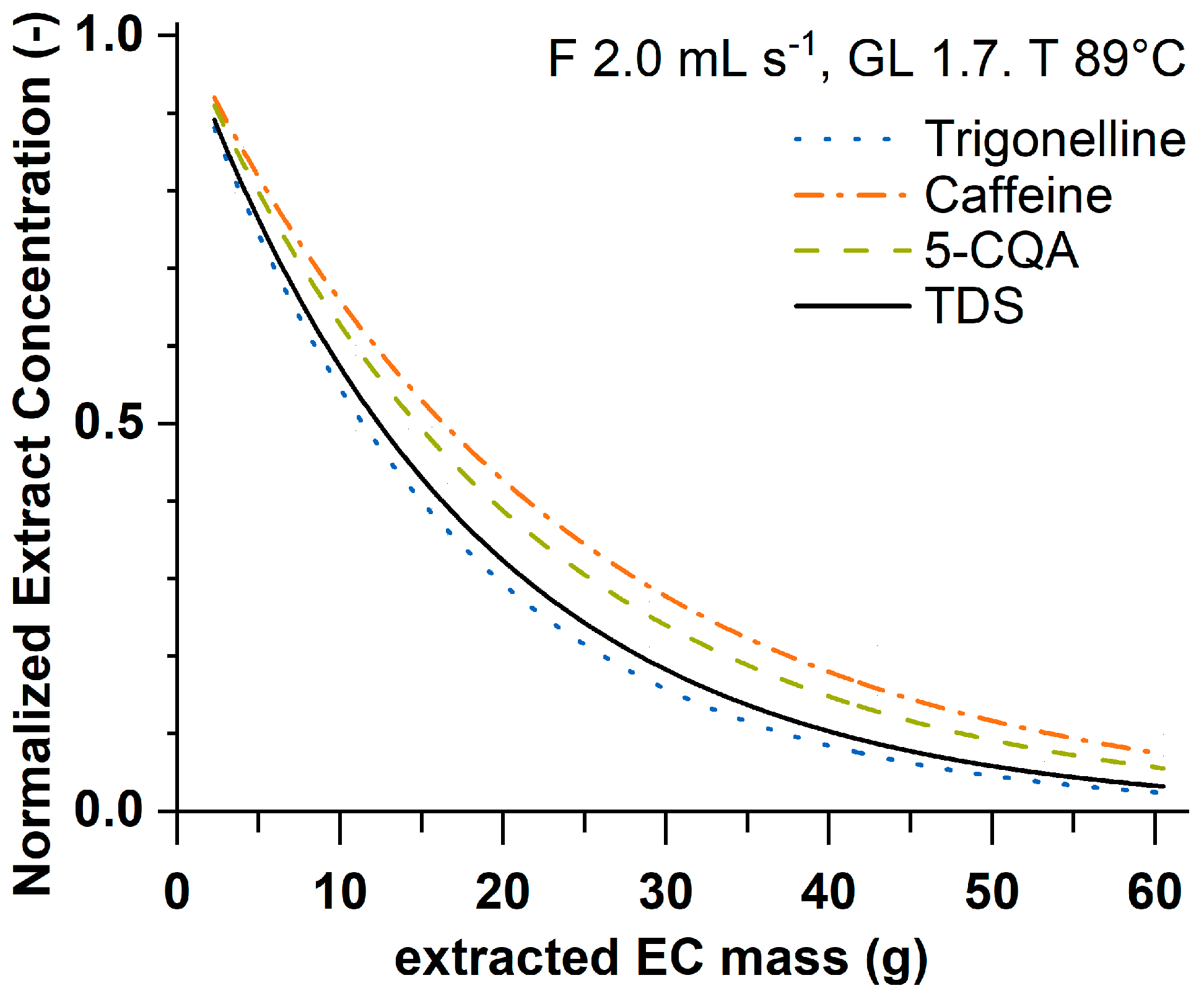
3.1. Extraction Kinetics
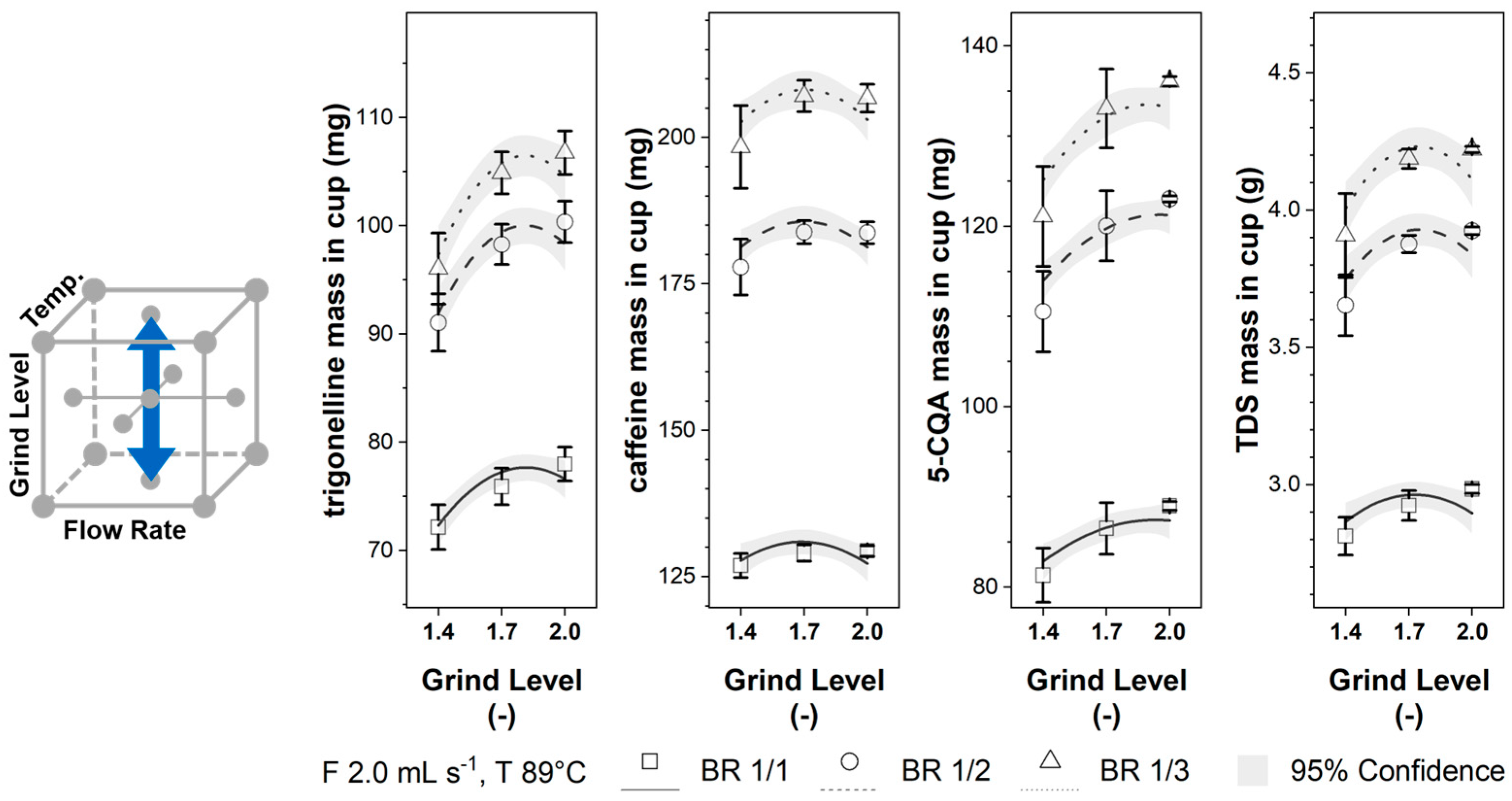
3.2. Extracted Component Mass in the Cup for Brew Ratios 1/1, 1/2, and 1/3
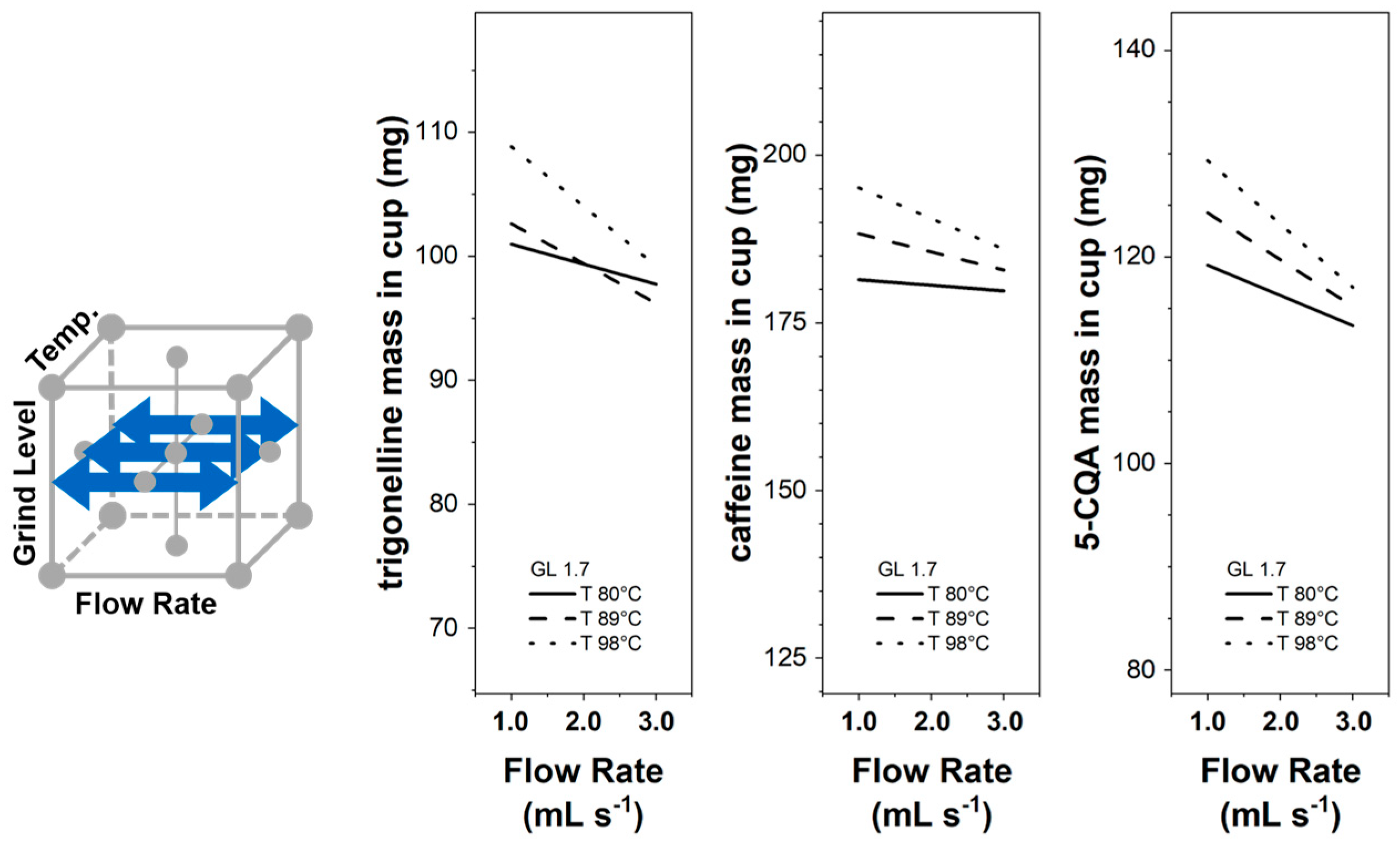
3.3. Influencing the EC Component Mass in the Cup
3.3.1. Linear Response Surfaces
3.3.2. Flow Rate Influence
3.3.3. Grinding Level Influence
3.3.4. Temperature Influence
3.3.5. Interactive Influences
3.3.6. Brew Ratio Influence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Experiment | c(m∑) = c0 exp(−m∑/λ) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigonelline | Caffeine | |||||||||||
| c0 (mg g−1) | λ (g) | Statistics | c0 (mg g−1) | λ (g) | Statistics | |||||||
| Value | SE | Value | SE | Red. Chi-Sqr | Adj. R2 | Value | SE | Value | SE | Red. Chi-Sqr | Adj. R2 | |
| 1 | 7.40899 | 0.12316 | 14.76344 | 0.42552 | 0.02561 | 0.99481 | 10.4887 | 0.14327 | 20.52467 | 0.5199 | 0.04752 | 0.99493 |
| 2 | 6.46932 | 0.23064 | 16.40303 | 0.86193 | 0.05312 | 0.9806 | 9.46765 | 0.28924 | 23.93518 | 1.18507 | 0.13117 | 0.97824 |
| 3 | 6.54716 | 0.23705 | 14.93682 | 0.84702 | 0.06872 | 0.97702 | 9.66427 | 0.27442 | 21.96458 | 1.0845 | 0.14334 | 0.97831 |
| 4 | 6.83473 | 0.11231 | 15.99516 | 0.42845 | 0.01714 | 0.99521 | 9.67546 | 0.15595 | 23.05736 | 0.66335 | 0.04944 | 0.99303 |
| 5 | 7.30706 | 0.16914 | 15.42548 | 0.57294 | 0.03804 | 0.9907 | 10.38155 | 0.30436 | 21.59864 | 1.08663 | 0.174 | 0.97851 |
| 6 | 6.95468 | 0.10214 | 15.7859 | 0.3698 | 0.01422 | 0.99616 | 10.04235 | 0.15921 | 22.39093 | 0.62077 | 0.05039 | 0.99327 |
| 7 | 6.69949 | 0.09587 | 16.08737 | 0.36681 | 2.47 × 10−2 | 0.99227 | 9.70981 | 0.13064 | 23.09434 | 0.5462 | 0.06781 | 0.9897 |
| 8 | 7.62541 | 0.09065 | 13.12089 | 0.27877 | 0.01422 | 0.9973 | 10.69172 | 0.15672 | 18.92703 | 0.52514 | 0.0595 | 0.99409 |
| 9 | 7.08449 | 0.1493 | 15.63306 | 0.56872 | 0.03722 | 0.99154 | 10.69195 | 0.19535 | 21.67685 | 0.73662 | 0.08821 | 0.99072 |
| 10 | 7.34872 | 0.18011 | 14.07208 | 0.54291 | 0.03783 | 0.99095 | 10.13843 | 0.25125 | 19.75409 | 0.82583 | 0.10739 | 0.98646 |
| 11 | 7.57984 | 0.13439 | 15.04329 | 0.43899 | 0.02663 | 0.99434 | 10.45723 | 0.1349 | 21.14279 | 0.4897 | 0.03828 | 0.99552 |
