Comparison of Caffeoylquinic Acids and Functional Properties of Domestic Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots with Established Overseas Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction
2.4. Total Phenols
2.5. Quantification of Different Phenolic Compounds
2.6. Antioxidant Properties
2.7. Statistical Analysis
3. Results and Discussion
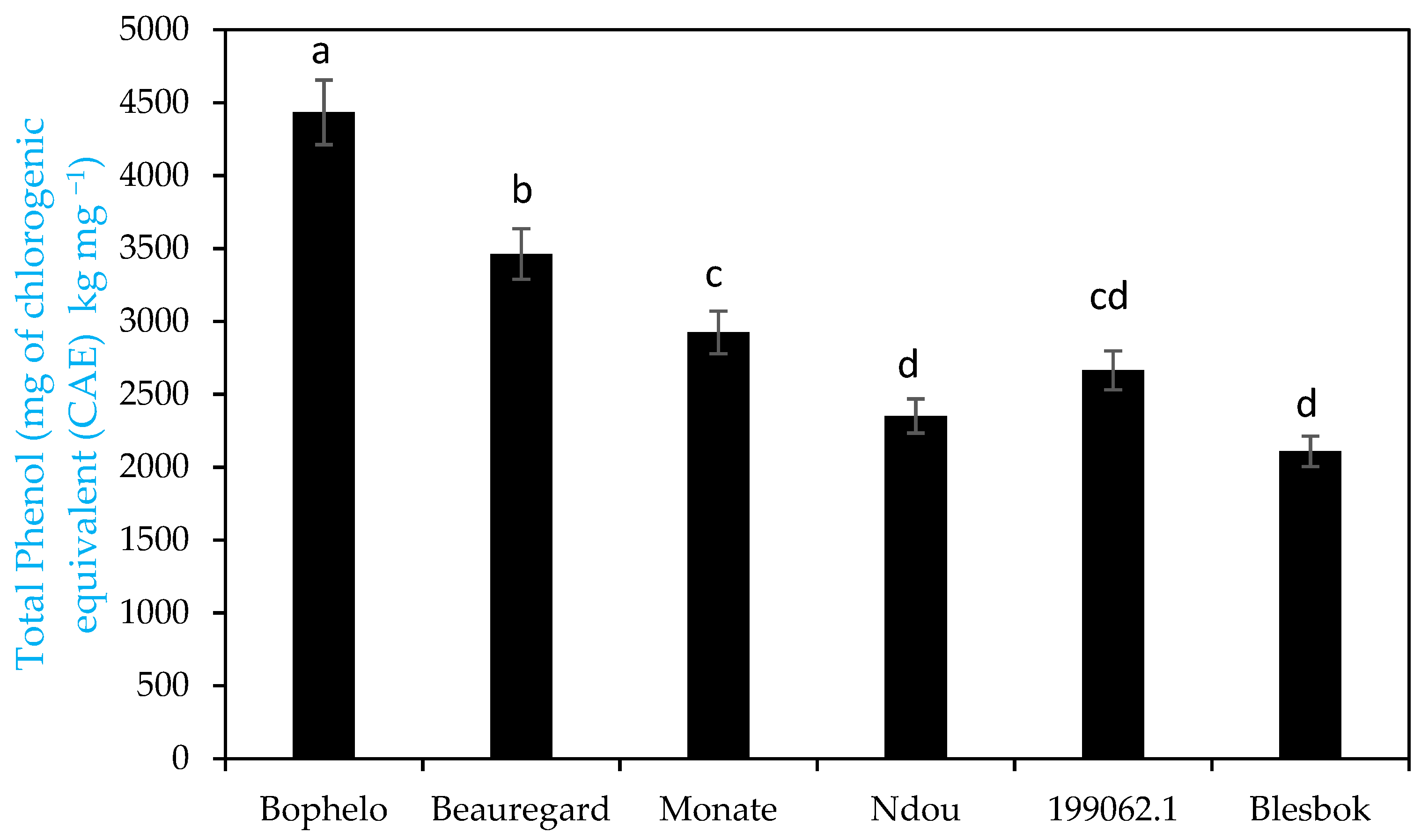
3.1. Total Phenols
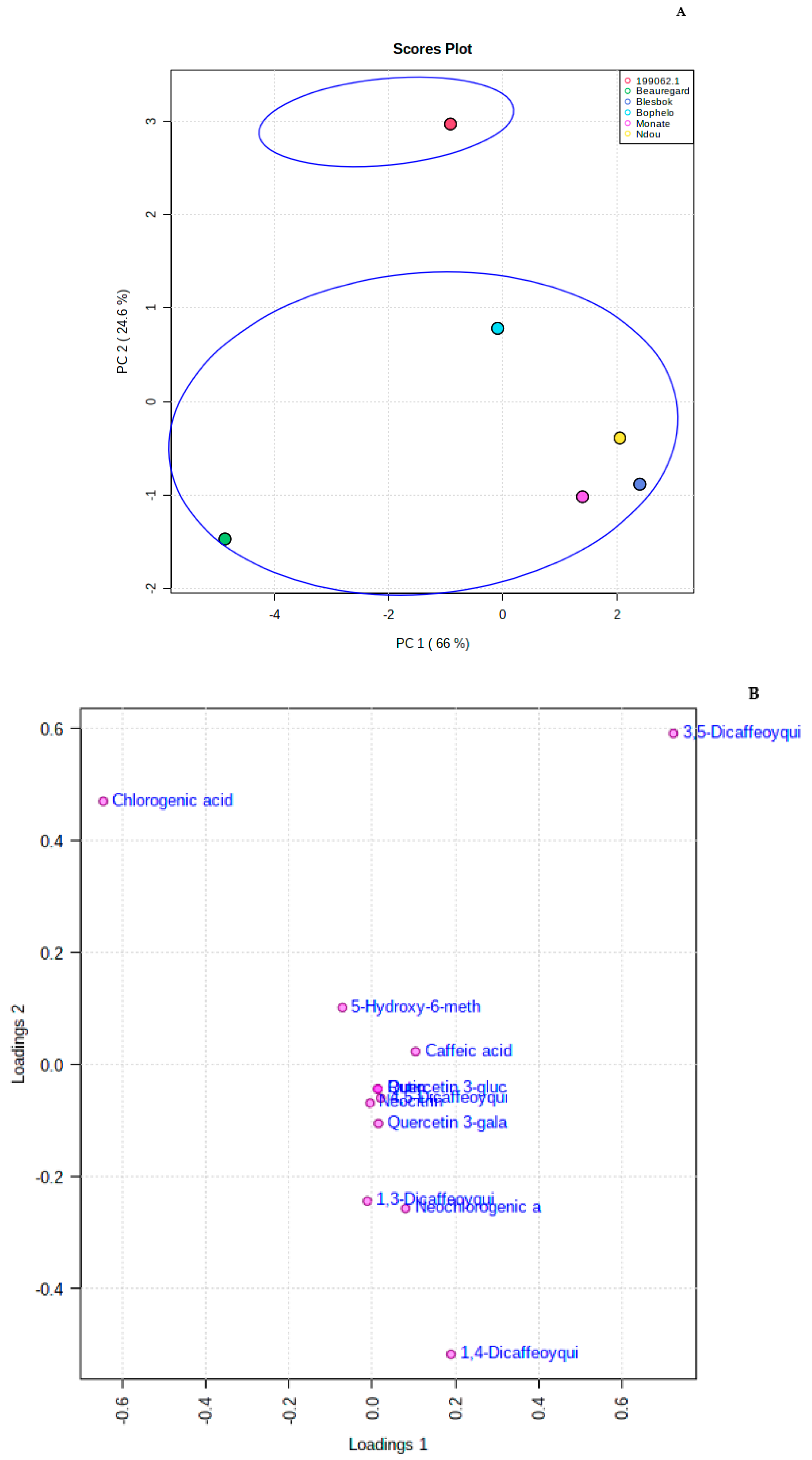
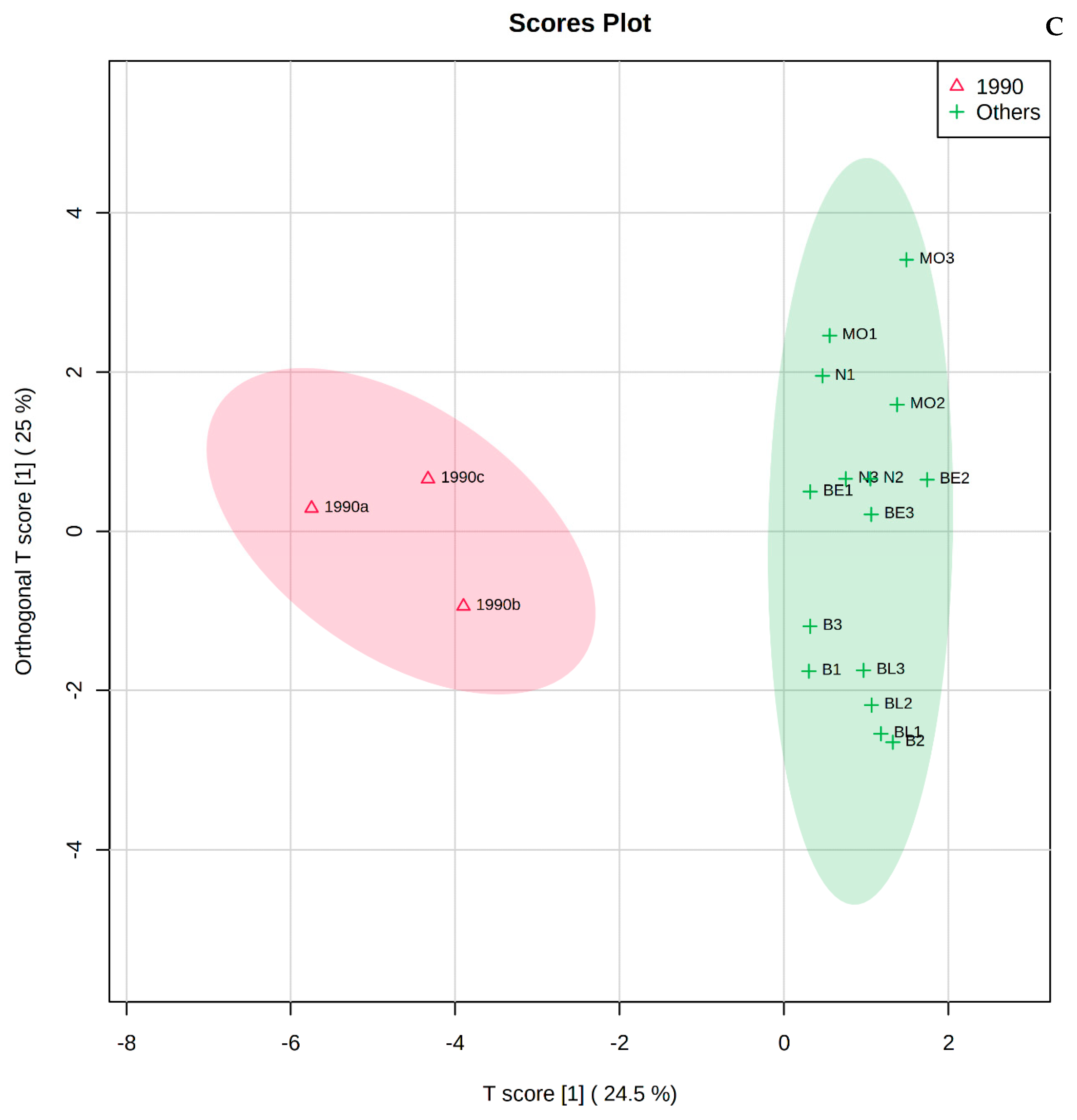
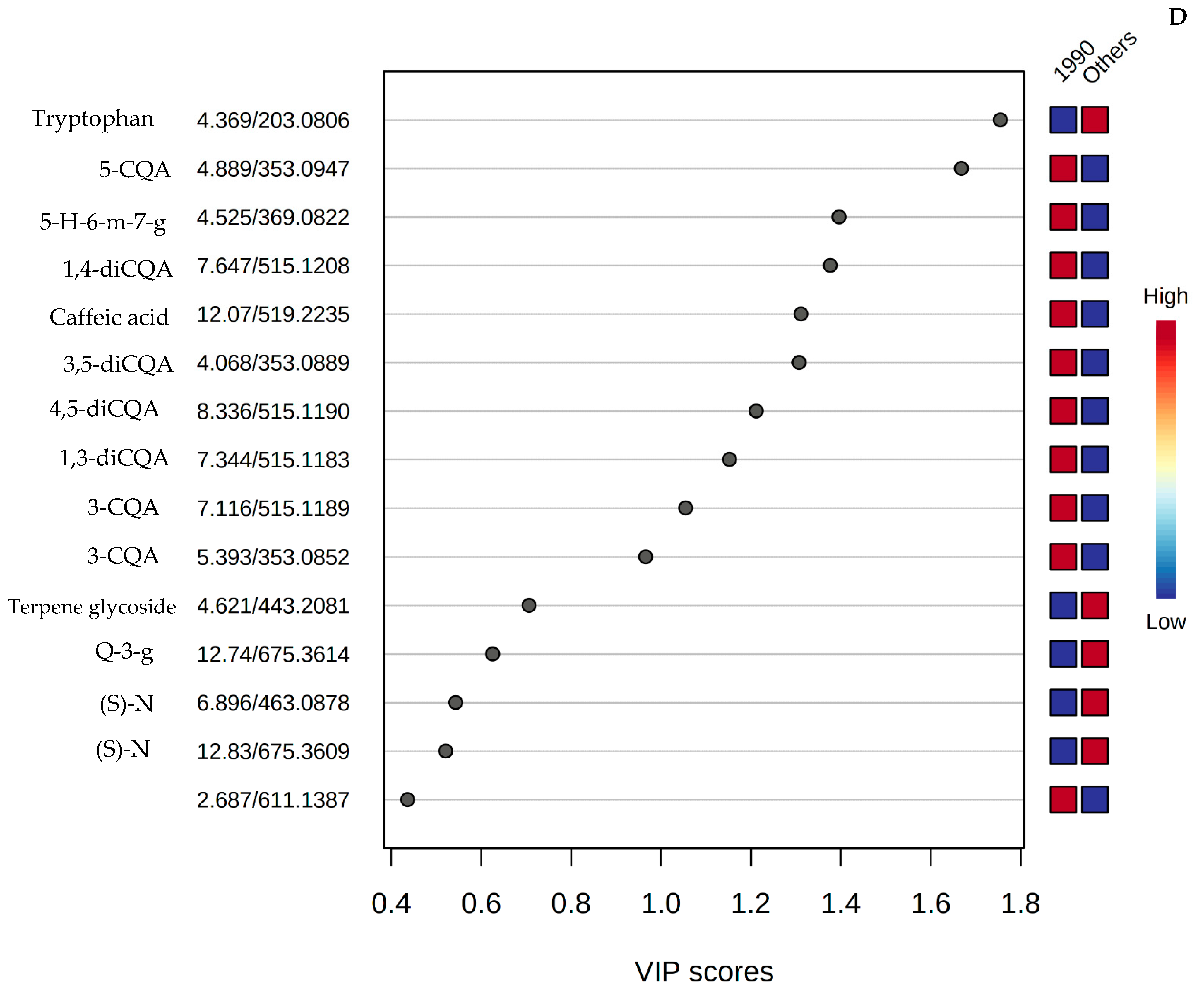
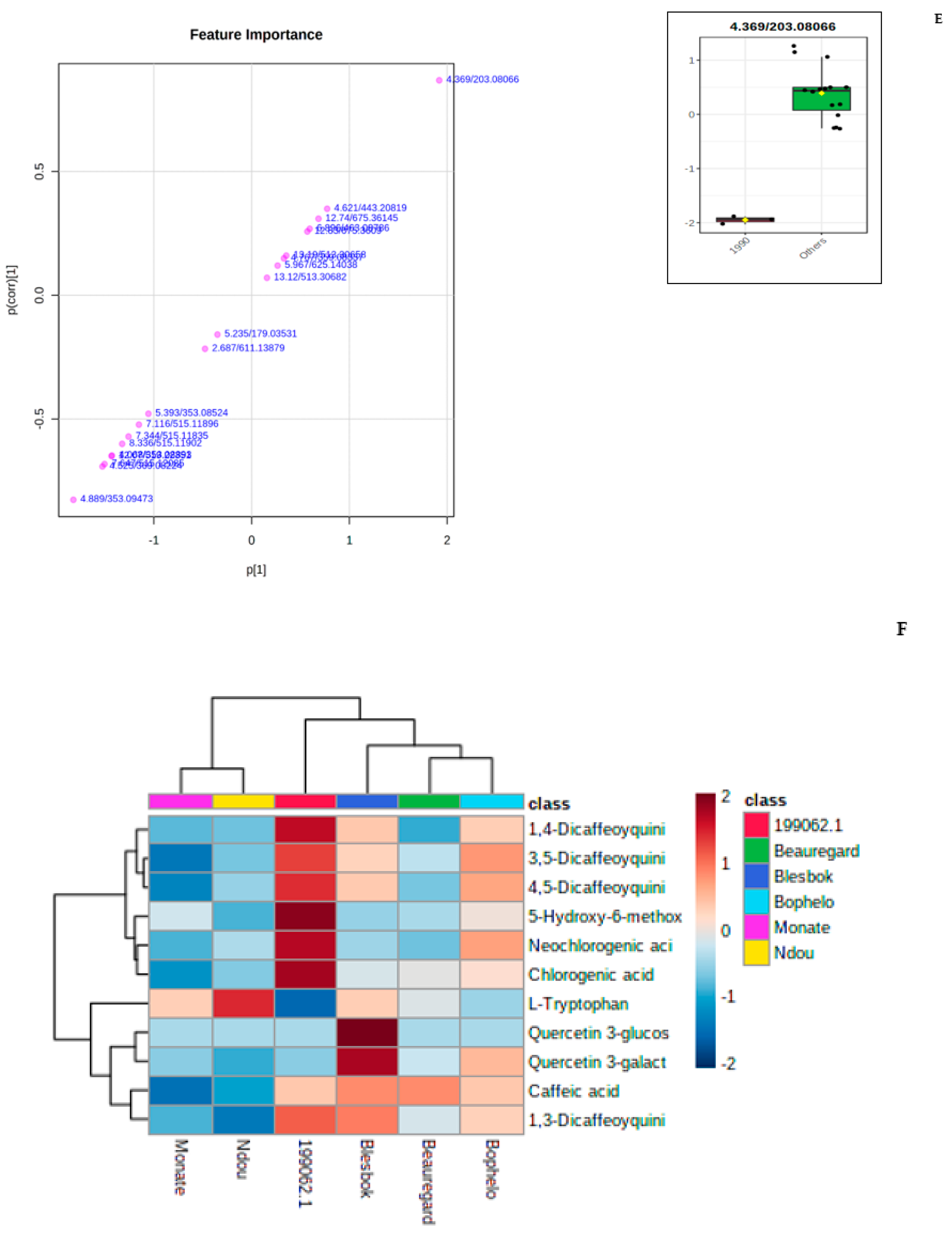
3.2. Untargeted Metabolitle Profile
3.3. Metabolomic and Chemometric Profiles
3.4. Quantified Concentrations of Phenolic Compounds
3.5. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yarmento, M.; Meamea, K. Effects of severity of apical shoot harvest on growth and tuber yield of two sweet potatoes varieties. Afr. J. Plant Sci. 2020, 14, 83–101. [Google Scholar] [CrossRef]