| 12 | 6.17348 | 0.332 | 15.48473 | 1.32764 | 0.13618 | 0.94866 | 8.97304 | 0.39549 | 23.57685 | 1.85949 | 0.3117 | 0.94656 |
| 13 | 6.50908 | 0.26715 | 15.53493 | 1.03374 | 0.09268 | 0.97024 | 9.67713 | 0.31126 | 23.15069 | 1.31948 | 0.19488 | 0.97264 |
| 14 | 6.47069 | 0.1751 | 16.63498 | 0.70499 | 0.03894 | 0.98677 | 9.03485 | 0.21274 | 24.1222 | 0.98821 | 0.08722 | 0.98465 |
| 15 | 6.62197 | 0.14144 | 16.37875 | 0.56426 | 0.02867 | 0.9911 | 9.45174 | 0.17933 | 23.78621 | 0.80464 | 0.06779 | 0.98933 |
| Experiment | 5-CQA | TDS | ||||||||||
| c0 (mg g−1) | λ (g) | Statistics | c0 (g g−1) | λ (g) | Statistics | |||||||
| Value | SE | Value | SE | Red. Chi-Sqr | Adj. R2 | Value | SE | Value | SE | Red. Chi-Sqr | Adj. R2 | |
| 1 | 7.32493 | 0.1108 | 19.11304 | 0.52774 | 0.02668 | 0.99429 | 0.28305 | 0.00386 | 15.70033 | 0.37567 | 2.68 × 10−5 | 0.9962 |
| 2 | 6.35357 | 0.22661 | 21.1787 | 1.18384 | 0.07051 | 0.97413 | 0.23924 | 0.00955 | 17.83024 | 1.06401 | 1.02 × 10−4 | 0.97201 |
| 3 | 6.47778 | 0.22434 | 19.62703 | 1.14453 | 0.08511 | 0.97164 | 0.24196 | 0.00933 | 16.59384 | 1.03039 | 1.21 × 10−4 | 0.97078 |
| 4 | 6.92748 | 0.11967 | 20.80018 | 0.62438 | 0.02622 | 0.99288 | 0.25164 | 0.00514 | 17.31233 | 0.58753 | 3.94 × 10−5 | 0.99185 |
| 5 | 7.08289 | 0.1861 | 19.75796 | 0.87311 | 0.05984 | 0.98429 | 0.2474 | 0.00636 | 17.98499 | 0.75292 | 6.37 × 10−5 | 0.98585 |
| 6 | 6.80018 | 0.12522 | 20.60333 | 0.6479 | 0.02871 | 0.9918 | 0.25713 | 0.00367 | 17.3144 | 0.40346 | 2.05 × 10−5 | 0.99593 |
| 7 | 6.79246 | 0.10682 | 20.77137 | 0.55683 | 4.08 × 10−2 | 0.98752 | 0.24827 | 0.00419 | 17.47261 | 0.48029 | 5.20 × 10−5 | 0.98811 |
| 8 | 7.31745 | 0.09572 | 17.29798 | 0.4219 | 0.02055 | 0.99572 | 0.30725 | 0.00999 | 13.16232 | 0.76497 | 1.73 × 10−4 | 0.97956 |
| 9 | 7.32619 | 0.12423 | 19.92738 | 0.61563 | 0.03302 | 0.99274 | 0.27778 | 0.00462 | 16.50264 | 0.47902 | 3.77 × 10−5 | 0.99428 |
| 10 | 7.18869 | 0.17832 | 18.48345 | 0.76212 | 0.05056 | 0.98742 | 0.2599 | 0.00608 | 15.62012 | 0.58694 | 4.89 × 10−5 | 0.99069 |
| 11 | 7.57346 | 0.10943 | 19.56499 | 0.49683 | 0.02338 | 0.99488 | 0.27975 | 0.00446 | 16.03038 | 0.42671 | 3.15 × 10−5 | 0.99499 |
| 12 | 6.02455 | 0.29418 | 20.79431 | 1.75317 | 0.15129 | 0.94255 | 0.22578 | 0.01287 | 17.38228 | 1.62994 | 2.36 × 10−4 | 0.93514 |
| 13 | 6.53399 | 0.25585 | 20.40671 | 1.37513 | 0.11588 | 0.96435 | 0.24308 | 0.01083 | 17.58935 | 1.30555 | 1.76 × 10−4 | 0.96041 |
| 14 | 6.35075 | 0.15431 | 21.56882 | 0.88151 | 0.04089 | 0.98564 | 0.22567 | 0.00635 | 18.4123 | 0.83369 | 5.79 × 10−5 | 0.98403 |
| 15 | 6.76424 | 0.14962 | 21.01016 | 0.79908 | 0.04192 | 0.98738 | 0.24533 | 0.00629 | 17.69504 | 0.74554 | 6.18 × 10−5 | 0.98599 |
References
- Petracco, M. Technology IV: Beverage Preparation: Brewing Trends for the New Millennium. In Coffee; Clarke, R.J., Vitzthum, O.G., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 140–164. ISBN 9780470690499. [Google Scholar]
- Bee, S.; Brando, C.; Brumen, G.; Carvalhaes, N.; Kölling-Speer, I.; Speer, K.; Suggi Liverani, F.; Teixeira, A.A.; Teixeira, R.; Thomaziello, R.A.; et al. The raw bean. In Espresso Coffee: The Science of Quality, 2nd ed.; Illy, A., Viani, R., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 87–178. [Google Scholar]
- Sanz-Uribe, J.R.; Sunalini, N.M.; Penuela, A.; Oliveros, C.; Husson, J.; Brando, C.; Rodriguez, A. Post-harvest Processing—Revealing the Green Bean. In The Craft and Science of Coffee; Folmer, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 51–75. ISBN 978-0-12-803520-7. [Google Scholar]
- Nicoli, M.C.; Savonitti, O. Storage and packaging. In Espresso Coffee: The Science of Quality, 2nd ed.; Illy, A., Viani, R., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 230–258. [Google Scholar]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
- Spiro, M.; Hunter, J.E. The kinetics and mechanism of caffeine infusion from coffee: The effect of roasting. J. Sci. Food Agric. 1985, 36, 871–876. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Navarini, L.; Rivetti, D. Water quality for Espresso coffee. Food Chem. 2010, 122, 424–428. [Google Scholar] [CrossRef]
- Hendon, C.H.; Colonna-Dashwood, L.; Colonna-Dashwood, M. The role of dissolved cations in coffee extraction. J. Agric. Food Chem. 2014, 62, 4947–4950. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the Aroma Profile and Main Key Odorants of Espresso Coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Stadler, R.H.; Varga, N.; Hau, J.; Vera, F.A.; Welti, D.H. Alkylpyridiniums. 