- Low, J.W.; Ortiz, R.; Vandamme, E.; Andrade, M.; Biazin, B.; Grüneberg, W.J. Nutrient-dense orange-fleshed Sweet potato: Advances in drought-tolerance breeding and understanding of management practices for sustainable next-generation cropping systems in sub-Saharan Africa. Front. Sustain. Food Syst. 2020, 4, 50. [Google Scholar] [CrossRef]
- Department of Agriculture Forestry and Fisheries (DAFF) Report. A Profile of the South African Sweet Potato Market Value Chain. Available online: http://webapps.daff.gov.za/AmisAdmin/upload/Sweet%20Potato%20Market%20Value%20Chain%20Profile%202019.pdf (accessed on 2 April 2019).
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.K.; Lee, S.U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Chapter 7-chlorogenic acids from sweet potato. In Sweet Potato Processing Technology, 1st ed.; Mu, T., Sun, H., Zhang, M., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 357–403. [Google Scholar]
- Wilson, W.D.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; Meester, F.D.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Noriega, L.G.; Delgadillo-Puga, C.; Tovar, A.N.; Navarro-Ocaña, A. Caffeoylquinic acid derivatives of purple sweet potato as modulators of mitochondrial function in mouse primary hepatocytes. Molecules. 2021, 26, 319. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Quantification of phenolic acids and antioxidant activity in sweet potato genotypes. Sci. Hortic. 2008, 119, 17–20. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference, Legacy Release. 2022. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 3 April 2022).
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the academy of nutrition and dietetics: Health implications of dietary fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef]
- Laurie, S.M.; Mtileni, M.M.; Mphela, W.M.; Tjale, S.S. Performance of informal market sweet potato cultivars in on-farm trials in South Africa. Open Agric. 2017, 2, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Nyathi, M.K.; Du Plooy, C.P.; Van Halsema, T.J.; Stomph, T.J.; Annandale, J.G.; Struik, P.C. The dual-pupose use of orange-fleshed sweet potato (Ipomoea batatas var. Bophelo) for improved nutritional food security. Agric. Water Manag. 2019, 21, 723–737. [Google Scholar] [CrossRef]
- Phahlane, C.J.; Laurie, S.M.; Shoko, T.; Manhivi, V.E.; Sivakuamar, D. An Evaluation of phenolic compounds, carotenoids, and antioxidant properties in leaves of South African cultivars, Peruvian 199062.1 and USA’s Beauregard. Front. Nutr. 2021, 8, 773550. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Seke, F.; Manhivi, V.E.; Shoko, T.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Effect of freeze drying and simulated gastrointestinal digestion on phenolic metabolites and antioxidant property of the Natal plum (Carissa macrocarpa). Foods 2021, 6, 1420. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Manhivi, V.; Shai, J.L.; Slabbert, R.M.; Sivakumar, D. Influence of different types of drying methods on color properties, phenolic metabolites and bioactivities of pumpkin leaves of var. Butternut squash (Cucurbita moschata Duchesne ex Poir) Front. Nutr. 2021, 8, 694649. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Commun. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Usuki, T.; Onda, S.; Yoshizawa-Fujita, M.; Rikukawa, M. Use of [C4mim]Cl for efficient extraction of caffeoylquinic acids from sweet potato leaves. Sci. Rep. 2017, 7, 6890. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.V.; Santana, L.F.; Diogenes, V.; da Silva, A.; Costa, L.; Zambotti-Villelae, L.; Colepicolo, P.; Ferraz, C.P.; Ribeiro, P.R. Combination of a multiplatform metabolite profiling approach and chemometrics as a powerful strategy to identify bioactive metabolites in Lepidium meyenii (Peruvian maca). Food Chem. 2021, 364, 130453. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, C. Antioxidant activities, phenolic and b-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional differences of phenolic compounds between black currant (Ribes nigrum L.) cultivars and their response to latitude and weather conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L.A. Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweet potato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef]
- Rabah, I.O.; Hou, D.X.; Komine, S.I.; Fujii, M. Potential chemopreventive properties of extract from baked sweet potato (Ipomoea batatas lam. Cv. Koganesengan). J. Agric. Food Chem. 2004, 23, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Padda, M.S.; Picha, D.H. Antioxidant activity and phenolic composition in ‘Beauregard’ sweet potato are affected by root size and leaf age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Yoshimoto, M.; Ishigure, K.; Okuno, S.; Yamakawa, O. Effect of artificial shading and temperature on radical scavenging activity and polyphenolic composition in sweet potato (Ipomoea batatas L.) leaves. J. Am. Soc. Hortic. 2003, 128, 182–187. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Lee, H.J.; Park, K.H.; Moon, J.H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, J.Y.; Lee, Y.G.; Lee, H.J.; Shim, H.J.; Lee, J.H.; Kim, S.J.; Ham, K.S.; Moon, J.H. Four new dicaffeoylquinic acid derivatives from glasswort (Salicornia herbacea L.) and their antioxidative activity. Molecules 2016, 21, 1097. [Google Scholar] [CrossRef] [Green Version]