1. Formation in model systems via thermal degradation of trigonelline. J. Agric. Food Chem. 2002, 50, 1192–1199. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Angeloni, S.; Giacomini, J.; Maponi, P.; Perticarini, A.; Vittori, S.; Cognigni, L.; Fioretti, L. Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives. Appl. Sci. 2023, 13, 2688. [Google Scholar] [CrossRef]
- Kuhn, M.; Lang, S.; Bezold, F.; Minceva, M.; Briesen, H. Time-resolved extraction of caffeine and trigonelline from finely-ground espresso coffee with varying particle sizes and tamping pressures. J. Food Eng. 2017, 206, 37–47. [Google Scholar] [CrossRef]
- Andueza, S.; de Peña, M.P.; Cid, C. Chemical and sensorial characteristics of espresso coffee as affected by grinding and torrefacto roast. J. Agric. Food Chem. 2003, 51, 7034–7039. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Wetzel, C.R.; Grand, A.N. Caffeine content in coffee as influenced by grinding and brewing techniques. Food Res. Int. 1996, 29, 785–789. [Google Scholar] [CrossRef]
- Spiro, M.; Selwood, R.M. The kinetics and mechanism of caffeine infusion from coffee: The effect of particle size. J. Sci. Food Agric. 1984, 35, 915–924. [Google Scholar] [CrossRef]
- Andueza, S.; Vila, M.A.; Paz de Peña, M.; Cid, C. Influence of coffee/water ratio on the final quality of espresso coffee. J. Sci. Food Agric. 2007, 87, 586–592. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Borsetta, G.; Xiao, J.; Maggi, F.; Sagratini, G.; Vittori, S.; Caprioli, G. Optimization of espresso coffee extraction through variation of particle sizes, perforated disk height and filter basket aimed at lowering the amount of ground coffee used. Food Chem. 2020, 314, 126220. [Google Scholar] [CrossRef]
- Fasano, A.; Talamucci, F.; Petracco, M. The Espresso Coffee Problem. In Complex Flows in Industrial Processes; Birkhäuser: Boston, MA, USA, 2000; pp. 241–280. [Google Scholar]
- Bear, J. Modeling Phenomena of Flow and Transport in Porous Media; Springer: Cham, Switzerland, 2018; pp. 263–282. ISBN 9783319728254. [Google Scholar]
- Giacomini, J.; Khamitova, G.; Maponi, P.; Vittori, S.; Fioretti, L. Water flow and transport in porous media for in-silico espresso coffee. Int. J. Multiph. Flow 2020, 126, 103252. [Google Scholar] [CrossRef]
- Andueza, S.; Maeztu, L.; Dean, B.; de Peña, M.P.; Bello, J.; Cid, C. Influence of water pressure on the final quality of arabica espresso coffee. Application of multivariate analysis. J. Agric. Food Chem. 2002, 50, 7426–7431. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: The influence of espresso machines and coffee cultivars. Int. J. Food Sci. Nutr. 2014, 65, 465–469. [Google Scholar] [CrossRef]
- Lee, W.T.; Smith, A.; Arshad, A. Uneven extraction in coffee brewing. Phys. Fluids 2023, 35, 054110. [Google Scholar] [CrossRef]
- Mestdagh, F.; Glabasnia, A.; Giuliano, P. The Brew—Extracting for Excellence. In The Craft and Science of Coffee; Elsevier: Amsterdam, The Netherlands, 2017; pp. 355–380. ISBN 9780128035207. [Google Scholar]
- Albanese, D.; Di Matteo, M.; Poiana, M.; Spagnamusso, S. Espresso coffee (EC) by POD: Study of thermal profile during extraction process and influence of water temperature on chemical–physical and sensorial properties. Food Res. Int. 2009, 42, 727–732. [Google Scholar] [CrossRef]
- Masella, P.; Guerrini, L.; Spinelli, S.; Calamai, L.; Spugnoli, P.; Illy, F.; Parenti, A. A new espresso brewing method. J. Food Eng. 2015, 146, 204–208. [Google Scholar] [CrossRef]
- Andueza, S.; Maeztu, L.; Pascual, L.; Ibáñez, C.; de Peña, M.P.; Cid, C. Influence of extraction temperature on the final quality of espresso coffee. J. Sci. Food Agric. 2003, 83, 240–248. [Google Scholar] [CrossRef]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; de Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Matias, A.F.V.; Valente-Matias, D.F.; Neng, N.R.; Nogueira, J.M.F.; Andrade, J.S.; Coelho, R.C.V.; Araújo, N.A.M. Continuum model for extraction and retention in porous media. arXiv 2023, arXiv:2304.03161. [Google Scholar]
- Severini, C.; Derossi, A.; Fiore, A.G.; de Pilli, T.; Alessandrino, O.; Del Mastro, A. How the variance of some extraction variables may affect the quality of espresso coffees served in coffee shops. J. Sci. Food Agric. 2016, 96, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Hemro International AG. Mahlkoenig_E65S_Espresso_Grinder_Product_Brochure_DE. Available online: https://downloads.mahlkoenig.de/Products/Mahlkoenig_E65S_Espresso_Grinder_Product_Brochure_DE.pdf (accessed on 4 April 2023).