| Phenolic Compounds (mg kg−1) | Bophelo | Beauregard | Monate | 199062.1 | Ndou | Blesbok |
|---|---|---|---|---|---|---|
| Neochlorogenic acid (5-CQA) | 6.39 ± 0.23 b | 1.91 ± 0.02 c | 1.46 ± 0.43 c | 9.60 ± 0.23 a | 2.93 ± 1.14 c | 2.69 ± 0.21 c |
| 5-Hydroxy-6-methoxycoumarin 7-glucoside | 0.44 ± 0.07 b | 0.33 ± 0.06 b | 0.39 ± 0.16 b | 1.04 ± 0.05 a | 0.22 ± 0.07 b | 0.30 ± 0.04 b |
| Chlorogenic acid (3-CQA) | 24.78 ± 0.14 b | 23.51 ± 0.50 c | 16.90 ± 0.17 e | 35.14 ± 0.45 a | 20.10 ± 0.45 d | 23.10 ± 0.47 c |
| Eriodictyol 7-O-neohesperidoside (Neoeriocitrin) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.01 a | 0.01 ± 0.01 a |
| Caffeic acid | 0.25 ± 0.05 b | 0.30 ± 0.02 a | 0.14 ± 0.02 | 0.27 ± 0.04 a | 0.17 ± 0.05 c | 0.28 ± 0.04 a |
| Quercetin 3-glucosyl-(1->2)-galactoside | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a |
| Quercetin-3-O-rutinoside (Rutin) | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a |
| Quercetin 3-galactoside | 0.16 ± 0.04 ab | 0.09 ± 0.02 b | 0.07 ± 0.02 b | 0.05 ± 0.01 b | 0.03 ± 0.01 c | 0.24 ± 0.05 a |
| 1,3-Dicaffeoyquinic acid (1,3-diCQA) | 30.79 ± 0.70 b | 23.79 ± 0.29 c | 19.37 ± 0.82 d | 31.97 ± 0.26 a | 16.03 ± 0.78 e | 26.83 ± 0.28 c |
| 1,4-Dicaffeoyquinic acid (1,4-diCQA) | 12.95 ± 0.80 b | 4.93 ± 0.16 c | 5.76 ± 0.39 c | 21.40 ± 0.93 a | 5.93 ± 3.01 c | 13.27 ± 0.31 b |
| 3,5-Dicaffeoyquinic acid (3,5-diCQA) | 31.52 ± 0.17 b | 21.00 ± 0.47 d | 10.02 ± 0.56 f | 36.77 ± 0.63 a | 16.57 ± 8.30 e | 26.83 ± 0.39 c |
| 4,5-Dicaffeoyquinic acid (4,5-diCQA) | 0.70 ± 0.11 a | 0.39 ± 0.08 d | 0.28 ± 0.03 d | 0.86 ± 0.08 a | 0.43 ± 0.03 c | 0.62 ± 0.07 b |
| Orange Fleshed Storage Roots | Cream Fleshed Roots | |||||
|---|---|---|---|---|---|---|
| ‘Bophelo’ | ‘Beauregard’ | ‘199062.1′ | ‘Monate’ | ‘Ndou’ | ‘Blesbok’ | |
| FRAP (µM TEAC g−1) | 525.63 ± 1.09 b | 459.53 ± 1.84 c | 532.06 ± 7.87 a | 184.20 ± 1.51 f | 231.58 ± 3.27 e | 272.82 ± 1.51 d |
| DPPH (IC50 mg mL−1) | 4.27 ± 0.10 b | 4.42 ± 0.03 b | 4.60 ± 0.03 b | 6.39 ± 0.02 a | 6.05 ± 0.10 a | 6.80 ± 0.02 a |
| ABTS (IC50 mg mL−1) | 4.32 ± 0.20 b | 4.32 ± 0.20 b | 4.73 ± 0.5 b | 6.67 ± 0.04 a | 6.12 ± 0.30 a | 6.90 ± 0.12 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phahlane, C.J.; Laurie, S.M.; Shoko, T.; Manhivi, V.E.; Sivakumar, D. Comparison of Caffeoylquinic Acids and Functional Properties of Domestic Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots with Established Overseas Varieties. Foods 2022, 11, 1329. https://doi.org/10.3390/foods11091329
Phahlane CJ, Laurie SM, Shoko T, Manhivi VE, Sivakumar D. Comparison of Caffeoylquinic Acids and Functional Properties of Domestic Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots with Established Overseas Varieties. Foods. 2022; 11(9):1329. https://doi.org/10.3390/foods11091329
Chicago/Turabian StylePhahlane, Charmaine J., Sunette M. Laurie, Tinotenda Shoko, Vimbainashe E. Manhivi, and Dharini Sivakumar. 2022. "Comparison of Caffeoylquinic Acids and Functional Properties of Domestic Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots with Established Overseas Varieties" Foods 11, no. 9: 1329. https://doi.org/10.3390/foods11091329
APA StylePhahlane, C. J., Laurie, S. M., Shoko, T., Manhivi, V. E., & Sivakumar, D. (2022). Comparison of Caffeoylquinic Acids and Functional Properties of Domestic Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots with Established Overseas Varieties. Foods, 11(9), 1329. https://doi.org/10.3390/foods11091329