- Deutsches Institut für Normung e.V. Analysis of Coffee and Coffee Products—Determination of Water-Soluble Extract—Method for Roasted Coffee; Beuth: Berlin, Germany, 2016. [Google Scholar]
- Moroney, K.M.; Lee, W.T.; O’Brien, S.; Suijver, F.; Marra, J. Modelling of coffee extraction during brewing using multiscale methods: An experimentally validated model. Chem. Eng. Sci. 2015, 137, 216–234. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. In Breakthroughs in Statistics; Springer: New York, NY, USA, 1992; pp. 270–310. [Google Scholar]
- Fahrmeir, L.; Kneib, T.; Lang, S.; Marx, B.D. Regression: Models, Methods and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 165–167. [Google Scholar]
- Vaca Guerra, M.; Harshe, Y.M.; Fries, L.; Pietsch-Braune, S.; Palzer, S.; Heinrich, S. Tuning the packed bed configuration for selective extraction of espresso non-volatiles based on polarity. J. Food Eng. 2023, 354. [Google Scholar] [CrossRef]
- Sánchez López, J.A.; Wellinger, M.; Gloess, A.N.; Zimmermann, R.; Yeretzian, C. Extraction kinetics of coffee aroma compounds using a semi-automatic machine: On-line analysis by PTR-ToF-MS. Int. J. Mass Spectrom. 2016, 401, 22–30. [Google Scholar] [CrossRef]
- Frank, O.; Blumberg, S.; Kunert, C.; Zehentbauer, G.; Hofmann, T. Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J. Agric. Food Chem. 2007, 55, 1945–1954. [Google Scholar] [CrossRef]
- Trugo, L.C.; Macrae, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chem. 1984, 15, 219–227. [Google Scholar] [CrossRef]
- Cameron, M.I.; Morisco, D.; Hofstetter, D.; Uman, E.; Wilkinson, J.; Kennedy, Z.C.; Fontenot, S.A.; Lee, W.T.; Hendon, C.H.; Foster, J.M. Systematically improving Espresso: Insights from mathematical modeling and experiment. Matter 2020, 2, 631–648. [Google Scholar] [CrossRef]




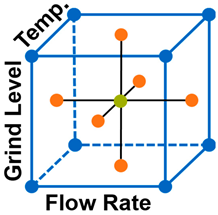 | Experiment | Replicates | Flow Rate (mL s−1) | Grinding Level (−) | Temp. (°C) | |
|---|---|---|---|---|---|---|
| DoE Axis Points | 1 | 3 | 1.0 | 1.7 | 89 | |
| 2 | 3 | 3.0 | 1.7 | 89 | ||
| 3 | 3 | 2.0 | 1.4 | 89 | ||
| 4 | 3 | 2.0 | 2.0 | 89 | ||
| 5 | 3 | 2.0 | 1.7 | 80 | ||
| 6 | 3 | 2.0 | 1.7 | 98 | ||
| DoE CP | 7 | 6 | 2.0 | 1.7 | 89 | |
| DoE Corner Points | 8 | 3 | 1.0 | 1.4 | 80 | |
| 9 | 3 | 1.0 | 1.4 | 98 | ||
| 10 | 3 | 1.0 | 2.0 | 80 | ||
| 11 | 3 | 1.0 | 2.0 | 98 | ||
| 12 | 3 | 3.0 | 1.4 | 80 | ||
| 13 | 3 | 3.0 | 1.4 | 98 | ||
| 14 | 3 | 3.0 | 2.0 | 80 | ||
| 15 | 3 | 3.0 | 2.0 | 98 |
 | Experiment | Flow Rate (mL s−1) | Grinding Level (−) | Temp. (°C) | Replicates | Mass in Cup; (RSD) | Pressure (Bar) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigonelline (mg) | Caffeine (mg) | 5-CQA (mg) | TDS (g) | |||||||||||||||
| Brew Ratio | Brew Ratio | Brew Ratio | Brew Ratio | |||||||||||||||
| 1/1 | 1/2 | 1/3 | 1/1 | 1/2 | 1/3 | 1/1 | 1/2 | 1/3 | 1/1 | 1/2 | 1/3 | |||||||
| DOE Axis Points | 1 | 1.0 | 1.7 | 87.3 | 3 | 81.2 | 102.1 | 107.5 | 134.0 | 184.6 | 203.7 | 90.8 | 122.7 | 133.9 | 3.20 | 4.10 | 4.35 | 2.7 |
| (7.5) | (0.2) | (1.1) | (1.1) | (1.2) | (1.9) | (2.4) | (2.7) | (1.7) | (2.1) | (2.3) | (0.7) | (0.6) | (0.6) | (4.0) | ||||
| 2 | 2.8 | 1.7 | 88.2 | 3 | 74.9 | 97.1 | 103.7 | 129.4 | 183.9 | 208.2 | 82.3 | 114.4 | 126.9 | 2.87 | 3.81 | 4.12 | 5.3 | |
| (1.0) | (0.3) | (1.9) | (2.4) | (2.8) | (3.6) | (2.9) | (2.8) | (3.3) | (3.3) | (3.3) | (1.7) | (1.5) | (1.3) | (35.3) | ||||
| 3 | 1.9 | 1.4 | 88.5 | 3 | 72.2 | 91.1 | 96.0 | 126.9 | 177.9 | 198.4 | 81.3 | 110.5 | 121.1 | 2.81 | 3.65 | 3.91 | 3.9 | |
| (0.3) | (0.1) | (2.8) | (2.9) | (3.4) | (1.6) | (2.7) | (3.6) | (3.7) | (4.1) | (4.6) | (2.4) | (3.0) | (3.9) | (5.1) | ||||
| 4 | 2.0 | 2.0 | 88.6 | 3 | 78.0 | 100.3 | 106.7 | 129.4 | 183.7 | 206.7 | 89.0 | 123.0 | 136.1 | 2.98 | 3.92 | 4.22 | 3.3 | |
| (0.2) | (0.1) | (2.0) | (1.9) | (1.9) | (0.7) | (1.0) | (1.1) | (0.5) | (0.3) | (0.4) | (0.5) | (0.4) | (0.3) | (3.4) | ||||
| 5 | 1.9 | 1.7 | 79.2 | 3 | 81.9 | 104.3 | 110.4 | 134.8 | 188.2 | 209.3 | 88.9 | 121.2 | 132.9 | 2.99 | 3.96 | 4.29 | 3.6 | |
| (0.7) | (0.1) | (1.8) | (2.1) | (2.6) | (5.3) | (3.3) | (2.0) | (3.1) | (1.3) | (0.6) | (1.7) | (3.1) | (4.0) | (7.7) | ||||
| 6 | 1.9 | 1.7 | 96.7 | 3 | 78.9 | 101.1 | 107.3 | 133.0 | 187.2 | 209.3 | 87.2 | 120.0 | 132.4 | 3.05 | 4.01 | 4.31 | 3.0 | |
| (1.0) | (0.2) | (1.9) | (1.6) | (1.9) | (3.5) | (3.5) | (3.6) | (4.4) | (4.0) | (3.8) | (2.4) | (1.8) | (1.8) | (4.6) | ||||
| DoE Central Point | 7 | 1.9 | 1.7 | 88.3 | 6 | 75.9 | 98.3 | 104.9 | 129.0 | 183.8 | 207.1 | 86.5 | 120.0 | 133.1 | 2.92 | 3.88 | 4.19 | 3.4 |
| (2.3) | (0.5) | (2.2) | (1.9) | (1.8) | (1.1) | (1.1) | (1.3) | (3.3) | (3.2) | (3.3) | (1.9) | (0.8) | (0.8) | (15.6) | ||||
| DOE Corner Points | 8 | 1.0 | 1.4 | 78.8 | 3 | 78.2 | 95.3 | 99.0 | 132.1 | 177.8 | 193.6 | 86.7 | 114.0 | 122.6 | 3.15 | 3.84 | 3.99 | 2.9 |
| (0.1) | (0.1) | (0.7) | (1.5) | (1.9) | (2.2) | (2.0) | (2.0) | (0.5) | (0.5) | (0.9) | (4.6) | (1.1) | (0.7) | (3.6) | ||||
| 9 | 0.9 | 1.4 | 96.2 | 3 | 80.1 | 102.2 | 108.4 | 139.8 | 195.2 | 217.2 | 92.6 | 126.4 | 138.8 | 3.22 | 4.18 | 4.46 | 2.8 | |
| (2.3) | (0.2) | (5.0) | (5.6) | (6.0) | (4.2) | (5.1) | (5.8) | (3.5) | (4.4) | (5.0) | (3.9) | (4.7) | (5.1) | (4.3) | ||||
| 10 | 1.0 | 2.0 | 78.7 | 3 | 78.0 | 96.6 | 101.1 | 127.0 | 172.9 | 189.6 | 87.5 | 117.0 | 126.9 | 2.92 | 3.72 | 3.94 | 2.9 | |
| (0.9) | (0.1) | (4.1) | (7.3) | (8.5) | (3.6) | (6.0) | (7.4) | (3.9) | (6.2) | (7.4) | (3.7) | (6.5) | (7.7) | (3.8) | ||||
| 11 | 0.9 | 2.0 | 96.1 | 3 | 83.9 | 106.1 | 111.9 | 135.3 | 187.8 | 208.2 | 94.9 | 129.0 | 141.3 | 3.19 | 4.11 | 4.38 | 2.6 | |
| (5.9) | (0.3) | (0.8) | (1.4) | (1.6) | (0.6) | (1.0) | (1.3) | (1.6) | (2.2) | (2.5) | (1.0) | (0.6) | (1.0) | (2.3) | ||||
| 12 | 2.7 | 1.4 | 79.8 | 3 | 69.0 | 88.3 | 93.8 | 120.4 | 172.4 | 195.0 | 77.1 | 106.9 | 118.4 | 2.67 | 3.53 | 3.81 | 8.4 | |
| (3.7) | (1.0) | (4.3) | (2.9) | (2.2) | (5.7) | (4.3) | (3.4) | (4.3) | (3.1) | (2.4) | (4.3) | (2.0) | (0.9) | (16.7) | ||||
| 13 | 2.7 | 1.4 | 97.1 | 3 | 72.8 | 93.2 | 99.0 | 129.2 | 184.0 | 207.2 | 83.0 | 114.4 | 126.3 | 2.89 | 3.83 | 4.13 | 7.6 | |
| (2.3) | (0.4) | (2.2) | (2.0) | (2.5) | (1.8) | (1.5) | (2.0) | (2.7) | (2.3) | (2.4) | (2.3) | (1.9) | (2.5) | (9.3) | ||||
| 14 | 2.9 | 2.0 | 79.1 | 3 | 75.1 | 97.8 | 104.7 | 122.6 | 176.3 | 199.8 | 81.8 | 115.5 | 128.7 | 2.75 | 3.68 | 3.99 | 3.6 | |
| (0.8) | (0.1) | (2.9) | (1.9) | (1.5) | (3.4) | (2.8) | (2.5) | (0.5) | (0.7) | (0.2) | (1.3) | (0.9) | (1.0) | (7.4) | ||||
| 15 | 2.8 | 2.0 | 96.4 | 3 | 76.2 | 98.9 | 105.7 | 127.5 | 182.7 | 206.6 | 86.9 | 120.7 | 131.4 | 2.93 | 3.88 | 4.19 | 3.5 | |
| (8.0) | (0.5) | (1.7) | (1.0) | (0.8) | (1.7) | (1.9) | (2.1) | (1.9) | (1.5) | (1.6) | (0.8) | (0.9) | (1.0) | (7.9) | ||||
| Average mass in cup: | 77.1 | 98.2 | 104.0 | 130.0 | 182.6 | 204.0 | 86.4 | 118.4 | 130.1 | 2.97 | 3.87 | 4.15 | ||||||
| Components | Brew Ratio | (mg) | (mg s mL−1) | (mg) | (mg °C−1) | (mg s2 mL−2) | (mg) | (mg °C−2) | (mg s mL−1) | (mg °C−1 s mL−1) | (mg °C−1) | R2 (adj.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigonelline | 1/1 | 185.8 | −9.17 | 105.90 | −4.48 | 0 | −30.96 | 0.03 | 3.24 | 0 | 0 | 0.66 |
| 1/2 | 134.6 | 4.28 | 163.33 | −4.40 | 0 | −47.80 | 0.03 | 4.92 | −0.18 | 0 | 0.60 | |
| 1/3 | 99.9 | 8.54 | 184.84 | −4.06 | 0 | −54.17 | 0.03 | 5.71 | −0.24 | 0 | 0.58 | |
| Caffeine | 1/1 | 192.0 | −13.85 | 118.83 | −3.59 | 0 | −38.45 | 0.02 | 5.52 | 0 | 0 | 0.50 |
| 1/2 | −9.2 | 2.92 | 149.27 | 0.97 | 0 | −48.37 | 0 | 7.57 | −0.21 | 0 | 0.41 | |
| 1/3 | −57.7 | 15.87 | 186.29 | 1.37 | 0 | −59.53 | 0 | 8.42 | −0.34 | 0 | 0.42 | |
| 5-CQA | 1/1 | 112.2 | −10.32 | 56.75 | −1.74 | 0 | −16.42 | 0.01 | 3.33 | 0 | 0 | 0.69 |
| 1/2 | −10.4 | 2.96 | 83.84 | 0.74 | 0 | −24.01 | 0 | 4.97 | −0.18 | 0 | 0.62 | |
| 1/3 | −54.7 | 13.94 | 119.15 | 1.02 | 0 | −34.02 | 0 | 4.93 | −0.30 | 0 | 0.57 | |
| (g) | (g s mL−1) | (g) | (g °C−1) | (g s2 mL−2) | (g) | (g °C−2) | (g s mL−1) | (g °C−1 s mL−1) | (g °C−1) | |||
| TDS | 1/1 | 3.65 | −0.70 | 2.78 | −0.06 | 0.05 | −0.91 | 0.001 | 0.19 | 0 | 0 | 0.75 |
| 1/2 | 4.19 | −0.50 | 4.75 | −0.10 | 0 | −1.48 | 0.001 | 0.21 | 0 | 0 | 0.64 | |
| 1/3 | 2.91 | 0.12 | 6.16 | −0.11 | 0 | −1.87 | 0.001 | 0.20 | −0.01 | 0 | 0.57 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmieder, B.K.L.; Pannusch, V.B.; Vannieuwenhuyse, L.; Briesen, H.; Minceva, M. Influence of Flow Rate, Particle Size, and Temperature on Espresso Extraction Kinetics. Foods 2023, 12, 2871. https://doi.org/10.3390/foods12152871
Schmieder BKL, Pannusch VB, Vannieuwenhuyse L, Briesen H, Minceva M. Influence of Flow Rate, Particle Size, and Temperature on Espresso Extraction Kinetics. Foods. 2023; 12(15):2871. https://doi.org/10.3390/foods12152871
Chicago/Turabian StyleSchmieder, Benedikt K. L., Verena B. Pannusch, Lara Vannieuwenhuyse, Heiko Briesen, and Mirjana Minceva. 2023. "Influence of Flow Rate, Particle Size, and Temperature on Espresso Extraction Kinetics" Foods 12, no. 15: 2871. https://doi.org/10.3390/foods12152871
APA StyleSchmieder, B. K. L., Pannusch, V. B., Vannieuwenhuyse, L., Briesen, H., & Minceva, M. (2023). Influence of Flow Rate, Particle Size, and Temperature on Espresso Extraction Kinetics. Foods, 12(15), 2871. https://doi.org/10.3390/foods12152871